Integrative Multi-Omics Analysis Identified Tissue-Specific Volatile Metabolites in Populus koreana
Abstract
1. Introduction
2. Results
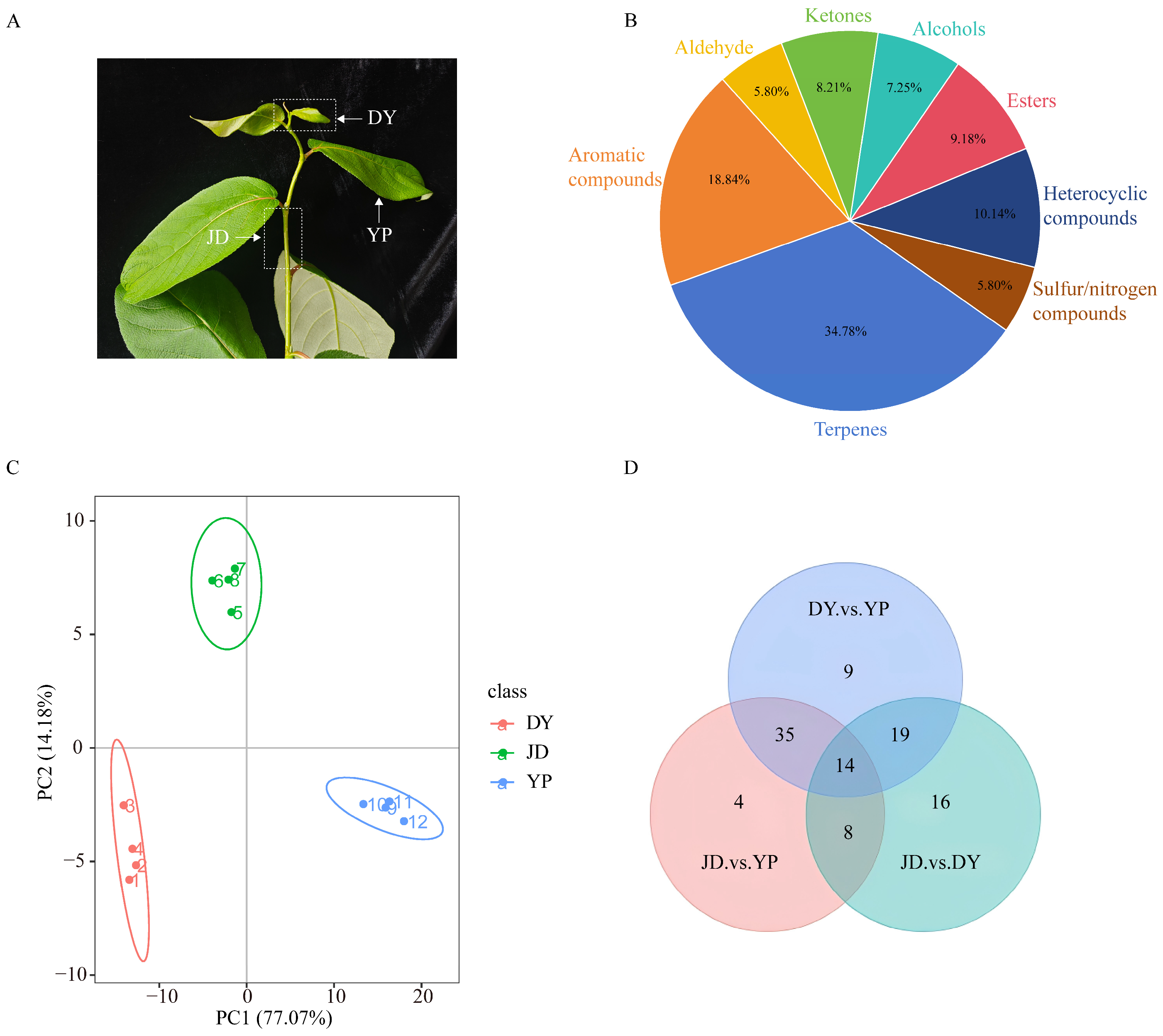
2.1. Identification of VOCs in Different Tissues of P. koreana
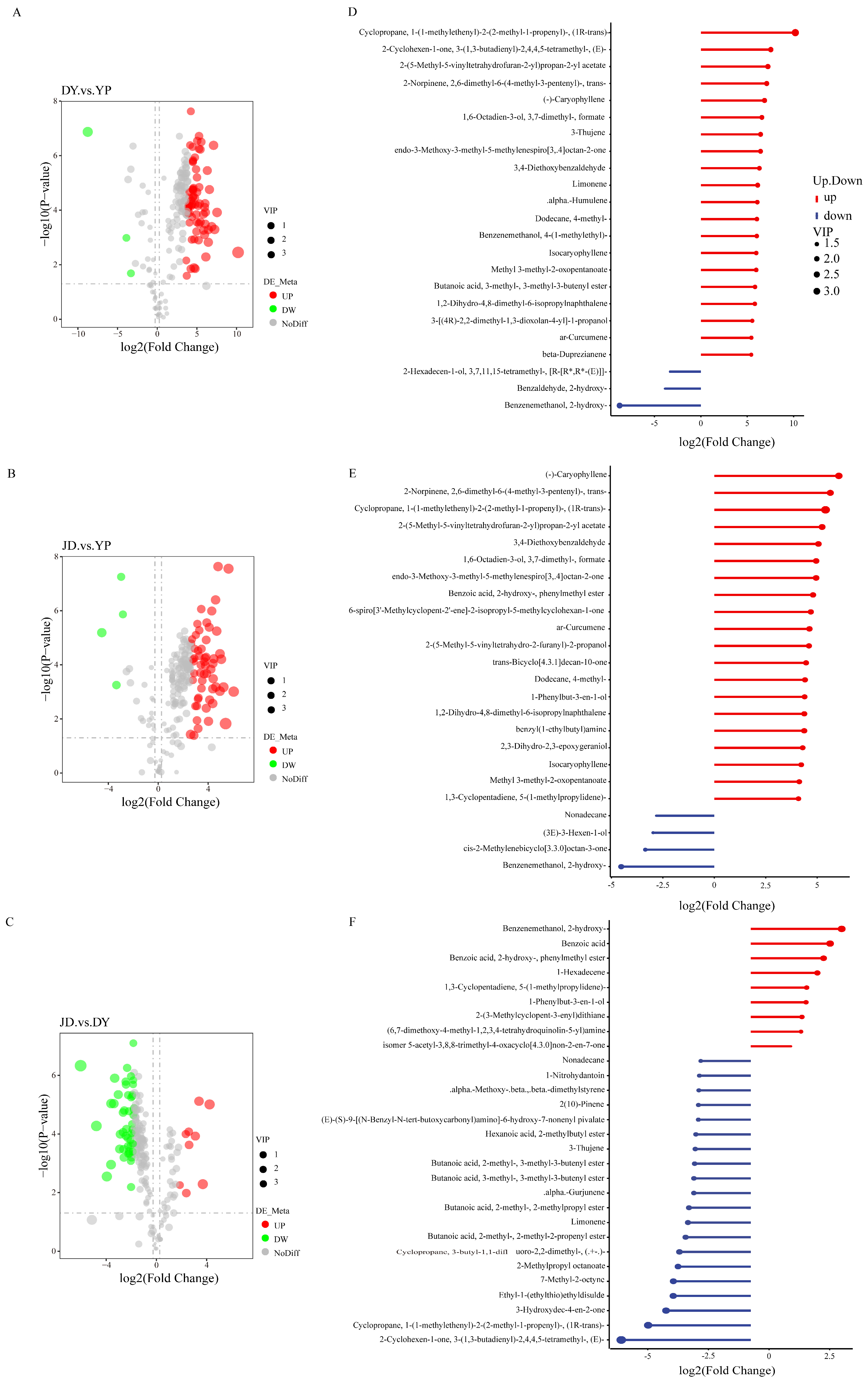
2.2. Identification of Tissue-Specific Differential VOCs in P. koreana
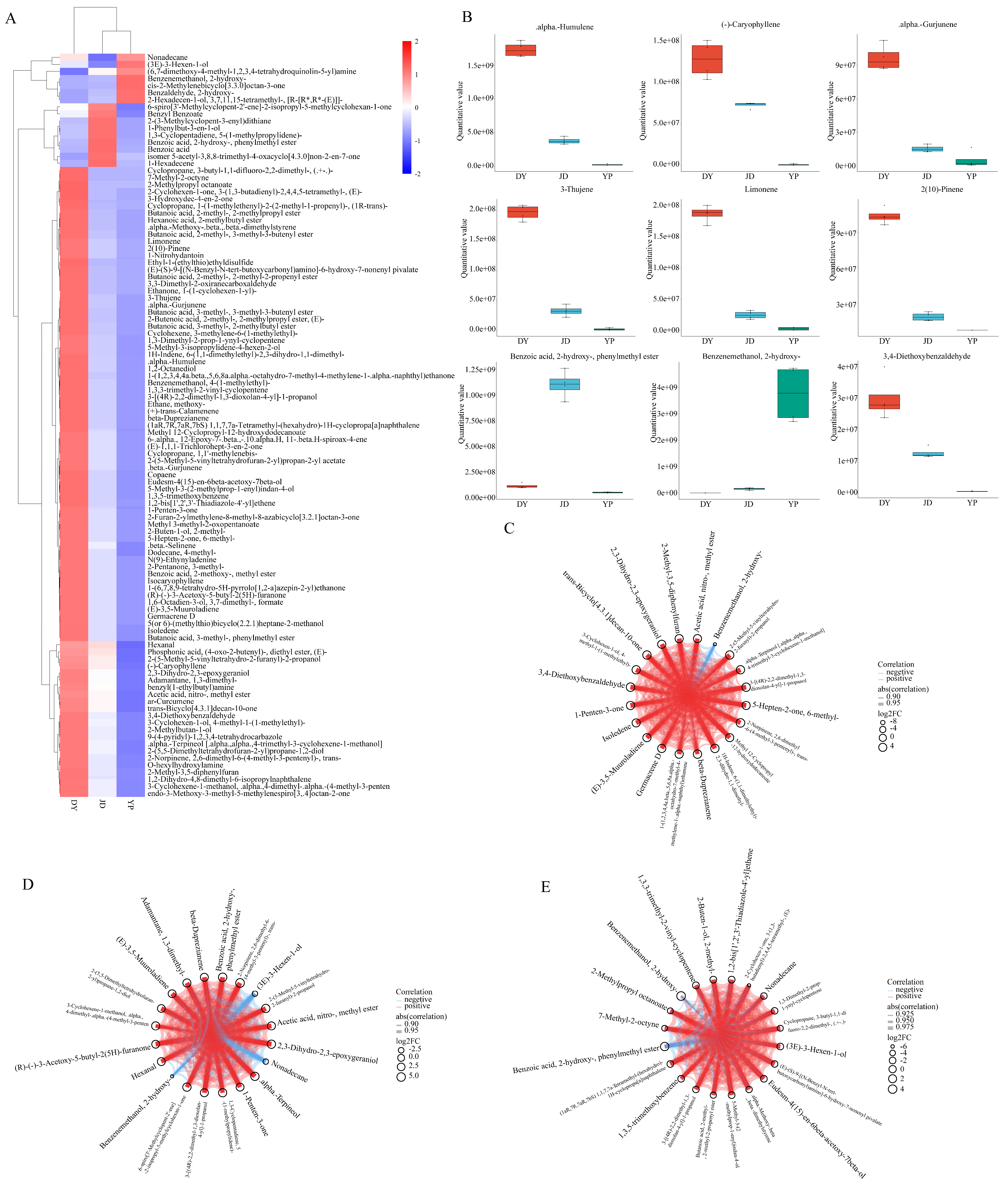
2.3. Quantitative and Correlation Analysis of Differential VOCs
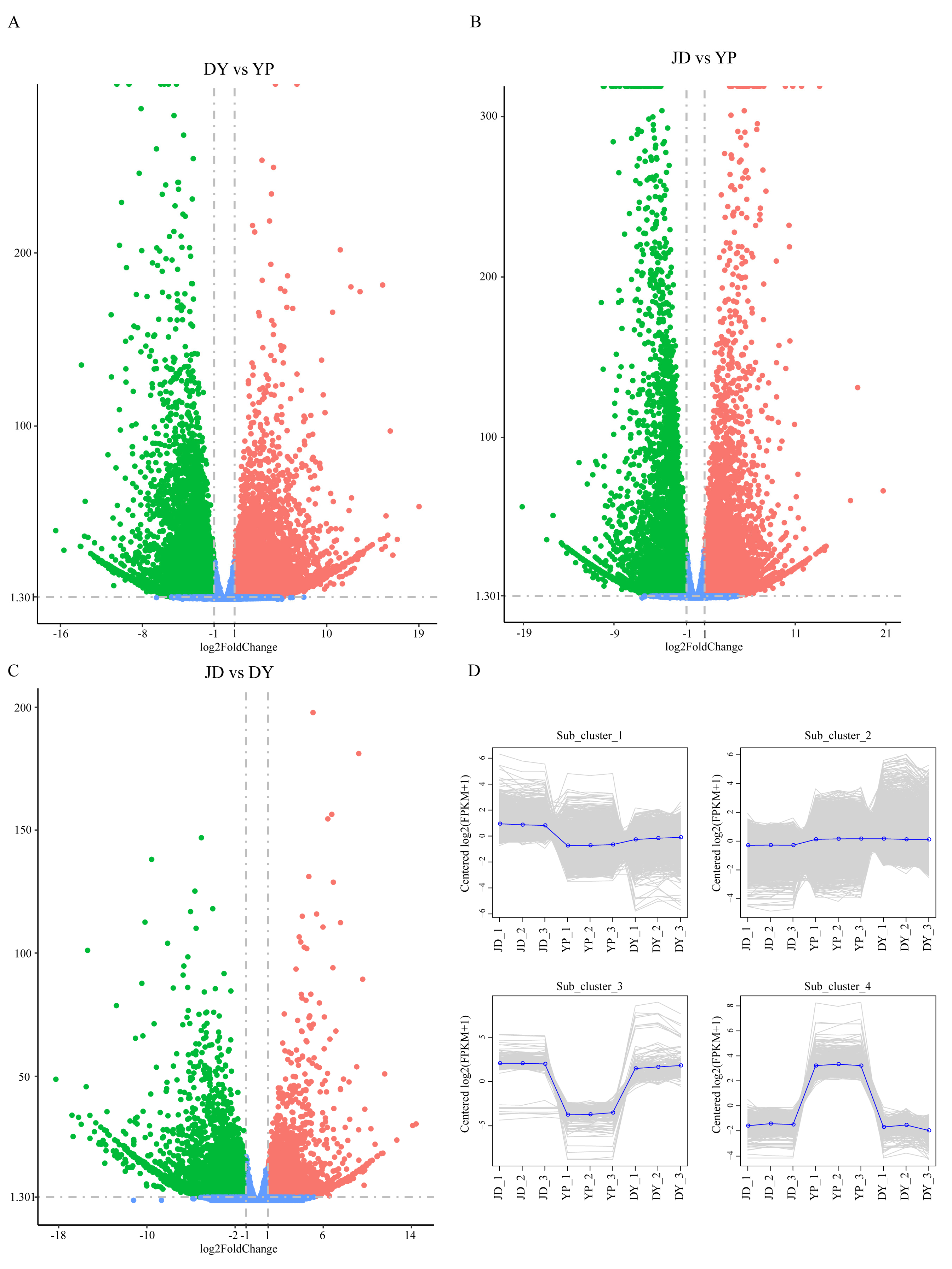
2.4. Identification of Differentially Expressed Genes (DEGs) in Different Tissues of P. koreana
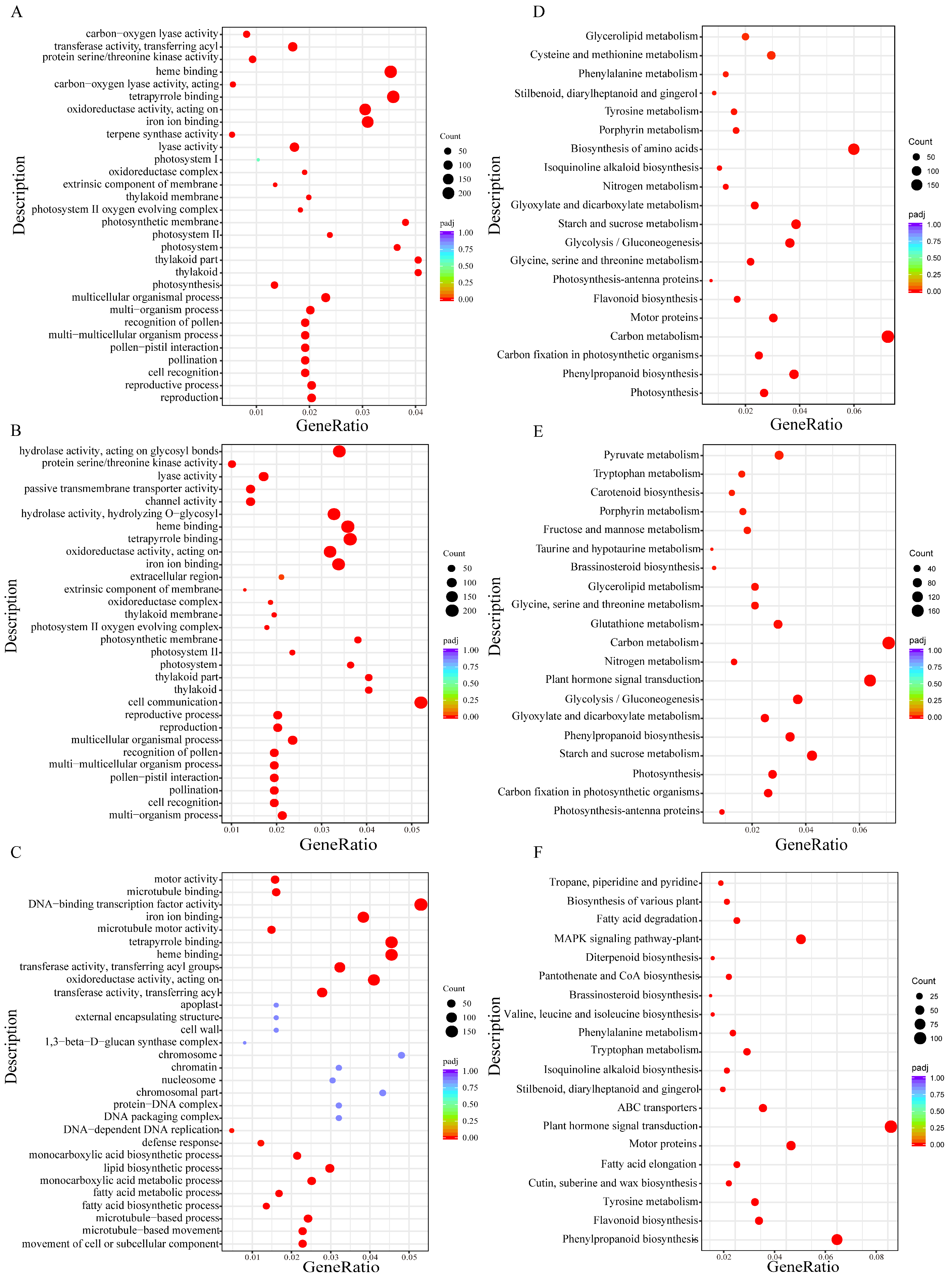
2.5. Gene Ontology (GO) Function and KEGG Enrichment Analysis of DEGs
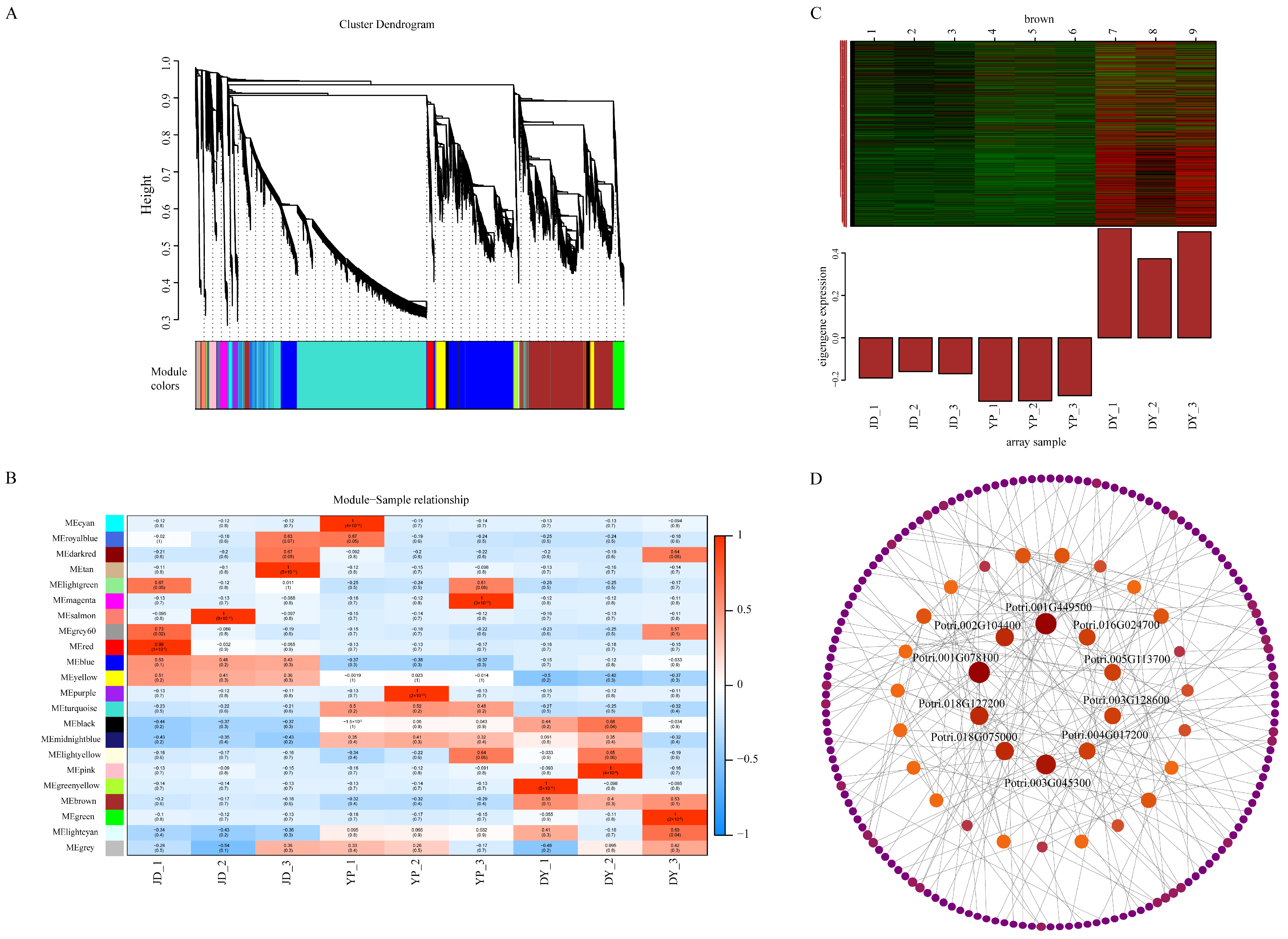
2.6. Weighted Gene Co-Expression Network Analysis (WGCNA) of VOC Biosynthesis
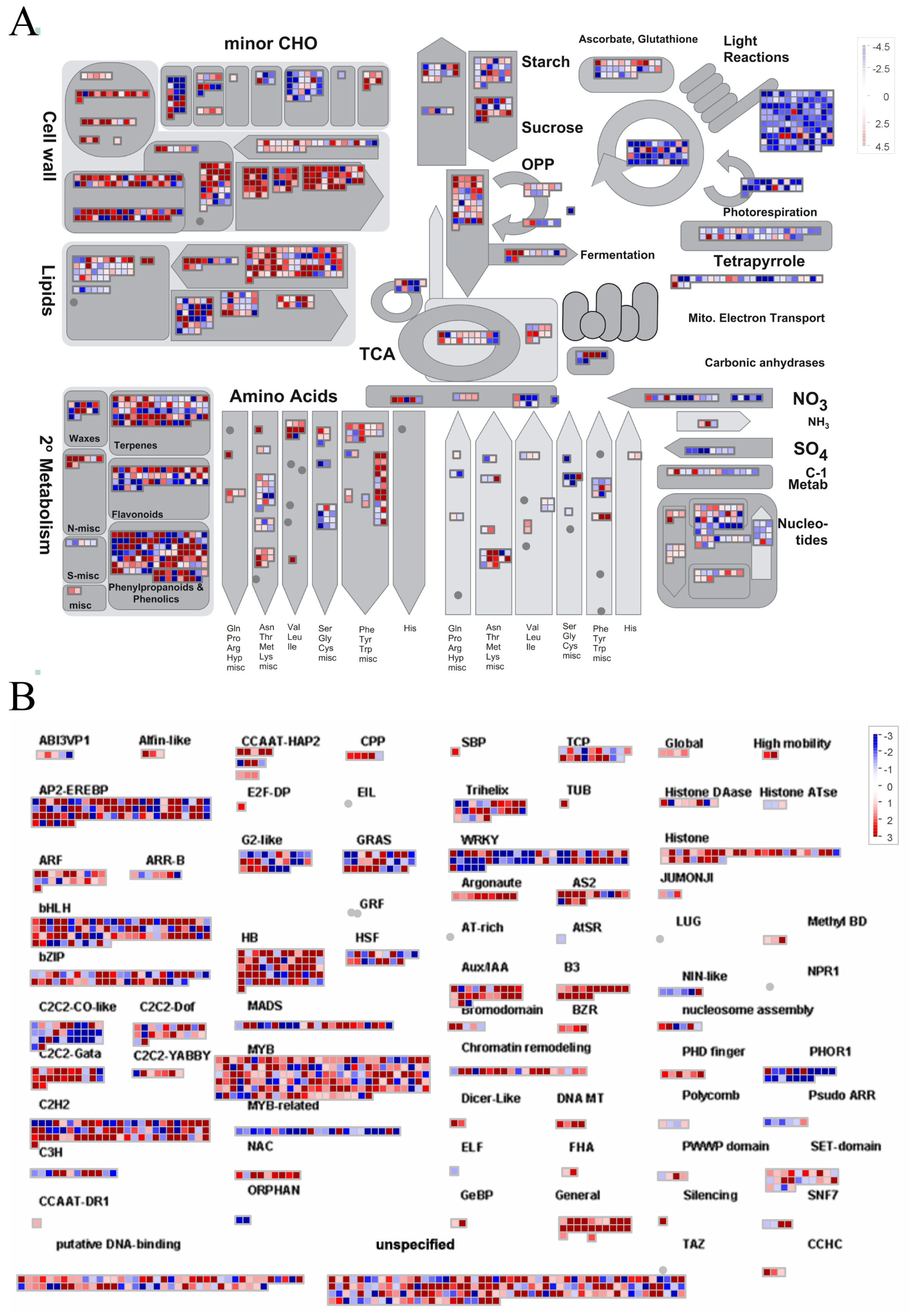
2.7. Analysis of DEG Expression Patterns Across Diverse Metabolic Pathways
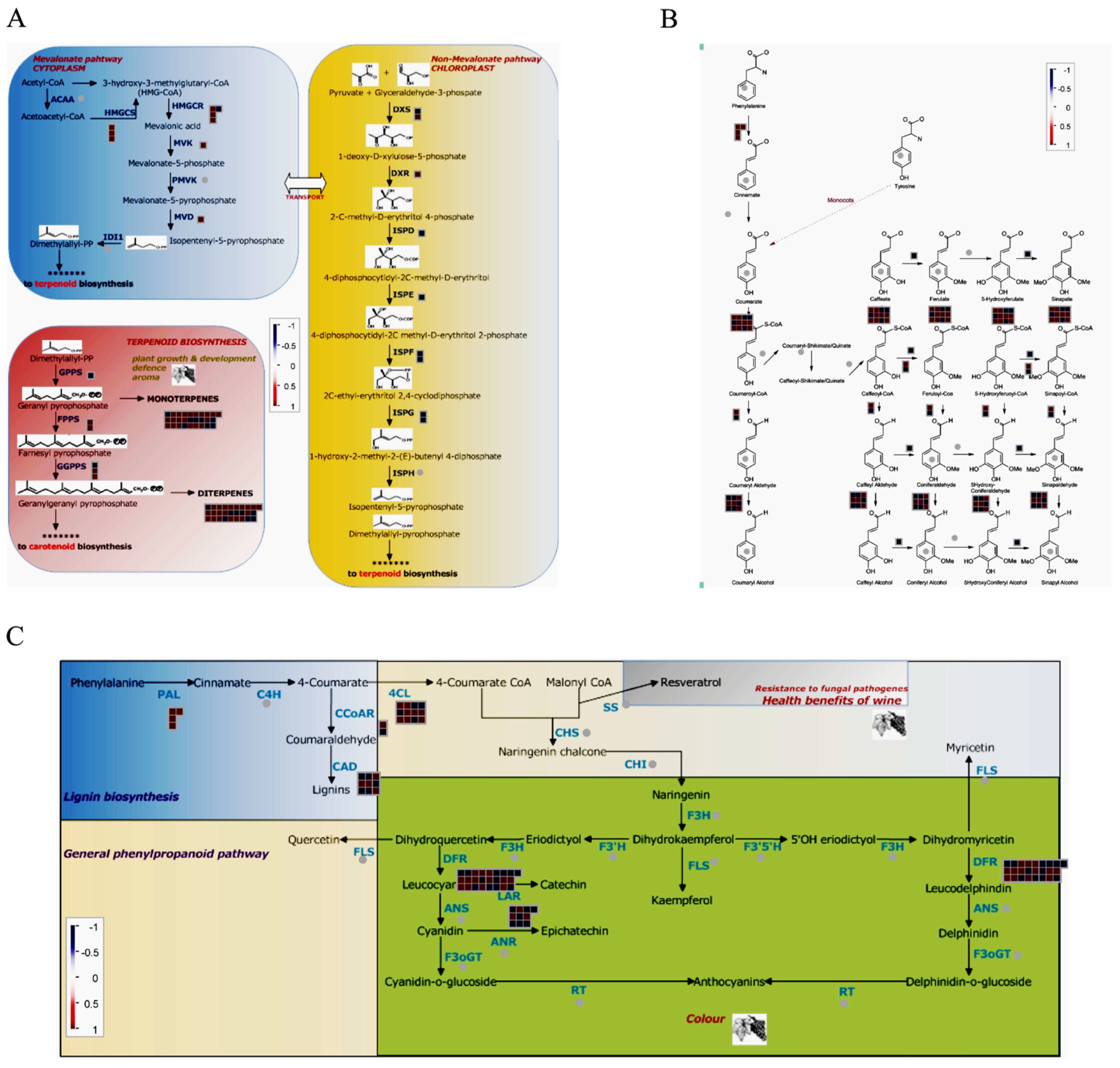
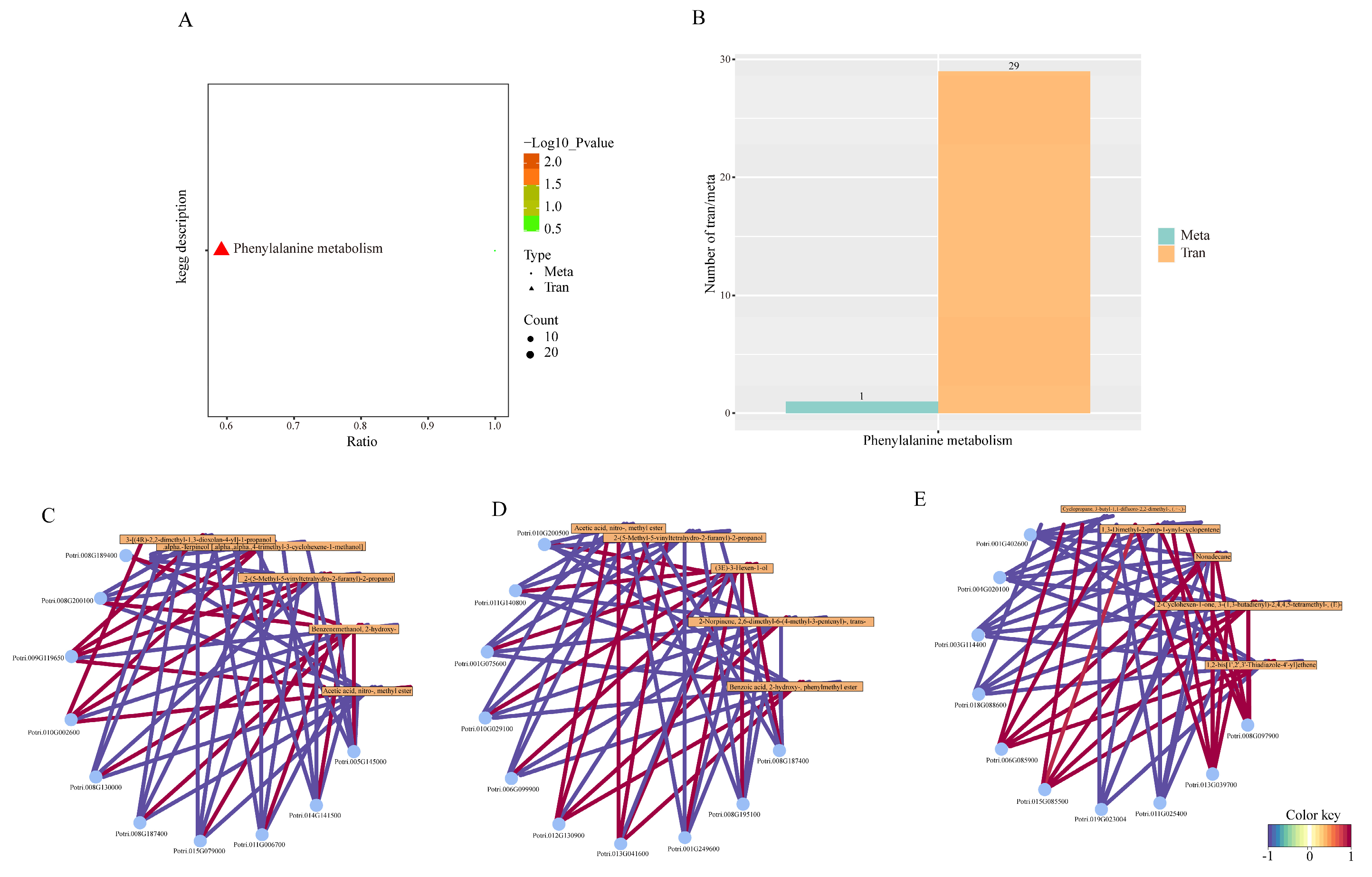
2.8. Combined Analysis of Transcriptome and Metabolome
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Volatile Compound Analysis by GC-MS
4.3. Total RNA Isolation, Library Construction and Transcriptome Sequencing
4.4. Estimation of Transcript Abundance and Differential Expression Analysis
4.5. Transcriptome Annotation and Functional Enrichment
4.6. Validation by RT-qPCR
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Z.; Han, J.; Liu, Z.; Zhang, X.; Chen, J.; Dong, A. First report of Pseudomonas aeruginosa causing tumor disease of Populus koreana in China. J. Plant Dis. Prot. 2019, 126, 485–488. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, W.; Diao, S.; Wu, X.; Lu, J.; Ding, C.; Su, X. The poplar pangenome provides insights into the evolutionary history of the genus. Commun. Biol. 2019, 2, 215. [Google Scholar] [CrossRef]
- Greenaway, W.; English, S.; May, J.; Whatley, F.R. Analysis of Phenolics o f Bud Exudates of Populus koreana, Populus maximowiczii and Populus suaveolens by GC-M S. Naturforsch 1992, 47, 313–317. [Google Scholar] [CrossRef]
- Bao, X.; Zhou, W.; Xu, L.; Zheng, Z. A meta-analysis on plant volatile organic compound emissions of different plant species and responses to environmental stress. Environ. Pollut. 2023, 318, 120886. [Google Scholar] [CrossRef]
- Montejano-Ramírez, V.; Ávila-Oviedo, J.L.; Campos-Mendoza, F.J.; Valencia-Cantero, E. Microbial volatile organic compounds: Insights into plant defense. Plants 2024, 13, 2013. [Google Scholar] [CrossRef] [PubMed]
- Bergman, M.E.; Huang, X.-Q.; Baudino, S.; Caissard, J.-C.; Dudareva, N. Plant volatile organic compounds: Emission and perception in a changing world. Curr. Opin. Plant Biol. 2025, 85, 102706. [Google Scholar] [CrossRef] [PubMed]
- Arimura, G.-I.; Uemura, T. Cracking the plant VOC sensing code and its practical applications. Trends Plant Sci. 2025, 30, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef]
- Degenhardt, J.; Köllner, T.G.; Gershenzon, J. Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry 2009, 70, 1621–1637. [Google Scholar] [CrossRef]
- Lee, S.; Badieyan, S.; Bevan, D.R.; Herde, M.; Gatz, C.; Tholl, D. Herbivore-induced and floral homoterpene volatiles are biosynthesized by a single P450 enzyme (CYP82G1) in Arabidopsis. Proc. Natl. Acad. Sci. USA 2010, 107, 21205–21210. [Google Scholar] [CrossRef]
- Irmisch, S.; McCormick, A.C.; Boeckler, G.A.; Schmidt, A.; Reichelt, M.; Schneider, B.; Block, K.; Schnitzler, J.-P.; Gershenzon, J.; Unsicker, S.B.; et al. Two herbivore-induced cytochrome P450 enzymes CYP79D6 and CYP79D7 catalyze the formation of volatile aldoximes involved in poplar defense. Plant Cell 2013, 25, 4737–4754. [Google Scholar] [CrossRef]
- Günther, J.; Lackus, N.D.; Schmidt, A.; Huber, M.; Stödtler, H.-J.; Reichelt, M.; Gershenzon, J.; Köllner, T.G. Separate pathways contribute to the herbivore-induced formation of 2-Phenylethanol in poplar. Plant Physiol. 2019, 180, 767–782. [Google Scholar] [CrossRef]
- Torrens-Spence, M.P.; Pluskal, T.; Li, F.-S.; Carballo, V.; Weng, J.-K. Complete pathway elucidation and heterologous reconstitution of rhodiola salidroside biosynthesis. Mol. Plant 2018, 11, 205–217. [Google Scholar] [CrossRef]
- Facchini, P.J.; Huber-Allanach, K.L.; Tari, L.W. Plant aromatic L-amino acid decarboxylases: Evolution, biochemistry, regulation, and metabolic engineering applications. Phytochemistry 2000, 54, 121–138. [Google Scholar] [CrossRef]
- Shen, L.; Ding, C.; Zhang, W.; Zhang, T.; Li, Z.; Zhang, J.; Chu, Y.; Su, X. The Populus koreana genome provides insights into the biosynthesis of plant aroma. Ind. Crops Prod. 2023, 197, 116453. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Li, Z.; Zhao, Y.-X.; Gao, M.; Wang, J.-Y.; Liu, K.-W.; Wang, X.; Wu, L.-W.; Jiao, Y.-L.; Xu, Z.-L.; et al. The Litsea genome and the evolution of the laurel family. Nat. Commun. 2020, 11, 1675. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Li, J.; Bo, W.; Yang, W.; Zuccolo, A.; Giacomello, S.; Chen, X.; Han, F.; Yang, J.; Song, Y.; et al. The Chinese pine genome and methylome unveil key features of conifer evolution. Cell 2022, 185, 204–217.e14. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Wang, W.; Liao, W.; Wang, H.; Zhang, D. Multiple glacial refugia for cool-temperate deciduous trees in northern East Asia: The Mongolian oak as a case study. Mol. Ecol. 2015, 24, 5676–5691. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Wang, X.-R.; Zeng, Q.-Y. De novo assembly of white poplar genome and genetic diversity of white poplar population in Irtysh River basin in China. Sci. China Life Sci. 2019, 62, 609–618. [Google Scholar] [CrossRef]
- Muhlemann, J.K.; Klempien, A.; Dudareva, N. Floral volatiles: From biosynthesis to function. Plant Cell Environ. 2014, 37, 1936–1949. [Google Scholar] [CrossRef] [PubMed]
- Farré-Armengol, G.; Filella, I.; Llusià, J.; Peñuelas, J. β-Ocimene, a key floral and foliar volatile involved in multiple interactions between plants and other organisms. Molecules 2017, 22, 1148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Shen, D.; Cao, Y.; Duan, X.; Sun, H. Widely targeted metabolomic approach reveals dynamic changes in non-volatile and volatile metabolites of peanuts during roasting. Food Chem. 2023, 412, 135577. [Google Scholar] [CrossRef] [PubMed]
- Isidorov, V.A.; Vinogorova, V.T. GC-MS Analysis of Compounds Extracted from Buds of Populus balsamifera and Populus nigra. Z. Fur Naturforschung Sect. C-A J. Biosci. 2003, 58, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.-C.; Lee, M.-C.; Chang, Y.-L.; Chen, W.-H.; Chen, H.-H. Diurnal regulation of the floral scent emission by light and circadian rhythm in the Phalaenopsis orchids. Bot. Stud. 2017, 58, 50. [Google Scholar] [CrossRef]
- Shalit, M.; Guterman, I.; Volpin, H.; Bar, E.; Tamari, T.; Menda, N.; Adam, Z.; Zamir, D.; Vainstein, A.; Weiss, D.; et al. Volatile ester formation in roses. Identification of an acetyl-coenzyme A. geraniol/citronellol acetyltransferase in developing rose petals. Plant Physiol. 2003, 131, 1868–1876. [Google Scholar] [CrossRef]
- Chen, G.; Mostafa, S.; Lu, Z.; Du, R.; Cui, J.; Wang, Y.; Liao, Q.; Lu, J.; Mao, X.; Chang, B.; et al. The Jasmine (Jasminum sambac) genome provides insight into the biosynthesis of flower fragrances and jasmonates. Genom. Proteom. Bioinform. 2022, 21, 127–149. [Google Scholar] [CrossRef]
- Irmisch, S.; Jiang, Y.; Chen, F.; Gershenzon, J.; Köllner, T.G. Terpene synthases and their contribution to herbivore-induced volatile emission in western balsam poplar (Populus trichocarpa). BMC Plant Biol. 2014, 14, 270. [Google Scholar] [CrossRef]
- Laia, S.; Yia, N.; Yina, S.; Huanga, Y.; Shena, T.; Daia, Q.; Gaob, L.; Jianga, X.; Xia, T. Biochemical characterization of CsCXEs: Carboxylesterase enhances the biosynthesis of green odor volatiles during tea processing. Hortic. Plant J. 2024, 11, 1684–1698. [Google Scholar] [CrossRef]
- Dao, M.; Zhang, Z.; Yin, X.; Wang, J.; Qin, Y.; Yu, L.; Wu, T. CsAFS1 Enhances the self-resistance of tea plants by promoting terpenoid accumulation. Physiol. Plant. 2025, 177, e70430. [Google Scholar] [CrossRef]
- Xie, Q.; Dong, W.; Wang, M.; Wang, J.; Sun, L.; Liu, Z.; Gao, C.; Cao, C. BpWRKY6 regulates insect resistance by affecting jasmonic acid and terpenoid synthesis in Betula platyphylla. Plant Biotechnol. J. 2025, 23, 3682–3696. [Google Scholar] [CrossRef]
- Pan, L.; Huang, R.; Lu, Z.; Duan, W.; Sun, S.; Yan, L.; Cui, G.; Niu, L.; Wang, Z.; Zeng, W. Combined transcriptome and metabolome analysis identifies triterpenoid-induced defense responses in Myzus persicae Sülzer-infested peach. J. Exp. Bot. 2024, 75, 6644–6662. [Google Scholar] [CrossRef]
- Wei, H.; Wang, Y.; Wang, X.; Zhou, X.; Zhang, H.; Mo, M.; Wang, L.; Li, Y.; Wu, Q. Untargeted metabolites profiling of volatile components of Chinese Antique Lotus (Nelumbo nucifera Gaertn.) using solid-phase microextraction (SPME) GC/MS. PeerJ 2025, 13, e19600. [Google Scholar] [CrossRef]
- He, L.; Hu, Q.; Wei, L.; Ge, X.; Yu, N.; Chen, Y. Unravelling dynamic changes in non-volatile and volatile metabolites of pulses during soaking: An integrated metabolomics approach. Food Chem. 2023, 422, 136231. [Google Scholar] [CrossRef]
- Jia, Q.; Brown, R.; Köllner, T.G.; Fu, J.; Chen, X.; Wong, G.K.-S.; Gershenzon, J.; Peters, R.J.; Chen, F. Origin and early evolution of the plant terpene synthase family. Proc. Natl. Acad. Sci. USA 2022, 119, e2100361119. [Google Scholar] [CrossRef]
- Abbas, F.; Ke, Y.; Zhou, Y.; Ashraf, U.; Li, X.; Yu, Y.; Yue, Y.; Ahmad, K.W.; Yu, R.; Fan, Y. Molecular cloning, characterization and expression analysis of LoTPS2 and LoTPS4 involved in floral scent formation in oriental hybrid Lilium variety ‘Siberia’. Phytochemistry 2020, 173, 112294. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Liu, B.; Li, M.; Gao, X.; Fang, Q.; Liu, C.; Ding, H.; Wang, L.; Gao, X. Identification and characterization of terpene synthase genes accounting for volatile terpene emissions in flowers of Freesia x hybrida. J. Exp. Bot. 2018, 69, 4249–4265. [Google Scholar] [CrossRef] [PubMed]
- Bao, T.; Kimani, S.; Li, Y.; Li, H.; Yang, S.; Zhang, J.; Wang, Q.; Wang, Z.; Ning, G.; Wang, L.; et al. Allelic variation of terpene synthases drives terpene diversity in the wild species of the Freesia genus. Plant Physiol. 2023, 192, 2419–2435. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Tian, J.; Cheng, H.; Yan, Q.; Li, L.; Jamal, A.; Xu, Z.; Xiang, L.; Saski, C.A.; Jin, S.; et al. The chromosome-level wintersweet (Chimonanthus praecox) genome provides insights into floral scent biosynthesis and flowering in winter. Genome Biol. 2020, 21, 200. [Google Scholar] [CrossRef]
- Scalliet, G.; Journot, N.; Jullien, F.; Baudino, S.; Magnard, J.L.; Channelière, S.; Vergne, P.; Dumas, C.; Bendahmane, M.; Cock, J.M.; et al. Biosynthesis of the major scent components 3,5-dimethoxytoluene and 1,3,5-trimethoxybenzene by novel rose O-methyltransferases. FEBS Lett. 2002, 523, 113–118. [Google Scholar] [CrossRef]
- Verdonk, J.C.; Ric de Vos, C.H.; Verhoeven, H.A.; Haring, M.A.; van Tunen, A.J.; Schuurink, R.C. Regulation of floral scent production in petunia revealed by targeted metabolomics. Phytochemistry 2003, 62, 997–1008. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Y.; Li, X.; Zhang, Y.; Chen, X.; Liu, J.; Qiua, Y.; Wang, A. Integration of metabolomics and transcriptomics reveals the regulation mechanism of the phenylpropanoid biosynthesis pathway in insect resistance traits in Solanum habrochaites. Hortic. Res. 2024, 11, uhad277. [Google Scholar] [CrossRef]
- Ma, Y.; Gao, Y.; Xu, Y.; Zhou, H.; Zhou, K.; Li, C.; Xu, B. Microbiota dynamics and volatile metabolite generation during sausage fermentation. Food Chem. 2023, 423, 136297. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuizen, N.J.; Chen, X.; Wang, M.Y.; Matich, A.J.; Perez, R.L.; Allan, A.C.; Green, S.A.; Atkinson, R.G. Natural variation in monoterpene synthesis in kiwifruit: Transcriptional regulation of terpene synthases by NAC and ETHYLENE-INSENSITIVE3-like transcription factors. Plant Physiol. 2015, 167, 1243–1258. [Google Scholar] [CrossRef] [PubMed]
- Picazo-Aragonés, J.; Terrab, A.; Balao, F. Plant volatile organic compounds evolution: Transcriptional regulation, epigenetics and polyploidy. Int. J. Mol. Sci. 2020, 21, 8956. [Google Scholar] [CrossRef] [PubMed]
- Abbas, F.; Ke, Y.; Zhou, Y.; Yu, Y.; Waseem, M.; Ashraf, U.; Wang, C.; Wang, X.; Li, X.; Yue, Y.; et al. Genome-wide analysis reveals the potential role of MYB transcription factors in floral scent formation in hedychium coronarium. Front. Plant Sci. 2021, 12, 623742. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Yu, R.; Fan, Y. Transcriptome profiling provides new insights into the formation of floral scent in Hedychium coronarium. BMC Genom. 2015, 16, 470. [Google Scholar] [CrossRef]
- Ke, Y.; Abbas, F.; Zhou, Y.; Yu, R.; Fan, Y. Auxin-responsive R2R3-MYB transcription factors HcMYB1 and HcMYB2 activate volatile biosynthesis in hedychium coronarium flowers. Front. Plant Sci. 2021, 12, 710826. [Google Scholar] [CrossRef]
- Yang, Y.-Y.; Ma, B.; Li, Y.-Y.; Han, M.-Z.; Wu, J.; Zhou, X.-F.; Tian, J.; Wang, W.-H.; Leng, P.-S.; Hu, Z.-H. Transcriptome analysis identifies key gene LiMYB305 involved in monoterpene biosynthesis in Lilium ‘Siberia’. Front. Plant Sci. 2022, 13, 1021576. [Google Scholar] [CrossRef]
- Aslam, M.Z.; Lin, X.; Li, X.; Yang, N.; Chen, L. Molecular cloning and functional characterization of CpMYC2 and CpBHLH13 transcription factors from wintersweet (Chimonanthus praecox L.). Plants 2020, 9, 785. [Google Scholar] [CrossRef]
- Chuang, Y.-C.; Hung, Y.-C.; Tsai, W.-C.; Chen, W.-H.; Chen, H.-H. PbbHLH4 regulates floral monoterpene biosynthesis in Phalaenopsis orchids. J. Exp. Bot. 2018, 69, 4363–4377. [Google Scholar] [CrossRef]
- Han, Y.; Lu, M.; Yue, S.; Li, K.; Dong, M.; Liu, L.; Wang, H.; Shang, F. Comparative methylomics and chromatin accessibility analysis in Osmanthus fragrans uncovers regulation of genic transcription and mechanisms of key floral scent production. Hortic. Res. 2022, 9, uhac096. [Google Scholar] [CrossRef]
- Abbas, F.; Ke, Y.; Zhou, Y.; Yu, Y.; Waseem, M.; Ashraf, U.; Li, X.; Yu, R.; Fan, Y. Genome-wide analysis of ARF transcription factors reveals HcARF5 expression profile associated with the biosynthesis of β-ocimene synthase in Hedychium coronarium. Plant Cell Rep. 2021, 40, 1269–1284. [Google Scholar] [CrossRef]
- Ding, W.; Ouyang, Q.; Li, Y.; Shi, T.; Li, L.; Yang, X.; Ji, K.; Wang, L.; Yue, Y. Genome-wide investigation of WRKY transcription factors in sweet osmanthus and their potential regulation of aroma synthesis. Tree Physiol. 2019, 40, 557–572. [Google Scholar] [CrossRef]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-McIntyre, S.; Anderson, N.; Brown, M.; Knowles, J.D.; Halsall, A.; Haselden, J.N.; et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef]
- Dai, W.; Xie, D.; Lu, M.; Li, P.; Lv, H.; Yang, C.; Peng, Q.; Zhu, Y.; Guo, L.; Zhang, Y.; et al. Characterization of white tea metabolome: Comparison against green and black tea by a nontargeted metabolomics approach. Food Res. Int. 2017, 96, 40–45. [Google Scholar] [CrossRef]
- Heischmann, S.; Quinn, K.; Cruickshank-Quinn, C.; Liang, L.-P.; Reisdorph, R.; Reisdorph, N.; Patel, M. Exploratory metabolomics profiling in the kainic acid rat model reveals depletion of 25-Hydroxyvitamin D3 during epileptogenesis. Sci. Rep. 2016, 6, 31424. [Google Scholar] [CrossRef] [PubMed]
- Haspel, J.A.; Chettimada, S.; Shaik, R.S.; Chu, J.-H.; Raby, B.A.; Cernadas, M.; Carey, V.; Process, V.; Hunninghake, G.M.; Ifedigbo, E.; et al. Circadian rhythm reprogramming during lung inflammation. Nat. Commun. 2014, 5, 5753. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Cheng, L.; Tang, L.; Yang, L.; Jiang, Z.; Song, X.; Kang, Q.; Yao, D.; Chen, S.; Ru, J.; et al. Transcriptome and endogenous hormone analysis reveals the molecular mechanism of callus hyperhydricity in flax (Linum usitatissimum L.). Int. J. Mol. Sci. 2025, 26, 5360. [Google Scholar] [CrossRef] [PubMed]
- Leng, X.; Wang, H.; Cao, L.; Chang, R.; Zhang, S.; Xu, C.; Yu, J.; Xu, X.; Qu, C.; Xu, Z.; et al. Overexpressing GLUTAMINE SYNTHETASE 1;2 maintains carbon and nitrogen balance under high-ammonium conditions and results in increased tolerance to ammonium toxicity in hybrid poplar. J. Exp. Bot. 2024, 75, 4052–4073. [Google Scholar] [CrossRef]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; Van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550–571. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Florea, L.; Song, L.; Salzberg, S.L. Thousands of exon skipping events differentiate among splicing patterns in sixteen human tissues. F1000Research 2013, 2, 188–213. [Google Scholar] [CrossRef]
- Zhu, L.; Liao, J.; Liu, Y.; Zhou, C.; Wang, X.; Hu, Z.; Huang, B.; Zhang, J. Integrative metabolome and transcriptome analyses reveal the molecular mechanism underlying variation in floral scent during flower development of Chrysanthemum indicum var. aromaticum. Front. Plant Sci. 2022, 13, 919151. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Huang, Q.; Zhang, B.; Ding, C.; Su, X. Expression and molecular evolution of two DREB1 genes in black poplar (Populus nigra). PLoS ONE 2014, 9, e98334. [Google Scholar] [CrossRef] [PubMed]
- Arocho, A.; Chen, B.; Ladanyi, M.; Pan, Q. Validation of the 2-DeltaDeltaCt calculation as an alternate method of data analysis for quantitative PCR of BCR-ABL P210 transcripts. Diagn. Mol. Pathol. 2006, 15, 56–61. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Wang, F.; Wu, J.; Xu, T.; Wang, Q.; Ju, Z.; Zhao, S.; Yang, J.; Leng, X. Integrative Multi-Omics Analysis Identified Tissue-Specific Volatile Metabolites in Populus koreana. Horticulturae 2025, 11, 1262. https://doi.org/10.3390/horticulturae11101262
Wang H, Wang F, Wu J, Xu T, Wang Q, Ju Z, Zhao S, Yang J, Leng X. Integrative Multi-Omics Analysis Identified Tissue-Specific Volatile Metabolites in Populus koreana. Horticulturae. 2025; 11(10):1262. https://doi.org/10.3390/horticulturae11101262
Chicago/Turabian StyleWang, Hanzeng, Fude Wang, Juan Wu, Tingting Xu, Qinhe Wang, Zhixin Ju, Shicheng Zhao, Jingli Yang, and Xue Leng. 2025. "Integrative Multi-Omics Analysis Identified Tissue-Specific Volatile Metabolites in Populus koreana" Horticulturae 11, no. 10: 1262. https://doi.org/10.3390/horticulturae11101262
APA StyleWang, H., Wang, F., Wu, J., Xu, T., Wang, Q., Ju, Z., Zhao, S., Yang, J., & Leng, X. (2025). Integrative Multi-Omics Analysis Identified Tissue-Specific Volatile Metabolites in Populus koreana. Horticulturae, 11(10), 1262. https://doi.org/10.3390/horticulturae11101262





