Abstract
Conventional propagation of the highly sought-after ornamental Philodendron erubescens ‘Pink Princess’ is constrained by slow multiplication rates, the risk of unstable variegation, and the limited availability of elite mother stock, making advanced in vitro techniques essential for large-scale production. This research aimed to establish an efficient micropropagation protocol by optimizing the shoot multiplication phase in a twin-flask Temporary Immersion Bioreactor (TIB) system (RITA-type) and subsequently assessing the genetic fidelity of the regenerated plants. Shoot induction was evaluated in a TIB system with an immersion frequency of 4 min every 8 h. Among the tested cytokinins, liquid Murashige and Skoog (MS) medium containing 1.0 mg/L 6-benzylaminopurine (BAP) provided the optimal conditions for shoot proliferation, accounting for approximately 21 shoots/explant. While the TIB system was highly effective for shoot multiplication, it proved suboptimal for root induction. Therefore, rooting was optimized on a semi-solid medium, where MS medium supplemented with 0.5 mg/L indole-3-acetic acid (IAA) was identified as the most effective treatment, yielding an average of 3.0 well-developed roots per explant (1.1 cm in length) within 30 days. For acclimatization, a substrate mix of peat moss, perlite, and vermiculite (2:1:1, v/v/v) ensured a 100% survival rate. Critically, genetic fidelity analysis using RAPD markers revealed monomorphic banding patterns between the micropropagated plantlets and the mother plant (100% similarity), confirming their genetic uniformity and true-to-type nature. The established protocol provides a robust and reliable method for the in vitro propagation of P. erubescens ‘Pink Princess’. This work offers a foundation for developing large-scale commercial production strategies and effectively overcomes many limitations of classical propagation techniques.
1. Introduction
Philodendron, one of the largest and most economically significant genera in the Araceae family, comprises over 500 species native to the tropical and subtropical Americas and the West Indies [1,2]. Renowned for their stunning foliage, diverse forms, and adaptability to indoor environments, Philodendrons are a cornerstone of the global ornamental plant industry. They hold a dominant position in the foliage trade, particularly in major production hubs like Taiwan and Thailand. The economic scale of this sector is substantial; for example, the wholesale value of the foliage plant market in the United States alone was approximately 631 million USD in 2013 [3]. This demonstrates the consistent and high consumer demand for interior landscaping plants. Among the vast array of species, Philodendron erubescens ‘Pink Princess’ is especially prized for its unique ornamental value and has emerged as a high-value commodity. This cultivar is distinguished by its dark purplish-green leaves that feature striking, unpredictable pink variegation, creating a dramatic visual contrast [4]. The high demand for ‘Pink Princess’ is driven by this exceptional beauty, which has elevated it to a collector’s item that commands premium retail prices. Therefore, developing efficient and reliable mass propagation methods is a critical goal for commercial growers seeking to capitalize on this profitable niche.
Despite high demand, the large-scale production of P. erubescens ‘Pink Princess’ is constrained by the significant limitations of conventional propagation methods. Traditional propagation through stem cuttings is slow, labor-intensive, and requires a large number of valuable mother plants to generate only a limited number of offspring. Propagation from seed is also impractical due to the short viability of Philodendron seeds. Furthermore, it carries a risk of genetic variation, which means the desirable pink variegation may not be passed on to the progeny. These traditional techniques are difficult to scale up and often yield a limited number of high-value plants, failing to meet market needs while also posing a risk for transmitting systemic pathogens [5,6].
To address these challenges, in vitro micropropagation has emerged as a superior alternative for the rapid and disease-free clonal multiplication of elite cultivars. This process involves growing plant tissues under sterile conditions and is critically dependent on plant growth regulators (PGRs) like auxins [e.g., indole-3-butyric acid (IBA), naphthyl-1-acetic acid (NAA), and indole-3-acetic acid (IAA)] and cytokinins [e.g., 6-benzylaminopurine (BAP), Kinetin (Kn), Thidiazuron (TDZ), and 2-isopentenyl adenine (2iP)] to direct development [7,8]. While effective micropropagation protocols have been established for P. erubescens ‘Pink Princess’ [4,5,6,9], these existing methods, while successful at a laboratory scale, predominantly rely on conventional semi-solid media. This reliance on semi-solid systems presents a major bottleneck for commercial production, as the process remains labor-intensive, costly, and difficult to automate for the large-scale output required to meet market demand. Although using liquid media can accelerate growth, the continuous immersion of explants can lead to hyperhydricity, a physiological disorder that has been a documented challenge in the micropropagation of aroids. For instance, hyperhydricity was specifically observed in liquid cultures of P. hastatum ‘Burgundy’ [10]. This condition results in weak, glassy plantlets that exhibit low survival rates upon transfer to soil [11,12], severely limiting the practicality of continuous liquid systems.
Consequently, a critical research gap exists: a scalable, automated, and fully integrated protocol that overcomes the limitations of both semi-solid and continuous liquid systems for Philodendron ‘Pink Princess’ has not yet been developed. Therefore, the primary novelty of this study lies in optimizing the first comprehensive and efficient protocol for this high-value cultivar by employing an advanced Temporary Immersion Bioreactor (TIB) system. This approach is designed to overcome the limitations of both semi-solid and continuous liquid culture by providing intermittent nutrient immersion and enhanced aeration [13], paving the way for industrial-scale production. While the successful application of TIBs for other economically important species within the Araceae family demonstrates the potential of this technology, a protocol specifically tailored to the unique requirements of Philodendron ‘Pink Princess’ has not been reported. For instance, the RITA system, a popular twin-flask bioreactor, has been effectively used to enhance the multiplication of Zantedeschia aethiopica and Anthurium andreanum [14]. Similarly, the RITA and Ebb-and-Flow bioreactors were successfully adapted for the in vitro propagation of A. andreanum, demonstrating the versatility of TIB technology for aroids [15]. However, optimizing conditions such as immersion condition and medium composition is species- and even cultivar-specific, highlighting the need for the current investigation.
A key challenge in TIB-based micropropagation is maintaining the genetic integrity of the cloned material. The stresses of the in vitro environment, including hormonal imbalances and rapid forced cell proliferation, can lead to somaclonal variation—genetic and epigenetic changes that can arise spontaneously. These variations pose a significant risk to commercial production, as they may not be observable phenotypically until the plants are mature, potentially leading to losses in yield, quality, or uniformity. Consequently, morphological observation alone is an unreliable method for confirming clonal fidelity. Molecular markers are therefore essential for a robust quality control system. They allow for the direct assessment of the DNA sequence, providing a highly sensitive and early detection tool to verify that micropropagated plants are genetically identical to the elite donor plant, thereby guaranteeing their ‘true-to-type’ status. Several PCR-based molecular marker systems are available to assess genetic fidelity, including Amplified Fragment Length Polymorphism (AFLP), Inter Simple Sequence Repeats (ISSR), Simple Sequence Repeats (SSR), and Start Codon Targeted (SCoT). The selection of a particular marker system often depends on a balance of factors, such as reliability, cost, ease of use, and the availability of genomic information for the species. For instance, highly stable and reproducible co-dominant markers, like SSRs, were considered; however, they require pre-existing sequence data for primer design, which can be a significant bottleneck and increase the cost and time of analysis. For this study, the Random Amplified Polymorphic DNA (RAPD) marker system was selected. Although RAPD markers are dominant and can be sensitive to experimental conditions, they offer compelling advantages for the purpose of verifying clonal fidelity: (1) they do not require any prior DNA sequence information, (2) the technique is simple, rapid, and cost-effective, and (3) it allows for the random screening of the entire genome, making it highly effective for detecting potential genetic variations. The utility of RAPD for this purpose is well-documented [16,17,18,19], and our choice is further supported by its successful application in a similar context for genetic stability assessment in P. bipinnatifidum by Alwahibi et al. [18].
To bridge the gap between existing laboratory-scale methods and a robust, commercially viable protocol, this study aimed to develop a fully integrated TIB-based system by systematically optimizing each critical stage of the micropropagation process. Specifically, our objectives were to address key bottlenecks in scaling up production. Our primary objective was to maximize the shoot multiplication rate within the TIB by identifying the optimal cytokinin treatment, which is essential for rapid and cost-effective scale-up. We then focused on establishing an effective and reliable protocol for high-frequency root induction, a critical prerequisite for successful plantlet development and a crucial step for streamlining the entire production pipeline. The practical feasibility of the protocol was assessed by targeting a high plantlet survival rate, ideally exceeding 90%, during acclimatization, a stage not previously documented for TIB-derived ‘Pink Princess’ plantlets. Finally, we confirmed the genetic stability and clonal fidelity of the TIB-regenerated plantlets using RAPD markers to ensure that the valuable ‘Pink Princess’ traits remained intact, thus validating its suitability for commercial application.
2. Materials and Methods
2.1. Plant Materials, In Vitro Establishment, and Chemicals
Stock cultures of P. erubescens ‘Pink Princess’ were initiated from established in vitro material provided by Mrs. Nuntipa Khumkarjorn of the Plant Propagation Center No. 10 (Udon Thani, Thailand). The identity of the source plant was confirmed morphologically based on its distinct variegated pink and dark green foliage, consistent with published descriptions of the cultivar. Although specific pathogen indexing was not conducted, strict aseptic techniques were maintained throughout all procedures. According to the provider, the cultures were initially established using axillary bud explants, which gave rise to adventitious shoot clusters. The establishment was performed on semi-solid Murashige and Skoog (MS) medium containing 2.0 mg/L BAP and 0.5 mg/L NAA. The cultures were incubated at 25 ± 2 °C with a 16 h photoperiod delivered by fluorescent lamps (45 µmol/m2/s). For the present study, these same media and culture conditions were used to maintain and proliferate the stock cultures. To ensure the availability of vigorous plant material, cultures were subcultured onto fresh medium every 30 days.
The MS basal medium was a product of PhytoTech Labs (Kansas, MO, USA). Sigma-Aldrich Corporation (St. Louis, MO, USA) supplied all plant growth regulators used in this study, including BAP, NAA, IBA, IAA, Kn, and TDZ. The substrates for the acclimatization stage (perlite, vermiculite, and peat moss) were obtained from the Agro Outlet at Khon Kaen University, Thailand.
2.2. The Temporary Immersion Bioreactor (TIB) System Setup
Micropropagation was carried out in a twin-flask TIB system (RITA-type) [20] consisting of two interconnected, 600 mL autoclavable glass vessels. The upper vessel was configured as the culture chamber to hold the plant explants, while the lower vessel served as a reservoir for the liquid medium, with a working volume of 300 mL. The entire TIB unit, including the medium, was autoclaved before use.
The system’s operation was automated. A pneumatic pump, regulated by an electronic timer and solenoid valves, delivered sterile-filtered air through a 0.2 µm syringe filter (Minisart NML, Sartorius Stedim Biotech GmbH, August-Spindler-Strasse 11, Goettingen, Germany) into the medium reservoir. The resulting positive pressure propelled the liquid medium through a stainless-steel tube into the culture chamber, immersing the explants for a pre-set duration. Upon completion of the immersion period, the timer deactivated the pump and opened the solenoid valve to release the air pressure. This allowed the medium to drain completely back into the reservoir via gravity. This design provides intermittent nutrient contact and a well-aerated environment, which is critical for promoting vigorous growth while preventing conditions like waterlogging and hyperhydricity.
2.3. Effect of Cytokinins on Shoot Induction of Philodendron ‘Pink Princess’
The effect of different cytokinins on shoot proliferation was investigated using a twin-flask TIB system. For each treatment, five healthy 30-day-old adventitious shoot clusters, measuring 1.0–1.5 cm in diameter, were used as explants, and each experimental treatment was performed in triplicate. This sample size was determined to be optimal based on preliminary studies, which showed that a higher explant density (>5 per TIB unit) caused intense competition for space and light, leading to stunted growth and confounding the treatment effects. Therefore, using five explants per replicate ensured uniform development and a reliable assessment of shoot proliferation. The explants were placed in the upper chamber of a TIB, with the lower chamber containing 300 mL of liquid MS medium. The basal medium was supplemented with 30 g/L sucrose and one of three cytokinins: BAP (0–8.0 mg/L), Kn (0–8.0 mg/L), or TDZ (0–2.0 mg/L) at the specified concentrations. The pH of all media was adjusted to 5.6–5.8 before use. All experimental treatments were performed in triplicate.
All TIB cultures were maintained for 30 days in a controlled growth room at a temperature of 25 ± 2 °C. The incubation environment included a 16 h photoperiod provided by fluorescent lighting at an intensity of 45 µmol/m2/s. The TIB system was programmed to flood the explants with liquid medium for 4 min every 8 h. At the end of the cultivation period, data on the number of shoots and shoot length were collected and recorded.
2.4. Effect of Auxins on Root Formation of Philodendron ‘Pink Princess’
This experiment was designed to compare the efficacy of two culture systems—a TIB and a conventional semi-solid medium—for inducing roots on shoots. The shoots used were healthy, well-established explants obtained from the optimal cytokinin treatment in the previous study. In both systems, the effects of three different auxins were evaluated—NAA, IBA, and IAA—each at concentrations of 0, 0.5, 1.0, 2.0, and 4.0 mg/L. The basal medium for all treatments was MS supplemented with 30 g/L sucrose. For the semi-solid cultures, the medium was solidified with a gelling agent (i.e., 8 g/L agar). The pH of all media was adjusted to 5.6–5.8 before use.
For the TIB treatments, individual shoots were placed in the upper growth chamber, with the lower storage chamber containing 300 mL of the corresponding liquid medium. For the semi-solid treatments, individual shoots were cultured in vessels on the surface of the solidified medium. All treatments were conducted in triplicate. All cultures were maintained in a single growth room at 25 ± 2 °C under a 16 h photoperiod and a light intensity of 45 µmol/m2/s. The TIB systems were programmed for an immersion frequency of 4 min every 8 h. After 30 days of cultivation, the number of roots and their lengths were recorded for comparison.
2.5. Acclimatization of the Plantlets
The acclimatization experiment began with the selection of uniform, rooted plantlets from the semi-solid culture system. Plantlets were chosen based on specific criteria: a height of 1.0–1.2 cm and the presence of at least three healthy roots. Before transplanting, these selected plantlets were carefully removed from the culture vessel, and their root systems were gently washed with sterile distilled water to remove any adhering agar. Following preparation, the plantlets were transferred into 7.5 cm plastic pots containing one of three different substrate mixtures for evaluation. The treatments consisted of: (i) peat moss, perlite, and vermiculite (PPV) (2:1:1, v/v/v); (ii) peat moss and vermiculite (PV) (2:1, v/v); and (iii) peat moss and perlite (PP) (2:1, v/v). Ten plantlets were used for each substrate treatment. All potted plants were maintained in a growth chamber under controlled conditions of 25 ± 2 °C, 45–55% relative humidity, and a 16 h photoperiod at a light intensity of approximately 45 µmol/m2/s. The growth performance and survival of the plantlets were evaluated at 30 and 45 days post-transplanting. The recorded parameters included survival rate (%), plant height, leaf count, root count, and root length.
2.6. Genetic Stability Assessment of the Plantlets
2.6.1. Genomic DNA Extraction
Genomic DNA was extracted from two sources to assess clonal fidelity: (1) fresh, young leaves from the one-year-old, field-grown mother plant (donor control), and (2) leaves from randomly selected plantlets generated through the TIB system. One mother plant and ten micropropagated plantlets were used in this study. For the micropropagated plantlets, the explants used were sourced from a stock culture that had been maintained through continuous monthly subculturing for approximately two years (corresponding to roughly 24 subculture cycles). The sampled plantlets were analyzed 45 days after successful acclimatization. DNA was extracted using the DNeasy Plant Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. The DNA concentration was measured with a BioDrop µLite spectrophotometer (Denville Scientific Inc., South Plainfield, NJ, USA), while its integrity and quality were verified by 1% agarose gel electrophoresis.
2.6.2. PCR Reaction and RAPD Analysis
DNA amplification was performed using ten RAPD primers (Table 1). The 25 µL PCR reaction mixture contained 25 ng of genomic DNA template, 2.5 µL of 10× PCR buffer, 0.5 µL of dNTPs (10 mM), 1.0 µL of MgCl2 (50 mM), 0.125 µL of Taq DNA polymerase (5 U/µL), and 2.5 µL of primer (10 µM), and was brought to a final volume of 25 µL with nuclease-free water.

Table 1.
List of RAPD primers used for genetic stability assessment of micropropagated Philodendron ‘Pink Princess’.
Amplification was conducted in a thermal cycler (C1000 Touch cycler, Bio-Rad Lab, Inc., Hercules, CA, USA) with the following program: an initial denaturation at 94 °C for 4 min, followed by 40 cycles of denaturation at 94 °C for 30 s, annealing at 35 °C for 1 min, and extension at 72 °C for 2 min; and a final extension at 72 °C for 7 min. The resulting PCR products were separated on a 1.5% agarose gel, visualized, and their sizes were determined against a molecular weight marker. For data analysis, the bands obtained by scoring the RAPD profiles were treated as binary characters (1 = presence, 0 = absence). The genotypes were then clustered using the Unweighted Paired Group with Arithmetic Average (UPGMA) method to construct a dendrogram illustrating their genetic relationships [18].
2.7. Experimental Design and Statistical Analysis
All experiments were conducted using a completely randomized design (CRD). The statistical approach varied based on the experimental stage: For the in vitro stages (shoot and root induction), the experimental unit was a single culture vessel (either a TIB or a tissue culture bottle) containing five explants. Each treatment was replicated three times (n = 3). The five explants within each vessel were considered subsamples, and their mean value was used as a single data point for analysis to avoid pseudoreplication. For the acclimatization stage, each individual plantlet was treated as a single, independent replicate, with ten replicates per treatment (n = 10). All collected data were analyzed using SPSS Statistics software (Version 28.0, IBM Corporation, Armonk, NY, USA). The data were subjected to a one-way Analysis of Variance (ANOVA), and treatment means were subsequently compared using Duncan’s Multiple Range Test (DMRT). Significance was determined at the p < 0.05 level. Results are expressed as mean ± standard deviation (SD).
3. Results and Discussion
3.1. Effect of Cytokinins on Shoot Induction and Proliferation
Cytokinins are a class of phytohormones essential for regulating numerous aspects of plant development and physiology. Their functions include promoting cell division and expansion, guiding chloroplast differentiation, controlling morphogenesis, managing nutrient allocation, and mediating cellular adaptation to abiotic stresses [21,22,23].
In plant micropropagation, various natural and synthetic cytokinins are used to stimulate growth, with their efficacy being highly dependent on the plant species, the type of explant used, and the specific developmental stage [23]. The literature documents the successful application of several cytokinins for shoot induction in the Philodendron genus. These primarily include adenine-type cytokinins such as BAP, BA, Kn, and 2-isopentenyl adenine (2iP), as well as the potent phenylurea-type cytokinin TDZ [5,6,24,25,26]. Of these, BAP, TDZ, and Kn are frequently reported as the most effective for inducing organogenesis in Philodendron. Building on these findings, the present study aimed to determine the optimal cytokinin and concentration for shoot proliferation of Philodendron ‘Pink Princess’. To achieve this, the effects of BAP, TDZ, and Kn were evaluated within a TIB culture system, with the results presented in Table 2 and Figure 1.

Table 2.
Effect of cytokinins on shoot induction of Philodendron erubescens ‘Pink Princess’ using the TIB system after cultivation for 30 days.

Figure 1.
Shoot induction of Philodendron erubescens ‘Pink Princess’ under different types and concentrations of cytokinins using the TIB system after cultivation for 30 days.
The results revealed significant differences in shoot proliferation and development based on the type and concentration of cytokinin used (Table 2, Figure 1). In terms of shoot multiplication, both BAP treatments and the hormone-free control were significantly more effective than either Kn or TDZ. While there was no statistically significant difference in the number of shoots produced between the hormone-free control (19.7 shoots/explant) and the optimal BAP treatments (21.4 shoots/explant at 1.0–2.0 mg/L), a critical qualitative assessment of shoot morphology revealed significant differences in plantlet quality. Shoots cultured on medium supplemented with 1.0 and 2.0 mg/L BAP were not only significantly longer (~2.40 cm) but were also visibly more vigorous. These shoots were characterized by robust stems and large, well-expanded leaves of a healthy dark purplish-green color, with no visible symptoms of hyperhydricity. In contrast, shoots grown on the hormone-free control medium were shorter (2.05 cm) and appeared less developed. This improvement in shoot elongation and vigor is a key factor for successful micropropagation, as longer and more robust shoots typically exhibit higher survival rates during the subsequent rooting and acclimatization stages. Short, less-developed shoots, like those from the control group and some other treatments, are often more difficult to handle and are more susceptible to stress. While the hormone-free medium is sufficient for inducing a high number of shoots, the addition of 1.0–2.0 mg/L BAP is crucial for producing high-quality, well-developed plantlets. This finding aligns with previous research, where 1.0–2.0 mg/L BAP was identified as the optimal concentration for shoot multiplication and development in P. bipinnatifidum in a semi-solid medium [5] and P. erubescens ‘Pink Princess’ in both semi-solid and liquid media [6].
In contrast to the potent effects of BAP, both Kn and TDZ demonstrated significantly lower efficacy. Treatment with 1.0 mg/L Kn produced the best result among kinetin concentrations, yielding 16.7 shoots per explant, but shoot elongation was limited, resulting in smaller plantlets. This observation aligns with reports that kinetin is often less effective than BAP, a phenomenon attributed to its slower translocation rates and weaker mitogenic activity [27]. Treatment with TDZ proved to be even less effective and, at higher concentrations, inhibitory. All TDZ treatments yielded fewer and shorter shoots than the BAP treatments (Table 2). More importantly, our qualitative observations confirmed the induction of severe physiological disorders by TDZ. Shoots treated with TDZ, particularly at 1.5 and 2.0 mg/L, exhibited distinct symptoms of undesirable compact growth and hyperhydricity. These plantlets were characterized by swollen, brittle stems and misshapen, translucent, water-soaked leaves that failed to expand properly, indicating poor leaf size and abnormal coloration (Figure 1). These morphological aberrations are classic indicators of phytotoxicity often attributed to potent phenylurea-type cytokinins. The glassy appearance suggests excessive water accumulation in the tissue and poorly developed epicuticular wax, while the stunted, compact growth points to an inhibitory effect on apical dominance and cell elongation. While a detailed histological or physiological analysis of tissue water content was not performed, these pronounced visual markers strongly support the conclusion that TDZ, under these conditions, induced a phytotoxic stress response rather than promoting healthy shoot development. This is consistent with the literature, indicating that while low TDZ levels can induce organogenesis, supra-optimal concentrations often disrupt hormonal balance and inhibit proper shoot development [9,28]. These divergent outcomes underscore that cytokinin efficacy is species-specific and highly dependent on factors such as absorption, translocation to meristematic zones, and metabolic pathways.
Based on our comprehensive results, considering the entire propagation-to-acclimatization pipeline as well as the quantitative data and crucial morphological assessments of leaf size, color, and hyperhydricity, a protocol using 1.0–2.0 mg/L BAP is recommended for producing vigorous shoots suitable for commercial-scale production. Although these concentrations did not increase the number of shoots beyond that of the hormone-free control, the significant improvement in shoot length justifies their use. To develop a protocol that balances enhanced plantlet quality with cost-effectiveness, the lower of these two optimal concentrations was selected. Consequently, a liquid MS medium supplemented with 1.0 mg/L BAP within the TIB system was established as the optimal condition for subsequent experiments.
To assess its efficacy, the TIB system was directly compared to our previous findings using semi-solid and continuous liquid culture systems for Philodendron ‘Pink Princess’ [6]. Under identical culture conditions, the TIB system yielded a shoot induction rate that was 2.7- and 1.8-fold higher, respectively. This result underscores the superior efficacy of the TIB system for micropropagating this cultivar.
3.2. Effect of Auxin on Root Induction
The application of exogenous auxins is a well-established and critical step for promoting adventitious root formation in Philodendron and other Araceae species [8,29,30]. The importance of this hormone supply is consistently demonstrated in both ex vitro and in vitro propagation. For instance, a recent study by Lee et al. [30] on P. hederaceum var. oxycardium cuttings confirmed that treatments with IAA, IBA, and NAA significantly enhanced rooting percentage, root number, and overall root quality. Similarly, numerous in vitro studies have documented the successful use of these same auxins for rooting various Philodendron species, though these efforts have consistently relied on solid or semi-solid culture media [5,6,24,25,26].
Building on this established knowledge, we sought to adapt this successful rooting strategy to a more efficient liquid-based TIB system. We therefore tested three common auxins—NAA, IBA, and IAA—across a concentration range of 0.5 to 4.0 mg/L for rooting Philodendron ‘Pink Princess’ shoots. However, it is important to acknowledge a key limitation of this experiment: our protocol was restricted to a single immersion setting of 4 min every 8 h. This setting was chosen as a preliminary starting point, but as demonstrated by the results, it was entirely unsuccessful after a 30-day period. As illustrated in Figure 2, root initiation was negligible across all auxin treatments. Furthermore, the plantlets exhibited severe signs of phytotoxicity, including extensive basal necrosis and a failure to thrive.
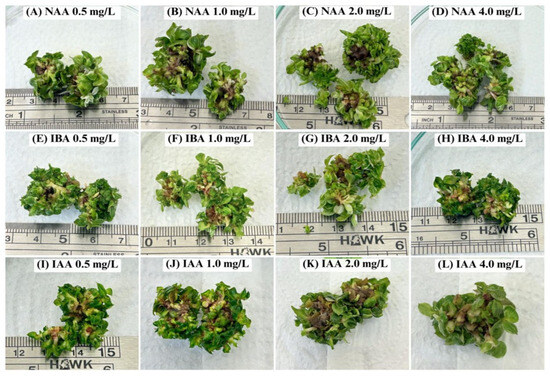
Figure 2.
Shoots of Philodendron erubescens ‘Pink Princess’ after cultivation using MS medium containing different types and concentrations of auxins in the TIB system for 30 days.
This outcome strongly suggests that conventional auxin application methods are not directly transferable to TIB systems for this species without first optimizing the physical culture parameters. The inhibitory effect can likely be attributed to the suboptimal physical conditions within the bioreactor rather than the hormonal treatments themselves. The TIB system’s efficacy relies on a delicate balance between nutrient solution immersion and gaseous exchange [31]. We hypothesize that the prolonged 8 h interval between immersions may have led to excessive aeration and dehydration stress at the explant base. While direct physiological measurements of plant water status were not performed to confirm this mechanism, the severe basal necrosis observed is a classic symptom consistent with desiccation in in vitro cultures. This stress may have caused the plantlets to enter a state of reduced metabolic activity, thereby preventing the energy-demanding process of root initiation [32]. Consequently, we cannot conclude that TIBs, in general, are unsuitable for rooting this cultivar. Instead, we conclude that the specific protocol tested here—combining this auxin range with a low-frequency, short-duration immersion—is inappropriate. Future work must focus on optimizing the physical parameters, such as immersion frequency and duration, likely through a factorial experimental design, to establish a successful TIB-based rooting protocol for this cultivar.
To isolate the effects of the auxins from the influence of the culture system, a parallel experiment was conducted on a semi-solid MS medium using the same hormonal treatments. In contrast to the TIB results, significant differences in rooting were observed (Table 3 and Figure 3). Both the hormone-free control and the 0.5 mg/L NAA treatment failed to produce roots. This indicates not only a requirement for exogenous auxin but also a strong specificity towards the type of auxin used, as NAA was ineffective at the tested concentration. While IBA induced rooting, consistent with its reported efficacy in other Philodendron species [26,33,34], its performance was suboptimal. The highest IBA concentration (4.0 mg/L) yielded the longest roots (~1.2 cm) but only an average of 2.96 roots per explant. The most effective treatment was unequivocally 0.5 mg/L IAA, which produced the highest number of roots (3.03 roots per explant) with substantial length (~1.1 cm). This demonstrates IAA’s high potency at low concentrations for this cultivar. However, IAA exhibited a classic dose-dependent inhibitory effect, as higher concentrations (e.g., 4.0 mg/L) significantly suppressed root elongation (~0.3 cm).

Table 3.
Effect of auxins on root formation of Philodendron ‘Pink Princess’ on a semi-solid MS medium after 30 days of cultivation.

Figure 3.
Root induction of Philodendron ’Pink Princess’ after cultivation on semi-solid MS medium containing different types and concentrations of auxins for 30 days.
Our finding that IAA yielded a superior root system compared to NAA and IBA can be explained by their fundamental biochemical differences. As the primary natural auxin, IAA is subject to the plant’s endogenous metabolic controls, allowing for rapid degradation after initiating root primordia. This transient action prevents accumulation to supraoptimal, inhibitory levels, thereby promoting the crucial subsequent stage of root elongation [8,35]. In contrast, the synthetic auxins NAA and IBA are more resistant to metabolic breakdown, leading to prolonged activity within the plant tissue. NAA, with its high potency and stability, frequently causes an initial burst of root primordia that subsequently fail to elongate due to persistent supraoptimal concentrations, resulting in a mass of short, callused, and non-functional roots [36]. IBA, while also stable, is generally considered less phytotoxic. It is thought to function as a pro-drug that is slowly converted into IAA by the plant, providing a more gradual rooting stimulus. However, this conversion process is still less finely tuned than the plant’s direct regulation of endogenous IAA, which accounts for the superior root morphology observed with direct IAA application in this study [35,37,38].
Based on the current results, 0.5 mg/L IAA in a semi-solid medium was identified as the optimal protocol for rooting Philodendron ‘Pink Princess’. It is noteworthy that this finding contrasts with results for P. bipinnatifidum, where NAA was superior [5]. This discrepancy underscores a critical principle in micropropagation: the optimal type and concentration of auxin are highly species- and even cultivar-dependent.
3.3. Acclimatization of the Plantlets
The transition from sterile, high-humidity in vitro conditions to a non-sterile, low-humidity ex vitro environment is a critical bottleneck in micropropagation. Successful acclimatization, which ensures plantlet survival and vigor, is highly dependent on the choice of growing substrate [26]. An ideal substrate must provide a balanced environment that supports fragile, newly formed roots with adequate aeration, moisture retention, and physical support. While numerous materials have been tested, such as sand, cocopeat, peat moss, perlite, vermiculite, and soilrite [6,34,39,40], composite substrates combining peat moss (for water/nutrient retention), perlite (for aeration and drainage), and vermiculite (for moisture retention and cation exchange) are often cited for superior performance [5,33].
Building on this knowledge, we evaluated three substrate mixtures for the acclimatization of Philodendron ‘Pink Princess’. The most effective formulation was unequivocally a 2:1:1 ratio of peat moss, perlite, and vermiculite (PPV). This substrate yielded a 100% survival rate. This result is consistent with the findings of Klanrit et al. [6], whose acclimatization protocol we adapted for this study, and it is particularly noteworthy given the relatively low ambient relative humidity (RH) of 45–55% maintained during the experiment. The PPV substrate also promoted significantly superior growth across all measured parameters, including leaf number, root number, root length, and plant height, at both 30 days and 45 days post-transplant (Table 4, Figure 4). We hypothesize that this exceptional success is due to the substrate’s ability to create a stable, high-humidity micro-environment directly around the developing root system, thereby decoupling the plant’s immediate environment from the drier ambient air. While the plantlets’ leaves were exposed to low RH, the superior water-holding capacity of the peat moss and vermiculite likely maintained near-saturated conditions at the substrate surface. This would have effectively buffered the fragile plantlets against severe transpiration stress during the critical initial phase [5,33]. While we acknowledge that physiological measurements like stomatal conductance would be needed to definitively confirm this mechanism, our growth and survival data strongly support this interpretation.

Table 4.
Effect of planting substrates during the acclimatization stage of Philodendron ’Pink Princess’ after 30 and 45 days.

Figure 4.
The Philodendron ‘Pink Princess’ plantlets after acclimatization for 30 and 45 days using peat moss, perlite, and vermiculite (PPV) at 2:1:1 (A,D); peat moss and vermiculite (PV) at 2:1 (B,E); and peat moss and perlite (PP) at 2:1 (C,F) as planting materials.
In comparison, substrate mixtures lacking one component were less effective. Both the 2:1 peat moss–vermiculite (PV) mixture and the 2:1 peat moss–perlite (PP) mixture resulted in a reduced survival rate of 90%. Notably, the plantlets in the PV mixture exhibited better overall growth than those in the PP mixture. This suggests that the water-retaining properties of vermiculite were more critical for this species’ initial establishment than the enhanced aeration provided by perlite, a finding that aligns with reports on other plants like tea clones [41], wasabi [42], and banana [11,20,43,44].
3.4. Assessment of Genetic Stability of Micropropagated Plantlets
Maintaining the genetic integrity of micropropagated plants is paramount, as the stresses of in vitro culture can induce somaclonal variation—genetic or epigenetic changes that may compromise desirable traits [45]. Factors such as explant source, culture type, culture duration, and the specific combinations of PGRs can disrupt cellular stability and lead to off-types, undermining the goal of clonal propagation [46,47,48]. Consequently, assessing the genetic fidelity of regenerated plants is a critical quality control measure.
In this study, RAPD analysis was employed to evaluate the genetic stability of TIB-regenerated Philodendron ‘Pink Princess’ plantlets against the original mother plant. A total of 10 primers was used to screen 10 randomly selected clones. The primers generated a total of 73 clear and reproducible bands ranging from 400 to 3500 bp, with an average of 7.3 bands per primer (Table 1). The amplified DNA profiles for all 10 clones were monomorphic and identical to that of the mother plant, revealing no detectable polymorphism (Figure 5). This result suggests that, within the limits of the RAPD technique and the sample size tested, the developed TIB protocol facilitates multiplication with a high degree of genetic stability. These findings align with previous reports where high genetic stability was confirmed in other micropropagated aroids, such as Anthurium andreanum (using ISSR markers) and P. bipinnatifidum (using RAPD markers) [5,18,49].
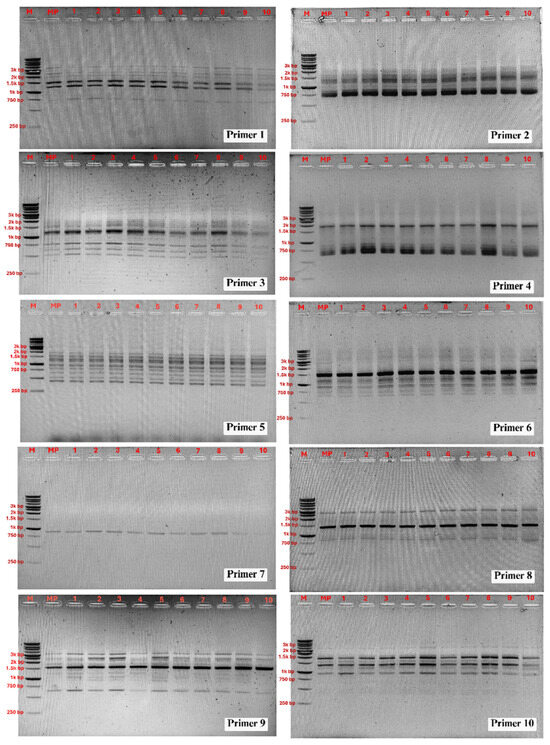
Figure 5.
PCR amplification products obtained with the RAPD primers. Lane M: DNA ladder; lane MP: DNA from mother plant; 1–10: DNA from micropropagated plantlets.
However, it is essential to interpret these results within the context of the methodology’s limitations. First, the sample size (n = 10) may not be sufficient to detect rare, low-frequency somaclonal variants that could become significant during large-scale production. Second, while RAPD markers can detect broad genomic changes, they have known issues with reproducibility and dominance [50,51]. Crucially, for a cultivar prized for its variegation, RAPD analysis is unable to assess the stability of this chimeric or epigenetically controlled trait, which is a primary concern for growers.
Therefore, for robust validation intended for commercial-scale production, a more comprehensive, multi-faceted approach is highly recommended [19,52]. Future work should focus on expanding the sample size significantly and corroborating these findings using a combination of marker systems. Co-dominant markers with higher reproducibility, such as ISSR and SCoT, would increase confidence in genomic fidelity [53,54,55]. Furthermore, to address the stability of the ‘Pink Princess’ phenotype, this molecular analysis should be coupled with rigorous phenotypic quantification of the variegated leaf area across a larger acclimatized population. For a deeper understanding, the use of methylation-sensitive markers could also be considered to investigate any epigenetic changes that might affect variegation patterns.
4. Conclusions
This research successfully establishes a comprehensive and highly efficient micropropagation protocol for the commercially valuable ornamental, P. erubescens ‘Pink Princess’. The study demonstrates the superiority of a hybrid approach, leveraging a TIB system for the shoot proliferation stage and a semi-solid medium for rooting. The TIB system, when supplied with MS medium containing 1.0 mg/L BAP, proved ideal for rapid shoot multiplication, yielding nearly 21 shoots per explant while preventing hyperhydricity. For the subsequent rooting stage, a transition to a semi-solid MS medium supplemented with 0.5 mg/L IAA was essential, indicating that constant, stable contact with the rooting hormone is more critical for adventitious root formation in this cultivar than the intermittent immersion provided by the TIB. This two-step process overcomes the common limitations of both static liquid and solid culture systems. The resulting plantlets were of high morphological quality, characterized by vigorous shoots, purplish-green leaves without any signs of hyperhydricity, and extensive root formation, in addition to being genetically stable, as confirmed by RAPD analysis, which showed 100% clonal fidelity with the mother plant. This genetic uniformity, combined with a 100% survival rate during acclimatization in a peat–perlite–vermiculite substrate, validates the reliability of the entire pipeline from lab to greenhouse. In summary, the integrated protocol developed herein provides an efficient and genetically stable method for propagating P. erubescens ‘Pink Princess’ at the laboratory scale. This work lays the groundwork for developing scalable production systems, offering a significant advancement for commercial growers seeking to meet market demand and providing a robust model for the conservation and propagation of other valuable Araceae species.
Author Contributions
Conceptualization, P.K., S.T. and P.T.; methodology, B.K.V., P.K., S.T. and P.T.; software, P.T.; validation, P.K., S.T. and P.T.; formal analysis, B.K.V., P.K., S.T. and P.T.; investigation, B.K.V. and S.T.; resources, P.K. and P.T.; data curation, P.K., S.T. and P.T.; writing—original draft preparation, B.K.V., S.T. and P.T.; writing—review and editing, P.K., S.T. and P.T.; visualization, B.K.V. and P.T.; supervision, P.K. and P.T.; project administration, P.T.; funding acquisition, P.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Research Program funding from the Research and Innovation Department, Khon Kaen University (grant number: RP68-6-Research Center-001). A part of this research was also supported by the International Affairs Division, Faculty of Technology, and Department of Biotechnology, Khon Kaen University, through a Khon Kaen University Scholarship for ASEAN and GMS country personnel.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors are grateful to Mamoru Yamada (Yamaguchi University) for his invaluable guidance and suggestions throughout the experimental work and manuscript preparation. The authors also acknowledge the Department of Biotechnology, Faculty of Technology, Khon Kaen University, for providing the necessary facilities and resources for this study, and the Ministry of Higher Education, Science, Research and Innovation through Reinventing University (2025), Khon Kaen University, for supporting Yamada’s appointment as a visiting professor.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mayo, S.J. A revision of Philodendron subgenus Meconostigma (Araceae). Kew Bull. 1991, 46, 601–681. [Google Scholar] [CrossRef]
- Croat, T.B. A revision of Philodendron subgenus Philodendron (Araceae) for Mexico and Central America. Ann. Mo. Bot. Gard. 1997, 84, 311–704. [Google Scholar] [CrossRef]
- Hovhannisyan, V.; Khachatryan, H. Ornamental plants in the United States: An econometric analysis of household-level demand system. Agribusiness 2017, 33, 226–241. [Google Scholar] [CrossRef]
- Chiewchan, N.; Saetiew, K.; Teerarak, M. The effect of BA on inducing shoots of Philodendron erubescens ‘Pink Princes’ in vitro. Int. J. Agric. Technol. 2023, 19, 2385–2398. [Google Scholar]
- Alawaadh, A.A.; Dewir, Y.H.; Alwihibi, M.S.; Aldubai, A.A.; El-Hendawy, S.; Naidoo, Y. Micropropagation of lacy tree Philodendron (Philodendron bipinnatifidum Schott ex Endl.). HortScience 2020, 55, 294–299. [Google Scholar] [CrossRef]
- Klanrit, P.; Kitwetcharoen, H.; Thanonkeo, P.; Thanonkeo, S. In Vitro propagation of Philodendron erubescens ‘Pink Princess’ and ex vitro acclimatization of the plantlets. Horticulturae 2023, 9, 688. [Google Scholar] [CrossRef]
- Yunita, R.; Nugraha, M.F.I. Effect of auxin type and concentration on the induction of Alternanthera Reineckii roots in vitro. IOP Conf. Ser. Earth Environ. Sci. 2021, 653, 012073. [Google Scholar] [CrossRef]
- Sosnowski, J.; Truba, M.; Vasileva, V. The impact of auxin and cytokinin on the growth and development of selected crops. Agriculture 2023, 13, 724. [Google Scholar] [CrossRef]
- Han, B.H.; Park, B.M. In vitro micropropagation of Philodendron cannifolium. J. Plant Biotechnol. 2008, 35, 203–208. [Google Scholar] [CrossRef]
- Ziv, M.; Ariel, T. Bud proliferation and plant regeneration in liquid-cultured Philodendron treated with ancymidol and paclobutrazol. J. Plant Growth Regul. 1991, 10, 53–57. [Google Scholar] [CrossRef]
- Bozkurt, T.; İnan, S.; Dündar, İ. Comparison of temporary immersion bioreactor (SETISTM) and classical solid culture in micropropagation of ‘Grand Naine’ (Musa spp.) banana cultivar. J. Agric. Sci. 2023, 15, 51–60. [Google Scholar] [CrossRef]
- Gupta, S.D.; Prasad, V.S.S. Matrix-supported liquid culture systems for efficient micropropagation of floricultural plants. Floric. Ornam. Plant Biotechnol. Adv. Trop. Issue 2006, 2, 488–495. [Google Scholar]
- Uma, S.; Karthic, R.; Kalpana, S.; Backiyarani, S.; Saraswathi, M.S. A novel temporary immersion bioreactor system for large scale multiplication of banana (Rasthali AAB-silk). Sci. Rep. 2021, 11, 20371. [Google Scholar] [CrossRef]
- Ruffoni, B.; Savona, M. The temporary immersion system (T.I.S) for the improvement of micropropagation of ornamental plants. Acta Hortic. 2005, 683, 445–454. [Google Scholar] [CrossRef]
- Ramírez-Mosqueda, M.A.; Cruz-Cruz, C.A.; Cano-Ricárdez, A.; Bello-Bello, J.J. Assessment of different temporary immersion systems in the micropropagation of anthurium (Anthurium andreanum). 3 Biotech 2019, 9, 307. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.K.; Phulwaria, M.; Harish; Gupta, A.K.; Shekhawat, N.; Jaiswal, U. Genetic homogeneity of guava plants derived from somatic embryogenesis using SSR and ISSR markers. Plant Cell Tiss. Org. Cult. 2012, 111, 259–264. [Google Scholar] [CrossRef]
- Gupta, A.K.; Harish; Rai, M.K.; Phulwaria, M.; Agarwal, T.; Shekhawat, N. In vitro propagation, encapsulation, and genetic fidelity analysis of Terminalia arjuna: A cardioprotective medicinal tree. Appl. Biochem. Biotechnol. 2014, 173, 1481–1494. [Google Scholar] [CrossRef] [PubMed]
- Alwahibi, M.S.; Alawaadh, A.A.; Dewir, Y.H.; Soliman, D.A.; Seliem, M.K. Assessment of genetic fidelity of lacy tree Philodendron (Philodendron bipinnatifidum Schott ex Endl.) micropropagated plants. Revis Bionatura 2022, 7, 10. [Google Scholar]
- Al-Aizari, A.A.; Dewir, Y.H.; Ghazy, A.-H.; Al-Doss, A.; Al-Obeed, R.S. Micropropagation and genetic fidelity of Fegra Fig (Ficus palmata Forssk.) and grafting compatibility of the regenerated plants with Ficus carica. Plants 2024, 13, 1278. [Google Scholar] [CrossRef]
- Thanonkeo, S.; Kitwetcharoen, H.; Thanonkeo, P.; Klanrit, P. Temporary immersion bioreactor (TIB) system for large-scale micropropagation of Musa sp. cv Kluai Numwa Pakchong 50. Horticulturae 2024, 10, 1030. [Google Scholar] [CrossRef]
- Kieber, J.J.; Schaller, G.E. Cytokinin signaling in plant development. Development 2018, 145, dev149344. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Mekureyaw, M.F.; Pandey, C.; Roitsch, T. Role of cytokinins for interactions of plants with microbial pathogens and pest insects. Front. Plant Sci. 2020, 10, 1777. [Google Scholar] [CrossRef]
- Emery, R.J.N.; Kisiala, A. The roles of cytokinins in plants and their response to environmental stimuli. Plants 2020, 9, 1158. [Google Scholar] [CrossRef] [PubMed]
- Khamrit, R.; Jongrungklang, N. Determining optimal mutation induction of Philodendron billietiae using gamma radiation and in vitro tissue culture techniques. Horticulturae 2024, 10, 1164. [Google Scholar] [CrossRef]
- Maikaeo, L.; Puripunyavanich, M.; Limtiyayotin, M.; Orpong, P.; Kongpeng, C. Micropropagation and gamma irradiation mutagenesis in Philodendron billietiae. Thai J. Agric. Sci. 2024, 57, 11–19. [Google Scholar]
- Kang, I.; Sivanesan, I. Micropropagation of Philodendron ‘White Knight’ via shoot regeneration from petiole explants. Plants 2025, 14, 1714. [Google Scholar] [CrossRef]
- Klaocheed, S.; Jehsu, W.; Choojun, W.; Thammasiri, K.; Prasertsongskun, S.; Rittirat, S. Induction of direct shoot organogenesis from shoot tip explants of an ornamental aquatic plant, Cryptocoryne wendtii. Walailak J. Sci. Technol. 2018, 17, 293–302. [Google Scholar] [CrossRef]
- Dewir, Y.H.; Nurmansyah; Naidoo, Y.; Teixeira da Silva, J.A. Thidiazuron-induced abnormalities in plant tissue cultures. Plant Cell Rep. 2018, 37, 1451–1470. [Google Scholar] [CrossRef]
- Wiesman, Z.; Riov, J.; Epstein, E. Comparison of movement and metabolism of indole-3-acetic acid and indole-3-butyric acid in mung bean cuttings. Physiol. Plant. 1988, 74, 556–560. [Google Scholar] [CrossRef]
- Lee, J.H.; Shin, E.J.; Kim, E.A.; Jang, I.T.; Lee, S.; Nam, S.Y. Effects of different concentrations of exogenous auxins (IAA, IBA, and NAA) on growth and rooting ability of Philodendron hederaceum var. oxycardium (Schott) Croat stem cuttings. J. People Plants Environ. 2024, 27, 279–289. [Google Scholar] [CrossRef]
- Ahmadian, M.; Babaei, A.; Shokri, S.; Hessami, S. Micropropagation of carnation (Dianthus caryophyllus L.) in liquid medium by temporary immersion bioreactor in comparison with solid culture. J. Genet. Eng. Biotechnol. 2017, 15, 309–315. [Google Scholar] [CrossRef]
- Etienne, H.; Berthouly, M. Temporary immersion systems in plant micropropagation. Plant Cell Tiss. Org. Cult. 2002, 69, 215–231. [Google Scholar] [CrossRef]
- Hassan, H.M.S.; Ali, M.A.M.; Soliman, D.A. Effect of low-cost gelling agents and some growth regulators on micropropagation of Philodendron selloum. J. Plant Prod. 2016, 7, 169–176. [Google Scholar] [CrossRef][Green Version]
- Akramian, M.; Khaleghi, A.R.; Salehi-Arjmand, H. Optimization of plant growth regulators for in vitro mass propagation of Philodendron cv. Birkin through shoot tip culture. Greenh. Plant Prod. J. 2024, 1, 55–62. [Google Scholar] [CrossRef]
- Woodward, A.W.; Bartel, B. Auxin: Regulation, action, and interaction. Ann. Bot. 2005, 95, 707–735. [Google Scholar] [CrossRef]
- Hausman, J. Changes in peroxidase activity, auxin level and ethylene production during root formation by poplar shoots raised in vitro. Plant Growth Regul. 1993, 13, 263–268. [Google Scholar] [CrossRef]
- Epstein, E.; Ludwig-Müller, J. Indole-3-butyric acid in plants: Occurrence, synthesis, metabolism and transport. Physiol. Plant. 1993, 88, 382–389. [Google Scholar] [CrossRef]
- Epstein, E.; Sagee, O.; Zelcer, A. Uptake and metabolism of indole-3-butyric acid and indole-3-acetic acid by petunia cell suspension culture. Plant Growth Regul. 1993, 13, 31–40. [Google Scholar] [CrossRef]
- Khan, M.I.; Ahmad, N.; Anis, M. The role of cytokinins on in vitro shoot production in Salix tetraspera Roxb.: A tree of ecological importance. Trees 2011, 25, 577–584. [Google Scholar] [CrossRef]
- Abdalla, N.; Ragab, M.; El-Miniawy, S.; Arafa, N.; Taha, H. A new aspect for in vitro propagation of Jerusalem artichoke and molecular assessment using RAPD, ISSR and SCoT marker techniques. Egypt. J. Bot. 2021, 61, 203–218. [Google Scholar] [CrossRef]
- Gonbad, R.A.; Moghaddam, S.S.; Sinniah, U.R.; Aziz, M.A.; Safarpour, M. Determination of potting media for effective acclimatization in micropropagated plants of tea clone Iran 100. Int. J. For. Soil Erosion. 2013, 3, 40–44. [Google Scholar]
- Hoang, N.N.; Kitaya, Y.; Shibuya, T.; Endo, R. Effects of supporting materials in in vitro acclimatization stage on ex vitro growth of wasabi plants. Sci. Hortic. 2020, 261, 109042. [Google Scholar] [CrossRef]
- Erol, M.H.; Dönmez, D.; Biçen, B.; Şimşek, Ö.; Kaçar, Y.A. Modern approaches to in vitro clonal banana production: Next-generation tissue culture systems. Horticulturae 2023, 9, 1154. [Google Scholar] [CrossRef]
- Uma, S.; Karthic, R.; Kalpana, S.; Backiyarani, S. Evaluation of temporary immersion bioreactors for in vitro micropropagation of banana (Musa spp.) and genetic fidelity assessment using flow cytometry and simple-sequence repeat markers. S. Afr. J. Bot. 2023, 157, 553–565. [Google Scholar] [CrossRef]
- Krishna, H.; Alizadeh, M.; Singh, D.; Singh, U.; Chauhan, N.; Eftekhari, M.; Sadh, R.K. Somaclonal variations and their applications in horticultural crops improvement. 3 Biotech 2016, 6, 54. [Google Scholar] [CrossRef]
- Lal, D.; Singh, N. Mass multiplication of Celastrus paniculatus Willd: An important medicinal plant under in vitro conditions via nodal segments. Int. J. Biodivers. Conserv. 2010, 2, 140–145. [Google Scholar]
- Bairu, M.W.; Aremu, A.O.; Van Staden, J. Somaclonal variation in plants: Causes and detection methods. Plant Growth Regul. 2011, 63, 147–173. [Google Scholar] [CrossRef]
- Premvaranon, P.; Vearasilp, S.; Thanapornpoonpong, S.N.; Karladee, D.; Gorinstein, S. In vitro studies to produce double haploid in Indica hybrid rice. Biologia 2011, 66, 1074–1081. [Google Scholar] [CrossRef]
- Gantait, S.; Mandal, N.; Bhattacharyya, S.; Das, P.K. In vitro mass multiplication with pure genetic identity in Anthurium andreanum Lind. Plant Tissue Cult. Biotechnol. 2009, 18, 113–122. [Google Scholar] [CrossRef]
- EL-Banna, A.N.; Khatab, I.A. Assessing genetic diversity of some potato (Solanum tuberosum L.) cultivars by protein and RAPD markers. Egypt. J. Genet. Cytol. 2013, 42, 89–101. [Google Scholar] [CrossRef]
- Salama, D.M.; Osman, S.A.; Abd EL-Aziz, M.; Abd ELwahed, M.S.; Shaaban, E. Effect of zinc oxide nanoparticles on the growth, genomic DNA, production and the quality of common dry bean (Phaseolus vulgaris). Biocatal. Agric. Biotechnol. 2019, 18, 101083. [Google Scholar] [CrossRef]
- Lakshmanan, V.; Venkataramareddy, S.R.; Neelwarne, B. Molecular analysis of genetic stability in long-term micropropagated shoots of banana using RAPD and ISSR markers. Electron. J. Biotechnol. 2007, 10, 106–113. [Google Scholar] [CrossRef]
- Cabo, S.; Ferreira, L.; Carvalho, A.; Martins-Lopes, P.; Martín, A.; Lima-Brito, J.E. Potential of start codon targeted (SCoT) markers for DNA fingerprinting of newly synthesized tritordeums and their respective parents. J. Appl. Genet. 2014, 55, 307–312. [Google Scholar] [CrossRef]
- Fang-Yong, C.; Ji-Hong, L. Germplasm genetic diversity of Myrica rubra in Zhejiang province studied using inter-primer binding site and start codon-targeted polymorphism markers. Sci. Hortic. 2014, 170, 169–175. [Google Scholar] [CrossRef]
- Thakur, J.; Dwivedi, M.D.; Sourabh, P.; Uniyal, P.L.; Pandey, A.K. Genetic homogeneity revealed using SCoT, ISSR and RAPD markers in micropropagated Pittosporum eriocarpum Royle-an endemic and endangered medicinal plant. PLoS ONE 2016, 11, e0159050. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).