A Composite Vase Solution Using Silicon (Si) and Other Preservatives Improved the Vase Quality of Cut Lily (Lilium ‘Siberia’) Flowers
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and General Processing
2.2. Treatments and Experimental Design
2.3. Flowering Stage Definitions and Observations
2.4. Daily Fresh Weight Change Measurements and Maximum Flower Diameter Determinations
2.5. Evaluations of Antioxidant Defense System of Cut Lily Flowers
2.5.1. Non-Enzymatic Antioxidant Compounds
2.5.2. Major Antioxidant Enzymes and ROS
2.6. Statistics and Graphs
3. Results
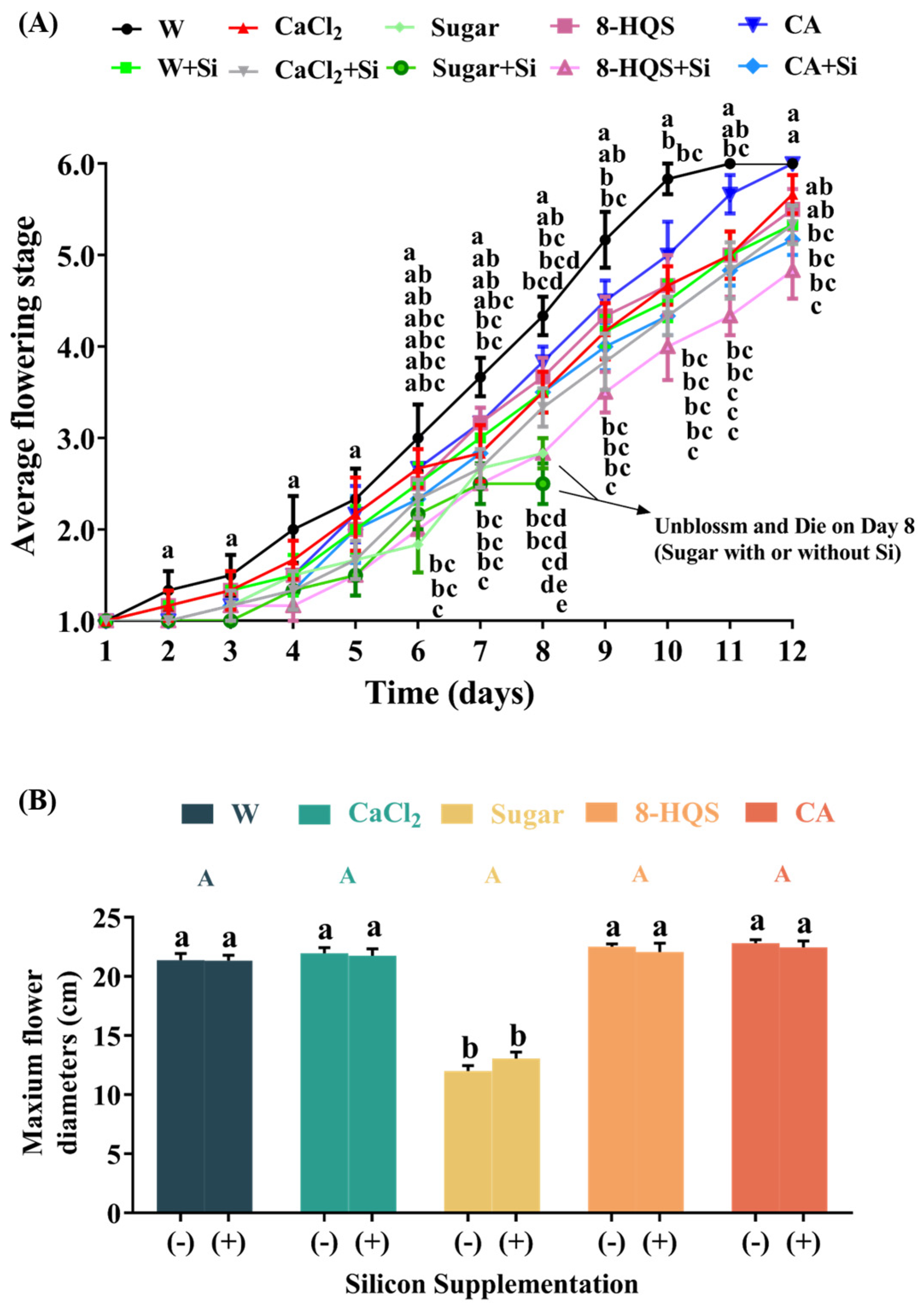
3.1. Vase Life and Maximum Flowering Diameter as Affected by Composite Vase Solutions
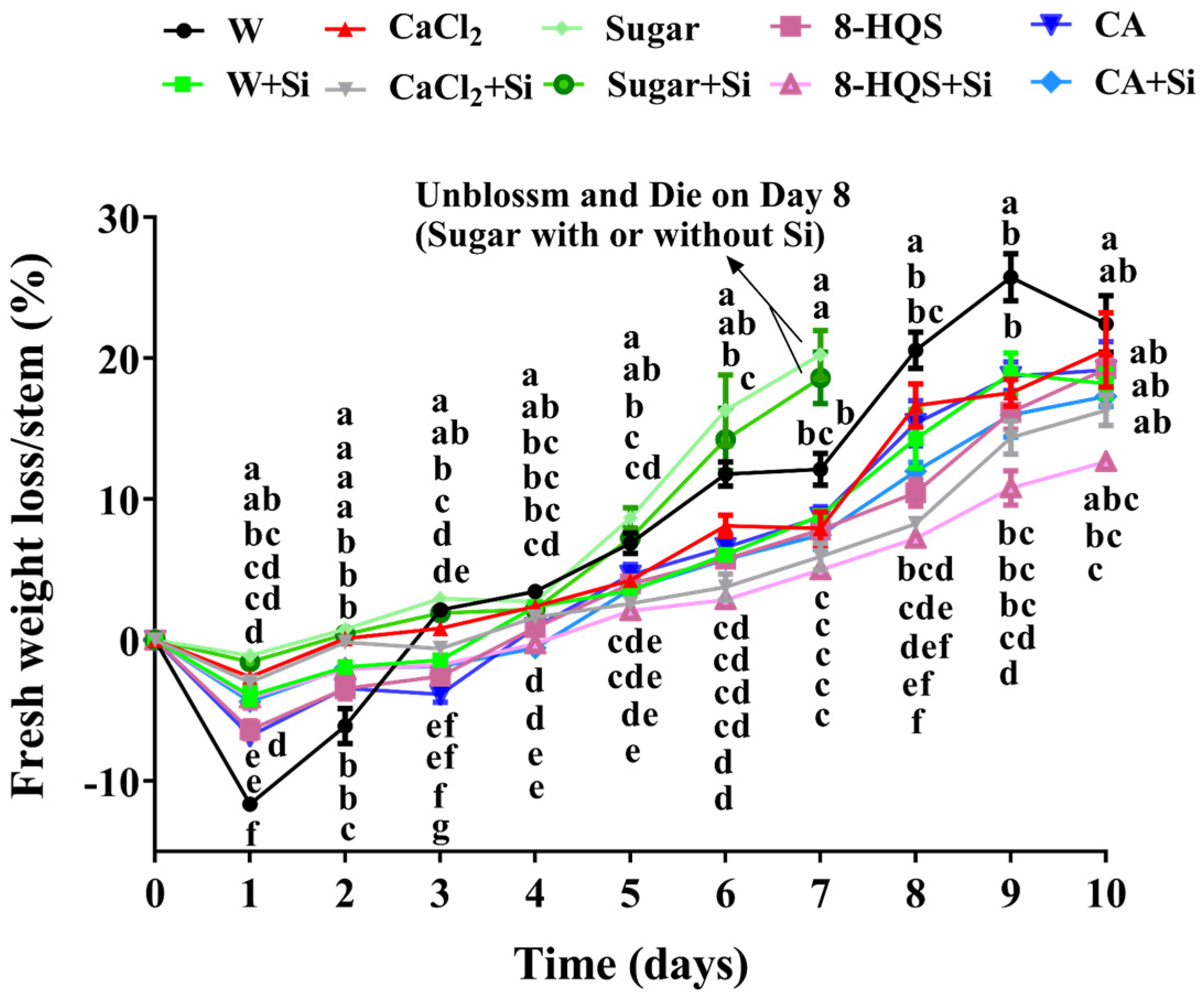
3.2. Fresh Weight Loss During Vase Life Was Delayed by Si and Other Preservatives
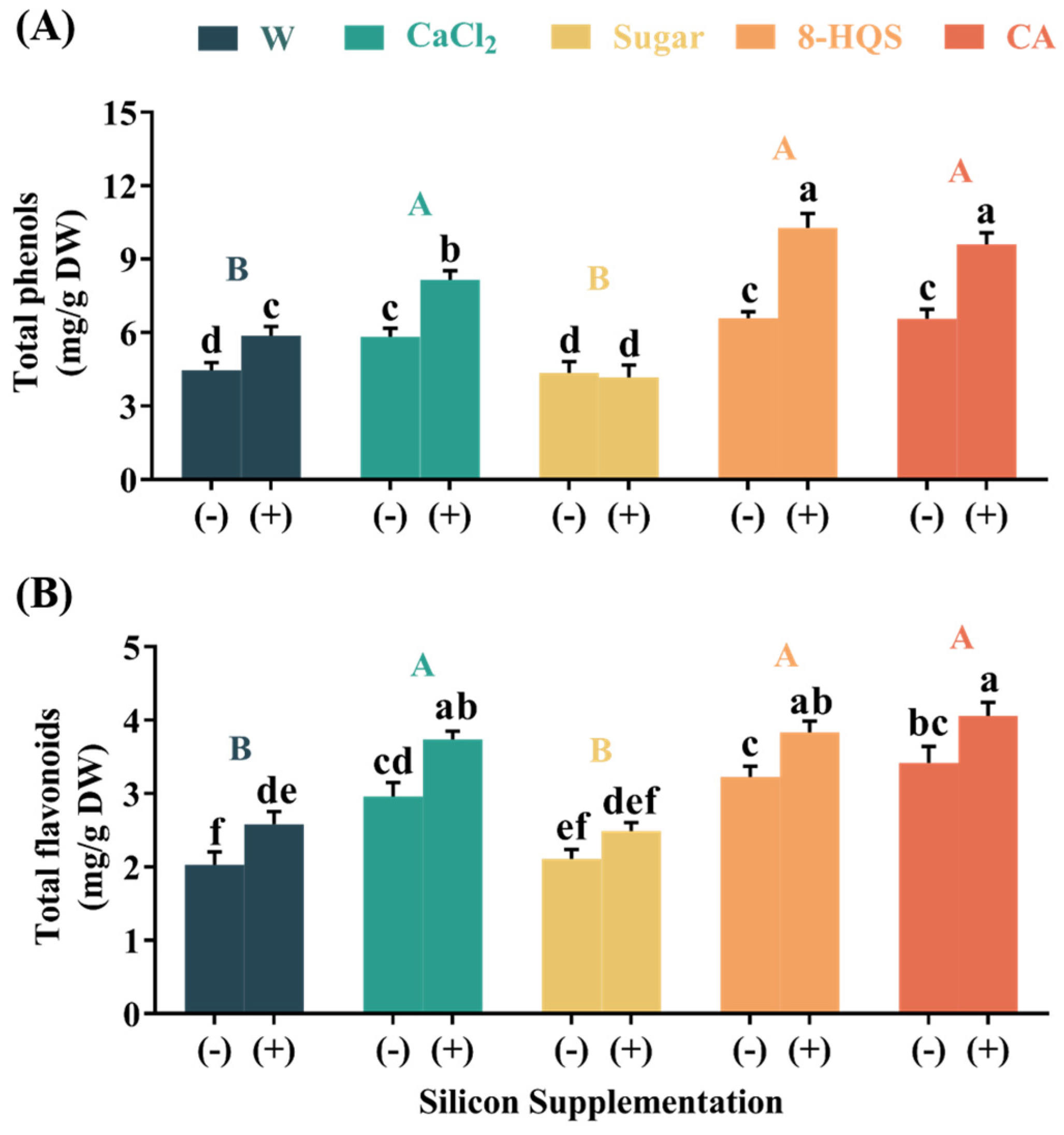
3.3. Enhanced Non-Enzymatic Antioxidant Compounds in Petals Due to Si and Other Preservatives
3.4. Enhanced Enzymatic Antioxidant Compounds in Petals Due to Si and Other Preservatives
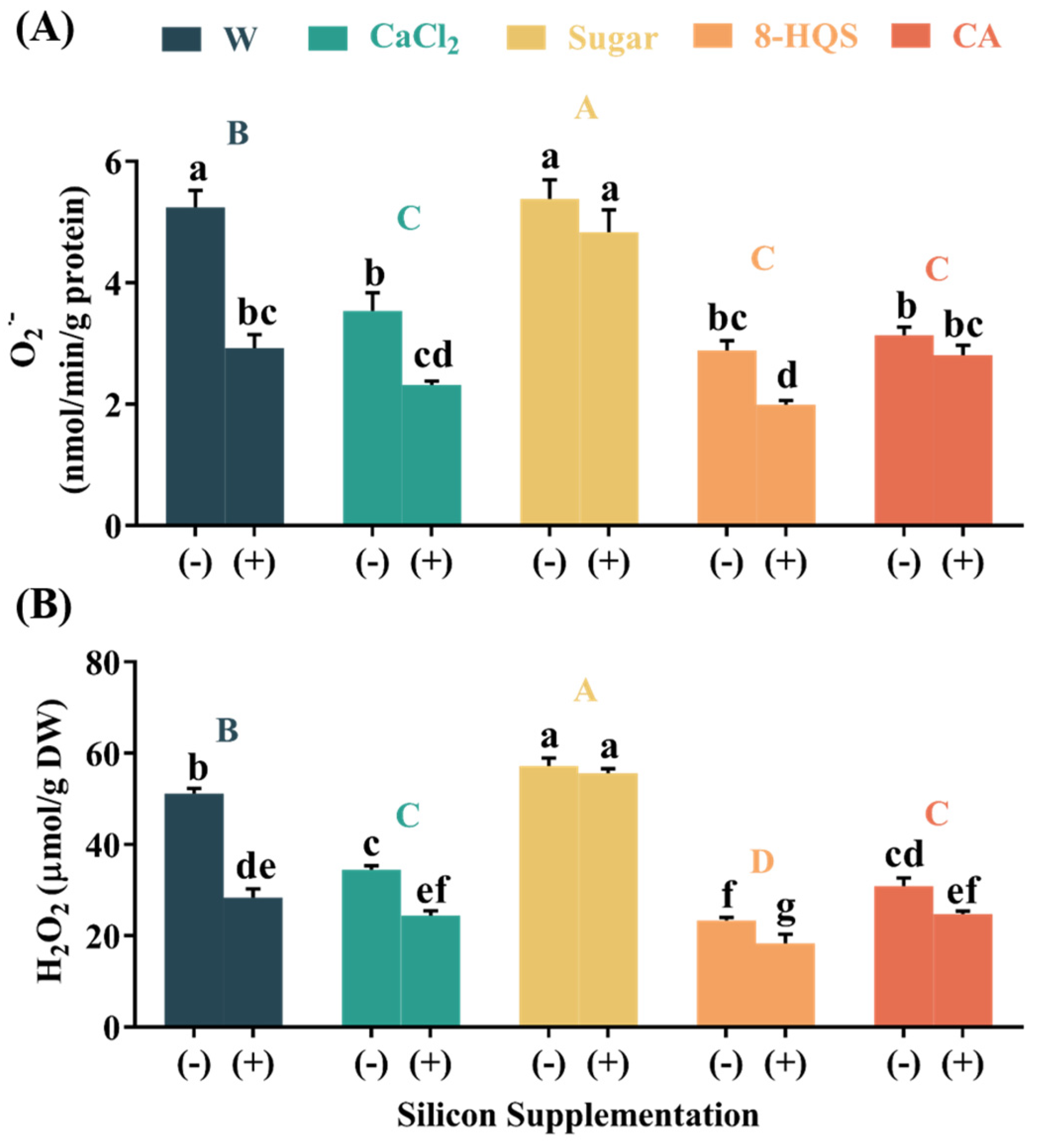
3.5. Declined ROS Contents in Petals Due to Si and Other Preservatives
4. Discussion
4.1. Co-Application of Si and a Preservative Prolonged Vase Life, Reduced Water Loss, and Delayed the Senescence of Cut Lily Flowers, but Did Not Improve the Maximum Flower Diameter
4.2. Co-Application of Si and a Preservative Reinforced the Antioxidant Defense System
4.3. The Combination of Si and 8-HQS May Be the Optimal Combination for Improving the Vase Quality of Cut Lily Flowers
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, J.; Chai, N.; Zhang, T.; Zhu, T.; Cheng, Y.; Sui, S.; Li, M.; Liu, D. Prediction of temperature tolerance in Lilium based on distribution and climate data. Iscience 2021, 24, 102794. [Google Scholar] [CrossRef] [PubMed]
- Aziz, S.; Younis, A.; Jaskani, M.J.; Ahmad, R. Effect of PGRs on antioxidant activity and phytochemical in delay senescence of lily cut flowers. Agronomy 2020, 10, 1704. [Google Scholar] [CrossRef]
- Shaheen, R.; Hassan, I.; Hafiz, I.A.; Jilani, G.; Abbasi, N.A. Balanced zinc nutrition enhances the antioxidative activities in Oriental lily cut-flower leading to improved growth and vase quality. Sci. Hortic. 2015, 197, 644–649. [Google Scholar] [CrossRef]
- Ren, P.-J.; Jin, X.; Liao, W.-B.; Wang, M.; Niu, L.-J.; Li, X.-P.; Xu, X.-T.; Zhu, Y.-C. Effect of hydrogen-rich water on vase life and quality in cut lily and rose flowers. Hortic. Environ. Biotechnol. 2017, 58, 576–584. [Google Scholar] [CrossRef]
- Cheng, G.; Wang, L.; Wu, H.; Yu, X.; Zhang, N.; Wan, X.; He, L.; Huang, H. Variation in petal and leaf wax deposition affects cuticular transpiration in cut lily flowers. Front. Plant Sci. 2021, 12, 781987. [Google Scholar] [CrossRef]
- Hassan, F.; Mazrou, R.; Gaber, A.; Hassan, M.M. Moringa extract preserved the vase life of cut roses through maintaining water relations and enhancing antioxidant machinery. Postharvest Biol. Technol. 2020, 164, 111156. [Google Scholar] [CrossRef]
- Sánchez-Navarro, J.F.; González-García, Y.; Benavides-Mendoza, A.; Morales-Díaz, A.B.; González-Morales, S.; Cadenas-Pliego, G.; García-Guillermo, M.d.S.; Juárez-Maldonado, A. Silicon nanoparticles improve the shelf life and antioxidant status of lilium. Plants 2021, 10, 2338. [Google Scholar] [CrossRef]
- Shabanian, S.; Esfahani, M.N.; Karamian, R.; Tran, L.-S.P. Physiological and biochemical modifications by postharvest treatment with sodium nitroprusside extend vase life of cut flowers of two gerbera cultivars. Postharvest Biol. Technol. 2018, 137, 1–8. [Google Scholar] [CrossRef]
- Song, J.; Li, Y.; Hu, J.; Lee, J.; Jeong, B.R. Pre- and/or postharvest silicon application prolongs the vase life and enhances the quality of cut peony (Paeonia lactiflora Pall.) flowers. Plants 2021, 10, 1742. [Google Scholar] [CrossRef] [PubMed]
- Dole, J.M.; Stamps, R.H.; Carlson, A.S.; Ahmad, I.; Greer, L. Postharvest Handling of Cut Flowers and Greens: A Practical Guide for Commercial Growers, Wholesalers, and Retailers; Association of Specialty Cut Flower Growers: Oberlin, OH, USA, 2017. [Google Scholar]
- Dung, C.D.; Seaton, K.; Singh, Z. Influence of type and concentration of sugars, supplemented with 8-hydroxyquinoline sulphate, on the vase life of waxflower. Folia Hortic. 2017, 29, 39–49. [Google Scholar] [CrossRef]
- Song, J.; Yang, J.; Jeong, B.R. Synergistic Effects of Silicon and Preservative on Promoting Postharvest Performance of Cut Flowers of Peony (Paeonia lactiflora Pall.). Int. J. Mol. Sci. 2022, 23, 13211. [Google Scholar] [CrossRef] [PubMed]
- Elhindi, K.M. Evaluation of several holding solutions for prolonging vase-life and keeping quality of cut sweet pea flowers (Lathyrus odoratus L.). Saudi J. Biol. Sci. 2012, 19, 195–202. [Google Scholar] [CrossRef]
- Uddina, A.J.; Khanb, P.; Mehrajc, H.; Taufiquea, T.; Shiama, I. Influence of different pulsing and holding solutions on vase life of tuberose. J. Biosci. Agric. Res. 2016, 7, 578–582. [Google Scholar] [CrossRef]
- De Silva, W.; Kirthisinghe, J.; Alwis, L. Extending the vase life of gerbera (Gerbera hybrida) cut flowers using chemical preservative solutions. Trop. Agric. Res. 2015, 24, 375–379. [Google Scholar] [CrossRef]
- Deshmukh, R.K.; Ma, J.F.; Belanger, R.R. Role of silicon in plants. Front. Plant Sci. 2017, 8, 1858. [Google Scholar] [CrossRef]
- Souri, Z.; Khanna, K.; Karimi, N.; Ahmad, P. Silicon and plants: Current knowledge and future prospects. J. Plant Growth Regul. 2021, 40, 906–925. [Google Scholar] [CrossRef]
- Gaur, S.; Kumar, J.; Kumar, D.; Chauhan, D.K.; Prasad, S.M.; Srivastava, P.K. Fascinating impact of silicon and silicon transporters in plants: A review. Ecotox. Environ. Saf. 2020, 202, 110885. [Google Scholar] [CrossRef] [PubMed]
- Tubana, B.S.; Babu, T.; Datnoff, L.E. A review of silicon in soils and plants and its role in US agriculture: History and future perspectives. Soil Sci. 2016, 181, 393–411. [Google Scholar] [CrossRef]
- Chen, D.; Wang, S.; Yin, L.; Deng, X. How does silicon mediate plant water uptake and loss under water deficiency? Front. Plant Sci. 2018, 9, 281. [Google Scholar] [CrossRef] [PubMed]
- Van Bockhaven, J.; De Vleesschauwer, D.; Höfte, M. Towards establishing broad-spectrum disease resistance in plants: Silicon leads the way. J. Exp. Bot. 2013, 64, 1281–1293. [Google Scholar] [CrossRef] [PubMed]
- Shohani, F.; Fazeli, A.; Sarghein, S.H. The effect of silicon application and salicylic acid on enzymatic and non-enzymatic reactions of Scophularia striata L. under drought stress. Sci. Hortic. 2023, 319, 112143. [Google Scholar] [CrossRef]
- SeyedHajizadeh, H.; Esmaili, S.; Zahedi, S.M.; Fakhrghazi, H.; Kaya, O. Silicon dioxide and selenium nanoparticles enhance vase life and physiological quality in black magic roses. Sci. Rep. 2024, 14, 22848. [Google Scholar] [CrossRef] [PubMed]
- Kamiab, F.; Shahmoradzadeh Fahreji, S.; Zamani Bahramabadi, E. Antimicrobial and physiological effects of silver and silicon nanoparticles on vase life of lisianthus (Eustoma grandiflora cv. Echo) flowers. Int. J. Hortic. Sci. Technol. 2017, 4, 135–144. [Google Scholar]
- Nguyen, T.K.; Lim, J.H. Do eco-friendly floral preservative solutions prolong vase life better than chemical solutions? Horticulturae 2021, 7, 415. [Google Scholar] [CrossRef]
- Geshnizjany, N.; Ramezanian, A.; Khosh-Khui, M. Postharvest life of cut gerbera (Gerbera jamesonii) as affected by nano-silver particles and calcium chloride. Int. J. Hortic. Sci. Technol. 2014, 1, 171–180. [Google Scholar]
- Tonooka, M.; Homma, Y.; Nukui, H.; Ichimura, K. The extension of vase life in cut gerbera flowers through pretreatment with gibberellin A3 in combination with calcium chloride. Horticulturae 2023, 9, 1106. [Google Scholar] [CrossRef]
- Rabiza-Świder, J.; Skutnik, E.; Jędrzejuk, A.; Rochala-Wojciechowska, J. Nanosilver and sucrose delay the senescence of cut snapdragon flowers. Postharvest Biol. Technol. 2020, 165, 111165. [Google Scholar] [CrossRef]
- Yang, H.; Lim, S.; Lee, J.-H.; Choi, J.-W.; Shin, I.-S. Influence of solution combination for postharvest treatment stage on vase life of cut hydrangea flowers (Hydrangea macrophylla cv. ‘Verena’). Horticulturae 2021, 7, 406. [Google Scholar] [CrossRef]
- Sheikh, F.; Neamati, S.H.; Vahdati, N.; Dolatkhahi, A. Study on effects of ascorbic acid and citric acid on vase life of cut lisianthus (Eustoma grandiflorum) ‘Mariachi Blue’. J. Ornam. Plants 2014, 4, 57–64. [Google Scholar]
- Balieiro, B.T.; Júnior, M.A.; Vieira, M.d.S.; Souza, A.d.; Moreira, S.d.O.; Nascimento, A.d.; Júnior, W.S.; Souza, G.d. Postharvest life of cut chrysanthemum flowers as affected by citric acid, boric acid and salicylic acid. Amaz. J. Plant Res. 2018, 2, 127–144. [Google Scholar] [CrossRef]
- Park, Y.G.; Muneer, S.; Kim, S.; Hwang, S.J.; Jeong, B.R. Foliar or subirrigational silicon supply modulates salt stress in strawberry during vegetative propagation. Hortic. Environ. Biotechnol. 2018, 59, 11–18. [Google Scholar] [CrossRef]
- Selvarajan, V.; Obuobi, S.; Ee, P.L.R. Silica nanoparticles—A versatile tool for the treatment of bacterial infections. Front. Chem. 2020, 8, 602. [Google Scholar] [CrossRef] [PubMed]
- AlFayad, A.; Othman, Y. Pre-Harvest Chemical Compounds Influence Lily (Lilium × elegans) Leaf and Flower Indigenous Phenols, Flavonoids and Gibberellic Acid Levels. Int. J. Plant Biol. 2024, 15, 551–560. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Arvouet-Grand, A.; Vennat, B.; Pourrat, A.; Legret, P. Standardization of propolis extract and identification of principal constituents. J. Pharm. Belg. 1994, 49, 462–468. [Google Scholar] [PubMed]
- Wu, Y.-X.; von Tiedemann, A. Impact of fungicides on active oxygen species and antioxidant enzymes in spring barley (Hordeum vulgare L.) exposed to ozone. Environ. Pollut. 2002, 116, 37–47. [Google Scholar] [CrossRef]
- Mukherjee, S.; Choudhuri, M. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant. 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Li, N.; Wang, K.; Lv, Y.; Zhang, Z.; Cao, B.; Chen, Z.; Xu, K. Silicon enhanced the resistance of Chinese cabbage (Brassica rapa L. ssp. pekinensis) to ofloxacin on the growth, photosynthetic characteristics and antioxidant system. Plant Physiol. Biochem. 2022, 175, 44–57. [Google Scholar] [CrossRef]
- Zhao, D.; Cheng, M.; Tang, W.; Liu, D.; Zhou, S.; Meng, J.; Tao, J. Nano-silver modifies the vase life of cut herbaceous peony (Paeonia lactiflora Pall.) flowers. Protoplasma 2018, 255, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, S.; Ali, S.; Ahmad, R.; Ercisli, S.; Anjum, M.A. Foliar application of silicon enhances growth, flower yield, quality and postharvest life of tuberose (Polianthes tuberosa L.) under saline conditions by improving antioxidant defense mechanism. Silicon 2021, 14, 1511–1518. [Google Scholar] [CrossRef]
- Islam, W.; Tayyab, M.; Khalil, F.; Hua, Z.; Huang, Z.; Chen, H.Y. Silicon-mediated plant defense against pathogens and insect pests. Pestic. Biochem. Phys. 2020, 168, 104641. [Google Scholar] [CrossRef] [PubMed]
- Thwala, M.; Wahome, P.K.; Oseni, T.O.; Masarirambi, M.T. Effects of floral preservatives on the vase life of Orchid (Epidendrum radicans L.) cut flowers. Hortic. Sci. Ornam. Plants 2013, 5, 22–29. [Google Scholar]
- Krasavina, M.S.; Burmistrova, N.A.; Raldugina, G.N. The role of carbohydrates in plant resistance to abiotic stresses. In Emerging Technologies and Management of Crop Stress Tolerance; Elsevier: Amsterdam, The Netherlands, 2014; pp. 229–270. [Google Scholar]
- Gong, B.; Huang, S.; Ye, N.; Yuan, X.; Ma, H. Pre-harvest ethylene control affects vase life of cut rose ‘Carola’ by regulating energy metabolism and antioxidant enzyme activity. Hortic. Environ. Biotechnol. 2018, 59, 835–845. [Google Scholar] [CrossRef]
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as natural phenolic compounds and their role in therapeutics: An overview. Futur. J. Pharm. Sci. 2021, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, K.A.; Mazrou, Y.S.; Hafez, Y.M. Silicon foliar application mitigates salt stress in sweet pepper plants by enhancing water status, photosynthesis, antioxidant enzyme activity and fruit yield. Plants 2020, 9, 733. [Google Scholar] [CrossRef] [PubMed]
- Sharifzadeh, K.; Asil, M.H.; Roein, Z.; Sharifzadeh, M. Effect of 8-Hydroxyquinoline Citrate, Sucrose and Peroxidase Inhibitors on Vase Life of Lisianthus (L.) Cut Flowers. J. Hortic. Res. 2014, 22, 41–47. [Google Scholar] [CrossRef]
- Vahdati Mashhadian, N.; Tehranifar, A.; Bayat, H.; Selahvarzi, Y. Salicylic and citric acid treatments improve the vase life of cut chrysanthemum flowers. J. Agric. Sci. Technol. 2012, 14, 879–887. [Google Scholar]
- Atefepour, E.; Saadatian, M.; Asil, M.H.; Rabiei, B. Effect of silver nano particles and 8-hydroxyquinoline citrate on the longer life of cut Gerbera (Gerbera jamesonii) ’Sunway’ flowers. Sci. Hortic. 2021, 289, 110474. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.; Yang, J.; Jeong, B.R. A Composite Vase Solution Using Silicon (Si) and Other Preservatives Improved the Vase Quality of Cut Lily (Lilium ‘Siberia’) Flowers. Horticulturae 2025, 11, 112. https://doi.org/10.3390/horticulturae11020112
Song J, Yang J, Jeong BR. A Composite Vase Solution Using Silicon (Si) and Other Preservatives Improved the Vase Quality of Cut Lily (Lilium ‘Siberia’) Flowers. Horticulturae. 2025; 11(2):112. https://doi.org/10.3390/horticulturae11020112
Chicago/Turabian StyleSong, Jinnan, Jingli Yang, and Byoung Ryong Jeong. 2025. "A Composite Vase Solution Using Silicon (Si) and Other Preservatives Improved the Vase Quality of Cut Lily (Lilium ‘Siberia’) Flowers" Horticulturae 11, no. 2: 112. https://doi.org/10.3390/horticulturae11020112
APA StyleSong, J., Yang, J., & Jeong, B. R. (2025). A Composite Vase Solution Using Silicon (Si) and Other Preservatives Improved the Vase Quality of Cut Lily (Lilium ‘Siberia’) Flowers. Horticulturae, 11(2), 112. https://doi.org/10.3390/horticulturae11020112







