Identification, Classification, and Expression Analysis of Leucine-Rich Repeat Extensin Genes from Brassica rapa Reveals Salt and Osmosis Stress Response Genes
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification of LRX Genes in the B. rapa Genome
2.2. Chromosomal Localization, Synteny, and Phylogenetic Analysis
2.3. Motif, Gene Structure, and Conserved Domain Analysis
2.4. Cis-Acting Element Analysis
2.5. Tissue-Specific Expression and GO Analysis
2.6. Stress Treatments and Transcriptome, Proteome, and RT-qPCR Analysis
2.7. PPI Network Analysis
2.8. Protein Secondary and Tertiary Structure Analysis
3. Results
3.1. Identification and Analysis of BrLRX Family Genes
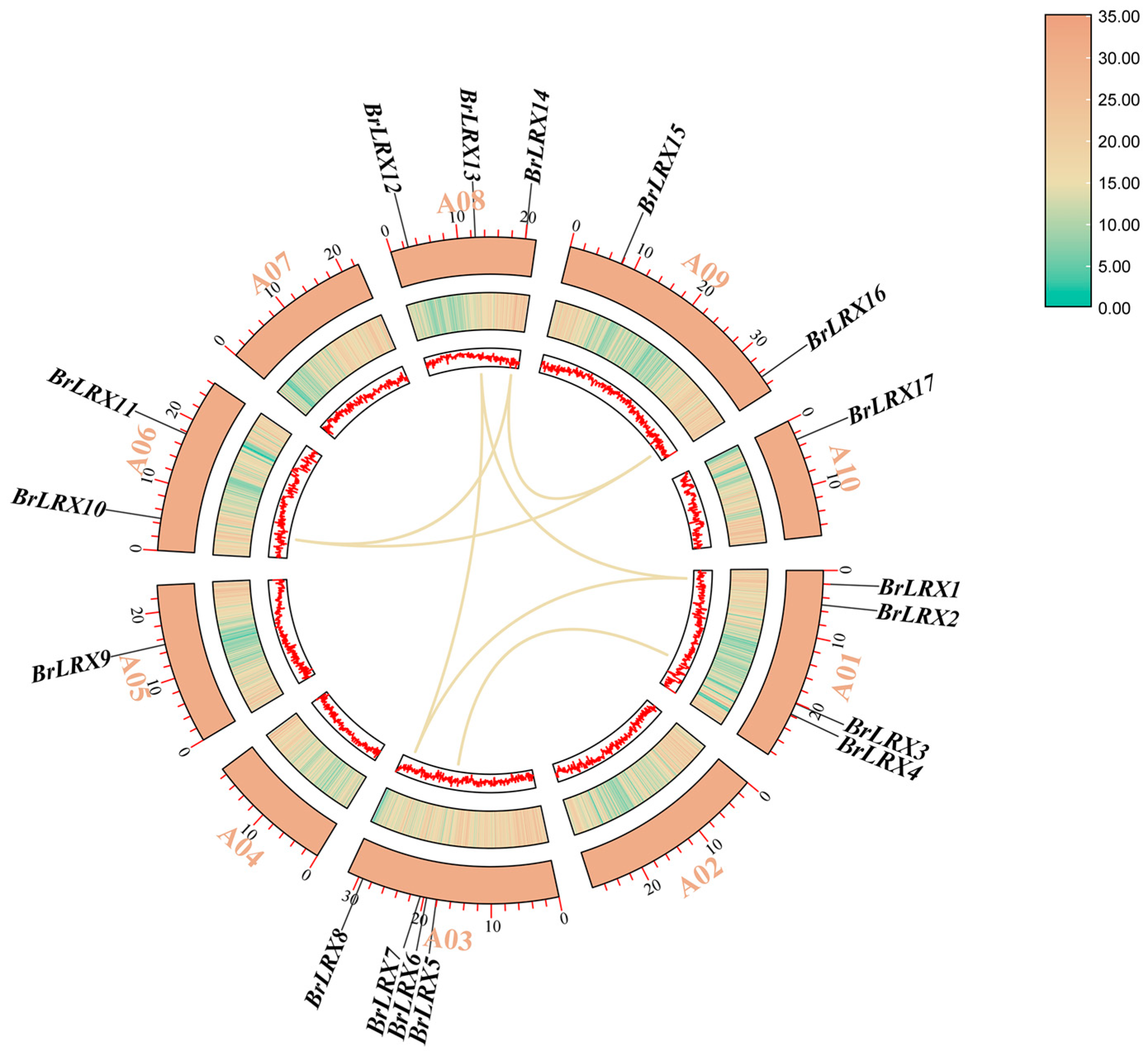
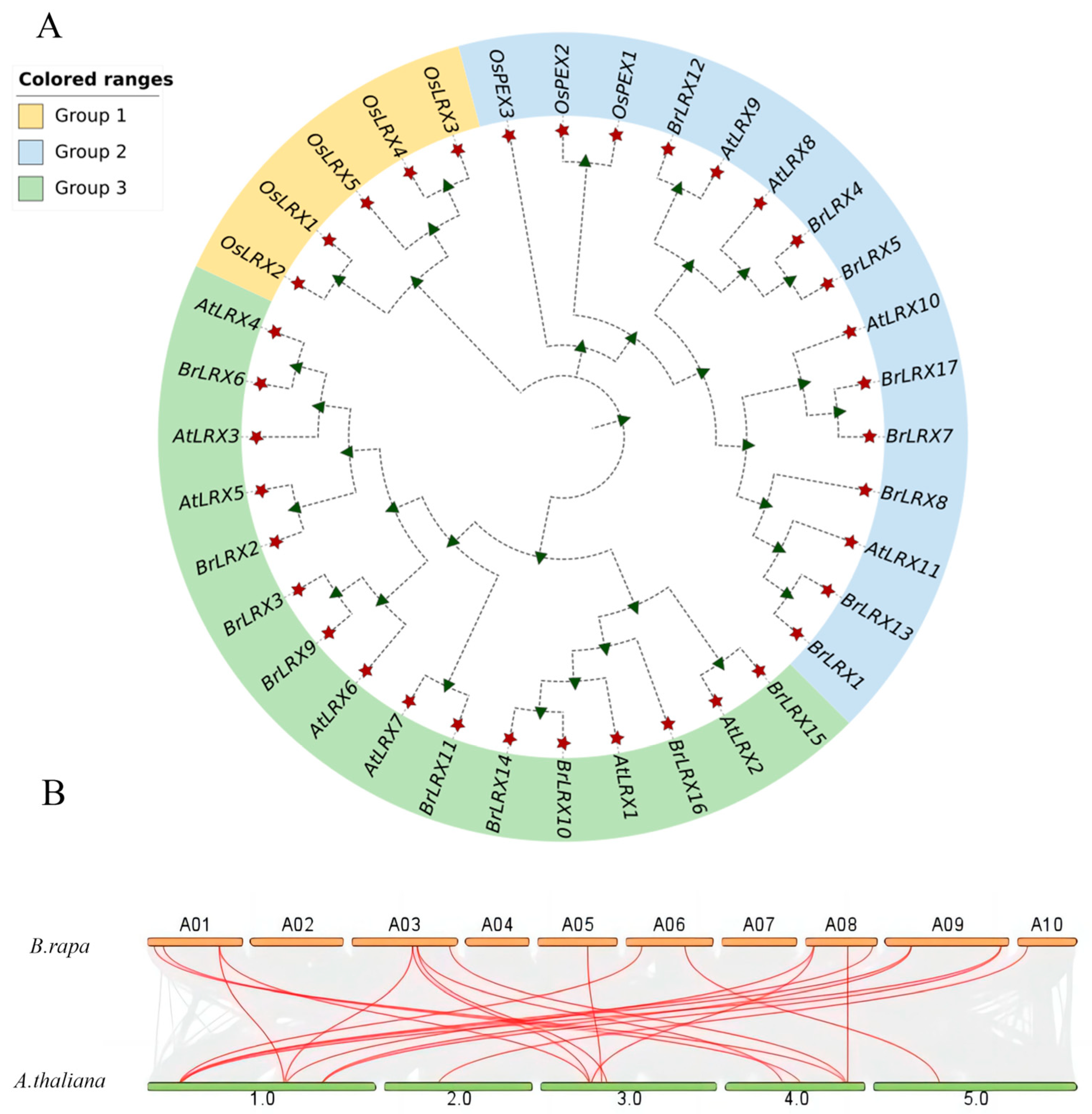
3.2. Chromosomal Localization, Synteny, and Phylogenetic Analysis of BrLRXs
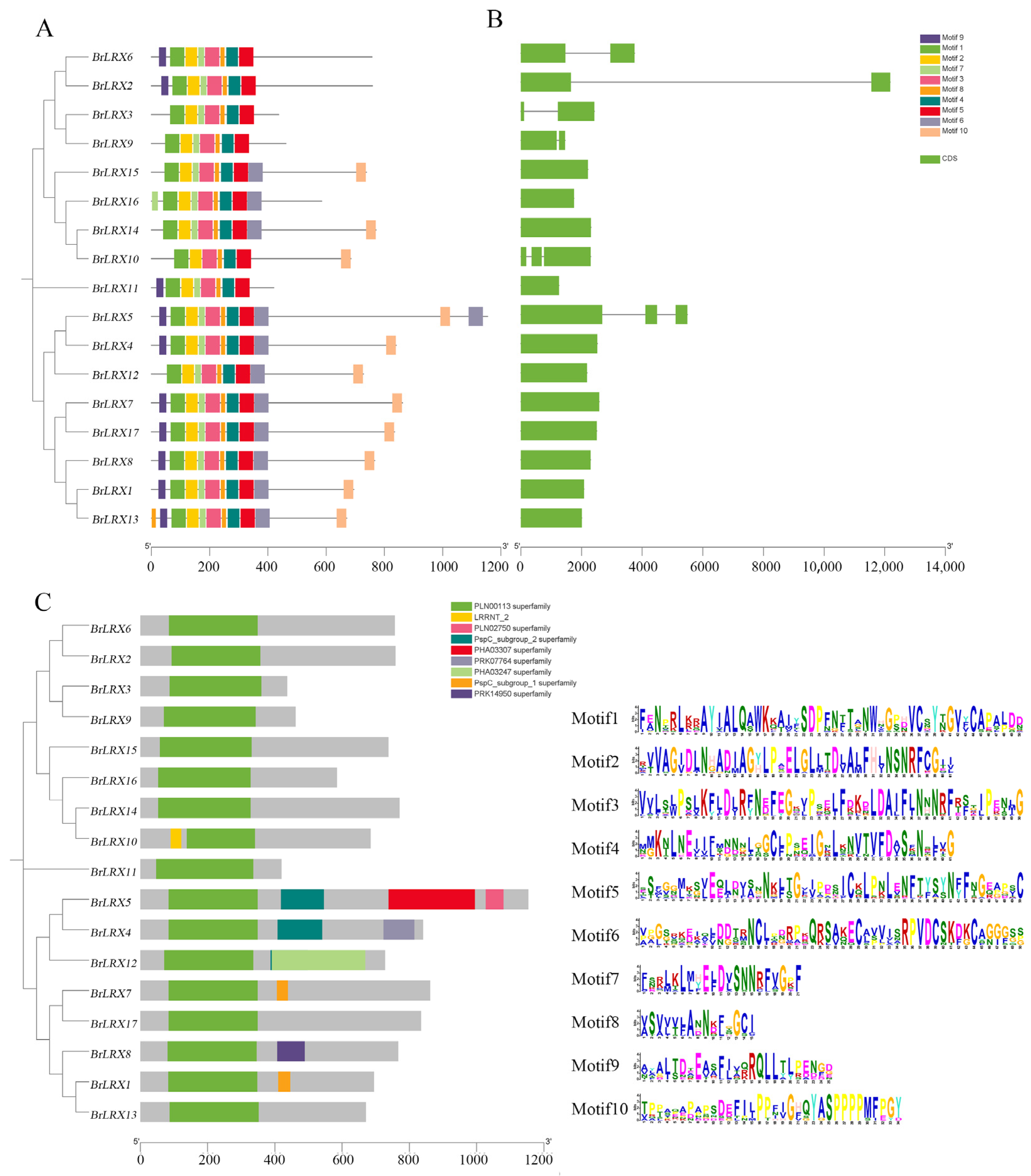
3.3. Motif Analysis, Gene Structure, and Conserved Domain Analysis of BrLRXs
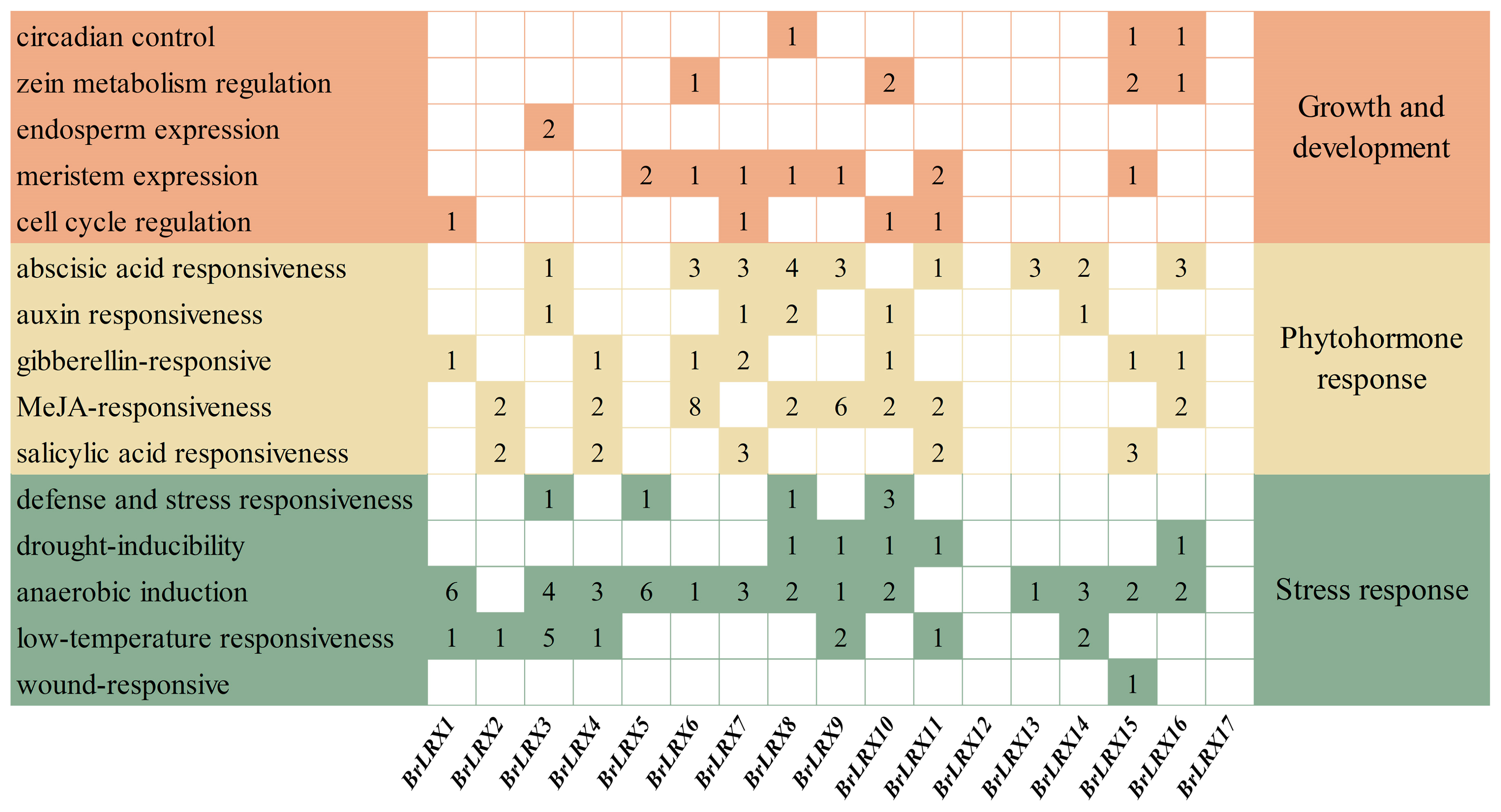
3.4. Cis-Acting Element Analysis of BrLRXs
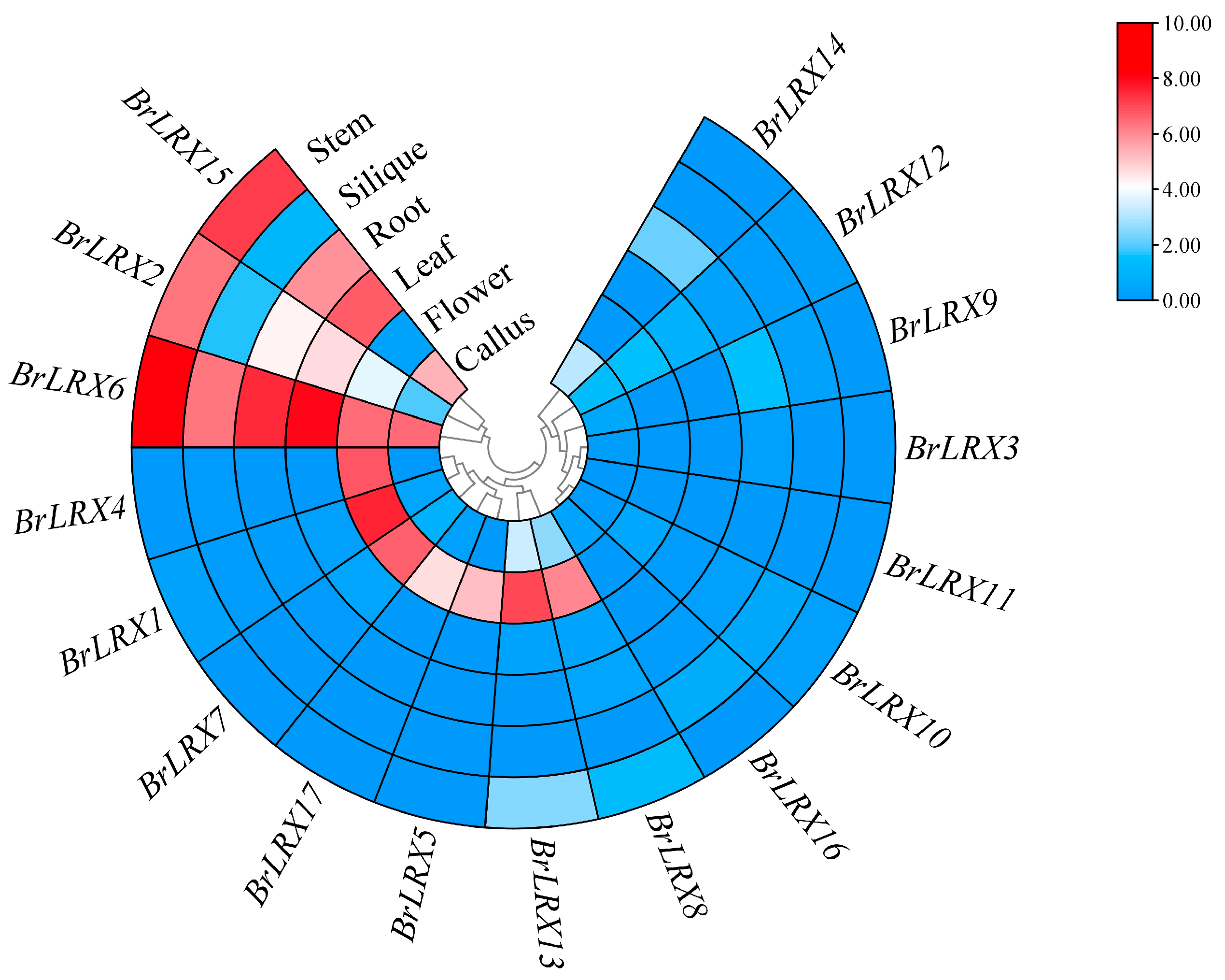
3.5. Tissue-Specific Expression of BrLRXs
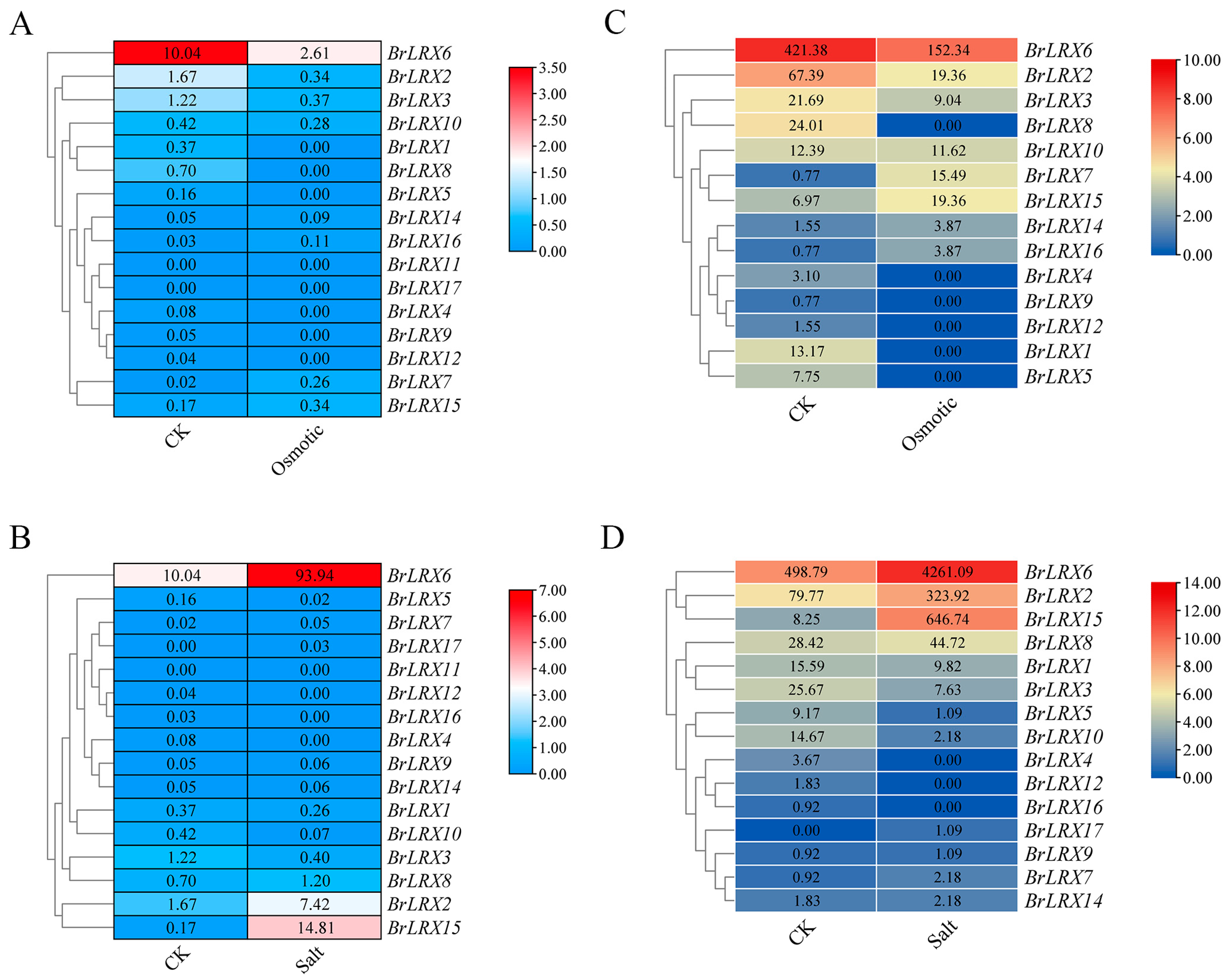
3.6. Proteome and Transcriptome Analysis of BrLRXs Involved in Responses to Abiotic Stresses
3.7. RT-qPCR Analysis of BrLRXs Involved in Responses to Abiotic Stresses
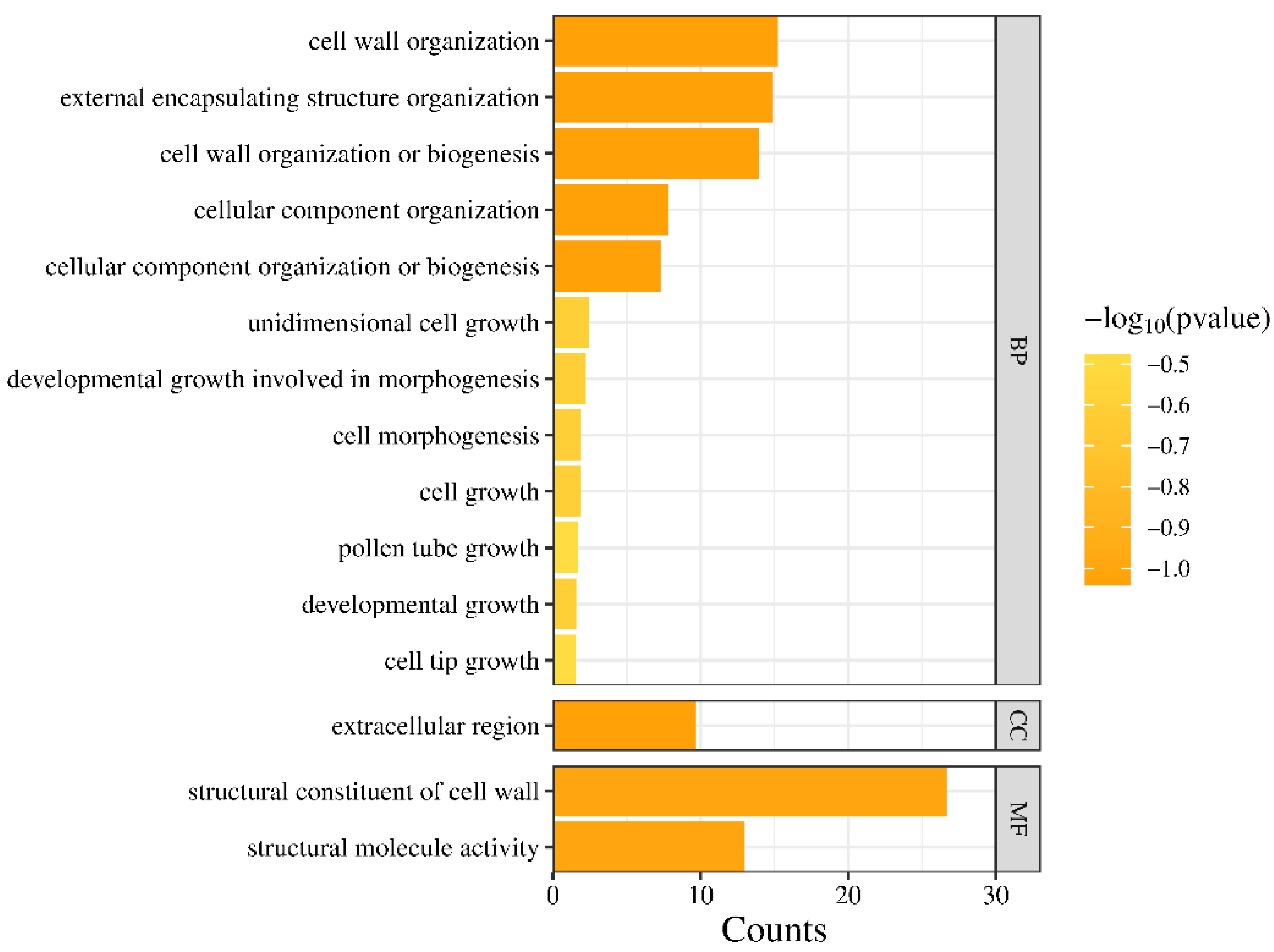
3.8. Gene Ontology (GO) Analysis of LRXs in A. thaliana
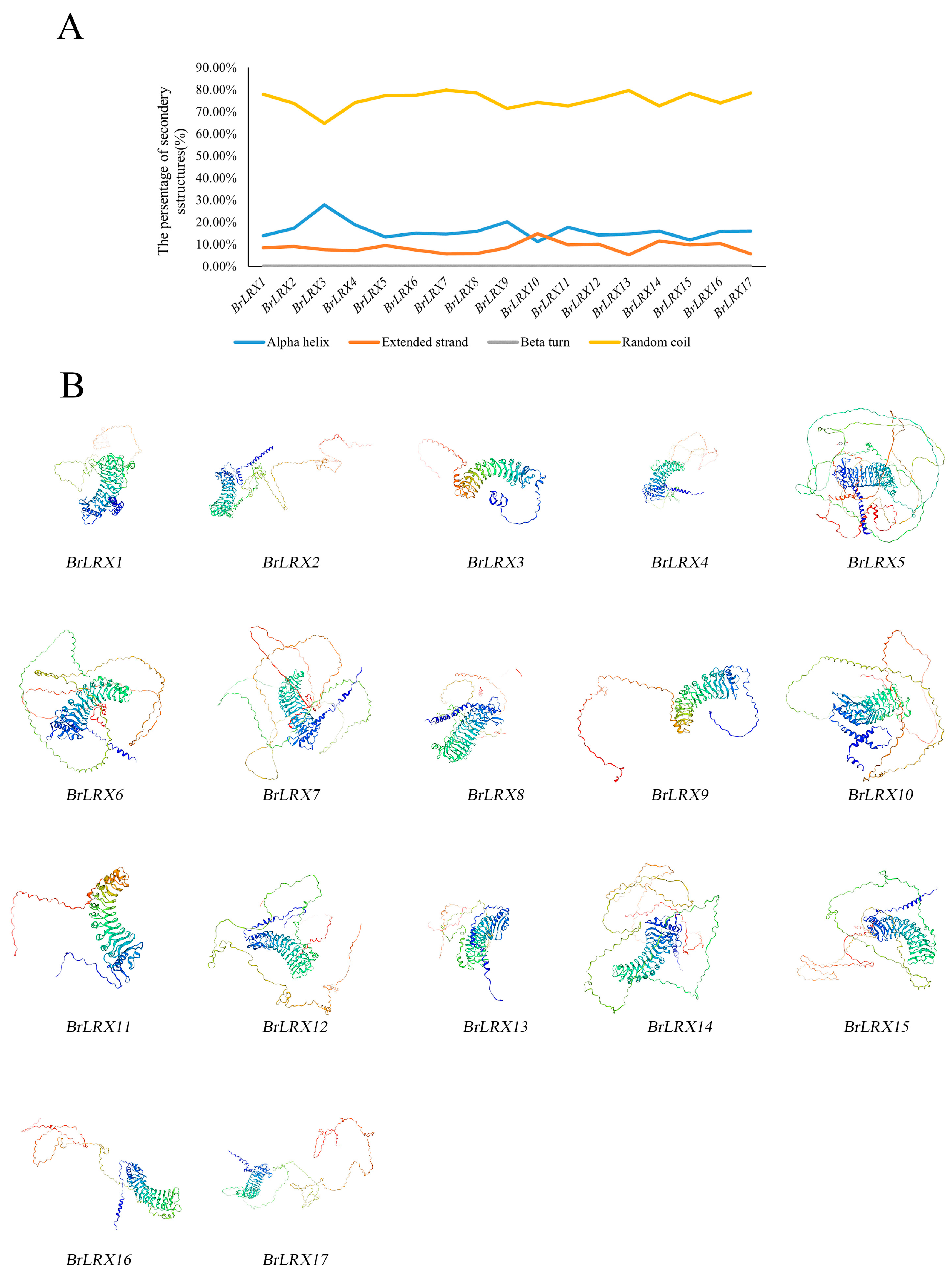
3.9. Protein Secondary and Tertiary Structure Analysis of BrLRXs
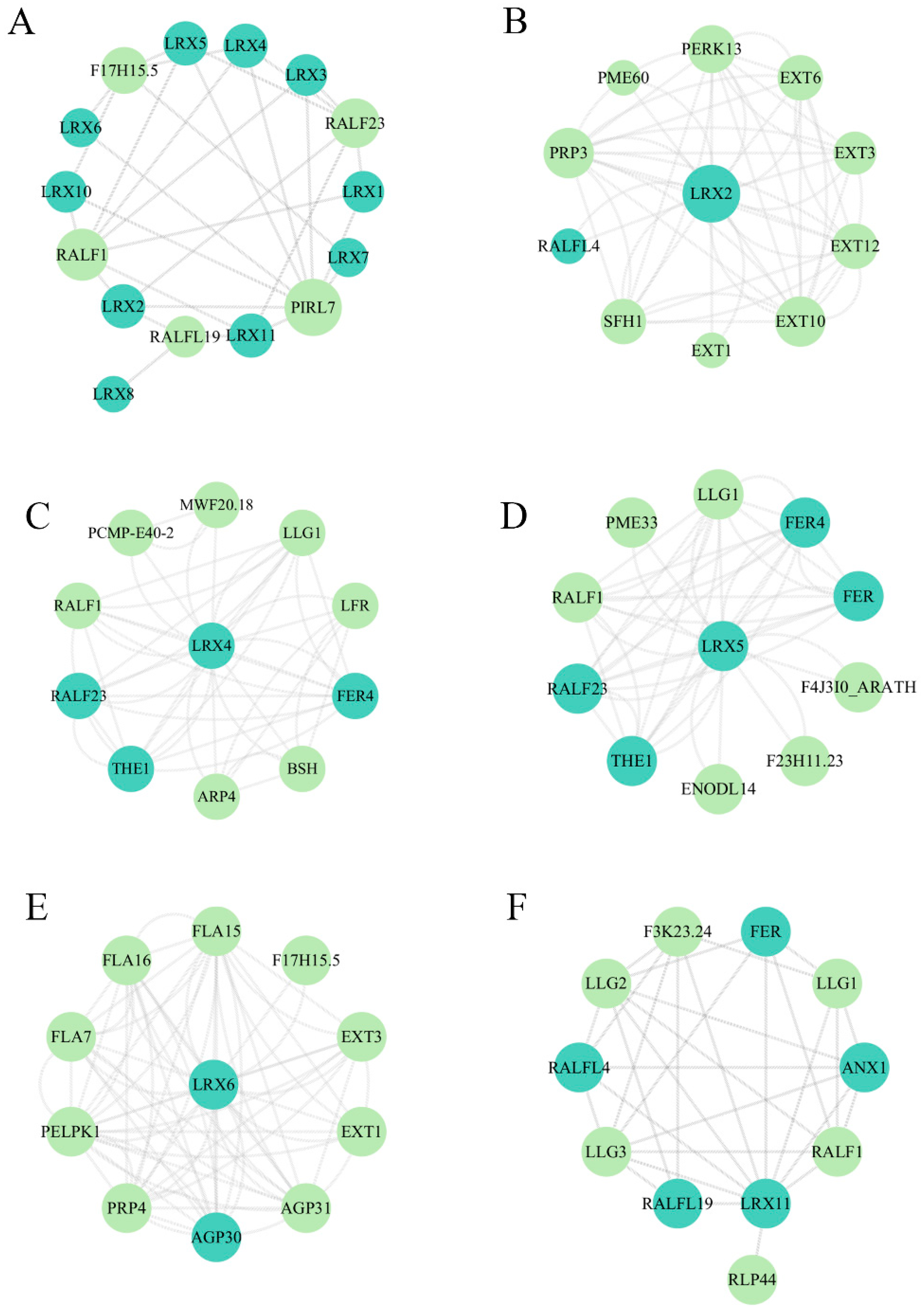
3.10. Protein–Protein Interaction (PPI) Network Prediction Analysis of BrLRXs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rubinstein, A.L.; Broadwater, A.H.; Lowrey, K.B.; Bedinger, P.A. Pex1, a pollen-specific gene with an extensin-like domain. Proc. Natl. Acad. Sci. USA 1995, 92, 3086–3090. [Google Scholar] [CrossRef] [PubMed]
- Baumberger, N.; Ringli, C.; Keller, B. The chimeric leucine-rich repeat/extensin cell wall protein LRX1 is required for root hair morphogenesis inArabidopsis thaliana. Genes. Dev. 2001, 15, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Stratford, S.; Barne, W.; Hohorst, D.L.; Sagert, J.G.; Cotter, R.; Golubiewski, A.; Showalter, A.M.; McCormick, S.; Bedinger, P. A leucine-rich repeat region is conserved in pollen extensin-like (Pex) proteins in monocots and dicots. Plant Mol. Biol. 2001, 46, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Lamport, D.T.; Kieliszewski, M.J.; Chen, Y.; Cannon, M.C. Role of the extensin superfamily in primary cell wall architecture. Plant Physiol. 2011, 156, 11–19. [Google Scholar] [CrossRef]
- Torii, K.U.; Mitsukawa, N.; Oosumi, T.; Matsuura, Y.; Yokoyama, R.; Whittier, R.F.; Komeda, Y. The Arabidopsis ERECTA gene encodes a putative receptor protein kinase with extracellular leucine-rich repeats. Plant Cell 1996, 8, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.E.; Williams, R.W.; Meyerowitz, E.M. The CLAVATA1 gene encodes a putative receptor kinase that controls shoot and floral meristem size in Arabidopsis. Cell 1997, 89, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chory, J. A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell 1997, 90, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Jinn, T.L.; Stone, J.M.; Walker, J.C. HAESA, an Arabidopsis leucine-rich repeat receptor kinase, controls floral organ abscission. Genes. Dev. 2000, 14, 108–117. [Google Scholar] [CrossRef]
- He, Z.; Wang, Z.Y.; Li, J.; Zhu, Q.; Lamb, C.; Ronald, P.; Chory, J. Perception of brassinosteroids by the extracellular domain of the receptor kinase BRI1. Science 2000, 288, 2360–2363. [Google Scholar] [CrossRef]
- Hou, B.Z.; Xu, C.; Shen, Y.Y. A leu-rich repeat receptor-like protein kinase, FaRIPK1, interacts with the ABA receptor, FaABAR, to regulate fruit ripening in strawberry. J. Exp. Bot. 2018, 69, 1569–1582. [Google Scholar] [CrossRef]
- Osakabe, Y.; Maruyama, K.; Seki, M.; Satou, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Leucine-rich repeat receptor-like kinase1 is a key membrane-bound regulator of abscisic acid early signaling in Arabidopsis. Plant Cell 2005, 17, 1105–1119. [Google Scholar] [CrossRef] [PubMed]
- Song, W.Y.; Wang, G.L.; Chen, L.L.; Kim, H.S.; Pi, L.Y.; Holsten, T.; Gardner, J.; Wang, B.; Zhai, W.X.; Zhu, L.H.; et al. A receptor kinase-like protein encoded by the rice disease resistance gene, Xa21. Science 1995, 270, 1804–1806. [Google Scholar] [CrossRef] [PubMed]
- Kieliszewski, M.J.; Lamport, D.T. Extensin: Repetitive motifs, functional sites, post-translational codes, and phylogeny. Plant J. 1994, 5, 157–172. [Google Scholar] [CrossRef]
- Kieliszewski, M.J.; Leykam, J.F.; Lamport, D.T. Structure of the Threonine-Rich Extensin from Zea mays. Plant Physiol. 1990, 92, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Showalter, A.M.; Keppler, B.; Lichtenberg, J.; Gu, D.; Welch, L.R. A bioinformatics approach to the identification, classification, and analysis of hydroxyproline-rich glycoproteins. Plant Physiol. 2010, 153, 485–513. [Google Scholar] [CrossRef] [PubMed]
- Elliott, K.A.; Shirsat, A.H. Promoter regions of the extA extensin gene from Brassica napus control activation in response to wounding and tensile stress. Plant Mol. Biol. 1998, 37, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Wycoff, K.L.; Powell, P.A.; Gonzales, R.A.; Corbin, D.R.; Lamb, C.; Dixon, R.A. Stress activation of a bean hydroxyproline-rich glycoprotein promoter is superimposed on a pattern of tissue-specific developmental expression. Plant Physiol. 1995, 109, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Carpita, N.C.; Gibeaut, D.M. Structural models of primary cell walls in flowering plants: Consistency of molecular structure with the physical properties of the walls during growth. Plant J. 1993, 3, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Hall, Q.; Cannon, M.C. The cell wall hydroxyproline-rich glycoprotein RSH is essential for normal embryo development in Arabidopsis. Plant Cell 2002, 14, 1161–1172. [Google Scholar] [CrossRef]
- Baumberger, N.; Doesseger, B.; Guyot, R.; Diet, A.; Parsons, R.L.; Clark, M.A.; Simmons, M.P.; Bedinger, P.; Goff, S.A.; Ringli, C.; et al. Whole-Genome Comparison of Leucine-Rich Repeat Extensins in Arabidopsis and Rice. A Conserved Family of Cell Wall Proteins Form a Vegetative and a Reproductive Clade. Plant Physiol. 2003, 131, 1313–1326. [Google Scholar] [CrossRef]
- Rubinstein, A.L.; Marquez, J.; Suarez-Cervera, M.; Bedinger, P.A. Extensin-like Glycoproteins in the Maize Pollen Tube Wall. Plant Cell 1995, 7, 2211–2225. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Rumeau, D.; Showalter, A.M. Isolation and characterization of two wound-regulated tomato extensin genes. Plant Mol. Biol. 1992, 20, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zayed, O.; Yu, Z.; Jiang, W.; Zhu, P.; Hsu, C.-C.; Zhang, L.; Tao, W.A.; Lozano-Durán, R.; Zhu, J.-K. Leucine-rich repeat extensin proteins regulate plant salt tolerance in Arabidopsis. Proc. Natl. Acad. Sci. USA 2018, 115, 13123–13128. [Google Scholar] [CrossRef] [PubMed]
- Baumberger, N.; Steiner, M.; Ryser, U.; Keller, B.; Ringli, C. Synergistic interaction of the two paralogous Arabidopsis genes LRX1 and LRX2 in cell wall formation during root hair development. Plant J. 2003, 35, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Draeger, C.; Ndinyanka Fabrice, T.; Gineau, E.; Mouille, G.; Kuhn, B.M.; Moller, I.; Abdou, M.T.; Frey, B.; Pauly, M.; Bacic, A.; et al. Arabidopsis leucine-rich repeat extensin (LRX) proteins modify cell wall composition and influence plant growth. BMC Plant Biol. 2015, 15, 155. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, K.; Yin, G.; Liu, X.; Liu, M.; Cao, N.; Duan, Y.; Gao, H.; Wang, W.; Ge, W.; et al. Pollen-Expressed Leucine-Rich Repeat Extensins Are Essential for Pollen Germination and Growth. Plant Physiol. 2018, 176, 1993–2006. [Google Scholar] [CrossRef] [PubMed]
- Sede, A.R.; Borassi, C.; Wengier, D.L.; Mecchia, M.A.; Estevez, J.M.; Muschietti, J.P. Arabidopsis pollen extensins LRX are required for cell wall integrity during pollen tube growth. FEBS Lett. 2018, 592, 233–243. [Google Scholar] [CrossRef]
- Fabrice, T.N.; Vogler, H.; Draeger, C.; Munglani, G.; Gupta, S.; Herger, A.G.; Knox, P.; Grossniklaus, U.; Ringli, C. LRX Proteins Play a Crucial Role in Pollen Grain and Pollen Tube Cell Wall Development. Plant Physiol. 2018, 176, 1981–1992. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Duvaud, S.; Gabella, C.; Lisacek, F.; Stockinger, H.; Ioannidis, V.; Durinx, C. Expasy, the Swiss Bioinformatics Resource Portal, as designed by its users. Nucleic Acids Res. 2021, 49, 216–227. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. g:Profiler: A web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019, 47, W191–W198. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Moussu, S.; Broyart, C.; Santos-Fernandez, G.; Augustin, S.; Wehrle, S.; Grossniklaus, U.; Santiago, J. Structural basis for recognition of RALF peptides by LRX proteins during pollen tube growth. Proc. Natl. Acad. Sci. USA 2020, 117, 7494–7503. [Google Scholar] [CrossRef] [PubMed]
- Alberts, B. The Cell as a Collection of Protein Machines: Preparing the next Generation of Molecular Biologists. Cell 1998, 92, 291–294. [Google Scholar] [CrossRef]
- Pearce, G.; Moura, D.S.; Stratmann, J.; Ryan, C.A. RALF, a 5-kDa ubiquitous polypeptide in plants, arrests root growth and development. Proc. Natl. Acad. Sci. USA 2001, 98, 12843–12847. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Tang, R.; Zhang, X.; Luan, S.; Yu, F. FERONIA Receptor Kinase at the Crossroads of Hormone Signaling and Stress Responses. Plant Cell Physiol. 2017, 58, 1143–1150. [Google Scholar] [CrossRef]
- Yu, F.; Qian, L.; Nibau, C.; Duan, Q.; Kita, D.; Levasseur, K.; Li, X.; Lu, C.; Li, H.; Hou, C.; et al. FERONIA receptor kinase pathway suppresses abscisic acid signaling in Arabidopsis by activating ABI2 phosphatase. Proc. Natl. Acad. Sci. USA 2012, 109, 14693–14698. [Google Scholar] [CrossRef]
- Wang, L.; Yang, T.; Wang, B.; Lin, Q.; Zhu, S.; Li, C.; Ma, Y.; Tang, J.; Xing, J.; Li, X.; et al. RALF1-FERONIA complex affects splicing dynamics to modulate stress responses and growth in plants. Sci. Adv. 2020, 6, 21. [Google Scholar] [CrossRef]
- Mecchia, M.A.; Santos-Fernandez, G.; Duss, N.N.; Somoza, S.C.; Boisson-Dernier, A.; Gagliardini, V.; Martínez-Bernardini, A.; Fabrice, T.N.; Ringli, C.; Muschietti, J.P.; et al. RALF4/19 peptides interact with LRX proteins to control pollen tube growth in Arabidopsis. Science 2017, 358, 1600–1603. [Google Scholar] [CrossRef] [PubMed]
- Gronnier, J.; Franck, C.M.; Stegmann, M.; DeFalco, T.A.; Abarca, A.; von Arx, M.; Dünser, K.; Lin, W.; Yang, Z.; Kleine-Vehn, J.; et al. Regulation of immune receptor kinase plasma membrane nanoscale organization by a plant peptide hormone and its receptors. eLife 2022, 11, e74162. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Shi, L.; Li, X.; Zheng, H.; He, L. AGP30: Cd tolerance related gene associate with mitochondrial pyruvate carrier 1. Plant Signal. Behav. 2019, 14, 1629269. [Google Scholar] [CrossRef] [PubMed]
- BBorassi, C.; Sede, A.R.; Mecchia, M.A.; Salgado Salter, J.D.; Marzol, E.; Muschietti, J.P.; Estevez, J.M. An update on cell surface proteins containing extensin-motifs. J. Exp. Bot. 2015, 67, 477–487. [Google Scholar] [CrossRef]
- Cannon, M.C.; Terneus, K.; Hall, Q.; Tan, L.; Wang, Y.; Wegenhart, B.L.; Chen, L.; Lamport, D.T.; Chen, Y.; Kieliszewski, M.J. Self-assembly of the plant cell wall requires an extensin scaffold. Proc. Natl. Acad. Sci. USA 2008, 105, 2226–2231. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Behrens, B.X.; West, P.R.; Mort, A.J. Solubilization and partial characterization of extensin fragments from cell walls of cotton suspension cultures. Evidence for a covalent cross-link between extensin and pectin. Plant Physiol. 1995, 108, 1691–1701. [Google Scholar] [CrossRef]
- Lewis, D.R.; Olex, A.L.; Lundy, S.R.; Turkett, W.H.; Fetrow, J.S.; Muday, G.K. A kinetic analysis of the auxin transcriptome reveals cell wall remodeling proteins that modulate lateral root development in Arabidopsis. Plant Cell 2013, 25, 3329–3346. [Google Scholar] [CrossRef] [PubMed]
- Abdi, N.; Van Biljon, A.; Steyn, C.; Labuschagne, M.T. Salicylic Acid Improves Growth and Physiological Attributes and Salt Tolerance Differentially in Two Bread Wheat Cultivars. Plants 2022, 11, 1853. [Google Scholar] [CrossRef] [PubMed]
- Ogunsiji, E.; Umebese, C.; Stabentheiner, E.; Iwuala, E.; Odjegba, V.; Oluwajobi, A. Salicylic Acid Enhances Growth, Photosynthetic Performance and Antioxidant Defense Activity Under Salt Stress in Two Mungbean [Vigna radiata (L.) R. Wilczek] Variety. Plant Signal. Behav. 2023, 18, 2217605. [Google Scholar] [CrossRef]
- Arruda, T.F.L.; Lima, G.S.; Silva, A.; Azevedo, C.A.V.; Souza, A.R.; Soares, L.; Gheyi, H.R.; Lima, V.L.A.; Fernandes, P.D.; Silva, F.A.D.; et al. Salicylic Acid as a Salt Stress Mitigator on Chlorophyll Fluorescence, Photosynthetic Pigments, and Growth of Precocious-Dwarf Cashew in the Post-Grafting Phase. Plants 2023, 12, 2783. [Google Scholar] [CrossRef]
- Ji, H.; Liu, L.; Li, K.; Xie, Q.; Wang, Z.; Zhao, X.; Li, X. PEG-mediated osmotic stress induces premature differentiation of the root apical meristem and outgrowth of lateral roots in wheat. J. Exp. Bot. 2014, 65, 4863–4872. [Google Scholar] [CrossRef] [PubMed]
- Colebrook, E.H.; Thomas, S.G.; Phillips, A.L.; Hedden, P. The role of gibberellin signalling in plant responses to abiotic stress. J. Exp. Biol. 2014, 217, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Azhar, M.T.; Hinze, L.; Qayyum, A.; Li, H.; Peng, Z.; Qin, G.; Jia, Y.; Pan, Z.; He, S.; et al. Insight into abscisic acid perception and signaling to increase plant tolerance to abiotic stress. J. Plant Interact. 2021, 16, 222–237. [Google Scholar] [CrossRef]
- Luo, X.; Li, C.; He, X.; Zhang, X.; Zhu, L. ABA signaling is negatively regulated by GbWRKY1 through JAZ1 and ABI1 to affect salt and drought tolerance. Plant Cell Rep. 2020, 39, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Jiang, W.; Zayed, O.; Liu, X.; Tang, K.; Nie, W.; Li, Y.; Xie, S.; Li, Y.; Long, T.; et al. The LRXs-RALFs-FER module controls plant growth and salt stress responses by modulating multiple plant hormones. Natl. Sci. Rev. 2021, 8, nwaa149. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Kita, D.; Peaucelle, A.; Cartwright, H.N.; Doan, V.; Duan, Q.; Liu, M.C.; Maman, J.; Steinhorst, L.; Schmitz-Thom, I.; et al. The FERONIA Receptor Kinase Maintains Cell-Wall Integrity during Salt Stress through Ca(2+) Signaling. Curr. Biol. 2018, 28, 666–675. [Google Scholar] [CrossRef]
- Bacete, L.; Schulz, J.; Engelsdorf, T.; Bartosova, Z.; Vaahtera, L.; Yan, G.; Gerhold, J.M.; Tichá, T.; Øvstebø, C.; Gigli-Bisceglia, N.; et al. THESEUS1 modulates cell wall stiffness and abscisic acid production in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2022, 119, e2119258119. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.W.; Baek, W.; Jung, J.; Kim, J.-H.; Lee, S.C. Function of ABA in Stomatal Defense against Biotic and Drought Stresses. Int. J. Mol. Sci. 2015, 16, 15251–15270. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef]
- Jing, H.; Wilkinson, E.G.; Sageman-Furnas, K.; Strader, L.C. Auxin and abiotic stress responses. J. Exp. Bot. 2023, 74, 7000–7014. [Google Scholar] [CrossRef]
- Xiong, B.; Wang, Y.; Zhang, Y.; Ma, M.; Gao, Y.; Zhou, Z.; Wang, B.; Wang, T.; Lv, X.; Wang, X.; et al. Alleviation of drought stress and the physiological mechanisms in Citrus cultivar (Huangguogan) treated with methyl jasmonate. Biosci. Biotechnol. Biochem. 2020, 84, 1958–1965. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Jiang, M.; Guo, C. Crop Pollen Development under Drought: From the Phenotype to the Mechanism. Int. J. Mol. Sci. 2019, 20, 1550. [Google Scholar] [CrossRef] [PubMed]

| Gene Name | Gene ID | Chromosome | pI | MW (Da) | Protein Length (aa) | Subcellular Location | A. thaliana ID | A. thaliana Name |
|---|---|---|---|---|---|---|---|---|
| BrLRX1 | Bra011483 | A01:1996205-1998289 | 5.32 | 74,239.31 | 694 | Nucleus, Chloroplast | AT4G33970 | AtLRX11 |
| BrLRX2 | Bra013339 | A01:5076635-5088816 | 6.69 | 80,732.58 | 758 | Chloroplast | AT4G18670 | AtLRX5 |
| BrLRX3 | Bra023805 | A01:20099064-20101488 | 6.54 | 48,666.94 | 436 | Chloroplast, Extracellular | AT3G22800 | AtLRX6 |
| BrLRX4 | Bra037543 | A01:21776633-21779155 | 5.54 | 90,368.26 | 840 | Vacuolar membrane | AT3G19020 | AtLRX8 |
| BrLRX5 | Bra001730 | A03:18114562-18120053 | 5.23 | 125,711.55 | 1153 | Vacuolar membrane | AT3G19020 | AtLRX8 |
| BrLRX6 | Bra001950 | A03:19552078-19555827 | 6.75 | 82,626.60 | 756 | Nucleus | AT3G24480 | AtLRX4 |
| BrLRX7 | Bra013092 | A03:20527348-20529933 | 6.89 | 92,180.92 | 860 | Vacuolar membrane, Extracellular | AT2G15880 | AtLRX10 |
| BrLRX8 | Bra017617 | A03:29343070-29345370 | 6.53 | 81,850.73 | 776 | Extracellular | AT4G33970 | AtLRX11 |
| BrLRX9 | Bra033914 | A05:15169415-15170875 | 5.75 | 51,109.69 | 461 | Extracellular | AT3G22800 | AtLRX6 |
| BrLRX10 | Bra019737 | A06:4713634-4715936 | 8.01 | 74,056.35 | 684 | Nucleus | AT1G12040 | AtLRX1 |
| BrLRX11 | Bra009850 | A06:17668994-17670253 | 4.91 | 46,466.39 | 419 | Vacuolar membrane | AT5G25550 | AtLRX7 |
| BrLRX12 | Bra014193 | A08:2658201-2660384 | 5.27 | 78,665.99 | 727 | Vacuolar membrane, Extracellular | AT1G49490 | AtLRX9 |
| BrLRX13 | Bra034594 | A08:12612559-12614571 | 5.28 | 71,304.18 | 670 | Nucleus | AT4G33970 | AtLRX11 |
| BrLRX14 | Bra016796 | A08:20029117-20031429 | 8.48 | 83,428.68 | 770 | Extracellular | AT1G12040 | AtLRX1 |
| BrLRX15 | Bra027044 | A09:7860356-7862569 | 8.47 | 79,946.18 | 737 | Vacuolar membrane, Extracellular | AT1G62440 | AtLRX2 |
| BrLRX16 | Bra026996 | A09:34684812-34686566 | 5.86 | 63,602.80 | 584 | Chloroplast, Extracellular, Vacuolar membrane | AT1G12040 | AtLRX1 |
| BrLRX17 | Bra015245 | A10:2972264-2974768 | 7.08 | 89,419.33 | 834 | Vacuolar membrane | AT2G15880 | AtLRX10 |
| Motif | Motif Consensus |
|---|---|
| Motif1 | FENPRLKRAYIALQAWKKAIYSDPFNTTANWHGPDVCSYTGVYCAPALDD |
| Motif2 | FENPRLKRAYIALQAWKKAIYSDPFNTTANWHGPDVCSYTGVYCAPALDD |
| Motif3 | VVLSLPSLKFLDLRFNEFEGKVPSELFDKDLDAIFLNNNRFRSTIPENLG |
| Motif4 | VVLSLPSLKFLDLRFNEFEGKVPSELFDKDLDAIFLNNNRFRSTIPENLG |
| Motif5 | VVLSLPSLKFLDLRFNEFEGKVPSELFDKDLDAIFLNNNRFRSTIPENLG |
| Motif6 | VPGSRKEIALDDTRNCLPDRPKQRSAKECAVVISRPVDCSKDKCAGGGSS |
| Motif7 | FSKLKLLHELDVSNNRFVGPF |
| Motif8 | VSVVVLABNKFGGCI |
| Motif9 | AYALTDEEASFJVQRQLLTLPENGD |
| Motif10 | TPPAEAPAPSDEFILPPFIGHQYASPPPPMFPGY |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hui, J.; Zhang, M.; Chen, L.; Wang, Y.; He, J.; Zhang, J.; Wang, R.; Jiang, Q.; Lv, B.; Cao, Y. Identification, Classification, and Expression Analysis of Leucine-Rich Repeat Extensin Genes from Brassica rapa Reveals Salt and Osmosis Stress Response Genes. Horticulturae 2024, 10, 571. https://doi.org/10.3390/horticulturae10060571
Hui J, Zhang M, Chen L, Wang Y, He J, Zhang J, Wang R, Jiang Q, Lv B, Cao Y. Identification, Classification, and Expression Analysis of Leucine-Rich Repeat Extensin Genes from Brassica rapa Reveals Salt and Osmosis Stress Response Genes. Horticulturae. 2024; 10(6):571. https://doi.org/10.3390/horticulturae10060571
Chicago/Turabian StyleHui, Jiyun, Meiqi Zhang, Luhan Chen, Yuexin Wang, Jiawei He, Jingjing Zhang, Ruolan Wang, Qiwei Jiang, Bingcan Lv, and Yunyun Cao. 2024. "Identification, Classification, and Expression Analysis of Leucine-Rich Repeat Extensin Genes from Brassica rapa Reveals Salt and Osmosis Stress Response Genes" Horticulturae 10, no. 6: 571. https://doi.org/10.3390/horticulturae10060571
APA StyleHui, J., Zhang, M., Chen, L., Wang, Y., He, J., Zhang, J., Wang, R., Jiang, Q., Lv, B., & Cao, Y. (2024). Identification, Classification, and Expression Analysis of Leucine-Rich Repeat Extensin Genes from Brassica rapa Reveals Salt and Osmosis Stress Response Genes. Horticulturae, 10(6), 571. https://doi.org/10.3390/horticulturae10060571







