Resveratrol in Grapevine Components, Products and By-Products—A Review
Abstract
1. Introduction
2. Resveratrol: Structure, Biological Properties, and Synthetic Factors
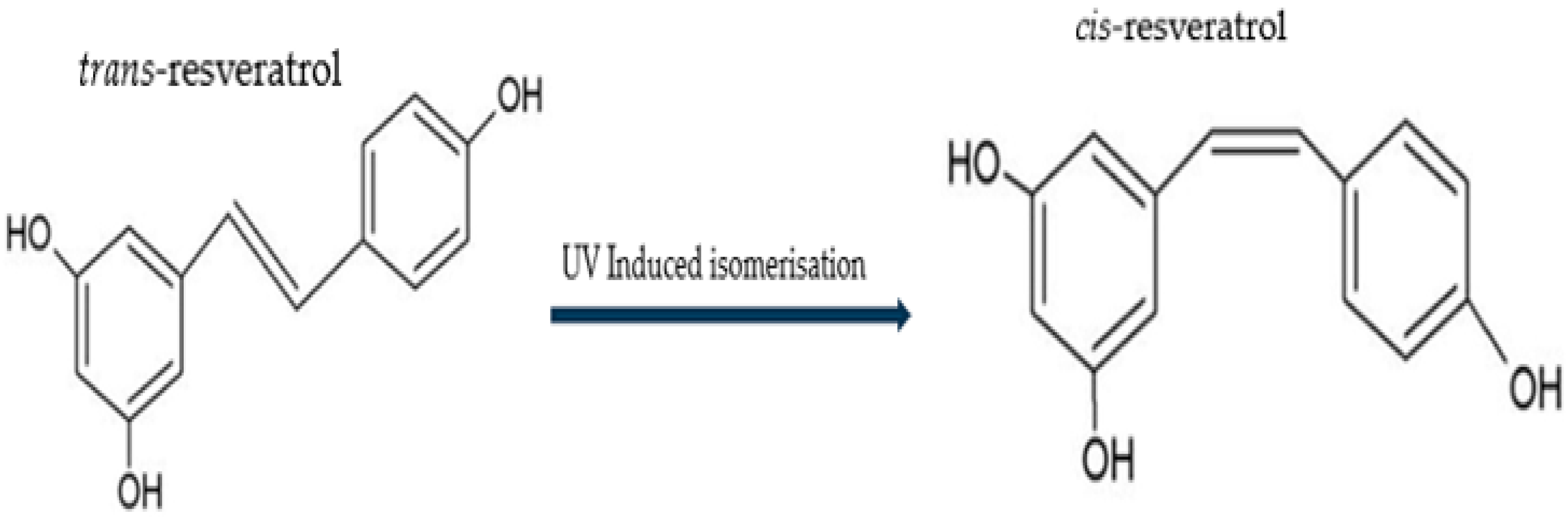
2.1. Chemical Structure and Isomers
2.2. Factors Influencing Resveratrol Synthesis and Content in Grapevine
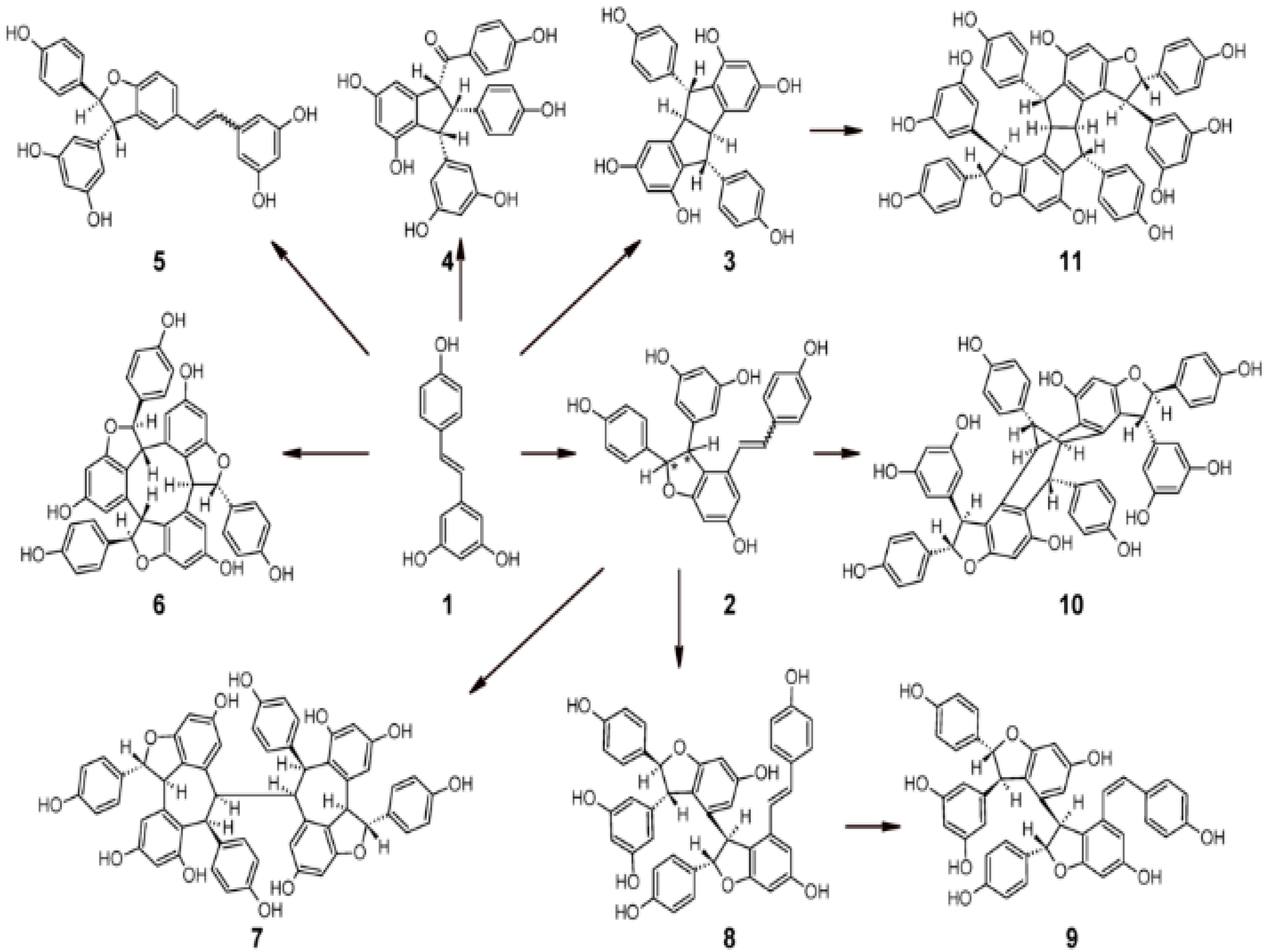
2.3. Biological Properties
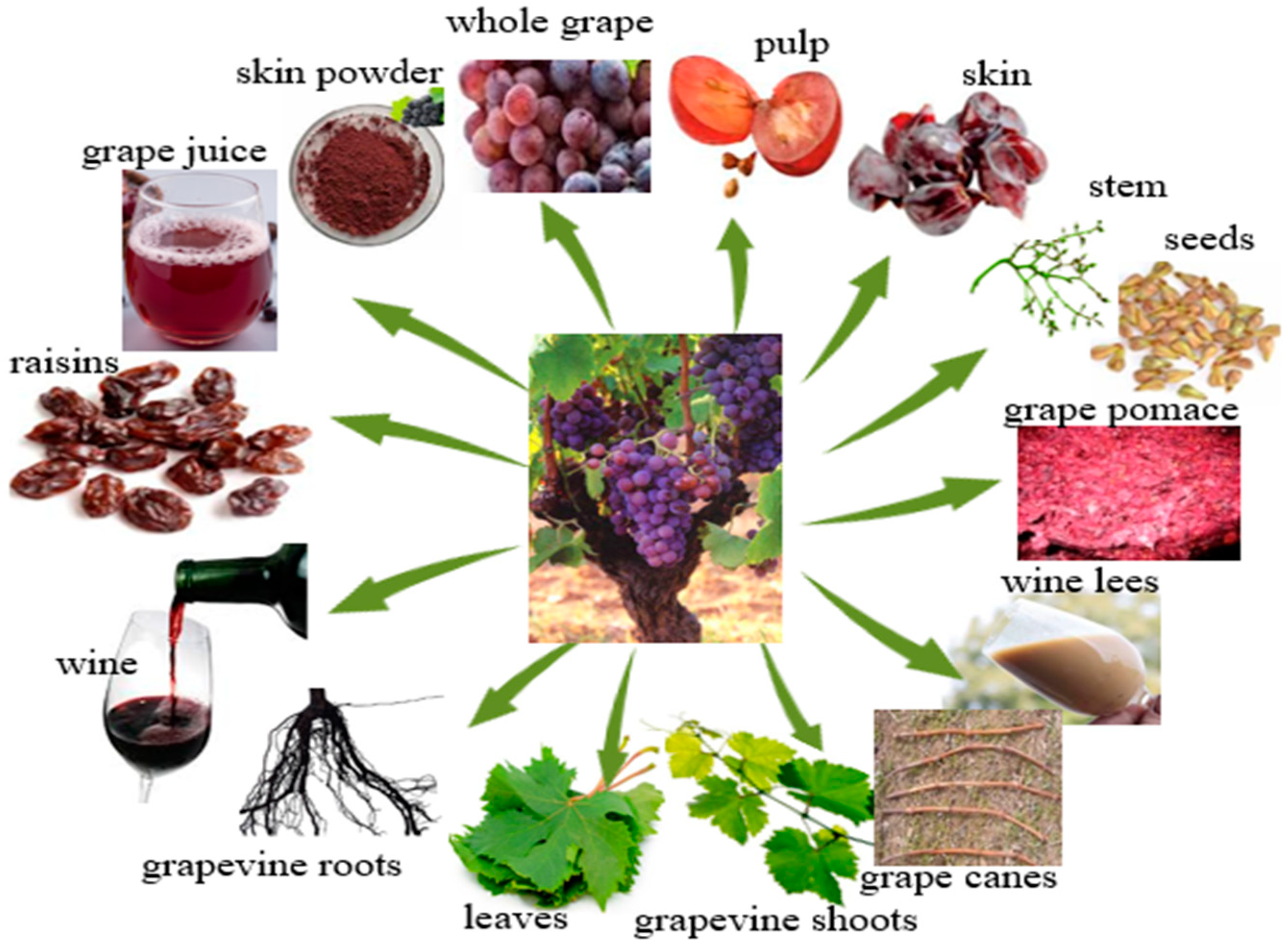
3. Sources of Resveratrol from the Grapevine
3.1. Sources of Resveratrol in Grapes and Other Grapevine Components
3.2. Sources of Resveratrol in Products and By-Products
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Valletta, A.; Iozia, L.M.; Leonelli, F. Impact of environmental factors on stilbene biosynthesis. Plants 2021, 10, 90. [Google Scholar] [CrossRef]
- Takaoka, M. Resveratrol, a new phenolic compound from Veratrum grandiflorum. J. Chem. Soc. Jpn. 1939, 60, 1090–1100. [Google Scholar]
- Tian, B.; Liu, J. Resveratrol: A review of plant sources, synthesis, stability, modification and food application. J. Sci. Food Agric. 2020, 100, 1392–1404. [Google Scholar] [CrossRef] [PubMed]
- Kupe, M.; Karatas, N.; Unal, M.S.; Ercisli, S.; Baron, M.; Sochor, J. Nutraceutical and Functional Properties of Peel, Pulp, and Seed Extracts of Six ‘Köhnü’ Grape Clones. Horticulturae 2021, 7, 346. [Google Scholar] [CrossRef]
- Balanov, P.E.; Smotraeva, I.V.; Abdullaeva, M.S.; Volkova, D.A.; Ivanchenko, O.B. Study on resveratrol content in grapes and wine products. E3S Web Conf. EDP Sci. 2021, 247, 01063. [Google Scholar] [CrossRef]
- Zhang, K.; Li, W.; Ju, Y.; Wang, X.; Sun, X.; Fang, Y.; Chen, K. Transcriptomic and metabolomic basis of short-and long-term post-harvest UV-C application in regulating grape berry quality development. Foods 2021, 10, 625. [Google Scholar] [CrossRef] [PubMed]
- Eshghi, S.; Karimi, R.; Shiri, A.; Karami, M.; Moradi, M. Effects of polysaccharide-based coatings on postharvest storage life of grape: Measuring the changes in nutritional, antioxidant and phenolic compounds. J. Food Meas. Charact. 2022, 16, 1159–1170. [Google Scholar] [CrossRef]
- Houillé, B.; Besseau, S.; Courdavault, V.; Oudin, A.; Glévarec, G.; Delanoue, G.; Guérin, L.; Simkin, A.J.; Papon, N.; Clastre, M.; et al. Biosynthetic origin of E-resveratrol accumulation in grape canes during postharvest storage. J. Agric. Food Chem. 2015, 63, 1631–1638. [Google Scholar] [CrossRef] [PubMed]
- Concenco, F.I.G.R.; Brotto, G.F.; Nora, L. Grape wine and juice: Comparison on resveratrol levels. Int. J. Adv. Res. Sci. Eng. Technol. 2019, 6, 368–386. [Google Scholar] [CrossRef]
- Crăciun, A.L.; Gutt, G. Study on kinetics of trans-resveratrol, total phenolic content, and antioxidant activity increase in vine waste during post-pruning storage. Appl. Sci. 2022, 12, 1450. [Google Scholar] [CrossRef]
- Brillante, L.; De Rosso, M.; Dalla Vedova, A.; Maoz, I.; Flamini, R.; Tomasi, D. Insights on the stilbenes in Raboso Piave grape (Vitis vinifera L.) as a consequence of postharvest vs on-vine dehydration. J. Sci. Food Agric. 2018, 98, 1961–1967. [Google Scholar] [CrossRef] [PubMed]
- Naiker, M.; Anderson, S.; Johnson, J.B.; Mani, J.S.; Wakeling, L.; Bowry, V. Loss of trans-resveratrol during storage and ageing of red wines. Aust. J. Grape Wine Res. 2020, 26, 385–387. [Google Scholar] [CrossRef]
- Jordão, A.M.; Correia, A.C.; DelCampo, R.; González SanJosé, M.L. Antioxidant capacity, scavenger activity, and ellagitannins content from commercial oak pieces used in winemaking. Eur. Food Res. Technol. 2012, 235, 817–825. [Google Scholar] [CrossRef]
- Fabjanowicz, M.; Płotka-Wasylka, J.; Namieśnik, J. Detection, identification and determination of resveratrol in wine. Problems and challenges. Trends Anal. Chem. 2018, 103, 21–33. [Google Scholar] [CrossRef]
- Stoenescu, A.M.; Trandafir, I.; Cosmulescu, S. Determination of phenolic compounds using HPLC-UV method in wild fruit species. Horticulturae 2022, 8, 84. [Google Scholar] [CrossRef]
- Biţă, C.E.; Scorei, I.R.; Vreju, A.F.; Muşetescu, A.E.; Mogoşanu, G.D.; Biţă, A.; Dinescu, V.C.; Dinescu, Ş.C.; Criveanu, C.; Bărbulescu, A.L.; et al. Microbiota-Accessible Boron-Containing Compounds in Complex Regional Pain Syndrome. Medicina 2023, 59, 1965. [Google Scholar] [CrossRef] [PubMed]
- Biţă, A.; Scorei, I.R.; Bălşeanu, T.A.; Ciocîlteu, M.V.; Bejenaru, C.; Radu, A.; Bejenaru, L.E.; Rău, G.; Mogoşanu, G.D.; Neamţu, J.; et al. New Insights into Boron Essentiality in Humans and Animals. Int. J. Mol. Sci. 2022, 23, 9147. [Google Scholar] [CrossRef] [PubMed]
- Ciocîlteu, M.V.; Scorei, I.R.; Rău, G.; Niculescu, C.; Biţă, A.; Ene, V.L.; Simionescu, A.; Turcu-Ştiolică, A.; Dinescu, V.C.; Neamţu, J.; et al. Zinc–Boron–PLGA biocomposite material: Preparation, structural characterization, and in vitro assessment. Rom. J. Morphol. Embryol. 2023, 64, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Salla, M.; Karaki, N.; El Kaderi, B.; Ayoub, A.J.; Younes, S.; Abou Chahla, M.N.; Baksh, S.; El Khatib, S. Enhancing the Bioavailability of Resveratrol: Combine It, Derivatize It, or Encapsulate It? Pharmaceutics 2024, 16, 569. [Google Scholar] [CrossRef]
- Moreno, J.; Peinado, R. Composition of Grape Must. In Enological Chemistry; Academic Press: Cambridge, MA, USA, 2012; pp. 13–22. [Google Scholar]
- Nawaz, W.; Zhou, Z.; Deng, S.; Ma, X.; Li, C.; Shu, X. Therapeutic Versatility of Resveratrol Derivatives. Nutrients 2017, 9, 1188. [Google Scholar] [CrossRef]
- Akinwumi, B.C.; Bordun, K.A.M.; Anderson, H.D. Biological activities of stilbenoids. Int. J. Mol. Sci. 2018, 19, 792. [Google Scholar] [CrossRef]
- Vitrac, X.; Bornet, A.; Vanderlinde, R.; Valls, J.; Tristan, R.; Delaunay, J.C.; Mérillon, J.M.; Teissédre, P.L. Determination of Stilbenes (delta-viniferin, trans-astringin, trans-piceid, cis- and trans-resveratrol, E-viniferin) in Brazilian Wines. J. Agric. Food Chem. 2005, 53, 5664–5669. [Google Scholar] [CrossRef] [PubMed]
- Lekli, I.; Ray, D.; Das, D.K. Longevity nutrients resveratrol, wines and grapes. Genes Nutr. 2010, 5, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Căpruciu, R.; Cichi, D.D.; Mărăcineanu, L.C.; Costea, D.C. The resveratrol content in black grapes skins at different development stages. Sci. Pap. Ser. B Hortic. 2022, LXVI, 245–252. [Google Scholar]
- Mikulski, D.; Molski, M. Quantitative structure–antioxidant activity relationship of trans-resveratrol oligomers, trans-4,4′-dihydroxystilbene dimer, trans-resveratrol-3-O-glucuronide, glucosides: Trans-piceid, cis-piceid, trans-astringin and trans-resveratrol-4′-O-β-D-glucopyranoside. Eur. J. Med. Chem. 2010, 45, 2366–2380. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; Harikumar, K.B.; Aggarwal, B.B. Resveratrol addiction: To die or not to die. Mol. Nutr. Food Res. 2009, 53, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Flamini, R.; Mattivi, F.; De Rosso, M.; Arapitsas, P.; Bavaresco, L. Advanced knowledge of three important classes of grape phenolics: Anthocyanins, stilbenes and flavonols. Int. J. Mol. Sci. 2013, 14, 19651–19669. [Google Scholar] [CrossRef] [PubMed]
- Flamini, R.; Zanzotto, A.; de Rosso, M.; Lucchetta, G.; Dalla Vedova, A.; Bavaresco, L. Stilbene oligomer phytoalexins in grape as a response to Aspergillus carbonarius infection. Physiol. Mol. Plant Pathol. 2016, 93, 112–118. [Google Scholar] [CrossRef]
- Gao, S.; Wei, D.; Pan, D.; Wang, F.; Kang, H.; Yao, Y. Melatonin Promotes Accumulation of Resveratrol and Its Derivatives through Upregulation of PAL, 4CL, C4H, and STS in Grape Seeds. Horticulturae 2024, 10, 65. [Google Scholar] [CrossRef]
- Latruffe, N.; Vervandier-Fasseur, D. Strategic syntheses of vine and wine resveratrol derivatives to explore their effects on cell functions and dysfunctions. Diseases 2018, 6, 110. [Google Scholar] [CrossRef] [PubMed]
- Šušnjar, A.; Šunjka, D.; Boškovic, D.; Lazic, S.; Vukovic, S. Resveratrol in grapes-benefits and potential use. Agrofor Int. J. 2022, 7, 110–122. [Google Scholar]
- Pajović, R.; Raičević, D.; Popović, T.; Sivilotti, P.; Lisjak, K.; Vanzo, A. Polyphenolic characterisation of Vranac, Kratosija and Cabernet Sauvignon (Vitis vinifera L. cv.) grapes and wines from different vineyard locations in Montenegro. S. Afr. J. Enol. Vitic. 2014, 35, 134–143. [Google Scholar] [CrossRef]
- Ochoa-Hueso, R.; Cantos-Villar, E.; Puertas, B.; Aguiar del Rio, J.F.; Belda, I.; Delgado-Baquerizo, M.; Fernández, V.; Gallardo, A.; García-Morales, J.L.; Garde-Cerdán, T.; et al. Nature-based strategies to regenerate the functioning and biodiversity of vineyards. J. Sustain. Agric. Environ. 2024, 3, e12088. [Google Scholar] [CrossRef]
- Geana, E.I.; Dinca, O.R.; Ionete, R.E.; Artem, V.; Niculescu, V.C. Monitoring trans-resveratrol in grape berry skins during ripening and in corresponding wines by HPLC. Food Technol. Biotechnol. 2015, 53, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Raičević, D.; Božinović, Z.; Petkov, M.; Ivanova, V.; Kodžulović, V.; Mugoša, M.; Sanja, S.; Maraš, V. Polyphenolic content and sensory profile of Montenegrin Vranac wines produced with different oenological products and maceration. Maced. J. Chem. Chem. Eng. 2017, 36, 229–238. [Google Scholar] [CrossRef]
- Rocchetti, G.; Ferrari, F.; Trevisan, M.; Bavaresco, L. Impact of climatic conditions on the resveratrol concentration in blend of Vitis vinifera L. cvs. Barbera and Croatina grape wines. Molecules 2021, 26, 401. [Google Scholar] [CrossRef] [PubMed]
- Cichi, D.D.; Căpruciu, R.; Gheorghiu, N.; Stoica, F. Agrobiological and technological characteristics of table grapes varieties, grown in the temperatecontinental climate from southwestern Romania. Sci. Pap. Ser. B Hortic. 2023, LXVII, 302–308. [Google Scholar]
- Sun, Y.; Xi, B.; Dai, H. Effects of Water Stress on Resveratrol Accumulation and Synthesis in ‘Cabernet Sauvignon’ Grape Berries. Agronomy 2023, 13, 633. [Google Scholar] [CrossRef]
- Constantin, O.E.; Stoica, F.; Rațu, R.N.; Stănciuc, N.; Bahrim, G.E.; Râpeanu, G. Bioactive Components, Applications, Extractions, and Health Benefits of Winery By-Products from a Circular Bioeconomy Perspective: A Review. Antioxidants 2024, 13, 100. [Google Scholar] [CrossRef]
- Bešlić, Z.; Pantelić, M.; Dabić, D.; Todić, S.; Natić, M.; Tešić, Ž. Effect of vineyard floor management on water regime, growth response, yield and fruit quality in Cabernet Sauvignon. Sci. Hortic. 2015, 197, 650–656. [Google Scholar] [CrossRef]
- Ingrà, C.; Del Frari, G.; Favole, M.; Tumminelli, E.; Rossi, D.; Collina, S.; Prati, M.; Ferreira, R.B.; Ferrandino, A. Effects of Growing Areas, Pruning Wound Protection Products, and Phenological Stage on the Stilbene Composition of Grapevine (Vitis vinifera L.) Canes. J. Agric. Food Chem. 2024, 72, 11465–11479. [Google Scholar] [CrossRef]
- Tzanova, M.; Atanasov, V.; Yaneva, Z.; Ivanova, D.; Dinev, T. Selectivity of current extraction techniques for flavonoids from plant materials. Processes 2020, 8, 1222. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Y.; Yan, Y. Bioproduction of Resveratrol. In Biotechnology of Natural Products; Schwab, W., Lange, B., Wüst, M., Eds.; Springer: Cham, Switzerland, 2018; pp. 61–79. [Google Scholar]
- Tıraş, Z.Ş.E.; Okur, H.H.; Günay, Z.; Yıldırım, H.K. Different approaches to enhance resveratrol content in wine. Cienc. Tec. Vitivinic. 2022, 37, 13–28. [Google Scholar] [CrossRef]
- Ispiryan, A.; Kraujutiene, I.; Viskelis, J. Retaining Resveratrol Content in Berries and Berry Products with Agricultural and Processing Techniques. Processes 2024, 12, 1216. [Google Scholar] [CrossRef]
- Guld, Z.S.; Rácz, A.; Tima, H.; Kállay, M.; Sárdy, D.N. Effects of aging in oak barrels on the trans-resveratrol and anthocyanin concentration of red wines from Hungary. Acta Aliment. 2019, 48, 349–357. [Google Scholar] [CrossRef]
- Uysal, R.S.; Issa-Issa, H.; Sendra, E.; Carbonell-Barrachina, Á.A. Changes in anthocyanin pigments, trans-resveratrol, and colorimetric characteristics of Fondillón wine and other “Monastrell” wines during the aging period. Eur. Food Res. Technol. 2023, 249, 1821–1831. [Google Scholar] [CrossRef]
- Fernández-Marín, M.I.; Guerrero, R.F.; García-Parrilla, M.C.; Puertas, B.; Ramírez, P.; Cantos-Villar, E. Terroir and variety: Two key factors for obtaining stilbene-enriched grapes. J. Food Compost. Anal. 2013, 31, 191–198. [Google Scholar] [CrossRef]
- Lubin, B.C.R.; Inbar, N.; Pinkus, A.; Stanevsky, M.; Cohen, J.; Rahimi, O.; Anker, Y.; Shoseyov, O.; Drori, E. Ecogeographic conditions dramatically affect trans-resveratrol and other major phenolics’ levels in wine at a semi-arid area. Plants 2022, 11, 629. [Google Scholar] [CrossRef]
- Noviello, M.; Caputi, A.F.; Squeo, G.; Paradiso, V.M.; Gambacorta, G.; Caponio, F. Vine shoots as a source of trans-resveratrol and ε-Viniferin: A Study of 23 Italian varieties. Foods 2022, 11, 553. [Google Scholar] [CrossRef] [PubMed]
- Paramithiotis, S.; Patra, J.K.; Kotseridis, Y.; Dimopoulou, M. Fermented beverages revisited: From terroir to customized functional products. Fermentation 2024, 10, 57. [Google Scholar] [CrossRef]
- Mucalo, A.; Maletić, E.; Zdunić, G. Positive Impact of Late Harvest Date on Polyphenolic Composition of Plavac Mali (Vitis vinifera L.) Wine Depends on Location. Foods 2024, 13, 2695. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.M.; Bae, H. An overview of stress-induced resveratrol synthesis in grapes: Perspectives for resveratrol-enriched grape products. Molecules 2017, 22, 294. [Google Scholar] [CrossRef] [PubMed]
- Erte, E.; Vural, N.; Mehmetoğlu, Ü.; Güvenç, A. Optimization of an abiotic elicitor (ultrasound) treatment conditions on trans-resveratrol production from Kalecik Karası (Vitis vinifera L.) grape skin. J. Food Sci. Tehnol. 2021, 58, 2121–2132. [Google Scholar] [CrossRef]
- Candar, S. How abiotic stress induced by artificial wounding changes maturity levels and berry composition of Merlot (Vitis vinifera L.). Eur. Food Res. Technol. 2023, 249, 2611–2623. [Google Scholar] [CrossRef]
- Aleynova, O.A.; Ogneva, Z.V.; Suprun, A.R.; Ananev, A.A.; Nityagovsky, N.N.; Beresh, A.A.; Dubrovina, A.S.; Kiselev, K.V. The Effect of External Treatment of Arabidopsis thaliana with Plant-Derived Stilbene Compounds on Plant Resistance to Abiotic Stresses. Plants 2024, 13, 184. [Google Scholar] [CrossRef] [PubMed]
- Ferreyra, S.; Bottini, R.; Fontana, A. Temperature and light conditions affect stability of phenolic compounds of stored grape cane extracts. Food Chem. 2023, 405, 134718. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuw, R.; Kevers, C.; Pincemail, J.; Defraigne, J.O.; Dommes, J. Antioxidant capacity and phenolic composition of red wines from various grape varieties: Specificity of Pinot Noir. J. Food Compos. Anal. 2014, 36, 40–50. [Google Scholar] [CrossRef]
- Yaman, Ü.R.; Adıgüzel, B.Ç.; Yücel, U.; Çetinkaya, N. Effect of vegetation time and climatic conditions on trans-resveratrol concentrations in Cabernet Sauvignon and Merlot wines from different regions in Turkey. S. Afr. J. Enol. Vitic. 2016, 37, 85–92. [Google Scholar] [CrossRef]
- Tříska, J.; Houška, M. Physical methods of resveratrol induction in grapes and grape products—A review. Czech J. Food Sci. 2012, 30, 489–502. [Google Scholar] [CrossRef]
- Filimon, R.V.; Bunea, C.I.; Bora, F.D.; Filimon, R.M.; Dunca, S.I.; Rózsa, S.; Ciurlă, L.; Patraș, A. Physico-Chemical Characterization, Phenolic Compound Extraction and Biological Activity of Grapevine (Vitis vinifera L.) Canes. Horticulturae 2023, 9, 1164. [Google Scholar] [CrossRef]
- Singh, C.K.; Liu, X.; Ahmad, N. Resveratrol, in its natural combination in whole grape, for health promotion and disease management. Ann. N. Y. Acad. Sci. 2015, 1348, 150–160. [Google Scholar] [CrossRef]
- Alañón, M.E.; Alarcón, M.; Marchante, L.; Díaz-Maroto, M.C.; Pérez-Coello, M.S. Extraction of Natural Flavorings with Antioxidant Capacity from Cooperage By-Products by Green Extraction Procedure with Subcritical Fluids. Ind. Crops Prod. 2017, 103, 222–232. [Google Scholar] [CrossRef]
- Venkat, R.; Verma, E.; Daimary, U.D.; Kumar, A.; Girisa, S.; Dutta, U.; Ahn, K.S.; Kunnumakkara, A.B. The Journey of Resveratrol from Vineyards to Clinics. Cancer Investig. 2022, 41, 183–220. [Google Scholar] [CrossRef]
- Mongioi, L.M.; La Vignera, S.; Cannarella, R.; Cimino, L.; Compagnone, M.; Condorelli, R.A.; Calogero, A.E. The role of resveratrol administration in human obesity. Int. J. Mol. Sci. 2021, 22, 4362. [Google Scholar] [CrossRef] [PubMed]
- Batista-Jorge, G.C.; Barala-Jorge, A.S.; Silveira, M.F.; Lelis, D.F.; Andrade, J.M.O.; de Paula, A.M.B.; Guimarães, A.L.S.; Santos, S.H.S. Oral resveratrol supplementation improves metabolic syndrome features in obese patients submitted to a lifestyle-changing program. Life Sci. 2020, 256, 117962. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, Y.; Gu, H.; Xu, Z.; Tang, Y.; He, H.; Peng, L.; Xiang, L. Adherence to the Paleolithic diet and Paleolithic-like lifestyle reduce the risk of colorectal cancer in the United States: A prospective cohort study. J. Transl. Med. 2023, 21, 482. [Google Scholar] [CrossRef] [PubMed]
- Wajima, C.S.; Pitol-Palin, L.; de Souza Batista, F.R.; Dos Santos, P.H.; Matsushita, D.H.; Okamoto, R. Morphological and biomechanical characterization of long bones and peri-implant bone repair in type 2 diabetic rats treated with resveratrol. Sci. Rep. 2024, 14, 2860. [Google Scholar] [CrossRef]
- Silva, A.F.; Silva-Reis, R.; Ferreira, R.; Oliveira, P.A.; Faustino-Rocha, A.I.; Pinto, M.D.L.; Coimbra, A.M.; Silva, A.M.S.; Cardoso, S.M. The impact of resveratrol-enriched bread on cardiac remodeling in a preclinical model of diabetes. Antioxidants 2023, 12, 1066. [Google Scholar] [CrossRef] [PubMed]
- Lacalle-Bergeron, L.; Izquierdo-Sandoval, D.; Fernández-Quintela, A.; Portillo, M.P.; Sancho, J.V.; Hernández, F.; Portolés, T. LC-IMS-HRMS for identification of biomarkers in untargeted metabolomics: The effects of pterostilbene and resveratrol consumption in liver steatosis, animal model. Food Res. Int. 2023, 165, 112376. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.; Brockmueller, A.; Buhrmann, C.; Shakibaei, M. Prevention and Co-management of breast cancer-related osteoporosis using resveratrol. Nutrients 2024, 16, 708. [Google Scholar] [CrossRef] [PubMed]
- Darby, J.R.T.; Saini, B.V.; Soo, J.Y.; Lock, M.C.; Holman, S.; Bradshaw, E.L.; McInnes, S.J.P.; Voelcker, N.H.; Macgowan, C.K.; Seed, M.; et al. Subcutaneous maternal resveratrol treatment increases uterine artery blood flow in the pregnant ewe and increases fetal but not cardiac growth. J. Physiol. 2019, 597, 5063–5077. [Google Scholar] [CrossRef] [PubMed]
- Novakovic, R.; Rajkovic, J.; Gostimirovic, M.; Gojkovic-Bukarica, L.; Radunovic, N. Resveratrol and Reproductive Health. Life 2022, 12, 294. [Google Scholar] [CrossRef] [PubMed]
- Gumus, R.; Ercan, N.; Özbilgin, A.; Moğulkoç, M.N.; İmik, H. The effect of dietary supplementation with natural antioxidants on growth performance, antioxidant capacity and intestinal microbial counts of broiler. J. Hell. Vet. Med. Soc. 2024, 75, 7441–7450. [Google Scholar] [CrossRef]
- Meng, X.; Zhou, J.; Zhao, C.N.; Gan, R.Y.; Li, H.B. Health benefits and molecular mechanisms of resveratrol: A narrative review. Foods 2020, 9, 340. [Google Scholar] [CrossRef] [PubMed]
- Rivière, C.; Pawlus, A.D.; Mérillon, J.M. Natural stilbenoids: Distribution in the plant kingdom and chemotaxonomic interest in Vitaceae. Nat. Prod. Rep. 2012, 29, 1317–1333. [Google Scholar] [CrossRef] [PubMed]
- Olivati, C.; de Oliveira Nishiyama, Y.P.; Teodoro de Souza, R.N.; Janzantti, S.; Mauro, M.A.; Gomes, E.; Hermosín-Gutiérrez, I.; da Silva, R.; Lago-Vanzela, E.S. Effect of the pre-treatment and the drying process on the phenolic composition of raisins produced with a seedless Brazilian grape cultivar. Food Res. Int. 2019, 116, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Šuković, D.; Knežević, B.; Gašić, U.; Sredojević, M.; Ćirić, I.; Todić, S.; Mutić, J.; Tešić, Ž. Phenolic Profiles of Leaves, Grapes and Wine of Grapevine Variety Vranac (Vitis vinifera L.) from Montenegro. Foods 2020, 9, 138. [Google Scholar] [CrossRef]
- de Peredo, A.V.G.; Vázquez-Espinosa, M.; Piñeiro, Z.; Espada-Bellido, E.; Ferreiro-González, M.; Barbero, G.F.; Palma, M. Development of a rapid and accurate UHPLC-PDA-FL method for the quantification of phenolic compounds in grapes. Food Chem. 2021, 334, 127569. [Google Scholar] [CrossRef]
- Milinčić, D.D.; Stanisavljević, N.S.; Kostić, A.Ž.; Soković Bajić, S.; Kojić, M.O.; Gašić, U.M.; Barać, M.B.; Stanojević, S.P.; Lj Tešić, Ž.; Pešić, M.B. Phenolic compounds and biopotential of grape pomace extracts from Prokupac red grape variety. LWT 2021, 138, 110739. [Google Scholar] [CrossRef]
- Kavgacı, M.; Yukunc, G.O.; Keskin, M.; Can, Z.; Kolaylı, S. Comparison of Phenolic Profile and Antioxidant Properties of Pulp and Seeds of Two Different Grapes Types (Vitis vinifera L. and Vitis labrusca L.) Grown in Anatolia: The Amount of Resveratrol of Grape Samples. Chem. Afr. 2023, 6, 2463–2469. [Google Scholar] [CrossRef]
- Li, X.; Wu, B.; Wang, L.; Li, S. Extractable amounts of trans-resveratrol in seed and berry skin in Vitis evaluated at the germplasm level. J. Agric. Food Chem. 2006, 54, 8804–8811. [Google Scholar] [CrossRef]
- Casas, L.; Mantell, C.; Rodríguez, M.; de la Ossa, E.J.; Roldán, M.; De Ory, A.I.; Caro, I.; Blandino, A. Extraction of resveratrol from the pomace of Palomino fino grapes by supercritical carbon dioxide. J. Food Eng. 2010, 96, 304–308. [Google Scholar] [CrossRef]
- Barcia, M.T.; Pertuzatti, P.B.; Gómez-Alonso, S.; Godoy, H.T.; Hermosín-Gutiérrez, I. Phenolic composition of grape and winemaking by-products of Brazilian hybrid cultivars BRS Violeta and BRS Lorena. Food Chem. 2014, 159, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Hua, L.H.; Stephen Inbaraj, B.; Chen, B.H. An improved analytical method for determination of trans-resveratrol and related stilbenes in grape skin by QuEChERS coupled with HPLC-PDA-MS. Int. J. Food Sci. Technol. 2021, 56, 6376–6387. [Google Scholar] [CrossRef]
- Takács, K.; Pregi, E.; Vági, E.; Renkecz, T.; Tátraaljai, D.; Pukánszky, B. Processing Stabilization of Polyethylene with Grape Peel Extract: Effect of Extraction Technology and Composition. Molecules 2023, 28, 1011. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Kaur, H.; Kaur, R.; Garg, R.; Prasad, R.; Assouguem, A.; Kara, M.; Bahhou, J. A Review on the Nutritional Value and Health Benefits of Different Parts of Grape (Vitis vinifera L.). Trop. J. Nat. Prod. Res. 2023, 7, 3874–3880. [Google Scholar]
- Fataliyev, H.; Lazgiyev, Y.; İmamguliyeva, M.; Haydarov, E.; Fataliyeva, S.; Huseynova, S.; Agayeva, S.; İsganderova, S.; Askarova, A.; Askarova, İ. Comparative evaluation and studing of some indigenous and introduced red grape varieties. Food Sci. Tehnol. 2023, 17, 2073–8684. [Google Scholar] [CrossRef]
- Inbaraj, B.S.; Hua, L.H.; Chen, B.H. Comparative study on inhibition of pancreatic cancer cells by resveratrol gold nanoparticles and a resveratrol nanoemulsion prepared from grape skin. Pharmaceutics 2021, 13, 1871. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Gonzaga, L.V.; Rizelio, V.M.; Gonçalves, A.E.D.S.S.; Genovese, M.I.; Fett, R. Phenolic compounds and antioxidant activity of seed and skin extracts of red grape (Vitis vinifera and Vitis labrusca) pomace from Brazilian winemaking. Food Res. Int. 2011, 44, 897–901. [Google Scholar] [CrossRef]
- Mohammadparast, B.; Rasouli, M.; Eyni, M. Resveratrol Contents of 27 Grape Cultivars. Appl. Fruit Sci. 2024, 66, 1053–1060. [Google Scholar] [CrossRef]
- Anastasiadi, M.; Pratsinis, H.; Kletsas, D.; Skaltsounis, A.L.; Haroutounian, S.A. Grape stem extracts: Polyphenolic content and assessment of their in vitro antioxidant properties. LWT Food Sci. Technol. 2012, 48, 316–322. [Google Scholar] [CrossRef]
- Jiménez-Moreno, N.; Volpe, F.; Moler, J.A.; Esparza, I.; Ancín-Azpilicueta, C. Impact of extraction conditions on the phenolic composition and antioxidant capacity of grape stem extracts. Antioxidants 2019, 8, 597. [Google Scholar] [CrossRef] [PubMed]
- Bzainia, A.; Dias, R.C.S.; Costa, M.R.P.F.N. A simple process to purify (E)-resveratrol from grape stems with a photo-molecularly imprinted sorbent. Food Bioprod. Process. 2023, 142, 1–16. [Google Scholar] [CrossRef]
- Timperio, A.M.; d’Alessandro, A.; Fagioni, M.; Magro, P.; Zolla, L. Production of the phytoalexins trans-resveratrol and delta-viniferin in two economy-relevant grape cultivars upon infection with Botrytis cinerea in field conditions. Plant Physiol. Biochem. 2012, 50, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Paczkowska-Walendowska, M.; Miklaszewski, A.; Michniak-Kohn, B.; Cielecka-Piontek, J. The antioxidant potential of resveratrol from red vine leaves delivered in an electrospun nanofiber system. Antioxidants 2023, 12, 1777. [Google Scholar] [CrossRef] [PubMed]
- Contreras, M.D.M.; Feriani, A.; Gómez-Cruz, I.; Hfaiedh, N.; Harrath, A.H.; Romero, I.; Castro, E.; Tlili, N. Grapevine shoot extract rich in trans-resveratrol and trans-εagronomy-viniferin: Evaluation of their potential use for cardiac health. Foods 2023, 12, 4351. [Google Scholar] [CrossRef] [PubMed]
- Hoseinpanahi, B.; Bahramnejad, B.; Majdi, M.; Dastan, D.; Ashengroph, M. The Effect of Different Elicitors on Hairy Root Biomass and Resveratrol Production in Wild Vitis vinifera. J. Appl. Biotechnol. Rep. 2020, 7, 25–31. [Google Scholar] [CrossRef]
- Zhou, D.D.; Li, J.; Xiong, R.G.; Saimaiti, A.; Huang, S.Y.; Wu, S.X.; Yang, Z.J.; Shang, A.; Zhao, C.N.; Gan, R.Y.; et al. Bioactive compounds, health benefits and food applications of grape. Foods 2022, 11, 2755. [Google Scholar] [CrossRef]
- Leena, M.M.; Yoha, K.S.; Moses, J.A.; Anandharamakrishnan, C.J.F.B. Edible coating with resveratrol loaded electrospun zein nanofibers with enhanced bioaccessibility. Food Biosci. 2020, 36, 100669. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, X.; Zhang, W.; Cao, D.; Ye, G.; Chen, J.; Lei, Y.; Wei, X. Transcriptomic and metabolomic analyses reveal mechanisms underpinning resistance of Chinese wild grape to Colletotrichum viniferum. Plant Physiol. Biochem. 2024, 215, 108851. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Panaite, S.A.; Tomé-Carneiro, J. Wine’s Phenolic Compounds and Health: A Pythagorean View. Molecules 2020, 25, 4105. [Google Scholar] [CrossRef]
- Bede, T.P.; Jesuz, V.A.; Souza, V.R.; Elias, M.B.; Oliveira, F.L.; Dias, J.F.; Teodoro, A.J.; Azeredo, V.B. Effects of grape juice, red wine and resveratrol on liver parameters of rat submitted high-fat diet. An. Acad. Bras. Cienc. 2020, 92, e20191230. [Google Scholar] [CrossRef]
- Williamson, G.; Carughi, A. Polyphenol content and health benefits of raisins. Nutr. Res. 2010, 30, 511–519. [Google Scholar] [CrossRef]
- Abdel-Hamid, G.A.; Ayuob, N.N. Can raisins ameliorate the hypercholesterolaemia-induced cardiac affection? Folia Morphol. 2015, 74, 106–117. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Anderson, J.W.; Weiter, K.M.; Christian, A.L.; Ritchey, M.B.; Bays, H.E. Raisins compared with other snack effects on glycemia and blood pressure: A randomized, controlled trial. Postgrad. Med. 2014, 126, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Muhlack, R.A.; Potumarthi, R.; Jeffery, D.W. Sustainable wineries through waste valorisation: A review of grape marc utilisation for value-added products. Waste Manag. 2018, 72, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Ghendov-Mosanu, A.; Cojocari, D.; Balan, G.; Patras, A.; Lung, I.; Soran, M.L.; Opriş, O.; Cristea, E.; Sturza, R. Chemometric Optimization of Biologically Active Compounds Extraction from Grape Marc: Composition and Antimicrobial Activity. Molecules 2022, 27, 1610. [Google Scholar] [CrossRef] [PubMed]
- Farhan, M.; Asim, R. The Pharmacological Properties of Red Grape Polyphenol Resveratrol: Clinical Trials and Obstacles in Drug Development. Nutrients 2023, 15, 4486. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Lei, J.; Shao, X.; Fan, Y.; Huang, W.; Lian, W.; Wang, C. Effect of Pretreatment and Drying on the Nutritional and Functional Quality of Raisins Produced with Seedless Purple Grapes. Foods 2024, 13, 1138. [Google Scholar] [CrossRef] [PubMed]
- Kosović, E.; Topiař, M.; Cuřínová, P.; Sajfrtová, M. Stability testing of resveratrol and viniferin obtained from Vitis vinifera L. by various extraction methods considering the industrial viewpoint. Sci. Rep. 2020, 10, 5564. [Google Scholar] [CrossRef]
- Negro, C.; Aprile, A.; Luvisi, A.; De Bellis, L.; Miceli, A. Antioxidant activity and polyphenols characterization of four monovarietal grape pomaces from Salento (Apulia, Italy). Antioxidants 2021, 10, 1406. [Google Scholar] [CrossRef] [PubMed]
- Sinrod, A.J.; Shah, I.M.; Surek, E.; Balanovarile, D. Uncovering the promising role of grape pomace as a modulator of the gut microbiome: An in-depth review. Heliyon 2023, 9, e20499. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Santos, P.; Nobre, C.; Rodrigues, R.M.; Genisheva, Z.; Botelho, C.; Teixeira, J.A. Extraction of phenolic compounds from grape pomace using ohmic heating: Chemical composition, bioactivity and bioaccessibility. Food Chem. 2024, 436, 137780. [Google Scholar] [CrossRef]
- Zemni, H.; Khiari, R.; Lamine, M.; Houimli, Y.; Chenenaoui, S.; Ben Salem, A. Grape Marc Skin Valorization: From Waste to Valuable Polyphenol Source. Chem. Afr. 2024, 7, 765–776. [Google Scholar] [CrossRef]
- Zwingelstein, M.; Draye, M.; Besombes, J.L.; Piot, C.; Chatel, C. trans-Resveratrol and trans-ε-Viniferin in Grape Canes and Stocks Originating from Savoie Mont Blanc Vineyard Region: Pre-extraction Parameters for Improved Recovery. ACS Sustain. Chem. Eng. 2019, 7, 8310–8316. [Google Scholar] [CrossRef]
- Kiene, M.; Zaremba, M.; Fellensiek, H.; Januschewski, E.; Juadjur, A.; Jerz, G.; Winterhalter, P. In Silico-Assisted Isolation of trans-Resveratrol and trans-ε-Viniferin from Grapevine Canes and Their Sustainable Extraction Using Natural Deep Eutectic Solvents (NADES). Foods 2023, 12, 4184. [Google Scholar] [CrossRef] [PubMed]
- Noronha, H.; Silva, A.; Garcia, V.; Billet, K.; Dias, A.C.; Lanoue, A.; Gallusci, P.; Gerós, H. Grapevine woody tissues accumulate stilbenoids following bud burst. Planta 2023, 258, 118. [Google Scholar] [CrossRef]
- Besrukow, P.; Orbach, A.; Schweiggert, R. Modulating the Photostability of (E)-Resveratrol in Grape Cane Extract Formulations. ACS Food Sci. Technol. 2024, 4, 625–632. [Google Scholar] [CrossRef]
- Duarte, H.; Aliaño-González, M.J.; Cantos-Villar, E.; Faleiro, L.; Romano, A.; Medronho, B. Sustainable extraction of polyphenols from vine shoots using deep eutectic solvents: Influence of the solvent, Vitis sp., and extraction technique. Talanta 2024, 267, 125135. [Google Scholar] [CrossRef]
- Fernández-Bedmar, Z.; Anter, J.; Alonso-Moraga, A.; Delgado de la Torre, P.; Luque de Castro, M.D.; Millán-Ruiz, Y.; Sánchez-Frías, M.; Guil-Luna, S. Red and White Wine Lees Show Inhibitory Effects on Liver Carcinogenesis. Mol. Nutr. Food Res. 2019, 63, 1800864. [Google Scholar] [CrossRef] [PubMed]
- Dujmić, F.; Kovačević Ganić, K.; Ćurić, D.; Karlović, S.; Bosiljkov, T.; Ježek, D.; Vidrih, R.; Hribar, J.; Zlatić, E.; Prusina, T.; et al. Non-thermal ultrasonic extraction of polyphenolic compounds from red wine lees. Foods 2020, 9, 472. [Google Scholar] [CrossRef] [PubMed]
- López-Fernández-Sobrino, R.; Margalef, M.; Torres-Fuentes, C.; Ávila-Román, J.; Aragonès, G.; Muguerza, B.; Bravo, F.I. Enzyme-assisted extraction to obtain phenolic-enriched wine lees with enhanced bioactivity in hypertensive rats. Antioxidants 2021, 10, 517. [Google Scholar] [CrossRef] [PubMed]
- Chioru, A.; Chiselita, N.; Suhodol, N.; Boiştean, A.; Paladi, D.; Capcanari, T.; Chirsanova, A. physico-chemical and microbiological profile of wine lees of red wines from local grapes varieties. Food Nutr. Sci. 2023, 14, 1133–1148. [Google Scholar] [CrossRef]
- Melo, F.D.O.; Ferreira, V.C.; Barbero, G.F.; Carrera, C.; Ferreira, E.D.S.; Umsza-Guez, M.A. Extraction of bioactive compounds from wine lees: A systematic and bibliometric review. Foods 2024, 13, 2060. [Google Scholar] [CrossRef]
- Gümüş, T.; Altan Kamer, D.D.; Kaynarca, G.B. Investigating the potential of wine lees as a natural colorant and functional ingredient in jelly production. J. Sci. Food Agric. 2024, 104, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, M.B.S.; Marques, M.P.; Correia, F.; Pires, P.C.; Correia, M.; Makvandi, P.; Varela, C.; Cefali, L.C.; Mazzola, P.G.; Veiga, F.; et al. Wine industry by-products as a source of active ingredients for topical applications. Phytochem. Rev. 2024, 1–35. [Google Scholar] [CrossRef]
- Gorena, T.; Mardones, C.; Vergara, C.; Saez, V.; von Baer, D. Evaluation of the Potential of Grape canes as a Source of Bioactive Stilbenoids. In Advances in Wine Research; American Chemical Society: Washington, DC, USA, 2015; Volume 1205, pp. 347–363. [Google Scholar]
- Labois, C.; Stempien, E.; Schneider, J.; Schaeffer-Reiss, C.; Bertsch, C.; Goddard, M.L.; Chong, J. Comparative study of secreted proteins, enzymatic activities of wood degradation and stilbene metabolization in grapevine botryosphaeria dieback fungi. J. Fungi 2021, 7, 568. [Google Scholar] [CrossRef]
- D’Eusanio, V.; Genua, F.; Marchetti, A.; Morelli, L.; Tassi, L. Characterization of Some Stilbenoids Extracted from Two Cultivars of Lambrusco—Vitis vinifera Species: An Opportunity to Valorize Pruning Canes for a More Sustainable Viticulture. Molecules 2023, 28, 4074. [Google Scholar] [CrossRef] [PubMed]
- Rajha, H.N.; Boussetta, N.; Louka, N.; Maroun, R.G.; Vorobiev, E. A comparative study of physical pretreatments for the extraction of polyphenols and proteins from vine shoots. Food Res. Int. 2014, 65, 462–468. [Google Scholar] [CrossRef]
- Taillis, D.; Becissa, O.; Pébarthé-Courrouilh, A.; Renouf, E.; Palos-Pinto, A.; Richard, T.; Cluzet, S. Antifungal activities of a grapevine byproduct extract enriched in complex stilbenes and stilbenes metabolization by Botrytis cinerea. J. Agric. Food Chem. 2023, 71, 4488–4497. [Google Scholar] [CrossRef] [PubMed]

| Abiotic Factors | References |
|---|---|
| Variety | [33,34] |
| Climatic conditions in the growing areas | [25,35,36,37,38,39] |
| Grapevine health | [40] |
| Vineyard management | [41,42] |
| Winemaking techniques | [36,43,44,45,46] |
| Wine ageing method | [12,45,47,48] |
| Terroir effect | [49,50,51,52] |
| Harvest time | [11,53] |
| Treatment with ultrasound (US), light-emitting diode (LED), ultraviolet (UV) irradiation, or macronutrients and fungicides | [54,55,56,57] |
| Storage conditions | [8,58] |
| Fractions | Content in Resveratrol | Method of Analysis * | References |
|---|---|---|---|
| Whole grape | 3.196349 mg/L | HPLC-MS | [54] |
| 3.06 ± 0.51 mg/kg FW | HPLC-ESI-MS/MS | [78] | |
| 4.8544547 mg/L-resveratrol hexoxide; 3.6558239 mg/L-RSV; 4.9643292 mg/L RSV tetramer; 4.5647856 mg/L-RSV dimer; 4.7645574 mg/LRSV trimer | UHPLC-LTQ-MS | [79] | |
| 0.2–9.1 mg/L (PDA) 0.04–9.1 mg/L (FL) | UPLC-PDA-FL | [80] | |
| 111.0 mg/kg DW | UHPLC-MS/MS | [81] | |
| 119 mg/K−1 FW | HPLC | [7] | |
| by 118.6 mg/kg up to 1018.9 mg/kg, DW depending on varieties | HPLC-UV | [82] | |
| Skin | 50–100 mg/kg FW | HPLC | [83] |
| 49,100 mg/kg DW | HPLC | [84] | |
| 48.99 ± 2.69 mg/kg DW | HPLC-DAD-ESI-MS | [85] | |
| 9.67 mg/L−1 (t-RSV) 1.83 mg/L−1 (t-RSV) | by QuEChERS method coupled with an HPLC-PDA-MS | [86] | |
| 57.7 mg/kg DW | UHPLC/MS | [81] | |
| 9.152 mg/L to 11.083 mg/L (trans-resveratrol depending on variety and period); from 7.119 mg/L to 8.071 mg/L (cis-resveratrol depending on variety and period) | HPLC | [25] | |
| 21.7 mg/L | HPLC | [87] | |
| 11.7 to 12.96 mg/kg−1 FW | HPLC | [88] | |
| 0.75–8.25 mg/kg FW | HPLC | [89] | |
| 11.02 mg/L | HPLC-MS | [90] | |
| Seed | 8300 mg/kg DW | HPLC | [84] |
| 37.5 ± 0.08 mg/kg DW (Isabel Variety) 11.1 ± 0.02 mg/kg DW (Sangiovese Variety) 14.2 ± 0.07 mg/kg DW (Negro Amaro Variety) | HPLC | [91] | |
| 2.8 mg/kg DW | UHPLC-orbitrap MS | [81] | |
| 0.31–5.7 mg/kg FW | HPLC | [89] | |
| from 36 mg/kg to 375 mg/kg DW, depending on the variety | HPLC-UV | [82] | |
| 92.312.43 ± 2404.19 mg/kg, DW | HPLC | [30] | |
| 4.97 mg/kg−1 FW | HPLC | [92] | |
| Pulp | 45 mg/kg, DW | HPLC-UV | [82] |
| Stem | 900 mg/kg DW | HPLC | [84] |
| 5–0.078 mg/L | HPLC/LC-MS-MS | [93] | |
| 122 ± 16 mg/kg DW | HPLC-DAD | [94] | |
| 3700 mg/kg DW | HPLC | [95] | |
| Leaf | 0.18 mg/L | HPLC-MS | [96] |
| 3060 ± 90 mg/kg DW | HPLC | [97] | |
| 0.01–0.25 mg/kg FW | HPLC | [89] | |
| Grapevine shoots | 27,400 ± 300 mg/kg DW | HPLC-QTOF-MS | [98] |
| 190 ± 0.34 mg/kg DW | TLC and HPLC | [99] |
| Fractions | CONTENT in Resveratrol | Method of Identification/Determination * | References | |
|---|---|---|---|---|
| Grape products | Wine | 6.9 to 12.6 mg/L | HPLC | [5] |
| 64 mg/L | HPLC | [110] | ||
| Juice | 4.4–7.0 mg/L (grape juice) 12.4–21.3 mg/L (concentrated juice) | HPLC | [5] | |
| Grape skin powder | 17.87 mg/L | HPLC-MS | [89] | |
| 0.313 mg/kg DW (Stir) 0.044 mg/kg DW (Sox) 0.178 mg/kg DW (LC-MS/MS) | LC-MS/MS | [86] | ||
| Raisins | 0.40 ± 0.04 mg/kg FW | HPLC-ESI-MS/MS | [77] | |
| 8993 ± 391 mg/kg DW 16.544 ± 440 mg/kg DW 8798 ± 137 mg/kg DW | UPLC-VION-IMS-QTOF—the physical pretreatment using a motorised rotating drum (PT) the drying agent treatment group (DT) in the control group (CK), the grape samples received no pretreatment | [111] | ||
| By-products | Grape pomace | 16,100 mg/kg DS | HPLC | [83] |
| 0.042–0.653 mg/L | HPLC-DAD/MS | [112] | ||
| 90 ± 0.04 mg/g DW | HPLC/MS | [113] | ||
| 0.7–21.7 mg/kg DM | UHPLC-MS/MS | [80] | ||
| 26.3 ± 0.5 mg/kg DW | HPLC | [114] | ||
| 2.38 ± 0.2 mg/L | HPLC | [115] | ||
| 0.80 mg/kg DM | UPLC | [116] | ||
| Grape canes | 3450 mg/kg−1 DW (Pinot Noir) 5361 mg/kg−1 DW (Gewurztraminer) | HPLC-UV | [117] | |
| 5298.1 mg/kg−1 DW | HPLC | [51] | ||
| 419.01–425.60 mg/kg DW (Pinot Gris) 282.19 ± 4.14 mg/kg DW (Sauvignon Blanc) 425.60 ± 5.98 mg/kg DW (Cabernet Sauvignon) | HPLC | [62] | ||
| 550–396 mg/kg DW | UPLC (HPLC-ESI-MS) | [118] | ||
| 69.1 to 436.5 mg/kg DW−1 | HPLC—DAD | [119] | ||
| 37 ± 0.2 mg/kg DW | HPLC—DAD | [120] | ||
| 9.50 mg·L−1 | HPLC | [121] | ||
| Wine lees | 104 mg/kg (Red wine lees) 30 mg/kg (White wine lees) | HPLC-DAD | [122] | |
| 40 ± 0.00 mg/kg DM (Merlot) 110 ± 0.01 mg/kg DM (Vranac) | HPLC-MS/MS | [123] | ||
| 2.95 ± 0.01 mg/kg (RSV) 4.60 ± 0.02 mg/kg (t-RSV) | UHPLC | [124] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Căpruciu, R. Resveratrol in Grapevine Components, Products and By-Products—A Review. Horticulturae 2025, 11, 111. https://doi.org/10.3390/horticulturae11020111
Căpruciu R. Resveratrol in Grapevine Components, Products and By-Products—A Review. Horticulturae. 2025; 11(2):111. https://doi.org/10.3390/horticulturae11020111
Chicago/Turabian StyleCăpruciu, Ramona. 2025. "Resveratrol in Grapevine Components, Products and By-Products—A Review" Horticulturae 11, no. 2: 111. https://doi.org/10.3390/horticulturae11020111
APA StyleCăpruciu, R. (2025). Resveratrol in Grapevine Components, Products and By-Products—A Review. Horticulturae, 11(2), 111. https://doi.org/10.3390/horticulturae11020111





