Abstract
Drought, exacerbated by climate change, represents a growing challenge for agriculture, significantly impacting on crops such as chili peppers (Capsicum), essential in the global diet. This work evaluated the response to water stress by suspending irrigation in Siete Caldos chili pepper plants (Capsicum frutescens). Control plants were watered every 48 h, while stress was induced in the test plants by withholding irrigation for 14 days, followed by an evaluation of recovery through rehydration on day 15. Growth parameters such as the fresh weight of the aerial part, root length, and number of flower buds showed significant differences between the two groups from the eighth day onwards. However, physiologically and biochemically stress-induced decreased relative water content, membrane stability, and chlorophyll content, coupled with increased electrolyte leakage, proline content, and antioxidant activity (catalases and peroxidases), were observed starting on the third day. These effects were more severe on day 14. At the molecular level, the expression of stress response genes (AP2, LOX2, CAT, CuSOD, MnSOD, and P5CS) was quantified at days 3, 14, and 15, revealing differences in transcript levels between the treatments. Finally, rehydration in the stressed plants resulted in the recovery of the evaluated parameters and a survival rate of 100%. Therefore, chili pepper has tolerance mechanisms that allow it to withstand a period of 14 days without irrigation, without reaching its permanent wilting point, and it can recover if conditions improve. This study underscores the complexity of plant responses and tolerance mechanisms to drought, providing insights into the behavior of semi-domesticated species.
1. Introduction
In recent years, climate change has intensified drought, leading to more significant variations in rainfall, exacerbating water scarcity. This global challenge significantly impacts agriculture, which is crucially dependent on water for the productivity, growth, and optimal development of various groups, as each crop has specific water requirements that must be met. However, with approximately 70% of freshwater used for agriculture, there is a growing need to develop resilience to drought [1,2].
Plants respond to water deficit in a complex way through morphological, physiological, biochemical, and molecular adaptations that change considerably depending on the stage of development, the plant species, the duration, severity, and time of exposure to stress [3,4,5,6]. Plants use avoidance, escape, and tolerance strategies to cope with drought. These strategies range from speeding up its life cycle and optimizing water conservation processes to overcoming dehydration through osmotic adjustment, osmoprotectant accumulation, and increasing cell wall elasticity [6,7,8].
A plant’s ability to tolerate or succumb (susceptibility) to water stress may be related to its genetic variability, which allows different species and genotypes within the same species to grow under hostile conditions [9]. Therefore, plants undergo a series of metabolic alterations in response to drought to achieve cellular balance [8,10].
Capsicum, also known as chili, rocoto, ají, chili pepper, or bell pepper, is the second most consumed vegetable in the world [1], making it a crop of economic and gastronomic importance. However, it is a drought-affected crop and a moderately sensitive genus to drought and salinity [11,12]. Drought affects the different phenological stages of the crop, restricting its productivity [13]. The genus Capsicum is susceptible to the effects of drought in the vegetative stage [14], being more severe in the flowering stage and during fruit development [1]. Exposure to different degrees of drought conditions has been reported to induce a decrease in relative leaf tissue water content, growth rate, carotenoid, and chlorophyll a, b, and total content in vegetative C. chinense plants [1]. Similarly, in C. annuum, exposure to drought has been shown to significantly reduce the number of leaves and water content in the shoots, leaves, and roots [15]. In addition, an increase in the activity of antioxidant enzymes such as catalase (CAT), superoxide dismutase (SOD), and peroxidases to mitigate oxidative stress has been documented in pepper plants (C. annuum) [16]. This increase in their antioxidant and osmoprotective activity has been observed in other nightshades such as tomatoes (Sufan14, Jinlingmeiyu, Kashi Amrit, Kashi Anupam, EC-317-6-1 and WIR-4360) [17,18].
On the other hand, the synthesis of compatible or osmoprotective solutes that protect the cell from osmotic mismatch is a stress tolerance mechanism. Among these osmoprotectants is Proline (Pro), an amino acid that accumulates in plants in stress response, which is why it is considered a crucial component of the tolerance mechanism [19,20,21]. Besides being a cellular osmoprotectant, Pro is indispensable for the synthesis of proteins, stabilizes the structure of cell membranes, acts as a protective agent for enzymes, maintains the balance between NADPH/NADP+, and minimizes the effects of reactive oxygen species (ROS) [22,23]. The accumulation of osmoprotectants such as Pro to mitigate oxidative stress has been documented in pepper plants (C. annuum) [16]. Pro accumulation in tomato leaves of tolerant varieties has been observed to affect transpiration positively and the water potential of leaves under water deficit [24]. Under conditions of moderate drought (water deficit of 50% of field capacity) in the vegetative stage corresponding to the onset of flowering, plants of Capsicum annuum var. Paco exhibit increased potassium concentration, essential for physiological and biochemical processes, including osmotic balance and enzyme activation. In parallel, total phenols and proline are elevated, acting as antioxidants and osmoprotectants, respectively, reflecting their crucial role in the response to drought stress. This increase in proline, especially noticeable in the leaves, and a decrease in carotenoid synthesis underscores the complex biochemical modulation that facilitates the survival and adaptation of chili pepper plants to adverse conditions [25].
Some genes and transcription factors are essential in response to stress due to drought, salinity, temperature, and other factors. These include Δ-1-pyrroline-5-carboxylate synthetase (P5CS), catalase (CAT), superoxide dismutase (CuSOD and MnSOD), 2-lipoxygenase (LOX2), and ethylene transcription factor (AP2/ERF), which are involved in osmoprotectant synthesis, ROS detoxification, and developmental regulation. Antioxidant enzymes play a fundamental role in eliminating ROS to maintain a redox balance by regulating ROS levels and reducing oxidative damage to various structures in the cell.
Mexico, as the world’s second-largest producer of Capsicum [26] and home to a rich diversity of wildlife species, provides a unique context for studying these mechanisms [27]. Chiapas has a great diversity of wild and semi-domesticated species of chili peppers. The Siete Caldos pepper (C. frutescens), in particular, is considered a semi-domesticated species, which could present more efficient tolerance mechanisms to abiotic factors than other species grown in large areas. In this study, the effects of drought due to irrigation suspension on the tolerance of the Siete Caldos chili pepper were evaluated at the physiological, biochemical, and molecular levels through the expression of genes related to response to water stress, which play a crucial role in the adaptation of plants to these conditions. Understanding these processes is imperative for enhancing crop production under drought conditions and contributing to the conservation of genetic diversity and the survival of plant species in a changing and unfavorable environment.
2. Materials and Methods
2.1. Obtaining and Acclimatizing Seedlings
The seeds of Siete Caldos chili peppers (Capsicum frutescens) were obtained from ripe fruits collected in the ejido El Porvenir Agrarista (16°10′02″ N and 91°50′59″ W) at an altitude of 1488 m above sea level in the municipality of La Trinitaria, Chiapas, Mexico.
Germination was carried out in polystyrene seedbeds using peat moss (Peat Moss Premir Pro-mix®, Quebec, Canada) and agrolite (Termolita® Hortiperl, Nuevo León, México) as substrate in a ratio of 3:1 v/v. After the first pair of true leaves appeared, the seedlings were watered with Hoagland’s solution at one-fifth of its ionic strength (1/5 i.s.) for maintenance and development. Hoagland and Arnon’s [28] solution contains the following: 50 μM CaCl2, 12.5 μM H3BO3, 1 Μm MnSO4·H2O, 1 μM ZnSO4·7H2O, 0.5 μM CuSO4, 0.1 μM (NH4)·6Mo3O24·2H2O, 0.1 μM NiCl·6H2O, 10 μM Fe-EDTA, 1.2 mM de KNO3, 0.8 mM de Ca(NO3)2·4H2O, 0.2 mM KH2PO4, and 0.2 mM MgSO4·7H2O (Sigma-Aldrich®, Merck KGaA, Darmstadt, Germany) in distilled water. The growing conditions in the culture room were an average temperature of 26 ± 2 °C, with a 16 h light and 8 h dark photoperiod and a relative humidity of the air of ~50%.
Thirty days after emergence (dae), the seedlings were transplanted into low-density polyethylene containers with a mixture of soil and sieved compost (3:1 v/v) and watered to field capacity (FC) every 48 h with a Hoagland solution (1/5 i.s.) for ten days for acclimatization. The substrate FC was calculated gravimetrically (Equation (1)), following the methodology of Luna-Flores [29]. For this purpose, five samples of 100 g of the mixture were taken and kept at 105 °C until reaching a constant weight. Subsequently, the samples were irrigated to excess and allowed to drain for 24 h at room temperature.
where: FC = field capacity; Wws = weight of wet soil; Wfp = weight of filter paper; Wds = weight of dry soil.
2.2. Application of Water Stress
Two hundred and twenty seedlings of chili plants were evaluated to determine the effect of drought. Water stress was applied by suspending irrigation for 14 days; at the end of the stress period, this group of plants was irrigated to FC to observe their recovery capacity 24 h later (day 15). The control group received one irrigation every 48 h at FC calculated with the methodology of Luna-Flores [29] (Equation (1)). For each treatment, 110 plants were used.
The maintenance conditions were an average temperature of 33 ± 2 °C, with a photoperiod of 16:8 h light–dark and relative humidity of the air of ~50%, and irrigation was carried out with Hoagland nutrient solution. Samples were taken at different times during the stress period to determine the growth variables (0, 3, 8, 14, and 15 days; 22 plants). The growth variables determined were the aerial part’s height, the root length, the number of flower buds, and the fresh and dry weight.
2.3. Relative Water Content
The relative water content (RWC) was calculated in the shoots and roots with the following equation (Equation (2)), described by Jothimani and Arulbalachandran [30].
where: RWC = relative water content; Wf = fresh weight; Wd = dry weight
2.4. Electrolyte Leakage and Membrane Stability Index
To determine the electrolyte leakage (Equation (3)) and membrane stability index (MSI) (Equation (4)) in the leaves and roots, the protocol described by Restrepo et al. [31] and Semida et al. [32], respectively, with modifications, was followed. For this, three leaf discs of 1 cm diameter and 25 mg of root were taken and placed in test tubes with 15 mL of tri-distilled water. The samples were incubated at room temperature (30 ± 2 °C) for two h to determine the initial electrical conductivity (EC1). Subsequently, the samples were placed in a water bath at 121 °C for 20 min to determine the final electrical conductivity (EC2). The determinations were carried out using a conductivity probe (CON-BTA Vernier®).
2.5. Total Chlorophyll Content
The extraction and quantification of total chlorophyll were carried out using the method of Inskeep and Bloom [33], with modifications. For the extraction, 0.05 g of fresh leaf was macerated with 80% acetone and incubated for one hour at 4 °C. Subsequently, it was centrifuged at 10,000 rpm for 5 min. The quantification was determined spectrophotometrically by measuring the absorption at wavelengths (λ) of 664 and 647 nm; the results were calculated using Equation (5).
2.6. Proline Content in Leaf and Root
Proline content was quantified from fresh leaf and root tissue based on the protocol described by Bates et al. [34] with modifications reported by Escalante-Magaña [35]. The reaction was quantified at a wavelength of 520 nm using toluene as a blank, and the results were calculated using the following Equation (6).
2.7. Determination of Enzymatic Activity of Catalase and Peroxidase
2.7.1. Total Protein Extraction and Quantification
The protein extract was obtained from 0.5 g of fresh plant material (leaf and root), which was macerated with liquid nitrogen and homogenized with 4 mL of sodium phosphate buffer solution at 100 mM and a pH of 7.0, EDTA at 0.1 mM. Subsequently, it was centrifuged at 10,000 rpm at 4 °C for 20 min [36]. The protein content was determined by a colorimetric reaction following Bradford’s methodology [37].
2.7.2. Catalase Activity (CAT EC 1.11.1.6)
The methodology described by Góth [38] and Suárez et al. [36] was used. The reaction mixture consisted of 1 mL of sodium phosphate buffer (60 mM) with hydrogen peroxide (65 mM) at a pH of 7.4 and 200 μL of protein extract. This mixture was incubated for 4 min at 37 °C. Subsequently, 1 mL of ammonium molybdate (32.4 mM) was added to stop the reaction, and the yellow ammonium–hydrogen peroxide complex was measured at λ 405 nm. The results obtained are expressed in specific activity (U∙mg protein−1).
2.7.3. Peroxidase Activity (POX EC 1.11.1.11)
Peroxidase activity was determined using Hammerschmidt et al.’s [39] method. The reaction mixture contained 2.9 mL of sodium phosphate buffer (0.01 M) with guaiacol (0.25%) and hydrogen peroxide (0.1 M) at a pH of 6.0. Subsequently, 100 μL of protein extract was added, and activity at λ 470 nm was measured for 1 min. The results obtained are expressed in specific activity (U∙mg protein−1).
2.8. Gene Expression in Roots
2.8.1. Total RNA Extraction and First-Strand DNA (cDNA) Synthesis
Total RNA extraction from the roots was performed at different times during the treatment (3, 14, and 15 days), following the methodology of Chomczynski and Sacchi [40] and using the Direct-zol ™ RNA Miniprep Plus Extraction Kit (Zymo Research, BioSystems, CA, USA). For cDNA synthesis, 5 μg of RNA was used, and we employed the iScript Advanced cDNA Synthesis Kit for RT-qPCR (BIO-RAD®, California, USA). RNA and cDNA sample integrity and quality were verified by ethidium bromide-stained 1% agarose gel electrophoresis and NanoDrop ND-100 spectrophotometry (Thermo Scientific™, Waltham, MA, USA).
2.8.2. Relative Gene Expression in Roots by RT-qPCR
RT-qPCR amplification was conducted using Real-Time System CFX96TM (BIO-RAD©, USA). Specific oligos (Table 1) were used for genes involved in proline synthesis, oxidative stress, jasmonate synthesis, and ethylene transcription factor in Capsicum. The UBIQUITIN gene was used as a reference gene to normalize the samples. The iTaqTM Universal SYBR® Green Supermix Kit (BIO-RAD© Hercules, CA, USA was used according to the manufacturer’s specifications for amplification reactions. The reaction conditions of the thermal cycler were a cycle of 95 °C for 30 s, followed by 40 cycles of 95 °C for 0.05 s and 60 °C for 30 s. The acquired data were analyzed using the 2−ΔΔCT method to ascertain the differential expression of the genes [41].

Table 1.
Primer sequences for real-time quantification of Capsicum frutescens.
2.9. Statistical Analysis
A completely randomized factorial design was employed, and the data obtained were analyzed by analysis of variance (ANOVA). Mean differences (post hoc analysis) were determined using Tukey’s multiple rank test (p ≤ 0.05). Statistical analysis was carried out using Statgraphics Centurion XIX® (Statgraphics Technologies, Inc., Madrid, Spain).
3. Results
3.1. Growth Characteristics in Response to Water Stress
Growth variables in the shoots and roots (height and length, fresh and dry weight), as well as the number of flower buds were evaluated to determine the effect of water stress and to evaluate the recovery capacity of the plants. Visually, the plants exposed to drought from the eighth day onwards showed chlorosis and curling in some leaves (Figure 1b). However, the loss of turgor in the shoots (the loss of water) was observed in the plants up to the tenth day (Figure 1c), with 50% of the plants exhibiting turgor loss, as well as leaf senescence. At 14 days, all the plants had turgor loss (Figure 1e). The plants were exposed to drought by irrigation suspension (IS) for 14 days and rehydrated (RP) to field capacity (FC) to analyze their resilience. A complete turgor recovery was observed in the leaves (Figure 1f). This indicates that after 14 days of stress, the plants remained alive, recovering from wilting when watered again.

Figure 1.
Behavior of Siete Caldos chili plants at different times of exposure to drought stress. (a) Control irrigation on day 8 (WW); (b) Eight days without irrigation (IS); (c) 10 days without irrigation; (d) 14 days WW control; (e) 14 days IS; (f) Plant recovery 24 h after rehydration, day 15 (RP), number of experimental units = 22.
Drought stress, induced by the suspension of irrigation, significantly affected the shoot height, root length, and number of flower buds in the Siete Caldos pepper (Capsicum frutescens). An increase in shoot height concerning time was observed in both treatments; however, the plants without irrigation (IS) had a lower height compared to the plants with irrigation (WW) at 8 and 14 days, with values of 16.87 ± 1.6 cm and 18.63 ± 0.4 cm, respectively. This represents a height reduction of ~3 cm compared to the WW plants, while root length was longer in the IS plants compared to the WW plants at 8 and 14 days, reaching 19.28 ± 1.8 cm and 21.66 ± 1.6 cm, respectively, compared to 16.17 ± 1.4 cm and 17.97 ± 0.8 cm for the WW plants. Regarding the number of flower buds, no significant differences were observed during the first eight days in any treatments. However, at 14 and 15 days, plants subjected to water stress (IS) developed significantly more flower buds, with 34 buds, compared to 4 shoots in the control plants (Table 2).

Table 2.
Effect of drought stress by suspending irrigation on shoot height, root length, and the number of flower buds in Siete Caldos chili pepper (Capsicum frutescens) evaluated at different days of exposure.
Fresh biomass decreased by ~1 g (1.19 g) in the leaf and ~0.5 g (0.6 g) in the root 14 days after discontinuing irrigation, while the decrease in dry biomass occurred from the third day compared to control plants.
3.2. Effect of Drought Stress Induced by Irrigation Suspension on the Physiological and Biochemical Parameters
During water deficiency, physiological parameters (relative water content (RWC), electrolyte leakage, membrane stability index (MSI), and total chlorophyll content) are affected as part of the plant’s response to the stress challenge. RWC is an essential indicator of plant water status, as it reflects a balance between the water supply to the leaf and root tissue and the transpiration rate [49].
Data obtained from the RWC showed a gradual decrease in the plants without irrigation from the third day, showing a decrease of ~8 ± 2% in leaf tissue (79.64%) and root tissue (77.55%). At 14 days, the most significant reduction was observed, of ~30% in both tissues (51.36% leaf and 54.96% root). In contrast, the control group maintained a constant RWC of about 86 ± 2% throughout the experimental phase in both tissues (Table 3).

Table 3.
Effect of drought stress by suspending irrigation on the percentage of relative water content (RWC), electrolyte leakage, and membrane stability index (MSI) in aerial part and roots of Siete Caldos chili pepper plants evaluated at different days of exposure.
To determine the effect of drought on the membrane integrity of C. frutescens, electrolyte leakage and the membrane stability index (MSI), two indicators that reflect the membrane’s ability to resist water stress, were measured. The plants exposed to drought exhibited a progressive decrease in MSI and increased electrolyte leakage concerning exposure time. It should be noted that both the electrolyte leakage and MSI were higher in the aerial part’s tissues than in the root tissue. In the aerial part, the plants under stress showed a significant increase in electrolyte leakage at 3, 8, and 14 days, with values of 29.11%, 40.70%, and 58.01%, respectively, in contrast to ~19% in the control plants. On the other hand, the MSI of the stressed plants decreased significantly in the same periods, with values of 70.88%, 59.29%, and 41.98%, respectively, while the control plants maintained ~80% (Table 3).
At 15 days, the rehydrated plants (RPs) recovered in the measured parameters compared to the non-irrigated (IS) plants at 14 days, although they did not reach the levels observed in the control plants (WW). In the aerial part, the relative water content (RWC) was 84.78%, electrolyte leakage reached 25.01%, and the membrane stability index (MSI) reached 74.99%. Meanwhile, in the roots, the RWC was 81.51%, electrolyte leakage was 48.58%, and the MSI was 51.41% (Table 3).
Total chlorophyll content is one of the most affected physiological factors during drought stress due to the photooxidation of photosynthetic pigments and chlorophyll degradation [50,51]. Our results showed increased chlorophyll content in the irrigated plants over time, with the highest content at 15 days (74.08 μg·mL−1). In contrast, drought stress caused a gradual decrease in total chlorophyll content. Compared to the control group (WW), a ~40% reduction (31.67 μg·mL−1) was evidenced from the third day without irrigation. At eight days, this reduction was ~64% (22.63 μg·mL−1), and finally, at 14 days, the decrease reached ~90% (14.01 μg·mL−1).
However, when the plants were rehydrated (RPs), a 100% increase in total chlorophyll content was observed, reaching 28.31 μg·mL−1, compared to the value recorded before hydration, which was 14.01 μg·mL−1 on day 14 (IS). When compared to the control plants (WW) at 15 days, a reduction in chlorophyll content caused by drought ~61% (Table 4) was observed.

Table 4.
Effect of drought stress by suspending irrigation on total chlorophyll content and proline in Siete Caldos chili plants (Capsicum frutescens) evaluated at different days of exposure.
Under conditions of abiotic stress such as drought, preserving the integrity of cellular structures and maintaining an adequate balance of osmoprotective substances to improve survival is critical. One osmoprotector that stands out is the amino acid Pro, as it acts not only as an osmotic protector but also as a signaling and antioxidant molecule [52]. The basal content of proline in the leaf and root tissue of C. frutescens experienced an increase of ~26% in leaf tissue (44.40 μg·gFW·mL−1 on day 15) and ~55% in root tissue (40.49 μg·gFW·mL−1 on day 15) over time in the control plants (WW). However, drought stress led to a notable increase in proline accumulation in response to stress. Proline gradually increased by the third day (505.49 μg·gFW·mL−1 in leaves and 371.04 μg·gFW·mL−1 in roots), reaching its peak accumulation of 1489.27 μg·gFW·mL−1 in the leaves and 1219.76 μg·gFW·mL−1 in the roots by day 14. The accumulation of proline in the control plants (irrigated every 48 h) was considerably lower, both in the leaves (35.26 μg·gFW·mL−1 and 44.40 μg·gFW·mL−1) and the roots (26.14 μg·gFW·mL−1 and 40.54 μg·gFW·mL−1) at 3 and 14 days, respectively. The plant total was ~42% (698.22 μg·gFW·mL−1) compared to day 14. But when compared to the control plants on day 15 (WW), this content was ~17 times higher in the roots and ~21 times higher in the leaves (Table 4).
In the continuously irrigated plants, catalase and peroxidase activity was observed over time, reaching an increase of ~3 U∙mg protein−1 at day 15. On the other hand, in the plants subjected to drought, an increase in the enzymatic activity of catalase and peroxidase in the leaf and root tissues was observed from the third day of irrigation suspension compared to the control group (Table 5). Catalase activity in the leaves and roots was 15.48 and 16.82 U∙mg protein−1, respectively, while in the control plants, it was 10.88 and 13.35 U∙mg protein−1. At 14 days, the maximum catalase activity was recorded, with a value of 28.18 (leaf) and 32.28 (root) U∙mg protein−1. The peroxidase activity was similar to that of the catalase. On the third day without irrigation, values of 9.49 (leaf) and 19.51 (root) U∙mg protein−1 were obtained, compared to the control values of 2.15 (leaf) and 6.41 (root) U∙mg protein−1. After 14 days without irrigation, the activity was 20.13 (leaf) and 63.22 (root) U∙mg protein−1. Post-rehydration (day 15), the plants (PR) showed a significant decrease in peroxidase activity in both tissues compared to day 14 (IS), whereas for catalase activity, this decrease was only observed in the leaf tissue.

Table 5.
Effect of drought stress by suspending irrigation on antioxidant activity in Siete Caldos chili plants (Capsicum frutescens) evaluated at different days of exposure.
3.3. Analysis of Stress-Related Gene Expression in Plants with Irrigation
Alterations in gene expression during various types of stress have been demonstrated. However, the expression pattern and reproducibility of each gene depend on the plant species and the type of stress [53,54]. Differential expression of the CaAP2/ERF064, LOX2, CAT, CuSOD, MnSOD, and P5CS genes was observed in the C. frutescens plants subjected to drought stress compared to the controls.
The expression of the CAT gene increased in the roots of the plants exposed to stress on the third day compared to the controls. However, a decrease in gene expression was observed at day 14 in the drought-exposed plants, although it was still approximately 0.4 times greater than in the control plants at day 14. It should be noted that 24 h after rehydration, the expression was upregulated, approximately 5.40 times higher than in the control plants (Figure 2).
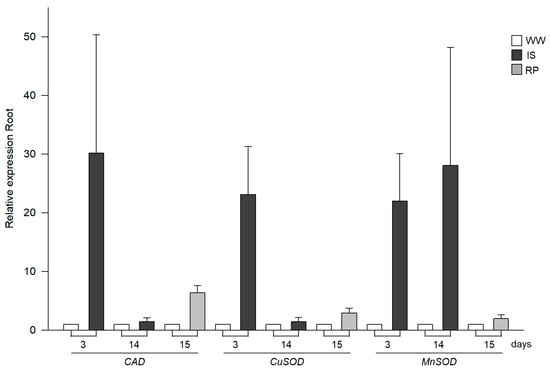
Figure 2.
Relative expression profiles by RT-qPCR of genes related to oxidative stress (CAT, CuSOD, and MnSOD) in roots of drought-exposed Siete Caldos chili pepper plants.
As for the CuSOD (copper–zinc superoxide dismutase) and MnSOD (manganese superoxide dismutase) genes (Figure 2), they showed upregulation on the third day during the initial phase of drought in the treatment plants, with increases of approximately 22.11 and 20.99 times, respectively, compared to the controls. However, on day 14, CuSOD showed an approximately 0.45 times higher expression than the control plants, significantly decreasing its transcription compared to the third day of treatment. MnSOD, at 14 days, showed an upward modulation approximately 27 times greater than in the control plants. Finally, rehydration induced an increase in CuSOD expression of roughly 1.9 times more than on day fourteen. In contrast, MnSOD, although showing an increase of approximately 0.97 times more than in the control plants, experienced a drastic decrease in gene expression from the previous day.
LOX2 is associated with several biological functions, contributing to the molecular regulation of jasmonate biosynthesis. Its expression has been reported in healthy, young, fast-growing plant tissues and under stressful conditions [55]. Additionally, transcription factors (AP2/ERF) integrate signaling cascades by regulating the expression of downstream genes related to the stress response and the flowering state [56]. It was observed that the LOX2 (2-lipoxygenase) gene showed an upward expression on the third day of the suspension of irrigation, which suggests a significant response to drought stress and that it could be involved in defense mechanisms or adaptation of the plant to the lack of water (Figure 3). However, the decrease in expression at 14 days could indicate downregulation after a sustained activation period, or the plant is entering a different response phase to the prolonged lack of water. It should be noted that after rehydration (day 15), an upward expression was observed, regulating the plant to restore its homeostasis.
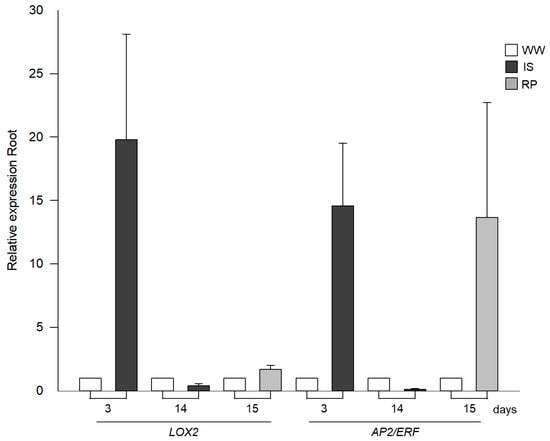
Figure 3.
Relative expression profiles by RT-qPCR of genes related to AP2/ERF and LOX2 in roots of drought-exposed Siete Caldos chili pepper plants.
The AP2/ERF gene in chili pepper plants exposed to drought (Figure 3) showed an upward expression from the third day, reaching approximately 13.5 times more than in the control plants. This increase is likely due to the regulation of osmoprotectant synthesis and increased root development. Nevertheless, at 14 days, the plants exposed to drought were characterized by downward modulation, approximately 0.9 times lower than in the control plants. However, upon rehydration, the modulation was again upward, with approximately 12.66 times more expression than in the control plants.
Proline accumulation is related to drought stress conditions. We have observed a significant increase in Pro content due to decreased water potential in Capsicum plants. Given this, we analyzed the expression of the P5CS gene, to see if it is consistent with an increase in its synthesis. The P5CS (Δ-pyrroline-5carboxylate synthetase) gene involved in Pro synthesis showed increased differential expression in the presence of stress (Figure 4). At three days without irrigation, gene expression increased 14-fold compared to the controls. By day 14, this increase in expression was reduced, being only four times more than in the control plants. Subsequently, upon rehydration (day 15), an upward expression in the plants was observed, approximately three times higher than that of the control group.
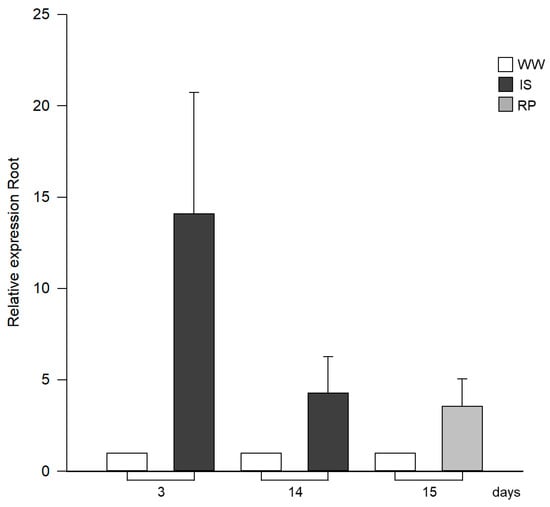
Figure 4.
Relative expression profiles by RT-qPCR of gene related to proline synthesis (P5CS) in roots of drought-exposed Siete Caldos chili pepper plants.
4. Discussion
4.1. Effect of Drought Stress on Growth Parameters
Siete Caldos chili plants (C. frutescens) exposed to drought conditions exhibited reduced height, an increase in root elongation, early flowering, and leaf curling eight days after the suspension of irrigation as adaptations to stress. Additionally, chlorosis was observed in the leaves as an adverse effect of stress (Figure 1). These observations are consistent with what Abobatta [7] describes, who points out that increasing root growth is a strategy to avoid dehydration during brief periods of drought stress. Molla et al. [57] highlight that genotypes with more extensive and voluminous root systems have more drought resistance than sensitive genotypes. Furthermore, vegetable roots play a crucial role under conditions of water deficit, enhancing water absorption from the lower soil layers, which contributes to their survival through elongation and thinning, and an increase in the extension of root hairs [58]. Meanwhile, Naikwade [6] mentions that drought avoidance also involves reversible physiological changes, such as decreased light absorption achieved by reducing leaf area through leaf curl and compact leaf architecture, for more efficient nutrient transport.
Regarding the height of the chili plants, a significant decrease was observed under drought stress compared to the controls (Table 1); similar results were observed by Zhou et al. [59], who reported a significant height reduction in three tomato varieties (Arvento, LA1994, and LA2093) under drought stress for five days. Meanwhile, Genie chili pepper plants (C. annuum L.), a moderately tolerant variety native to Indonesia, under mild drought conditions at an FC of 50%, presented a decrease of 21.87% in plant height [60]. This decrease is consistent with the results obtained in our study, where a 20% reduction was observed. It has been pointed out that the height of horticultural plants, a parameter highly influenced by drought, is closely associated with cell expansion and leaf senescence.
Another mechanism of escape from drought is an early onset of flowering, which has been reported in crops such as tomatoes, wheat, barley, and squash. Sivakumar and Srividhya [61] reported that flowering in tomato plants exposed to drought for 26 days occurred 4 days earlier than in plants with continuous irrigation (30 days). In this study, chili plants exposed to a 14-day drought period were observed to bloom early (up to 6 days earlier than the control plants). Several authors mention that this stress adaptation strategy, an escape, depends on early flowering for successful reproduction and a better distribution of assimilated products to develop fruits and seeds before severe drought occurs [6,62]. Regarding the fresh weight of the shoots of the chili plants exposed to drought, a reduction of ~51% was observed compared to the control plants. This decrease in growth is one of the main effects of drought. Zhou et al. [59] report a reduction of ~50% reduction in fresh weight in three tomato varieties (Solanum lycopersicum Arvento, LA1994, and LA2093) under stress for five days. In Genie pepper plants (C. annuum L.), the biomass of the shoots progressively decreased as the drought level increased until presenting a reduction of 68.94% of fresh weight under severe drought conditions (FC 35%) [60].
4.2. Physiological and Biochemical Responses
Cell membranes are one of the first to be affected by drought-induced water loss in many plants. The grade of cell membrane damage induced by water stress can be estimated by measuring electrolyte leakage and the membrane stability index [32]. In the present study, the RWC and MSI in the plants exposed to drought showed a significant decrease and increase in electrolyte leakage compared with the controls from the third day; these parameters increased as the time of exposure to drought was prolonged (Table 2). These results are similar to those reported by Sakya et al. [63], who reported an average decrease of 10% in the roots and 25% in the leaves of tomato plants under drought conditions with irrigation applied every eight days with an FC of 50%. The decrease in MSI indicates considerable damage in the cell membrane, which may be related to the increase in lipid peroxidation generated by the increase in reactive oxygen species (ROS) due to oxidative stress, especially in chloroplast membranes, which was observed in chlorotic leaves and is more evident at 14 days, which coincides with the maximum decrease in MSI [32]. Abbas et al. [58] point out that electrolyte leakage is a marker of drought tolerance capacity in horticultural plants. This is because tolerant genotypes maintain the integrity of their cell membranes, limiting electrolyte leakage in contrast to susceptible genotypes.
On the third day after stopping irrigation, the plants showed no visible damage, which could be attributed to the water-holding capacity of the tissues, which is consistent with the RWC values. On the other hand, Zhou et al. [59] report a ~20% decrease in RWC in three tomato varieties (Solanum lycopersicum Arvento, LA1994, and LA2093) exposed to drought for five days. Similar results were reported in three chili varieties, Genio, Romario, and Laris (C. annuum L.), under seven-day drought stress conditions; they registered an RWC of 67.51%, which increased to 80.58% after seven days of recovery [15]. Sahitya et al. [64] point out that a drought for seven days with an FC of 40% severely affected the RWC in two chili genotypes (C. annuum L.), with KCa-4884 being a tolerant genotype with 48.40% RWC and G-4 being a susceptible one with 18.33%. Molla et al. [57] suggest that RWC, as it correlates closely with cell volume, may accurately indicate water balance in the plant, i.e., between water absorption and loss through transpiration. RWC is considered a relevant marker for the selection of drought-tolerant varieties. In plants of susceptible chili bell pepper (C. annuum L.) genotypes, the decrease in RWC is more pronounced and did not show a significant recovery after rehydration.
The chlorophyll content indicates the plant’s health; a reduction of this pigment indicates effects on the photosystems. Singh and Thakur [65] mention that the decrease in total chlorophyll content is a common effect under drought conditions, as photosynthesis decreases mainly due to stomatal closure and cell membrane injury caused by increased ROS. Although the chili plants did not exhibit foliar chlorosis in the first three days of discontinued irrigation, a gradual reduction in total chlorophyll content was observed, reaching a ~80% decrease compared to the control plants at day 14 (Table 3). This suggests a degradation of photosynthetic pigments due to the increase in ROS, mainly by H2O2, which induces the inactivation/oxidation of pigments in chloroplasts. However, after rehydration (day 15), the chili plants recovered 100% of the total chlorophyll content compared to day 14. In a study conducted by Molla et al. [57], it was observed that in drought stress in chili pepper plants (C. annuum L.), tolerant chili pepper genotypes (BD-10906 and BD-10912) and susceptible ones (BD-10902 and RT-20) presented a significant decrease in the content of chlorophyll and carotenoids under irrigation suspension for ten days, this being more pronounced in the sensitive genotypes. However, only tolerant plants showed an increase in these pigments after the recovery phase. Widuri et al. [15] explain that the decrease in chlorophyll content limits light absorption by the leaf, thus reducing the energy available for photosynthesis and being associated with alterations in photosynthetic activities during drought exposure.
Another characteristic feature of the drought tolerance mechanism is the maintenance of cell turgor [66,67]. Our results show that the chili plants did not reach their point of permanent wilting as they could recover the turgor of the leaves and increase Pro content when rehydrated, indicating a tolerance to drought under these conditions.
Proline is reported as an osmoprotective amino acid commonly synthesized by plants in response to abiotic stress. The results showed a gradual increase in Pro content starting on the third day of stopping irrigation, which increased with extended periods of stress (Table 3). Mahmood et al. [5] report that the concentration of Pro varies significantly between the Pusajuala, Ghotki, Green Wonder, and PEE-311 genotypes of Capsicum spp. under drought conditions (FC 35% until fruit ripening); however, the highest concentration was obtained during the fruit formation stage in the Green Wonder genotype with an increase of ~200% compared to the controls. On the other hand, Wang et al. [23] report an increase in the content of Pro under water stress in watermelon roots, stems, and leaves (the tissue with the highest content).; they also point out that Pro was mainly synthesized in the leaves and was later transported to the roots to improve drought tolerance. Molla et al. [57] point out that in the recovery period, the Pro content in tolerant genotypes decreases rapidly and can reach values similar to the controls.
ROS are substances produced in various cellular compartments performing the function of signaling molecules. However, they are considered toxic byproducts of aerobic metabolism under stress conditions due to overaccumulation [68]. Antioxidant enzymes, such as catalase and peroxidase, play a key role in plants’ antioxidant defense system and help protect them against drought-induced oxidative stress. These enzymes scavenge free radicals and peroxides, reducing cell oxidative damage [69]. The study’s results showed that the activities of both enzymes gradually increased from the third day of the drought (Table 3). These results are consistent with those observed in different Solanaceae, where it has been observed that the activity of SOD, CAT, and POD increases as a defense against oxidative stress under conditions of water stress. Khan et al. [70] highlight progressive changes in the activity of antioxidant enzymes (SOD, POD, and CAT) in three varieties of tobacco in response to drought stress; in the case of POD and CAT, they observed significant changes from 48 and 72 h of drought, which coincides with our results. Mahmood et al. [5] report higher CAT and guaiac peroxidase activity in four chili varieties exposed to drought during the fruit formation stage than in the control plants and stressed plants in the flowering stage. On the other hand, Kopta et al. [71] indicate more excellent activity of CAT and peroxidases in the fruits and leaves of four chili varieties exposed to drought; they also point out that guaiac peroxidase and CAT are activated more quickly and firmly in tolerant genotypes compared to susceptible varieties.
4.3. Gene Regulation of Proline and Antioxidant Synthesis
Roots are the first organ to perceive water scarcity in the soil, playing an essential role in the perception of drought stress. Through complex signaling cascades, they transmit chemical signals, triggering a series of molecular responses that induce the expression of stress-responsive genes, including those related to the prevention of drought-induced damage such as oxidative stress (genes encoding enzymes that eliminate ROS) and osmotic stress (Pro biosynthesis genes), as well as genes related to ABA and JA signaling pathways [72].
In this study, the exposure of plants to three-day drought stress induced a positive regulation in the expression of the CAT and MnSOD genes and, to a lesser degree of magnitude, in the CuSOD gene. However, on day 14 of drought stress, CAT gene expression decreased, while MnSOD gene expression increased. These results are consistent with those observed in different plant species; in Camellia sinensis L. and Daucus carota, it was observed that expression values are higher for most of the genes of antioxidant enzymes, such as superoxide dismutase and catalase, under drought conditions. However, the expression profiles of each gene depended on each species [73,74]. For example, in Daucus carota under drought stress conditions, the expression of genes of the CuSOD family is higher compared to MnSODs [74]. On the other hand, in the roots of Kentucky bluegrass plants under drought stress conditions, cytosolic Cu/ZnSOD and MnSOD were suppressed, while the catalase gene showed no change in its expression [75]. This evidence could indicate that the expression profile of the family of genes related to antioxidant enzymes depends on the plant species. Additionally, this work observed that the expression of these genes correlates with the enzymatic activity of catalase and peroxidase (Table 3) at three days of stress, suggesting that the ROS clearance mechanism is active at the molecular and biochemical levels. However, on day 14 of stress, despite an increase in enzyme activity, the expression of some of the genes related to antioxidant enzymes was reduced. These results could be explained with the hypothesis that the low transcription of some of the genes related to antioxidant mechanisms, even though the activity of antioxidant enzymes is high, could indicate that it is leading to a disturbance of the balance between the production and elimination of ROS, producing oxidative damage to the cell [76].
Regarding genes related to the mitigation of osmotic damage, in this work, we focused on one of the genes of the glutamate pathway, the enzyme Δ1-pyrroline-5-carboxylase synthetase (P5CS). Our results show an upregulation of the P5CS gene under drought conditions for three days (Figure 4), which coincides with the accumulation of Pro in the root tissue (Table 3). However, a downward regulation was observed at 14 days without irrigation, which continued to decrease despite rehydration (day 15); at these points of the analysis, there is no correlation between the expression of the P5CS gene and the accumulation of Pro. These results are similar to those observed by Khan et al. [70], who reported an increase in the regulation of pro-synthesis (P5CS and OAT) genes in response to stress in three tobacco varieties. On the other hand, in a study of the Wisconsin 38 variety of tobacco plant (Nicotiana tabacum) exposed to mild (24 h) and severe (six days) drought stress after having suspended irrigation, it was observed that the P5CS-A gene was positively regulated in both the leaf and root tissue at 24 h after applying stress. The expression did not increase on the sixth day of stress. When tobacco plants were rehydrated, a downregulation of P5CS was observed in the roots of the rehydrated plants, with a decrease in the concentration of Pro [77].
Several studies underlined the strong interconnection between abscisic acid (ABA) and jamonic acid (JA) signaling pathways as a crucial response to the water stress response of plants. On the other hand, ABA and ethylene (ET) act antagonistically in growth control [78]. In the present study, a positive regulation of the CaLOX2 (JA response gene) and CaAP2/ERF064 (regulated by JA and ET) genes was observed in the early period of stress (three days without irrigation). However, it was downregulated due to prolonged and severe stress (14 days without irrigation). These results are consistent with previous reports in which it has been observed that some members of the large AP2/ERF family of Helianthus annuus L. are positively regulated against drought stress in early periods (24 h); however, after 48 h from the onset of stress, the expression falls [79]. Likewise, the JA biosynthesis gene, linoleate 13S-lipoxygenase 2-1 (CaLOX2), presented an expression profile similar to that observed in CaAP2/ERF064, which could indicate crosstalk of ABA with the modulation of JA and ET signaling during water stress [78].
5. Conclusions
In Siete Caldos chili pepper plants (Capsicum frutescens) exposed to drought conditions, significant changes in growth and responses at the physiological, biochemical, and molecular levels were observed. Early flowering, root elongation, and leaf turgor loss suggest an escape and avoidance strategy to cope with the water deficit. The decrease in the RWC and MSI and an increase in the percentage of electrolyte leakage indicate a gradual adaptation to drought. On the other hand, gene expression revealed variations in the levels of transcripts associated with proline synthesis, antioxidant enzymes, and developmental regulators, indicating a complex stress response. The plants’ ability to survive (100%) and to recover turgor, improve chlorophyll content, and restore all parameters evaluated after rehydration demonstrate their tolerance and maintenance of photosynthetic function, thus evidencing their ability to maintain the key processes to restore their cellular balance. These findings on adaptive responses to drought stress in C. frutescens enrich our understanding of the resilience and behavior of semi-domesticated chili pepper species.
Author Contributions
Conceptualization, N.R.-L.; Formal analysis, B.O.T.-P.; Investigation, B.O.T.-P. and N.R.-L.; Methodology, B.O.T.-P., M.G.C.-C. and N.R.-L.; Resources, M.G.C.-C., R.I.C.-R. and N.R.-L.; Supervision, M.G.C.-C. and N.R.-L.; Visualization, R.I.C.-R. and A.L.-G.; Writing—original draft, B.O.T.-P.; Writing—review & editing, M.G.C.-C., R.I.C.-R., A.L.-G. and N.R.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We gratefully acknowledge Consejo Nacional de Humanidades Ciencias y Tecnologías (CONAHCYT) for providing the Master’s scholarship of B.O.T.-P., and we extend our appreciation to Angel Edgardo Carrillo García, Centro de Investigación Biológicas del Noroeste S.C., for his invaluable technical assistance during the B.O.T.-P stay.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Okunlola, G.O.; Olatunji, O.A.; Akinwale, R.O.; Tariq, A.; Adelusi, A.A. Physiological response of the three most cultivated pepper species (Capsicum spp.) in Africa to drought stress imposed at three stages of growth and development. Sci. Hortic. 2017, 224, 198–205. [Google Scholar] [CrossRef]
- Organización de las Naciones Unidas para la Alimentación y la Agricultura. (FAO) Día Mundial de Lucha Contra la Desertificación y la Sequía 2020: El Director General de la FAO Pide una Nueva Estrategia para Frenar la Pérdida de Suelo|Alianza Mundial por el Suelo. Available online: https://www.fao.org/global-soil-partnership/resources/highlights/detail/es/c/1294379/ (accessed on 3 March 2024).
- Jalil, S.U.; Ansari, M.I. Stress implications and crop productivity. In Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives I: General Consequences and Plant Responses; Springer: Singapore, 2020; pp. 73–86. [Google Scholar] [CrossRef]
- Taiwo, A.F.; Daramola, O.; Sow, M.; Semwal, V.K. Ecophysiology and responses of plants under drought. In Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives I: General Consequences and Plant Responses; Springer: Singapore, 2020; pp. 231–268. [Google Scholar] [CrossRef]
- Mahmood, T.; Rana, R.M.; Ahmar, S.; Saeed, S.; Gulzar, A.; Khan, M.A.; Wattoo, F.M.; Wang, X.; Branca, F.; Mora-Poblete, F.; et al. Effect of drought stress on capsaicin and antioxidant contents in pepper genotypes at reproductive stage. Plants 2021, 10, 1286. [Google Scholar] [CrossRef] [PubMed]
- Naikwade, P.V. Plant Responses to Drought Stress: Morphological, Physiological, Molecular Approaches, and Drought Resistance. In Plant Metabolites under Environmental Stress; Apple Academic Press: Waretown, NJ, USA, 2023; pp. 149–183. [Google Scholar] [CrossRef]
- Abobatta, W.F. Drought adaptive mechanisms of plants—A review. Adv. Agric. Environ. Sci. Open Access 2019, 2, 42–45. [Google Scholar] [CrossRef]
- Parveen, A.; Rai, G.K.; Bagati, S.; Rai, P.K.; Singh, P. Morphological, Physiological, Biochemical and Molecular Responses of Plants to Drought Stress. In Abiotic Stress Tolerance Mechanisms in Plants; CRC Press: Boca Raton, FL, USA, 2021; pp. 321–339. [Google Scholar] [CrossRef]
- López-Serrano, L.; Penella, C.; Bautista, A.S.; López-Galarza, S.; Calatayud, A. Physiological changes of pepper accessions in response to salinity and water stress. Span. J. Agric. Res. 2017, 15, 15. [Google Scholar] [CrossRef]
- Jangid, K.K.; Dwivedi, P. Physiological Responses of Drought stress in Tomato: A Review. Int. J. Agric. Environ. Biotechnol. 2016, 9, 53. [Google Scholar] [CrossRef]
- Malika, L.Y.; Deshabandu, K.S.H.T.; De Costa, W.A.J.M.; Ekanayake, S.; Herath, S.; Weerakoon, W.M.W. Physiological traits determining tolerance to intermittent drought in the Capsicum annuum complex. Sci. Hortic. 2019, 246, 21–33. [Google Scholar] [CrossRef]
- Lim, C.W.; Bae, Y.; Lee, S.C. Differential role of Capsicum annuum fantastic four-like gene CaFAF1 on drought and salt stress responses. Environ. Exp. Bot. 2022, 199, 104887. [Google Scholar] [CrossRef]
- Momo, J.; Islam, K.; Kumar, N.; Ramchiary, N. Molecular Approaches for Breeding Abiotic Stress Tolerance Traits in Capsicum Species. In Genomic Designing for Abiotic Stress Resistant Vegetable Crops; Springer International Publishing: Cham, Switzerland, 2022; pp. 77–114. [Google Scholar] [CrossRef]
- Phimchan, P.; Techawongstien, S.; Chanthai, S.; Bosland, P.W. Impact of Drought Stress on the Accumulation of Capsaicinoids in Capsicum Cultivars with Different Initial Capsaicinoid Levels. HortScience 2012, 47, 1204–1209. [Google Scholar] [CrossRef]
- Widuri, L.I.; Lakitan, B.; Sakagami, J.; Yabuta, S.; Kartika, K.; Siaga, E. Short-term drought exposure decelerated growth and photosynthetic activities in chili pepper (Capsicum annuum L.). Ann. Agric. Sci. 2020, 65, 149–158. [Google Scholar] [CrossRef]
- Mardani, S.; Tabatabaei, S.H.; Pessarakli, M.; Zareabyaneh, H. Physiological responses of pepper plant (Capsicum annuum L.) to drought stress. J. Plant Nutr. 2017, 40, 1453–1464. [Google Scholar] [CrossRef]
- Zhou, R.; Kong, L.; Yu, X.; Ottosen, C.O.; Zhao, T.; Jiang, F.; Wu, Z. Oxidative damage and antioxidant mechanism in tomatoes responding to drought and heat stress. Acta Physiol. Plant. 2019, 41, 20. [Google Scholar] [CrossRef]
- Rai, G.K.; Parveen, A.; Jamwal, G.; Basu, U.; Kumar, R.R.; Rai, P.K.; Sharma, J.P.; Alalawy, A.I.; Al-Duais, M.A.; Hossain, M.A.; et al. Leaf Proteome Response to Drought Stress and Antioxidant Potential in Tomato (Solanum lycopersicum L.). Atmosphere 2021, 12, 1021. [Google Scholar] [CrossRef]
- Forlani, G.; Trovato, M.; Funck, D.; Signorelli, S. Regulation of Proline Accumulation and Its Molecular and Physiological Functions in Stress Defence. In Osmoprotectant-Mediated Abiotic Stress Tolerance in Plants: Recent Advances and Future Perspectives; Springer: Cham, Switzerland, 2019; pp. 73–97. [Google Scholar] [CrossRef]
- Hossain, A.; Garai, S.; Mondal, M.; Hamed Abdel Latef, A.A. The Key Roles of Proline against Heat, Drought and Salinity-Induced Oxidative Stress in Wheat (Triticum aestivum L.). In Organic Solutes, Oxidative Stress, and Antioxidant Enzymes Under Abiotic Stressors; Springer: Cham, Switzerland, 2021; pp. 171–190. [Google Scholar] [CrossRef]
- Mohammadi Alagoz, S.; Asgari Lajayer, B.; Ghorbanpour, M. Proline and soluble carbohydrates biosynthesis and their roles in plants under abiotic stresses. In Plant Stress Mitigators; Academic Press: Cambridge, MA, USA, 2023; pp. 169–185. [Google Scholar] [CrossRef]
- Bandurska, H.; Niedziela, J.; Pietrowska-Borek, M.; Nuc, K.; Chadzinikolau, T.; Radzikowska, D. Regulation of proline biosynthesis and resistance to drought stress in two barley (Hordeum vulgare L.) genotypes of different origin. Plant Physiol. Biochem. 2017, 118, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, Y.; Yadav, V.; Zhao, W.; He, Y.; Zhang, X.; Wei, C. Drought-induced proline is mainly synthesized in leaves and transported to roots in watermelon under water deficit. Hortic. Plant J. 2022, 8, 615–626. [Google Scholar] [CrossRef]
- Patanè, C.; Scordia, D.; Testa, G.; Cosentino, S.L. Physiological screening for drought tolerance in Mediterranean long-storage tomato. Plant Sci. 2016, 249, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Fiasconaro, M.L.; Lovato, M.E.; Antolín, M.C.; Clementi, L.A.; Torres, N.; Gervasio, S.; Martín, C.A. Role of proline accumulation on fruit quality of pepper (Capsicum annuum L.) grown with a K-rich compost under drought conditions. Sci. Hortic. 2019, 249, 280–288. [Google Scholar] [CrossRef]
- División de Estadística de la Organización de las Naciones Unidas para la Agricultura y la Alimentación (FAOSTAT). Crops and Livestock Products. 2024. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 3 March 2024).
- Aguilar-Meléndez, A.; Lira Noriega, A. ¿Dónde crecen los chiles en México? In Los Chiles Que le dan Sabor al Mundo; Veracruz University: Veracruz, México, 2018; pp. 75–92. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. Preparing the nutrient solution. Water-Cult. Method Grow. Plants Soil 1950, 347, 29–31. [Google Scholar]
- Luna-Flores, W. Efecto del estrés hídrico sobre el crecimiento y eficiencia del uso del agua en plántulas de tres especies arbóreas caducifolias. Rev. Terra Latinoam. 2012, 30, 343–353. Available online: https://www.terralatinoamericana.org.mx/index.php/terra/article/view/1090 (accessed on 25 June 2023).
- Jothimani, K.; Arulbalachandran, D. Physiological and biochemical studies of black gram (Vigna mungo L.) Hepper) under polyethylene glycol induced drought stress. Biocatal. Agric. Biotechnol. 2020, 29, 101777. [Google Scholar] [CrossRef]
- Restrepo, H.; Gómez, M.I.; Garzón, A.; Alzate, F.; López, J. Respuesta bioquímica de plántulas de maíz (Zea mays L.) a diferentes condiciones de temperaturas nocturnas. Rev. Colomb. Cienc. Hortícolas 2013, 7, 252–262. [Google Scholar] [CrossRef]
- Semida, W.M.; Abdelkhalik, A.; Rady MO, A.; Marey, R.A.; Abd El-Mageed, T.A. Exogenously applied proline enhances growth and productivity of drought stressed onion by improving photosynthetic efficiency, water use efficiency and up-regulating osmoprotectants. Sci. Hortic. 2020, 272, 109580. [Google Scholar] [CrossRef]
- Inskeep, W.P.; Bloom, P.R. Extinction Coefficients of Chlorophyll a and b in N,N-Dimethylformamide and 80% Acetone. Plant Physiol. 1985, 77, 483–485. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Escalante-Magaña, C.A. Tesis que Presenta Camilo Andres Escalante Magaña En opción al título de Doctorado en Ciencias (Ciencias Biológicas: Opción Bioquímica y Biología Molecular; Centro de Investigación Científica de Yucatán: Mérida, México, 2020. [Google Scholar]
- Suárez, D.F.; Mattos, A.D.P.; Broti Rissato, B.; Schwan-Estrada KR, F.; Suárez, D.F.; Mattos A do, P.; Broti Rissato, B.; Schwan-Estrada, K.R.F. Activación de mecanismos de defensa en maíz pira mediante el uso del abono orgánico Microgeo®. Rev. Mex. De Cienc. Agrícolas 2020, 11, 965–977. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Góth, L. A simple method for determination of serum catalase activity and revision of reference range. Clin. Chim. Acta 1991, 196, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Hammerschmidt, R.; Nuckles, E.M.; Kuć, J. Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol. Plant Pathol. 1982, 20, 73–82. [Google Scholar] [CrossRef]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Nieto-Garibay, A.; Barraza, A.; Caamal-Chan, G.; Murillo-Amador, B.; Troyo-Diéguez, E.; Burgoa-Cruz, C.A.; Jaramillo-Limón, J.N.; Loera-Muro, A. Habanero pepper (Capsicum chinense) adaptation to water-deficit stress in a protected agricultural system. Funct. Plant Biol. 2022, 49, 295–306. [Google Scholar] [CrossRef]
- Jin, J.H.; Zhang, H.X.; Ali, M.; Wei, A.M.; Luo, D.X.; Gong, Z.H. The CaAP2/ERF064 Regulates Dual Functions in Pepper: Plant Cell Death and Resistance to Phytophthora capsici. Genes 2019, 10, 541. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Lu, J.P.; Zhai, Y.F.; Chai, W.G.; Gong, Z.H.; Lu, M.H. Genome-wide analysis, expression profile of heat shock factor gene family (CaHsfs) and characterisation of CaHsfA2 in pepper (Capsicum annuum L.). BMC Plant Biol. 2015, 15, 151. [Google Scholar] [CrossRef] [PubMed]
- Caamal-Chan, M.G.; Loera-Muro, A.; Romero-Geraldo RD, J.; Ramírez-Serrano, R. Bacterial Strains from Saline Environment Modulate the Expression of Saline Stress-Responsive Genes in Pepper (Capsicum annuum). Plants 2023, 12, 3576. [Google Scholar] [CrossRef] [PubMed]
- Mejía-Teniente, L.; de Dalia Durán-Flores, F.; Chapa-Oliver, A.M.; Torres-Pacheco, I.; Cruz-Hernández, A.; González-Chavira, M.M.; Ocampo-Velázquez, R.V.; Guevara-González, R.G. Oxidative and molecular responses in Capsicum annuum L. after hydrogen peroxide, salicylic acid and chitosan foliar applications. Int. J. Mol. Sci. 2013, 14, 10178–10196. [Google Scholar] [CrossRef] [PubMed]
- Sziderics, A.H.; Rasche, F.; Trognitz, F.; Sessitsch, A.; Wilhelm, E. Bacterial endophytes contribute to abiotic stress adaptation in pepper plants (Capsicum annuum L.). Can. J. Microbiol. 2007, 53, 1195–1202. [Google Scholar] [CrossRef]
- Wan, H.; Yuan, W.; Ruan, M.; Ye, Q.; Wang, R.; Li, Z.; Zhou, G.; Yao, Z.; Zhao, J.; Liu, S.; et al. Identification of reference genes for reverse transcription quantitative real-time PCR normalization in pepper (Capsicum annuum L.). Biochem. Biophys. Res. Commun. 2011, 416, 24–30. [Google Scholar] [CrossRef]
- Lugojan, C.; Ciulca, S. Evaluation of relative water content in winter wheat. J. Hortic. For. Biotechnol. 2011, 15, 173–177. [Google Scholar]
- Kamatchi KA, M.; Anitha, K.; Kumar, K.A.; Senthil, A.; Kalarani, M.K.; Djanaguiraman, M. Impacts of combined drought and high-temperature stress on growth, physiology, and yield of crops. Plant Physiol. Rep. 2024, 29, 28–36. [Google Scholar] [CrossRef]
- Khan, T.A.; Saleem, M.; Fariduddin, Q. Differential Drought Stress Tolerance in Five Varieties of Tomato (Solanum lycopersicum L.): An Evaluation of Photosynthesis and Antioxidant System. J. Soil Sci. Plant Nutr. 2023, 23, 2810–2831. [Google Scholar] [CrossRef]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Cao, X.; Khan MA, R. Proline, a multifaceted signalling molecule in plant responses to abiotic stress: Understanding the physiological mechanisms. Plant Biol. 2022, 24, 227–239. [Google Scholar] [CrossRef]
- Bhargava, S.; Sawant, K. Drought stress adaptation: Metabolic adjustment and regulation of gene expression. Plant Breed. 2013, 132, 21–32. [Google Scholar] [CrossRef]
- Shirvani, H.; Mehrabi, A.A.; Farshadfar, M.; Safari, H.; Arminian, A.; Fatehi, F.; Pouraboughadareh, A.; Poczai, P. Investigation of the morphological, physiological, biochemical, and catabolic characteristics and gene expression under drought stress in tolerant and sensitive genotypes of wild barley [Hordeum vulgare subsp. spontaneum (K. Koch) Asch. & Graebn.]. BMC Plant Biol. 2024, 24, 214. [Google Scholar] [CrossRef]
- Viswanath, K.K.; Varakumar, P.; Pamuru, R.R.; Basha, S.J.; Mehta, S.; Rao, A.D. Plant Lipoxygenases and Their Role in Plant Physiology. J. Plant Biol. 2020, 63, 83–95. [Google Scholar] [CrossRef]
- Licausi, F.; Ohme-Takagi, M.; Perata, P. APETALA2/Ethylene Responsive Factor (AP2/ERF) transcription factors: Mediators of stress responses and developmental programs. New Phytol. 2013, 199, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Molla, M.R.; Rohman, M.M.; Monsur, M.B.; Hasanuzzaman, M.; Hassan, L. Screening and Assessment of Selected Chilli (Capsicum annuum L.) Genotypes for Drought Tolerance at Seedling Stage. Phyton-Int. J. Exp. Bot. 2021, 90, 1425–1443. [Google Scholar] [CrossRef]
- Abbas, K.; Li, J.; Gong, B.; Lu, Y.; Wu, X.; Lü, G.; Gao, H. Drought Stress Tolerance in Vegetables: The Functional Role of Structural Features, Key Gene Pathways, and Exogenous Hormones. Int. J. Mol. Sci. 2023, 24, 13876. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Yu, X.; Ottosen, C.O.; Rosenqvist, E.; Zhao, L.; Wang, Y.; Yu, W.; Zhao, T.; Wu, Z. Drought stress had a predominant effect over heat stress on three tomato cultivars subjected to combined stress. BMC Plant Biol. 2017, 17, 24. [Google Scholar] [CrossRef]
- Lestari, P.; Syukur, M.; Trikoesoemaningtyas Widiyono, W.; Muchdar Davis, L.O.M. The Effect of Mild and Severe Drought on Genie Chili (Capsicum annuum L. var. genie) Leaf Cell Growth. AIP Conf. Proc. 2023, 2606, 040010. [Google Scholar] [CrossRef]
- Sivakumar, R.; Srividhya, S. Impact of drought on flowering, yield and quality parameters in diverse genotypes of tomato (Solanum lycopersicum L.). Adv. Hortic. Sci. 2016, 30, 3–11. [Google Scholar]
- Shavrukov, Y. Pathway to the Molecular Origins of Drought Escape and Early Flowering Illuminated via the Phosphorylation of SnRK2-Substrate 1 in Arabidopsis. Plant Cell Physiol. 2024, 65, 179–180. [Google Scholar] [CrossRef]
- Sakya, A.T.; Sulistyaningsih, E.; Indradewa, D.; Purwanto, B.H. Physiological characters and tomato yield under drought stress. IOP Conf. Ser. Earth Environ. Sci. 2018, 200, 012043. [Google Scholar] [CrossRef]
- Sahitya, U.L.; Krishna MS, R.; Suneetha, P. Integrated approaches to study the drought tolerance mechanism in hot pepper (Capsicum annuum L.). Physiol. Mol. Biol. Plants 2019, 25, 637–647. [Google Scholar] [CrossRef]
- Singh, J.; Thakur, J.K. Photosynthesis and abiotic stress in plants. In Biotic and Abiotic Stress Tolerance in Plants; Springer: Singapore, 2018; pp. 27–46. [Google Scholar] [CrossRef]
- Oguz, M.C.; Aycan, M.; Oguz, E.; Poyraz, I.; Yildiz, M. Drought Stress Tolerance in Plants: Interplay of Molecular, Biochemical and Physiological Responses in Important Development Stages. Physiologia 2022, 2, 180–197. [Google Scholar] [CrossRef]
- Waqas, M.A.; Kaya, C.; Riaz, A.; Farooq, M.; Nawaz, I.; Wilkes, A.; Li, Y. Potential Mechanisms of Abiotic Stress Tolerance in Crop Plants Induced by Thiourea. Front. Plant Sci. 2019, 10, 1336. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Mirza, H.; Kamrun, N.; Masayuki, F.; Hirosuke, O.; Islam, T.M. Approaches for Enhancing Abiotic Stress Tolerance in Plants. In Approaches for Enhancing Abiotic Stress Tolerance in Plants; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar] [CrossRef]
- Khan, R.; Zhou, P.; Ma, X.; Zhou, L.; Wu, Y.; Ullah, Z.; Wang, S. Transcriptome Profiling, Biochemical and Physiological Analyses Provide New Insights towards Drought Tolerance in Nicotiana tabacum L. Genes 2019, 10, 1041. [Google Scholar] [CrossRef]
- Kopta, T.; Sekara, A.; Pokluda, R.; Ferby, V.; Caruso, G. Screening of Chilli Pepper Genotypes as a Source of Capsaicinoids and Antioxidants under Conditions of Simulated Drought Stress. Plants 2020, 9, 364. [Google Scholar] [CrossRef]
- Janiak, A.; Kwaśniewski, M.; Szarejko, I. Gene expression regulation in roots under drought. J. Exp. Bot. 2016, 67, 1003–1014. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, M.; Kordrostami, M.; Mohamadhasani, F.; Chaeikar, S.S. Antioxidant gene expression analysis and evaluation of total phenol content and oxygen-scavenging system in tea accessions under normal and drought stress conditions. BMC Plant Biol. 2021, 21, 494. [Google Scholar] [CrossRef]
- Zameer, R.; Fatima, K.; Azeem, F.; Algwaiz HI, M.; Sadaqat, M.; Rasheed, A.; Batool, R.; Shah, A.N.; Zaynab, M.; Shah, A.A.; et al. Genome-Wide Characterization of Superoxide Dismutase (SOD) Genes in Daucus carota: Novel Insights Into Structure, Expression, and Binding Interaction With Hydrogen Peroxide (H2O2) Under Abiotic Stress Condition. Front. Plant Sci. 2022, 13, 870241. [Google Scholar] [CrossRef]
- Bian, S.; Jiang, Y. Reactive oxygen species, antioxidant enzyme activities and gene expression patterns in leaves and roots of Kentucky bluegrass in response to drought stress and recovery. Sci. Hortic. 2009, 120, 264–270. [Google Scholar] [CrossRef]
- Li, J.; Yang, Y.; Sun, K.; Chen, Y.; Chen, X.; Li, X. Exogenous Melatonin Enhances Cold, Salt and Drought Stress Tolerance by Improving Antioxidant Defense in Tea Plant (Camellia sinensis L.) O. Kuntze. Molecules 2019, 24, 1826. [Google Scholar] [CrossRef] [PubMed]
- Dobrá, J.; Vanková, R.; Havlová, M.; Burman, A.J.; Libus, J.; Štorchová, H. Tobacco leaves and roots differ in the expression of proline metabolism-related genes in the course of drought stress and subsequent recovery. J. Plant Physiol. 2011, 168, 1588–1597. [Google Scholar] [CrossRef] [PubMed]
- Pirona, R.; Frugis, G.; Locatelli, F.; Mattana, M.; Genga, A.; Baldoni, E. Transcriptomic analysis reveals the gene regulatory networks involved in leaf and root response to osmotic stress in tomato. Front. Plant Sci. 2023, 14, 1155797. [Google Scholar] [CrossRef]
- Najafi, S.; Sorkheh, K.; Nasernakhaei, F. Characterization of the APETALA2/Ethylene-responsive factor (AP2/ERF) transcription factor family in sunflower. Sci. Rep. 2018, 8, 11576. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).