Dendrobium huoshanense In Vitro Culture and Selenium Metabolism: Speciation Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Optimization of the Tissue Culture System for D. huoshanense
2.2.1. Influence of Different Culture Media on D. huoshanense Development
2.2.2. Effects of pH Variation on the Growth of D. huoshanense
2.2.3. Effects of Plant Growth Regulators on Rooting and Seedling Vigor in D. huoshanense
2.3. Physiological and Metabolic Responses of D. huoshanense Under Exogenous Selenium Gradient Regulation
2.3.1. Effects of Sodium Selenite Concentration on Growth, Physiological Characteristics, Selenium Accumulation, and Polysaccharide Biosynthesis in Tissue-Cultured D. huoshanense
2.3.2. Determination of Selenium Content in D. huoshanense
2.3.3. Determination of Polysaccharide Content in D. huoshanense
2.3.4. Determination of Physiological Indices in D. huoshanense
2.4. Spatiotemporal Distribution Patterns of Selenium in D. huoshanense
2.5. Speciation Analysis of Selenium in Tissue-Cultured Plantlets of D. huoshanense
2.5.1. Determination of Organic and Inorganic Selenium Content
2.5.2. Extraction and Quantification of Selenium-Containing Polysaccharides
2.5.3. Quantification of Selenoproteins
2.5.4. Analysis of Selenoamino Acid Content
2.6. Statistical Analyses
3. Results
3.1. Investigation of Factors Influencing Rooting and Seedling Vigor in D. huoshanense
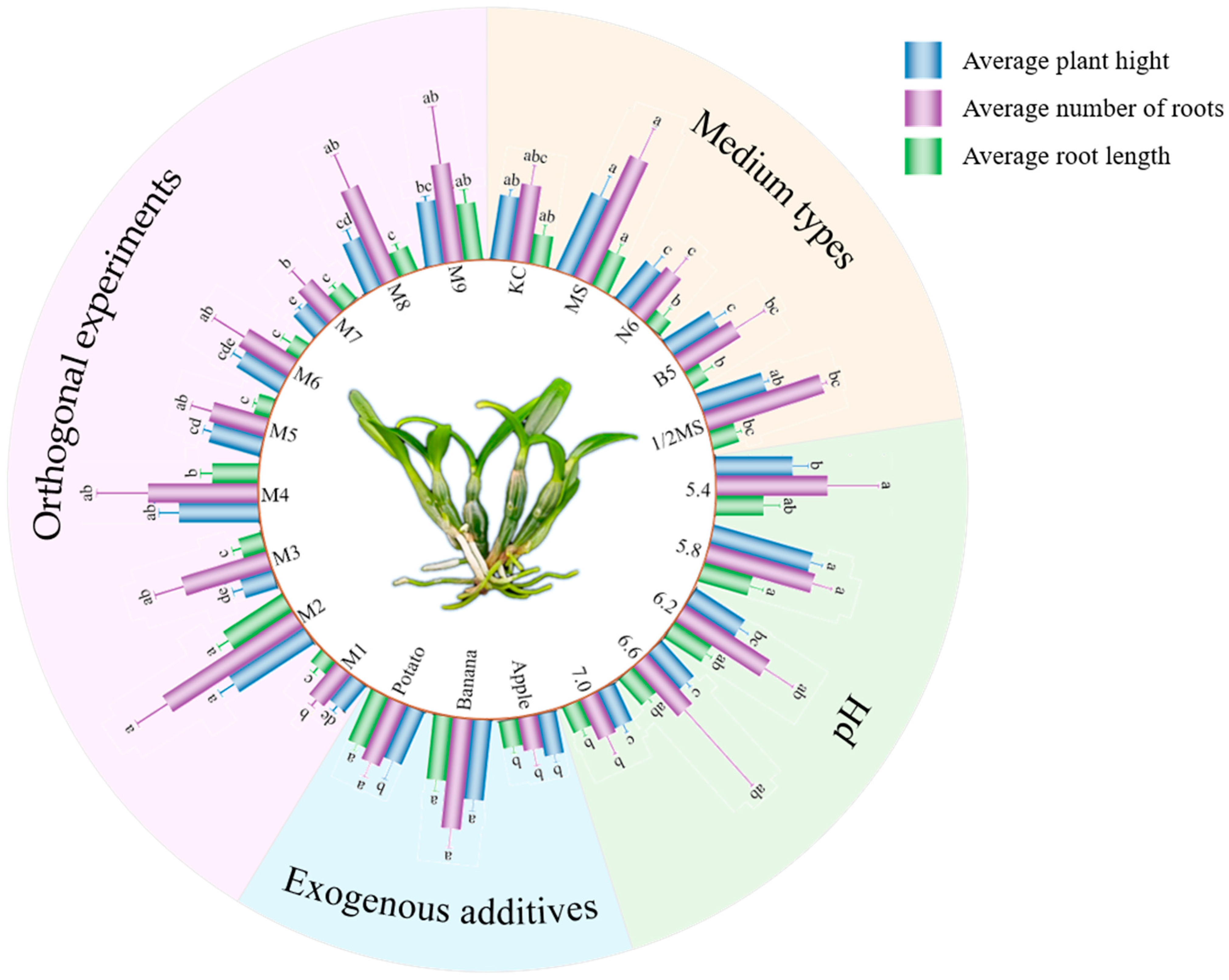
3.1.1. Effects of Culture Medium Types on Growth Performance of D. huoshanense
3.1.2. Impact of pH Variation on In Vitro Growth of D. huoshanense
3.1.3. Effects of Exogenous Additives on the Growth of D. huoshanense
3.1.4. Orthogonal Array Analysis of Phytohormone Combinations on Seedling Growth
3.2. Physiological and Metabolic Responses to Graded Selenium Supplementation
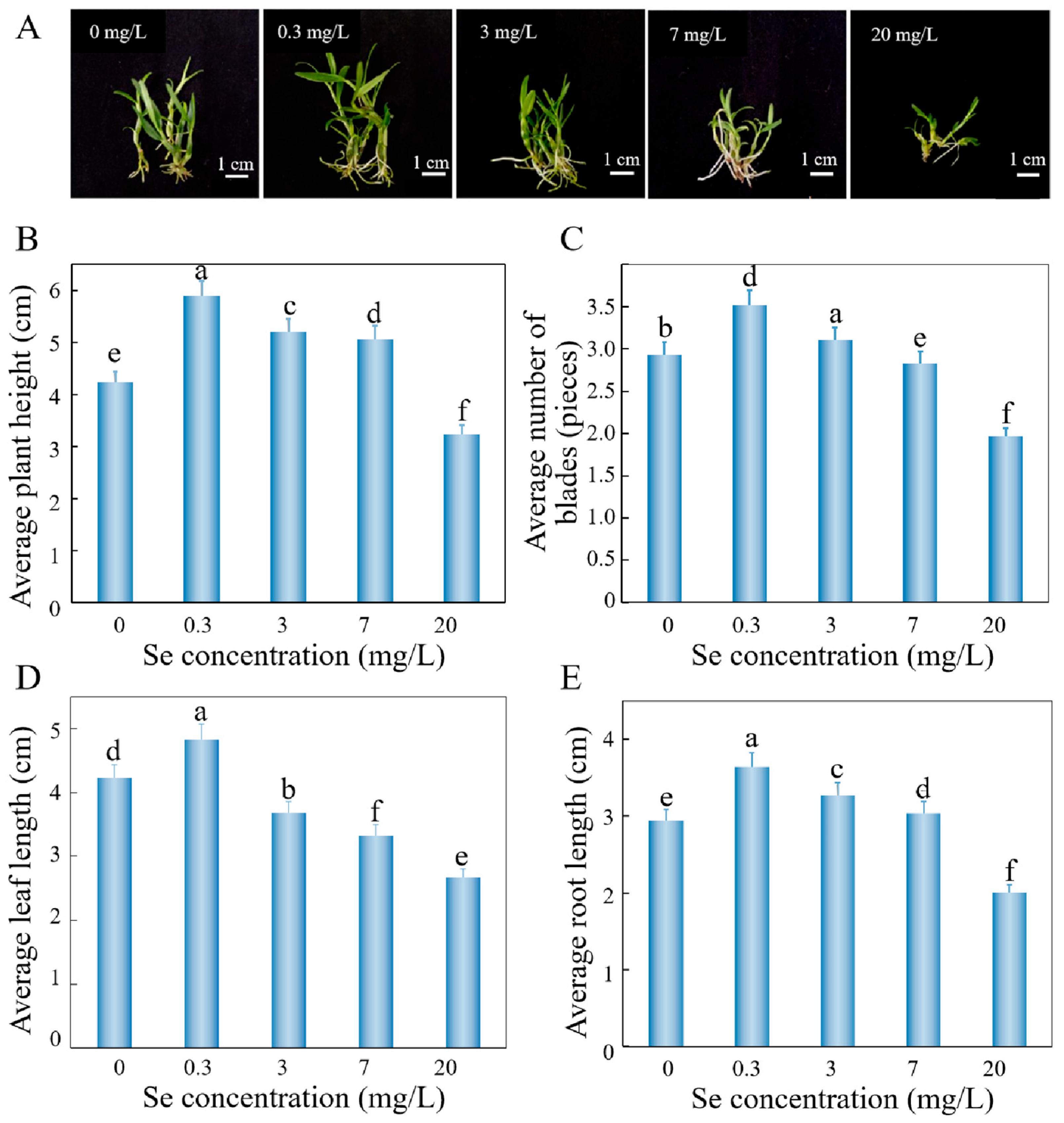
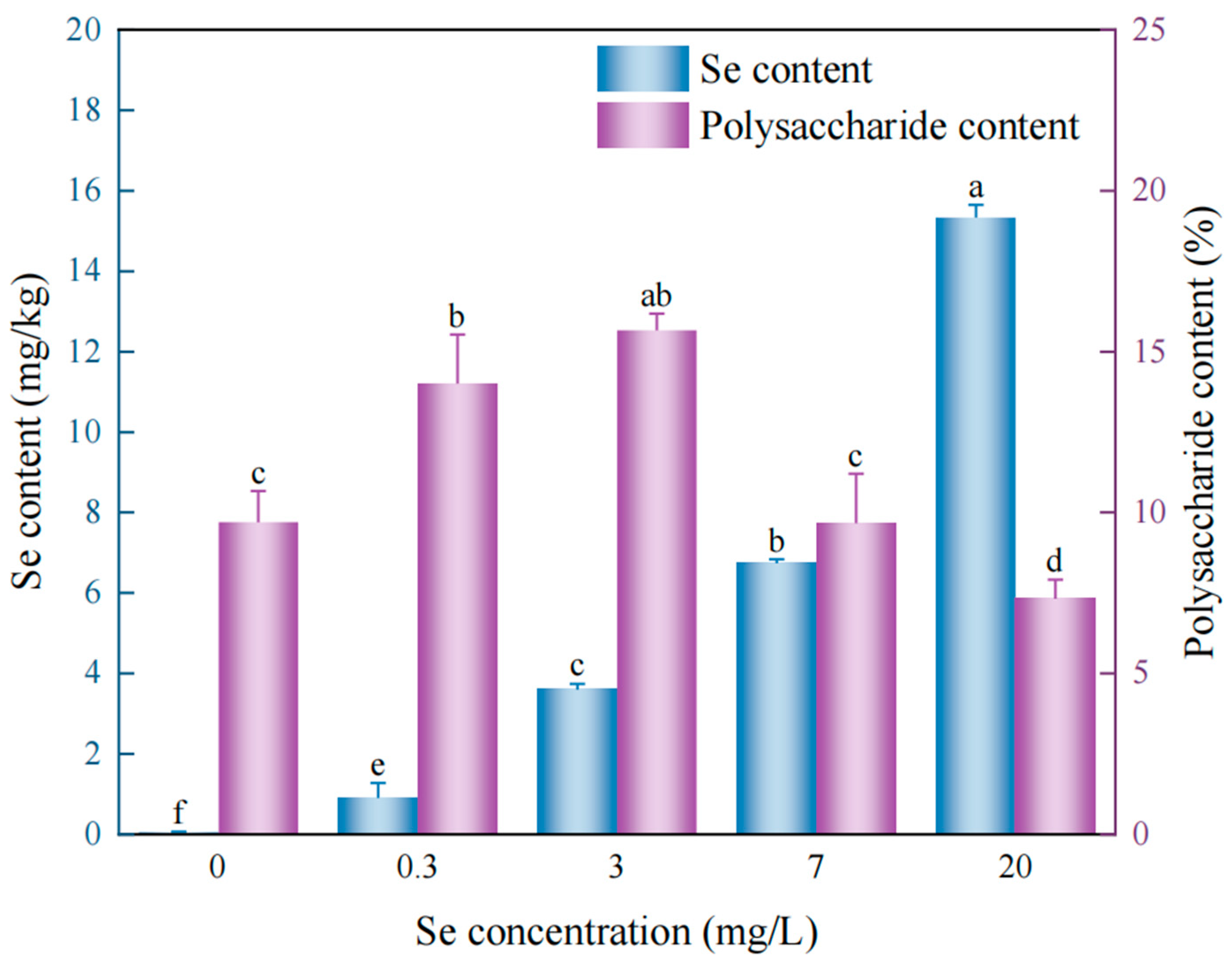
3.2.1. Concentration-Dependent Effects of Sodium Selenite on Growth, Polysaccharide Biosynthesis, and Selenium Accumulation in D. huoshanense
3.2.2. Impact of Sodium Selenite Gradients on Physiological Parameters in D. huoshanense
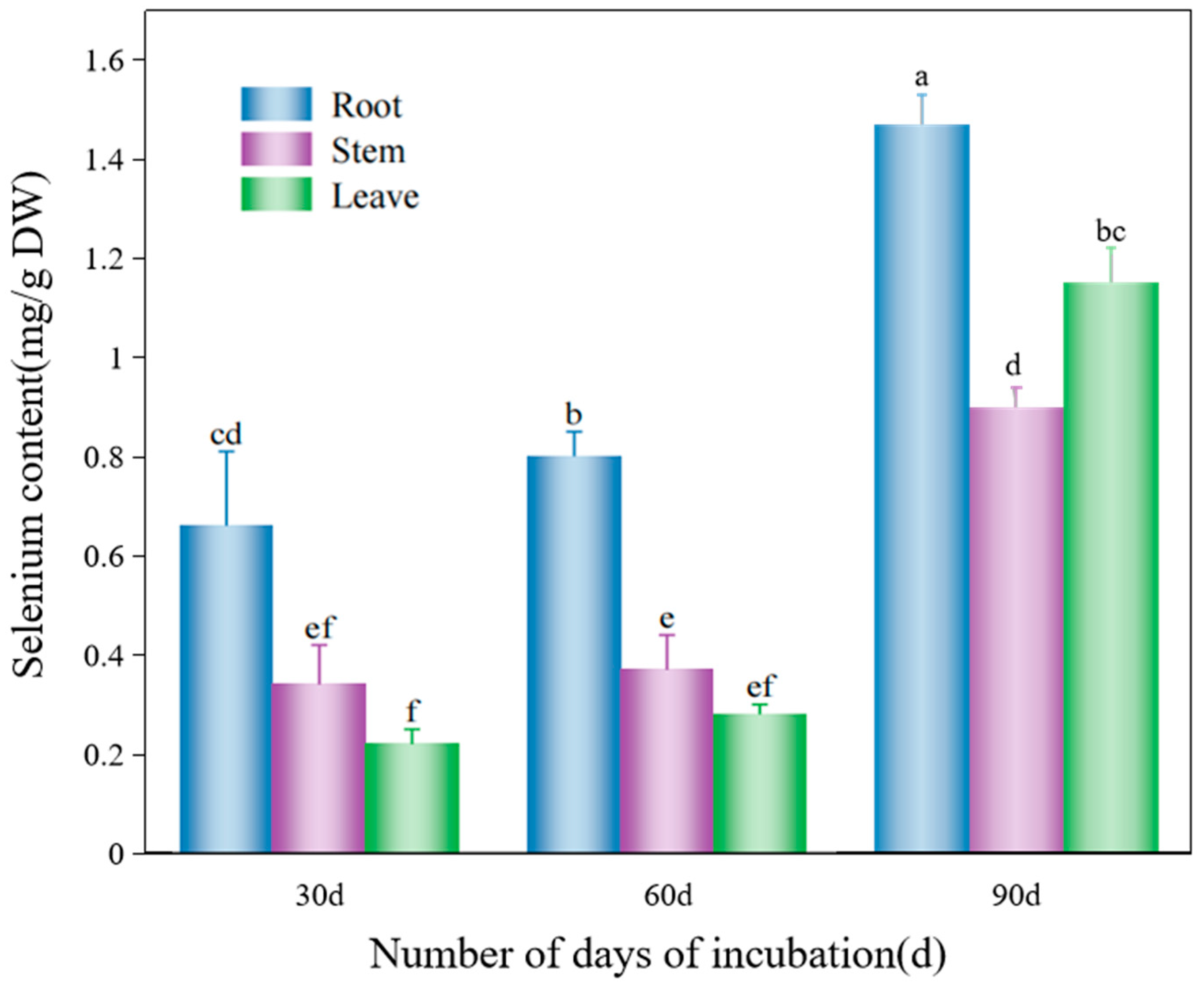
3.3. Spatiotemporal Dynamics of Selenium Uptake and Accumulation in D. huoshanense
3.4. Chemical Speciation of Selenium in D. huoshanense
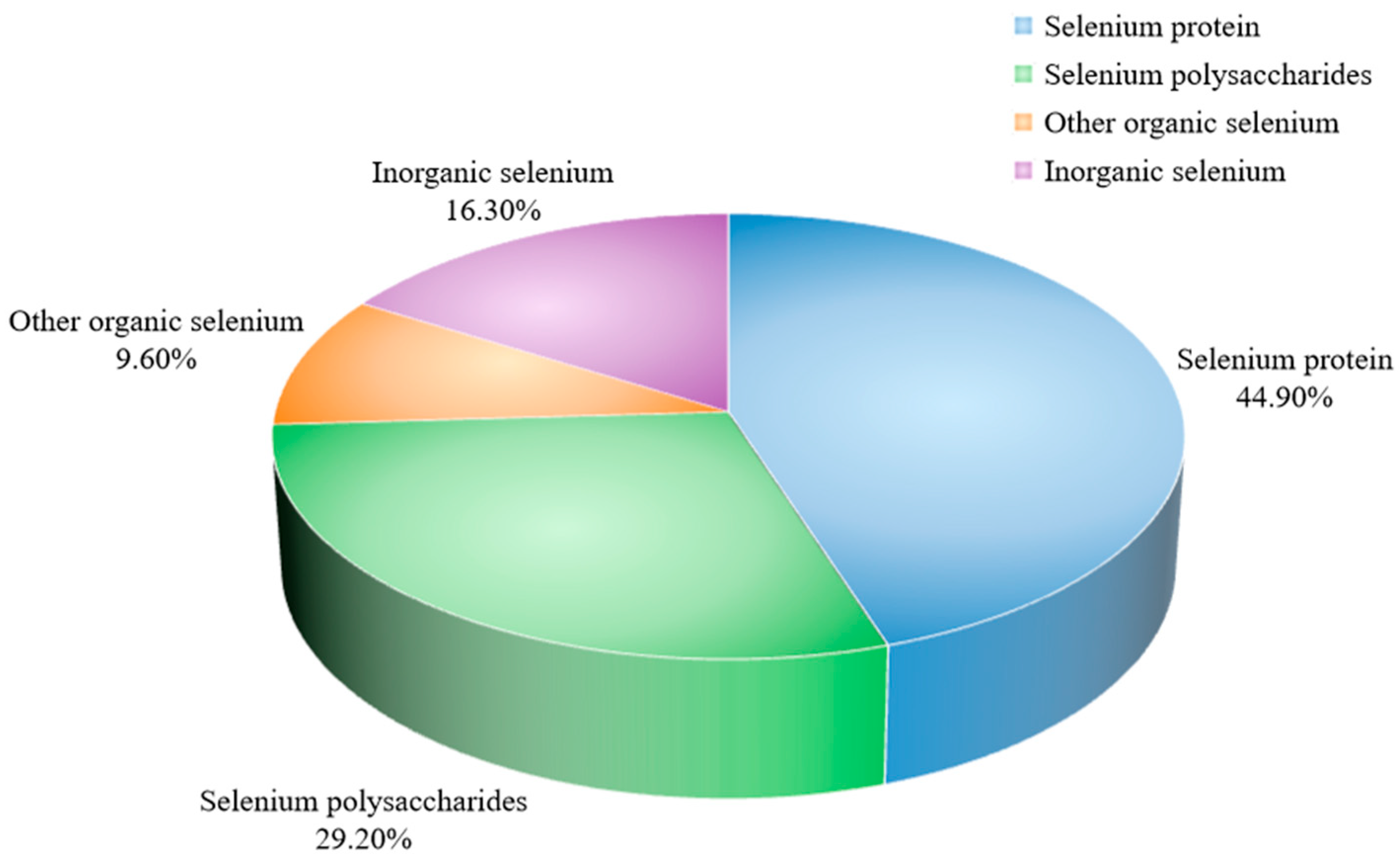
3.4.1. Quantification of Selenium Species in Selenium-Enriched D. huoshanense
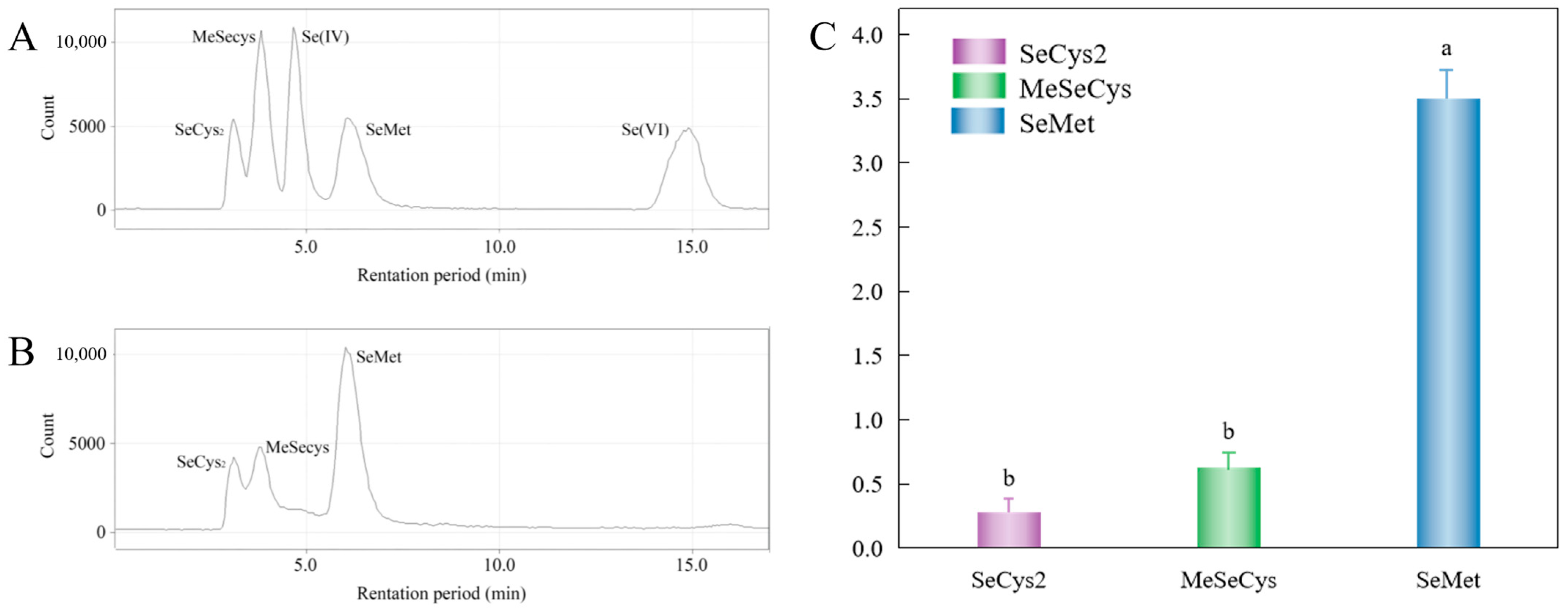
3.4.2. Distribution Profile of Selenoamino Acids in Selenium-Biofortified D. huoshanense
4. Discussion
4.1. Synergistic Effects of Phytohormones and Organic Supplements on Rooting and Seedling Vigor in D. huoshanense Plantlets
4.2. Physiological and Metabolic Response Mechanisms to Graded Selenium Supplementation in D. huoshanense
4.3. Spatiotemporal Dynamics and Organ-Specific Allocation Mechanisms of Selenium Accumulation in D. huoshanense
4.4. Chemical Speciation and Biotransformation Pathways of Selenium in Selenium-Enriched D. huoshanense
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SeMet | Selenomethionine |
| MeSeCys | Methylselenocysteine |
| SeCys2 | Selenocysteine |
| NAA | 1-Naphthaleneacetic acid |
| IBA | Indole-3-butyric acid |
| SOD | Superoxide dismutase |
| POD | Peroxidase |
| CAT | Catalase |
| ROS | Reactive oxygen species |
| MS | Murashige and Skoog medium |
| KC | Knudson C medium |
| B5 | Gamborg B5 medium |
| N6 | Chu’s N-6 medium |
References
- Sarsaiya, S.; Jain, A.; Shu, F.; Jia, Q.; Gong, Q.H.; Wu, Q.; Shi, J.S.; Chen, J.S. Unveiling the potential of dendrobine: Insights into bioproduction, bioactivities, safety, circular economy, and future prospects. Crit. Rev. Biotechnol. 2025, 25, 1268–1286. [Google Scholar] [CrossRef]
- Wan, J.; Gong, X.; Wang, F.; Wen, C.; Wei, Y.; Han, B.; Ouyang, Z. Comparative analysis of chemical constituents by HPLC–ESI–MSn and antioxidantactivities of Dendrobium huoshanense and Dendrobium officinale. Biomed. Chromatogr. 2022, 36, e5250. [Google Scholar] [CrossRef]
- Hao, Q.; Jiang, L.; Ma, J.; Wang, H.K.; Liu, Y.; Xu, Q.C.; Li, S.Z.; Han, S.; Zheng, Q.S.; Fan, X.C.; et al. Dendrobium huoshanense C. Z. Tang and S. J. Cheng can be prepared as a food with the ability to prevent and treat hyperuricaemia. Front. Nutr. 2025, 12, 1518014. [Google Scholar] [CrossRef]
- Xu, H.J.; Liu, Z.; Xu, W.; Zhang, Y.F. Beneficial in vitro effects of polysaccharide and non-polysaccharide components of Dendrobium huoshanense on gut microbiota of rats with type 1 diabetes as opposed to metformin. Molecules 2024, 29, 2791. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Ge, R.P.; Wu, J.; Cai, X.; Deng, G.H.; Lv, J.H.; Ma, M.Z.; Yu, N.J.; Yao, L.; Peng, D.Y. Structural characterization and improves cognitive disorder in ageing mice of a glucomannan from Dendrobium huoshanense. Int. J. Biol. Macromol. 2024, 269, 131995. [Google Scholar] [CrossRef]
- Gao, L.L.; Wang, F.; Hou, T.T.; Geng, C.Y.; Xu, T.; Han, B.X.; Liu, D. Dendrobium huoshanense C.Z. Tang et S.J. Cheng: A review of its traditional uses, phytochemistry, and pharmacology. Front. Pharmacol. 2022, 13, 920823. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.A.; Cardoso, J.C.; Dobránszki, J.; Zeng, S.J. Dendrobium micropropagation: A review. Plant Cell Rep. 2015, 34, 671–704. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y. Research on the In Vitro Rapid Propagation of Dendrobium huoshanense. Master’s Thesis, Guizhou University, Guiyang, China, May 2024. [Google Scholar] [CrossRef]
- Jin, R.; An, C.; Wang, B.R.; Liu, C.B.; Fan, X.P.; Zhang, Z.Z. Bioreactor scale-up and bioactivity evaluation of Psammosilene tunicoides hairy roots. Asian J. Agric. Biol. 2025, 4, e2025067. [Google Scholar] [CrossRef]
- Omid, A.; Somayeh, S.; Azita, H.; Foad, A.; Esmaeil, Y.R.; Shadab, S. Effects of selenium supplementation on serum C reactive protein level: A systematic review and meta-analysis of randomized controlled clinical trials. Obes. Med. 2020, 17, 100182. [Google Scholar] [CrossRef]
- Huang, Z.; Rose, A.H.; Hoffmann, P.R. The role of selenium in inflammation and immunity: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signaling 2012, 16, 705–743. [Google Scholar] [CrossRef]
- Tan, L.C.; Nancharaiah, Y.V.; van Hullebusch, E.D.; Lens, P.N.L. Selenium: Environmental significance, pollution, and biological treatment technologies. Biotechnol. Adv. 2016, 34, 886–907. [Google Scholar] [CrossRef]
- Gu, L.Y.; Zhao, W.Z.; Hu, J.C.; Jye, M.W.; Yu, Z.P. Selenium-Containing Peptides from Foodstuff: Preparation, Bioavailability, Bioactivities, and Future Perspectives. J. Agric. Food Chem. 2025, 73, 24501–24516. [Google Scholar] [CrossRef]
- Li, H.A.; Liu, H.Q.; Tang, X.R.; Deng, Z.Y.; Li, H.Y. From Soil to Table: A Comprehensive Review of Selenium-Fortified Foods. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70250. [Google Scholar] [CrossRef]
- Tangjaidee, P.; Swedlund, P.; Xiang, J.Q.; Yin, H.Q.; Quek, S.Y. Selenium-enriched plant foods: Selenium accumulation, speciation, and health functionality. Front. Nutr. 2023, 9, 962312. [Google Scholar] [CrossRef]
- Xie, M.H.; Sun, X.Y.; Li, P.; Shen, X.C.; Fang, Y. Selenium in cereals: Insight into species of the element from total amount. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2914–2940. [Google Scholar] [CrossRef]
- Hachemi, M.A.; Cardoso, D.; De Marco, M.; Geraert, P.A.; Briens, M. Inorganic and organic selenium speciation of selenoyeasts used as feed additives: New insights from elemental selenium determination. Biol. Trace Elem. Res. 2023, 201, 5839–5847. [Google Scholar] [CrossRef]
- Cheng, K.X.; Sun, Y.; Liu, B.W.; Ming, J.J.; Wang, L.L.; Xu, C.F.; Xiao, Y.Y.; Zhang, C.; Shang, L.C. Selenium modification of natural products and its research progress. Foods 2023, 12, 3773. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.Z.; Yan, J.P.; Qin, Y.; Xu, J.M.; Shohag, M.J.I.; Wei, Y.N.; Gu, M.H. Effect of different forms of selenium on the physiological response and the cadmium uptake by rice under cadmium stress. Int. J. Environ. Res. Public Health 2020, 17, 6991. [Google Scholar] [CrossRef] [PubMed]
- Puccinelli, M.; Malorgio, F.; Terry, L.A.; Tosetti, R.; Rosellini, I.; Pezzarossa, B. Effect of selenium enrichment on metabolism of tomato (Solanum lycopersicum) fruit during post-harvest ripening. J. Sci. Food Agric. 2018, 99, 2463–2472. [Google Scholar] [CrossRef]
- Yang, W.Q.; Jiang, T.T.; Wang, Y.Q.; Wang, X.J.; Wang, R. Combined transcriptomics and metabolomics analysis reveals the effect of selenium fertilization on Lycium barbarum fruit. Molecules 2023, 28, 8088. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.L.; Zhou, M.G.; Li, B.; Fu, Y.A.; Shi, Z.Y.; Ji, W.Q.; Zhang, R.; Wang, Z.H. Humic acid chelated selenium is suitable for wheat biofortification. J. Sci. Food Agric. 2023, 103, 4887–4898. [Google Scholar] [CrossRef]
- Zhang, H.B.; He, D.; Li, X.L.; Dun, B.C.; Wu, D.; Huang, G.Y. The establishment of rapid propagation system of ‘RED SUN’ Phalaenopsis aphrodite. Sustainability 2022, 14, 15305. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.W.; Li, N.Q.; Jin, Q.; Anbazhakan, R.; Dai, Y.F.; Xiang, Z.X.; Gao, J.Y. Ecological specificity of fungi on seedling establishment in Dendrobium huoshanense: A narrow distributed medicinal orchid. Mycorrhiza 2025, 35, 41. [Google Scholar] [CrossRef] [PubMed]
- Tie, M.; Zang, S.L.; Zhang, W.; Li, J.; Sun, T.B.; Li, H.W. Study on the Method of Using ICP-MS to Determine Se in the Edible Fungi. Spectrosc. Spectr. Anal. 2006, 26, 551–553. [Google Scholar]
- Zhao, Y.Q.; Zheng, J.P.; Yang, M.W.; Yang, G.D.; Wu, Y.N.; Fu, F.F. Speciation analysis of selenium in rice samples by using capillary electrop hores is inductively coupled plasma mass spectrometry. Talanta 2011, 84, 983–988. [Google Scholar] [CrossRef]
- Xu, M.Y. Optimization of Industrial Seedling Technology System of Dendrobium houshanense. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, June 2023. [Google Scholar] [CrossRef]
- Zhang, F.W. Study on the Tissue Culture and Temperature Stress Response Mechanisms of Dendrobium huoshanense. Master’s Thesis, Nanjing Normal University, Nanjing, China, March 2022. [Google Scholar] [CrossRef]
- Lee, P.L.; Chen, J.T. Plant regeneration via callus culture and subsequent in vitro flowering of Dendrobium huoshanense. Acta Physiol. Plant 2014, 36, 2619–2625. [Google Scholar] [CrossRef]
- Liu, Z.K.; Min, C.; Dong, H.; Zhang, Z.S. Improvement of adventitious root formation in Sedum aizoon L. and the production of flavonoids. S. Afr. J. Bot. 2021, 137, 483–491. [Google Scholar] [CrossRef]
- Klanrit, P.; Lila, K.; Netsawang, P.; Siangsanor, P.; Thanonkeo, P.; Thanonkeo, S. Effect of organic additives on the micropropagation of Asparagus officinalis. Horticulturae 2023, 9, 1244. [Google Scholar] [CrossRef]
- Nambiar, N.; Tee, C.S.; Maziah, M. Effects of organic additives and different carbohydrate sources on proliferation of protocorm like bodies in Dendrobium alya Pink. Plant Omics 2012, 5, 10–18. [Google Scholar]
- Rao, S.; Yu, T.; Cong, X.; Zhang, W.W.; Zhu, Z.Z.; Liao, Y.L.; Ye, J.B.; Cheng, S.Y.; Xu, F. Effects of selenate applied at two growth stages on the nutrient quality of Cardamine violifolia. Sci. Hortic. 2021, 288, 110352. [Google Scholar] [CrossRef]
- Xu, M.M.; Zhu, S.; Li, Y.R.; Xu, S.; Shi, G.Y.; Ding, Z.Y. Effect of selenium on mushroom growth and metabolism: A review. Trends Food Sci. Technol. 2021, 118, 328–340. [Google Scholar] [CrossRef]
- Lanza, M.; dos Reis, A.R. Roles of selenium in mineral plant nutrition: ROS scavenging responses against abiotic stresses. Plant Physiol. Biochem. 2021, 164, 27–43. [Google Scholar] [CrossRef]
- Li, L.X.; Qiao, Z.; Cai, J.Y.; Liang, Y.; Lin, Y.; Wei, G.L.; Hou, X.L.; Miao, J.H.; Wei, K.H. Effects of selenium on growth and biochemical characters of tissue culture seedlings of Sophora tonkinensis. Pharmacogn. Mag. 2023, 19, 772–781. [Google Scholar] [CrossRef]
- Ozturk, M.; Unal, B.T.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and its actions during the drought stress in plants. Physiol. Plant 2020, 172, 1321–1335. [Google Scholar] [CrossRef]
- Zhong, Y.; Li, Y.; Cheng, J.J. Effects of selenite on chlorophyll fluorescence, starch content and fatty acid in the duckweed Landoltia punctata. J. Plant Res. 2016, 129, 997–1004. [Google Scholar] [CrossRef]
- Jiang, Y.; Zeng, Z.H.; Bu, Y.; Ren, C.Z.; Li, J.Z.; Han, J.J.; Tao, C.; Zhang, K.; Wang, X.X.; Lu, G.X.; et al. Effects of selenium fertilizer on grain yield, Se uptake and distribution in common buckwheat (Fagopyrum esculentum Moench). Plant Soil Environ. 2015, 61, 371–377. [Google Scholar] [CrossRef]
- Wang, M.K.; Peng, Q.; Zhou, F.; Yang, W.X.; Dinh, Q.T.; Liang, D.L. Uptake kinetics and interaction of selenium species in tomato (Solanum lycopersicum L.) seedlings. Environ. Sci. Pollut. Res. 2019, 26, 9730–9738. [Google Scholar] [CrossRef]
- Wu, H.Z.; Zhang, D.X.; Wu, X.M.; Tian, X.S.; Hu, G.; Liu, S.L.; Jie, X.L.; Wang, D.C. Uptake and transport of selenium in a soil-tea plant–tea Infusion system: A study of typical tea plantations in a selenium-rich area of China. Forests 2024, 15, 914. [Google Scholar] [CrossRef]
- Freeman, J.L.; Marcus, M.A.; Fakra, S.C.; Devonshire, J.; McGrath, S.P.; Quinn, C.F.; Pilon-Smits, E.A.H. Selenium Hyperaccumulator plants Stanleya pinnata and Astragalus bisulcatus are colonized by Se-resistant, Se-excluding wasp and beetle seed herbivores. PLoS ONE 2012, 7, e50516. [Google Scholar] [CrossRef] [PubMed]
- Both, E.B.; Stonehouse, G.C.; Lima, L.W.; Fakra, S.C.; Aguirre, B.; Wangeline, A.L.; Xiang, J.Q.; Yin, H.Q.; Jókai, Z.; Soós, A.; et al. Selenium tolerance, accumulation, localization and speciation in a Cardamine hyperaccumulator and a non-hyperaccumulator. Sci. Total Environ. 2020, 703, 135041. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Wei, Y.; Zhou, P.; Zhu, J.X.; Peng, L.L.; Cheng, L.Z.; Wang, Y.F.; Wei, X.L. Preparation, characterization, and antioxidant properties of selenium-enriched tea peptides. Foods 2023, 12, 4105. [Google Scholar] [CrossRef]
- Zhang, X.; He, H.; Xiang, J.Q.; Yin, H.Q.; Hou, T. Selenium-containing proteins/peptides from plants: A review on the structures and functions. J. Agric. Food Chem. 2020, 68, 15061–15073. [Google Scholar] [CrossRef]
- Fang, Y.; Catron, B.; Zhang, Y.F.; Zhao, L.Y.; Caruso, J.A.; Hu, Q.H. Distribution and in Vitro availability of selenium in seleniumcontaining storage protein from selenium-enriched rice utilizing optimized extraction. J. Agric. Food Chem. 2010, 58, 9731–9738. [Google Scholar] [CrossRef] [PubMed]
- Torres, S.; Cerutti, S.; Raba, J.; Pacheco, P.; Silva, M.F. Preconcentration of seleno-amino acids on a XAD resin and determination in regional olive oils by SPE UPLC-ESI-MS/MS. Food Chem. 2014, 159, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Gou, Y.Y.; Yu, T.; Cong, X.; Gui, J.Y.; Zhu, Z.Z.; Zhang, W.W.; Liao, Y.L.; Ye, J.B.; Cheng, S.Y.; et al. Effects of selenate on Se, flavonoid, and glucosinolate in broccoli florets by combined transcriptome and metabolome analyses. Food Res. Int. 2021, 146, 110463. [Google Scholar] [CrossRef] [PubMed]

| Medium Number | IBA (mg/L) | NAA (mg/L) | Exogenous Additives (g/L) |
|---|---|---|---|
| M1 | 0.1 | 0.1 | 0 |
| M2 | 0.1 | 0.6 | 100 |
| M3 | 0.1 | 1.5 | 200 |
| M4 | 0.6 | 0.1 | 100 |
| M5 | 0.6 | 0.6 | 200 |
| M6 | 0.6 | 1.5 | 0 |
| M7 | 1.5 | 0.1 | 200 |
| M8 | 1.5 | 0.6 | 0 |
| M9 | 1.5 | 1.5 | 100 |
| Parameter Name | Value |
|---|---|
| RF Power | 1200 W |
| Plasma Gas Flow Rate | 12 L/min |
| Carrier Gas Flow Rate | 0.80 L/min |
| Auxiliary Gas Flow Rate | 0.40 L/min |
| Peristaltic Pump Speed | 1.0 L/min |
| Nebulizer Gas Flow Rate | 0.5 L/min |
| Argon Pressure | 0.65 MPa |
| Normal Sampling Time | 15 s |
| Fast Pump Flow Rate | 4.0 L/min |
| Rinse Time | 5 s |
| Integration Time | 1 s |
| Detection Wavelength | 196.026 nm |
| Number of Replicates | 3 |
| Parameter Name | Specification |
|---|---|
| SAX Strong Anion Exchange Column | 1000 mg/6 mL |
| Negative High Voltage | 300 V |
| Lamp Current | 80 mA |
| Furnace Height | 8 mm |
| Carrier Gas Flow Rate | 400 mL/min |
| Shield Gas Flow Rate | 1000 mL/min |
| Parameter | Specification |
|---|---|
| Column | Hamilton PRPX-100 analytical column, 250 mm × 4.1 mm, 10 μm |
| Guard Column | Hamilton PRPX-100 guard column, 20 mm × 2.1 mm, 10 μm |
| Mobile Phase | 1.05 g citric acid monohydrate + 20 mL methanol, diluted to 1000 mL with water |
| Flow Rate | 0.8 mL/min |
| pH | 5.3 |
| Injection Volume | 100 µL |
| Parameter | Operating Condition |
|---|---|
| RF Power | 1400 W |
| Nebulizer Gas Flow Rate | 1.05 L/min |
| Helium Flow Rate | 2.0 L/min |
| Integration Mode | Peak Area |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; An, C.; Wang, Y.; Sun, Y.; Liu, C.; Wang, B.; Qin, Y.; Zhang, Z. Dendrobium huoshanense In Vitro Culture and Selenium Metabolism: Speciation Mechanisms. Horticulturae 2025, 11, 1263. https://doi.org/10.3390/horticulturae11101263
Wu Y, An C, Wang Y, Sun Y, Liu C, Wang B, Qin Y, Zhang Z. Dendrobium huoshanense In Vitro Culture and Selenium Metabolism: Speciation Mechanisms. Horticulturae. 2025; 11(10):1263. https://doi.org/10.3390/horticulturae11101263
Chicago/Turabian StyleWu, Yulai, Chang An, Yanjie Wang, Yuqi Sun, Changbin Liu, Bingrui Wang, Yuan Qin, and Zongshen Zhang. 2025. "Dendrobium huoshanense In Vitro Culture and Selenium Metabolism: Speciation Mechanisms" Horticulturae 11, no. 10: 1263. https://doi.org/10.3390/horticulturae11101263
APA StyleWu, Y., An, C., Wang, Y., Sun, Y., Liu, C., Wang, B., Qin, Y., & Zhang, Z. (2025). Dendrobium huoshanense In Vitro Culture and Selenium Metabolism: Speciation Mechanisms. Horticulturae, 11(10), 1263. https://doi.org/10.3390/horticulturae11101263










