Exogenous Application of Gamma Aminobutyric Acid Improves the Morpho-Physiological and Biochemical Attributes in Lavandula dentata L. under Salinity Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Growth and Experimental Conditions
2.2. Salinity Stress Treatments
2.3. Measurement of Growth Parameters
2.4. Determination of Photosynthetic Pigments
2.5. Relative Water Content
2.6. Measurement of Proline Content
2.7. Essential Oil Extraction
2.8. Antioxidant Enzyme Activities
2.9. Statistical Analysis
3. Results and Discussion
3.1. Vegetative Growth Characteristics
3.2. Roots Characteristics
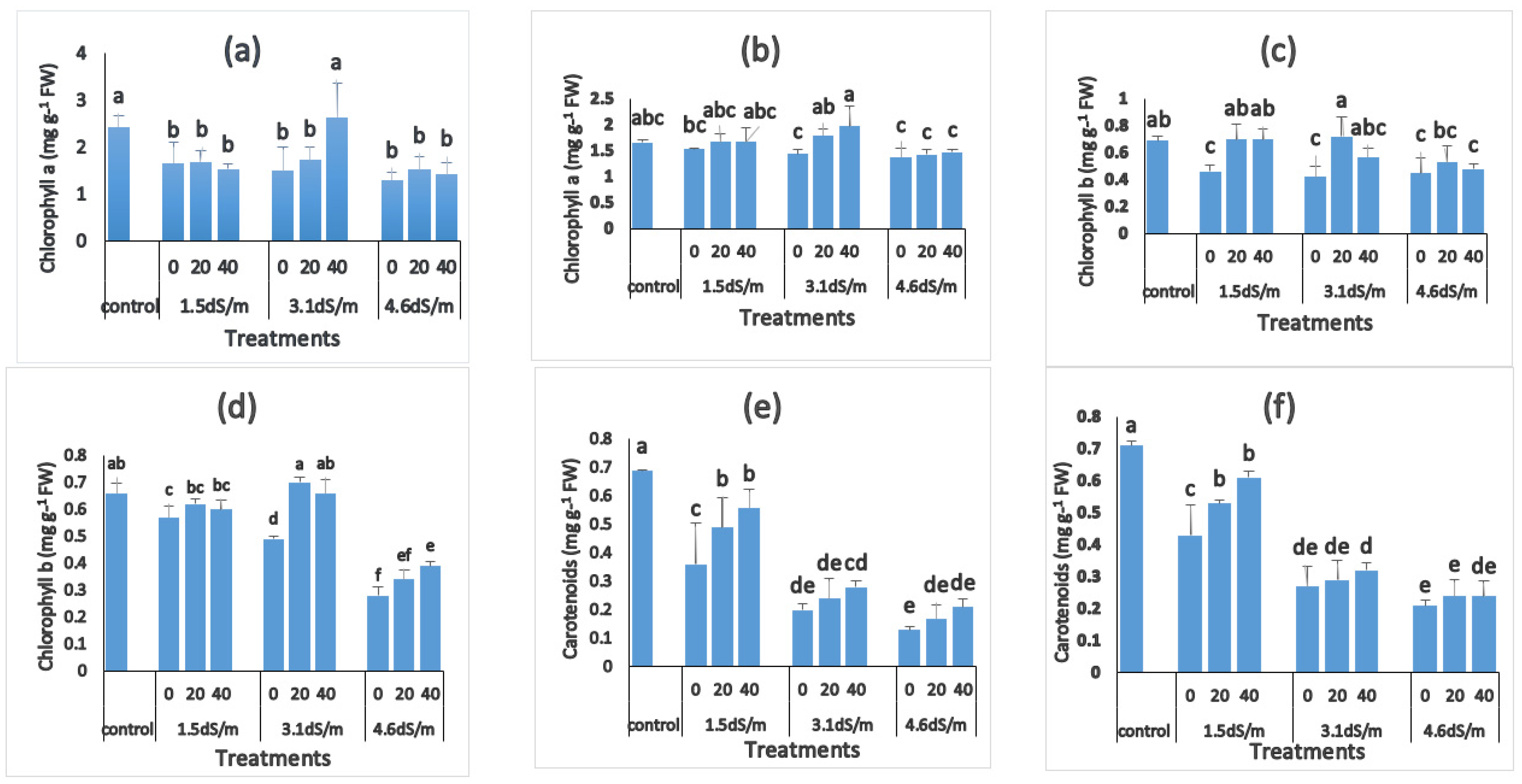
3.3. Photosynthetic Pigments
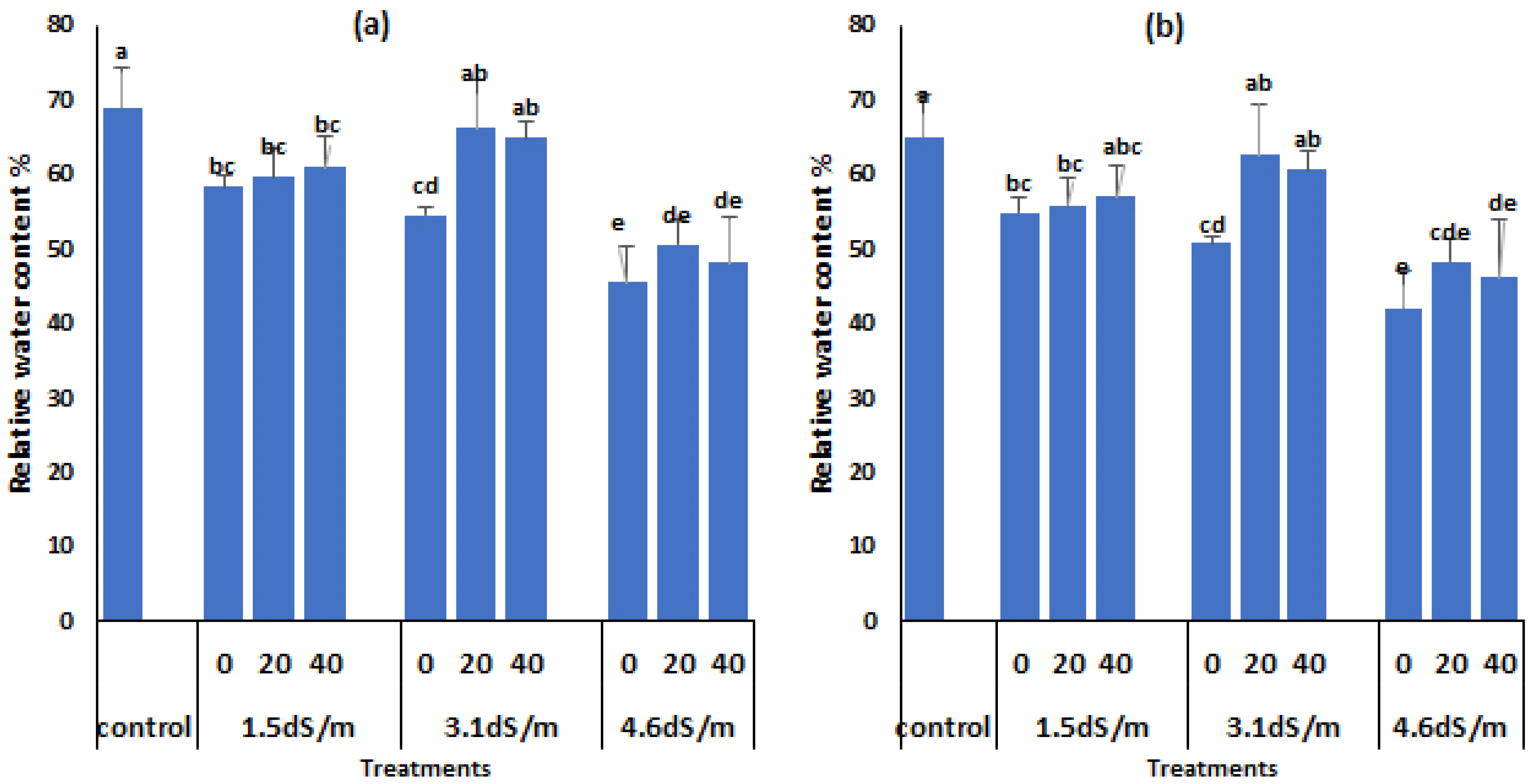
3.4. Relative Water Content
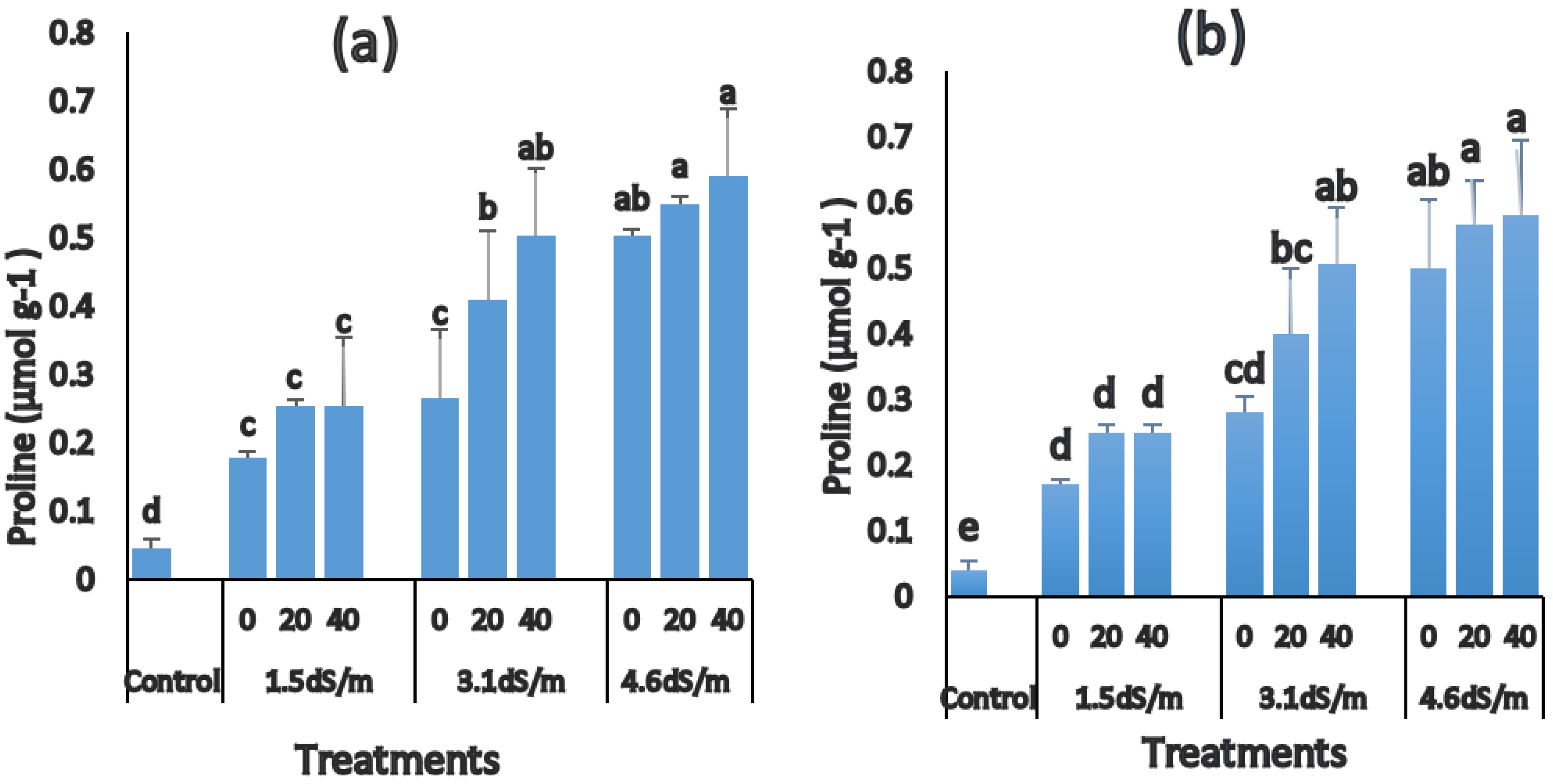
3.5. Changes in Proline Accumulation
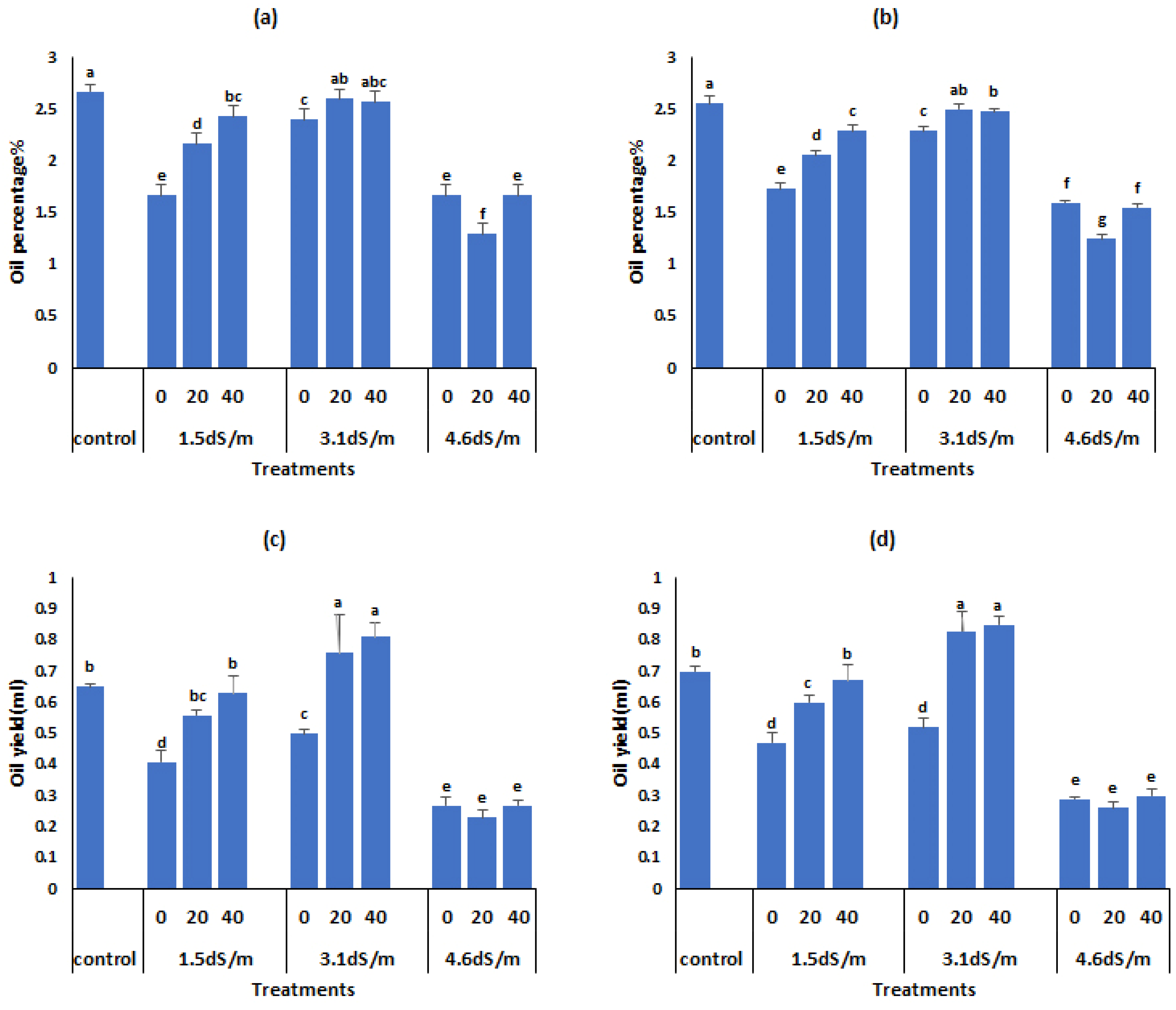
3.6. Changes in Essential Oil Yield
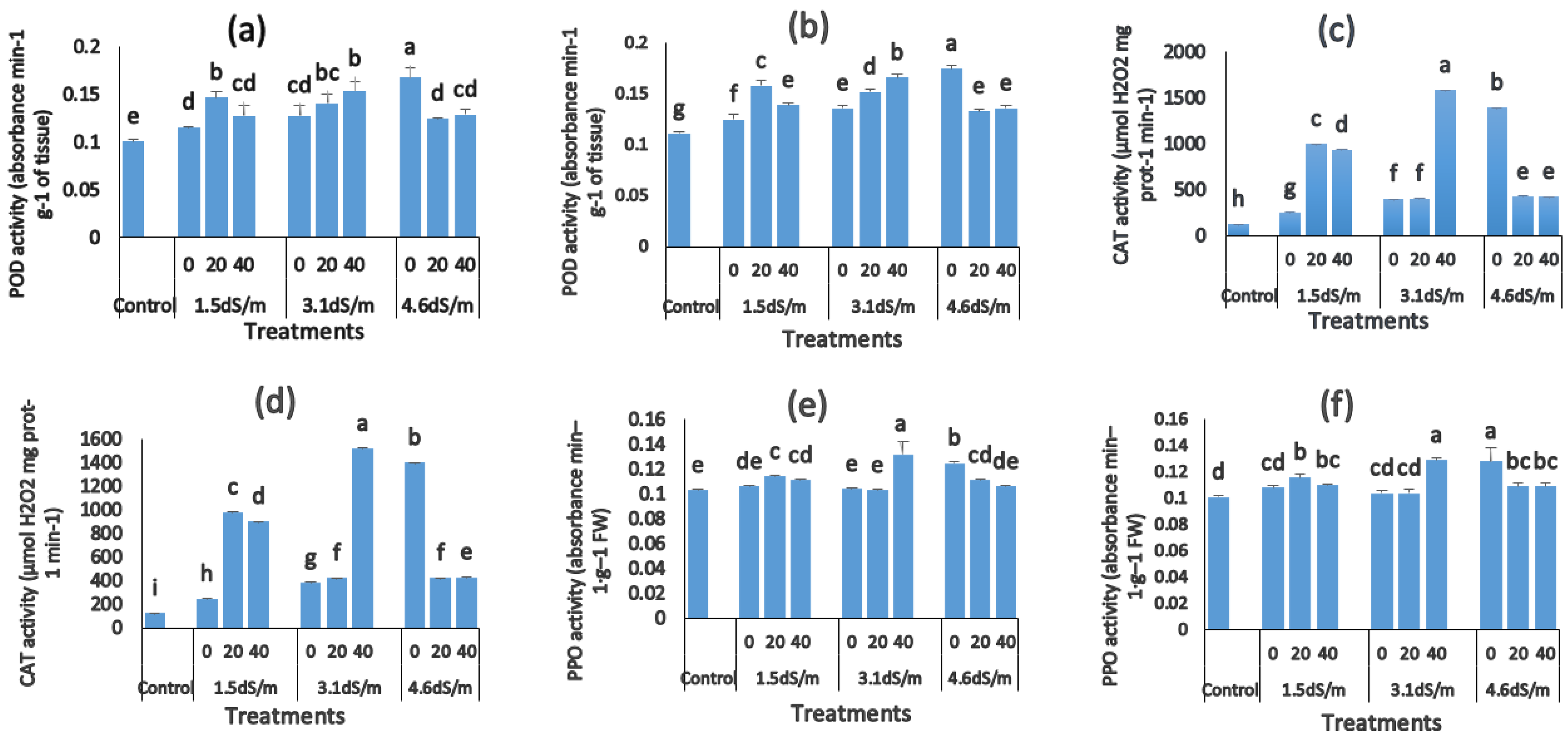
3.7. Changes in Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abou El Fadl, I.A.; Abd-Ella, M.K.; Hussein, E.H. Effect of saline irrigation water on the growth and some principal compounds of peppermint and spearmint in two types of soil. J. Agric. Res. Tanta Univ. 1990, 16, 276–295. [Google Scholar]
- Cordovilla, M.P.; Bueno, M.; Aparicio, C.; Urrestarazu, M. Effects of salinity and the interaction between Thymus vulgaris and Lavandula angustifolia on growth, ethylene production and essential oil contents. J Plant Nutr. 2014, 37, 875–888. [Google Scholar] [CrossRef]
- Moghith, W.M.A.; Youssef, A.S.M.; El-Wahab, M.A.A.; Mohamed, Y.F.Y.; Eman, M.A.E.-G. Effect of saline water stress in the presence of silicon foliar application on growth, productivity and chemical constituents of chia (Salvia hispanica L.) under Egyptian conditions. Asian Plant Res. J. 2020, 4, 28–45. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Michailidi, E.; Tzortzakis, N. Physiological and biochemical responses of Lavandula angustifolia to salinity under mineral foliar application. Front. Plant Sci. 2018, 9, 489. [Google Scholar] [CrossRef] [PubMed]
- Gururani, M.A. In vivo assessment of salinity stress tolerance in transgenic Arabidopsis plants expressing Solanum tuberosum D200 gene. Biol. Plant 2022, 66, 123–131. [Google Scholar] [CrossRef]
- Kappachery, S.; Sasi, S.; Alyammahi, O.; Alyassi, A.; Venkatesh, J.; Gururani, M.A. Overexpression of cytoplasmic Solanum tuberosum glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene improves PSII efficiency and alleviates salinity stress in Arabidopsis. J. Plant Interact. 2021, 16, 398–410. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, W.; Meng, Y.; Xie, T.; Li, L.; Li, J.; Wei, S. γ-Aminobutyric acid imparts partial protection from salt stress injury to maize seedlings by improving photosynthesis and upregulating osmoprotectants and antioxidants. Sci. Rep. 2017, 7, 43609. [Google Scholar] [CrossRef] [PubMed]
- Golnari, S.; Vafaee, Y.; Nazari, F.; Ghaderi, N. Gamma-aminobutyric acid (GABA) and salinity impacts antioxidative response and expression of stress-related genes in strawberry cv. Aromas. Braz. J. Bot. 2021, 44, 639–651. [Google Scholar] [CrossRef]
- Gururani, M.A.; Venkatesh, J.; Ghosh, R.; Strasser, R.J.; Ponpandian, L.N.; Bae, H. Chlorophyll-a fluorescence evaluation of PEG-induced osmotic stress on PSII activity in Arabidopsis plants expressing SIP1. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2017, 3504, 945–952. [Google Scholar] [CrossRef]
- Akilan, S.; Halima, T.H.; Sasi, S.; Kappachery, S.; Baniekal-Hiremath, G.; Venkatesh, J.; Gururani, M.A. Evaluation of osmotic stress tolerance in transgenic Arabidopsis plants expressing Solanum tuberosum D200 gene. J. Plant Interact. 2019, 14, 79–86. [Google Scholar] [CrossRef]
- Szekely-Varga, Z.; González-Orenga, S.; Cantor, M.; Jucan, D.; Boscaiu, M.; Vicente, O. Effects of drought and salinity on two commercial varieties of Lavandula angustifolia mill. Plants 2020, 9, 673. [Google Scholar] [CrossRef] [PubMed]
- Eisa, E.A.; Honfi, P.; Tilly-Mándy, A.; Gururani, M.A. Exogenous application of melatonin alleviates drought stress in Ranunculus asiaticus by improving its morphophysiological and biochemical attributes. Horticulturae 2023, 9, 262. [Google Scholar] [CrossRef]
- Gururani, M.A.; Mohanta, T.K.; Bae, H. Current understanding of the interplay between phytohormones and photosynthesis under environmental stress. Int. J. Mol. Sci. 2015, 16, 19055–19085. [Google Scholar] [CrossRef] [PubMed]
- Ghahremani, Z.; Mikaealzadeh, M.; Barzegar, T.; Ranjbar, M.E. Foliar application of ascorbic acid and gamma aminobutyric acid can improve important properties of deficit irrigated cucumber plants (Cucumis sativus cv. Us). Gesunde Pflanz. 2021, 73, 77–84. [Google Scholar] [CrossRef]
- Vijayakumari, K.; Puthur, J.T. γ-Aminobutyric acid (GABA) priming enhances the osmotic stress tolerance in Piper nigrum Linn. plants subjected to PEG-induced stress. Plant Growth Regul. 2016, 78, 57–67. [Google Scholar] [CrossRef]
- Rezaei-Chiyaneh, E.; Seyyedi, S.M.; Ebrahimian, E.; Moghaddam, S.S.; Damalas, C.A. Exogenous application of gamma-aminobutyric acid (GABA) alleviates the effect of water deficit stress in black cumin (Nigella sativa L.). Ind. Crops Prod. 2018, 112, 741–748. [Google Scholar] [CrossRef]
- Jamshideyni, M.; Behdani, M.A.; Parsa, S.; Khoramdel, S. Evaluation of yield and water use efficiency of Quinoa under irrigation regimes, gamma aminobutyric acid, and vermicompost application. Acta Agric. Slov. 2023, 119, 1–15. [Google Scholar] [CrossRef]
- Kaur, R.; Zhawar, V.K. Regulation of secondary antioxidants and carbohydrates by gamma-aminobutyric acid under salinity–alkalinity stress in rice (Oryza sativa L.). Biol. Futur. 2021, 72, 229–239. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Shao, H.; Qi, W.; Hamoud, Y.A.; Shaghaleh, H.; Khan, N.U.; Yang, R.; Tang, B. GABA-alleviated oxidative injury induced by salinity, osmotic stress and their combination by regulating cellular and molecular signals in rice. Int. J. Mol. Sci. 2019, 20, 5709. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, P.; Chen, Z.; Gu, Z.; Yang, R. GABA enhances physio-biochemical metabolism and antioxidant capacity of germinated hulless barley under NaCl stress. J. Plant Physiol. 2018, 231, 192–201. [Google Scholar] [CrossRef]
- Feizi, H.; Moradi, R.; Pourghasemian, N.; Sahabi, H. Assessing saffron response to salinity stress and alleviating potential of gamma amino butyric acid, salicylic acid and vermicompost extract on salt damage. S. Afr. J. Bot. 2021, 141, 330–343. [Google Scholar] [CrossRef]
- Peter, K.V.; Shylaja, M.R. Introduction to herbs and spices: Definitions, trade and applications. In Handbook of Herbs and Spices; Woodhead Publishing: Cambridge, UK, 2012; pp. 1–24. [Google Scholar]
- Barkaoui, H.; Chafik, Z.; Benabbas, R.; Chetouani, M.; El Mimouni, M.; Hariri, E. Antifungal activity of the essential oils of Rosmarinus officinalis, Salvia officinalis, Lavandula dentata and Cymbopogon citratus against the mycelial growth of Fusarium oxysporum f.sp.Albedinis. Arab. J. Med. Aromat. Plants 2022, 8, 108–133. [Google Scholar] [CrossRef]
- El Abdali, Y.; Agour, A.; Allali, A.; Bourhia, M.; El Moussaoui, A.; Eloutassi, N.; Salamatullah, A.M.; Alzahrani, A.; Ouahmane, L.; Aboul-Soud, M.A.; et al. Lavandula dentata L.: Phytochemical analysis, antioxidant, antifungal and insecticidal activities of its essential oil. Plants 2022, 11, 311. [Google Scholar] [CrossRef]
- Hammam, K.A.; Awadalla, S.S.S. Mitigation of saline water stress on French lavender (Lavandula dentata L.) plants. J. Hortic. Sci. Ornam. Plants 2020, 12, 8–16. [Google Scholar]
- Page, A.L. Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; American Society of Agronomy, Inc.: Madison, WI, USA; Soil Science Society of America, Inc.: Madison, WI, USA, 1983. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. In Current Protocols in Food Analytical Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2001; pp. F421–F426. [Google Scholar]
- Whetherley, P.E. Studies in the water relations of cotton plants. I. The field measurement of water deficit in leaves. New Phytol. 1950, 49, 81–87. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Pharmacopoeia, B. Determination of Volatile Oil in Drugs; The Pharmaceutical Press: London, UK, 1963. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Hammerschmidt, R.; Nuckles, E.M.; Kuć, J. Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol. Plant Pathol. 1982, 20, 73–82. [Google Scholar] [CrossRef]
- Malik, C.P.; Singh, M.B. Plant Enzymology and Histoenzymology. A Text Manual; Kalyani Publishers: New Delhi, India, 1980; p. 286. [Google Scholar]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 7th ed.; The Iowa State University Press: Ames, IA, USA, 1980. [Google Scholar]
- Kalhor, M.S.; Aliniaeifard, S.; Seif, M.; Asayesh, E.J.; Bernard, F.; Hassani, B.; Li, T. Enhanced salt tolerance and photosynthetic performance: Implication of ɤ-amino butyric acid application in salt-exposed lettuce (Lactuca sativa L.) plants. Plant Physiol. Biochem. 2018, 130, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Tariq, A.; Zeng, F.; Noor, J.; Sardans, J.; Asghar, M.A.; Zhang, Z.; Peñuelas, J. Application of GABA (γ-aminobutyric acid) to improve saline stress tolerance of chufa (Cyperus esculentus, L. var. sativus Boeck) plants by regulating their antioxidant potential and nitrogen assimilation. S. Afr. J. Bot. 2023, 157, 540–552. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Yousef, E.A.A.; Nasef, I.N. Growth and yield response of garlic genotypes to foliar application of γ-aminobutyric acid. Hortsci. J. Suez Canal Univ. 2019, 8, 35–43. [Google Scholar] [CrossRef]
- Ramzan, M.; Shah, A.A.; Ahmed, M.Z.; Bukhari, M.A.; Ali, L.; Casini, R.; Elansary, H.O. Exogenous application of glutathione and gamma amino-butyric acid alleviates salt stress through improvement in antioxidative defense system and modulation of CaXTHs stress-related genes. S. Afr. J. Bot. 2023, 157, 266–273. [Google Scholar] [CrossRef]
- Rashed, N.M.; Shala Awad, Y.; Mahmoud, M.A. Alleviation of salt stress in Nigella sativa, L. by gibberellic acid and rhizobacteria. Alex. Sci. Exch. J. 2017, 38, 785–799. [Google Scholar] [CrossRef]
- Alqarawi, A.A.; Hashem, A.; Abd Allah, E.F.; Al-Huqail, A.A.; Alshahrani, T.S.; Alshalawi, S.R.; Egamberdieva, D. Protective role of gamma amminobutyric acid on Cassia italica Mill under salt stress. Legume Res. 2016, 39, 396–404. [Google Scholar] [CrossRef]
- Szekely-varga, Z.; González-orenga, S.; Cantor, M.; Boscaiu, M.; Vicente, O. Antioxidant responses to drought and salinity in Lavandula angustifolia Mill. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 1980–1992. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Z.; Fan, Y.; Liu, C.; Wang, H.; Li, Y.; Xin, Y.; Gai, Y.; Ji, X. Characterization of GABA-transaminase gene from mulberry (Morus multicaulis) and its role in salt stress tolerance. Genes 2022, 13, 501. [Google Scholar] [CrossRef]
| Soil Depth (cm) | Field Capacity (%) | Wilting Point (%) | Bulk Density (Mg m−3) | Total Porosity (%) | Sand (%) | Silt (%) | Clay (%) | Texture Class | pH |
|---|---|---|---|---|---|---|---|---|---|
| 0–15 | 45.37 | 22.91 | 1.19 | 55.09 | 19.42 | 24.97 | 55.61 | Clayey | 7.92 |
| ECe (dS m−1) | Inions concentration (meq L−1) | Cation concentration (meq L−1) | |||||||
| CO3− | HCO3− | Cl− | SO4− | Ca++ | Mg++ | Na+ | K+ | ||
| 0–15 | 2.74 | --- | 2.52 | 14.94 | 12.28 | 5.02 | 6.84 | 17.22 | 0.66 |
| Treatments | Plant Height (cm) | No. of Branches | Herb Fresh Weight (g/Plant) | Herb Dry Weight (g/Plant) | ||||
|---|---|---|---|---|---|---|---|---|
| 1st Season | 2nd Season | 1st Season | 2nd Season | 1st Season | 2nd Season | 1st Season | 2nd Season | |
| Control (fresh water) | 44.00 a | 47.33 a | 23.67 a | 21.67 abc | 68.09 cd | 78.03 c | 24.51 c | 27.45 b |
| Sea water at 1000 ppm | 42.33 ab | 46.00 ab | 21.33 ab | 19.67 abc | 64.30 d | 70.63 d | 24.57 c | 27.38 b |
| Sea water at 2000 ppm | 39.67 cd | 44.00 bc | 20.33 ab | 18.33 c | 57.13 e | 61.24 f | 20.78 d | 22.61 c |
| Sea water at 3000 ppm | 37.00 e | 38.67 e | 13.00 d | 11.33 d | 50.20 f | 60.73 f | 15.96 e | 18.63 e |
| 1000 ppm sea water + 20 mM GABA | 43.00 a | 47.00 a | 24.00 a | 22.00 ab | 68.65 cd | 77.94 c | 26.27 bc | 29.19 b |
| 1000 ppm sea water + 40 mM GABA | 42.67 ab | 46.33 ab | 22.00 ab | 20.00 abc | 71.27 c | 80.32 c | 26.15 bc | 29.00 b |
| 2000 ppm sea water + 20 mM GABA | 40.33 bc | 44.00 bc | 21.33 ab | 18.67 bc | 83.59 b | 91.43 b | 29.34 ab | 33.29 a |
| 1000 ppm sea water + 40 mM GABA | 42.33 ab | 45.67 ab | 24.33 a | 22.67 a | 90.50 a | 96.02 a | 31.37 a | 34.20 a |
| 3000 ppm sea water + 20 mM GABA | 37.67 de | 41.00 de | 16.00 cd | 13.67 d | 51.91 ef | 67.72 de | 17.93 de | 21.10 cd |
| 3000 ppm sea water + 40 mM GABA | 39.67 cd | 42.00 cd | 18.33 bc | 14.00 d | 55.83 ef | 64.36 ef | 16.10 e | 19.14 de |
| Treatments | Root Length (cm) | Root Fresh Weight (g/Plant) | Root Dry Weight (g/Plant) | |||
|---|---|---|---|---|---|---|
| 1st Season | 2nd Season | 1st Season | 2nd Season | 1st Season | 2nd Season | |
| Control (fresh water) | 12.33 a | 14.00 ab | 4.04 ab | 5.87 ab | 1.30 b | 1.72 c |
| Sea water at 1000 ppm | 10.67 bc | 13.50 abc | 3.30 b | 5.10 b | 1.30 b | 1.90 bc |
| Sea water at 2000 ppm | 10.33 bc | 12.00 c | 2.37 c | 4.08 c | 1.01 c | 1.54 d |
| Sea water at 3000 ppm | 8.33 d | 10.00 d | 1.90 c | 2.55 d | 0.71 d | 0.90 f |
| 1000 ppm sea water + 20 mM GABA | 11.5 ab | 13.33 abc | 4.09 a | 6.23 a | 1.57 a | 2.27 a |
| 1000 ppm sea water + 40 mM GABA | 11.67 ab | 13.5 abc | 4.20 a | 6.17 a | 1.61 a | 2.23 a |
| 2000 ppm sea water + 20 mM GABA | 12.17 a | 14.00 ab | 3.90 ab | 5.44 ab | 1.49 a | 2.01 b |
| 2000 ppm sea water + 40 mM GABA | 12.5 a | 15.00 a | 3.82 ab | 5.23 ab | 1.45 ab | 1.94 bc |
| 3000 ppm sea water + 20 mM GABA | 10.00 c | 12.50 bc | 2.32 c | 3.13 cd | 1.02 c | 1.22 e |
| 3000 ppm sea water + 40 mM GABA | 9.50 cd | 12.67 bc | 2.12 c | 2.97 d | 0.97 c | 1.21 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shala, A.Y.; Aboukamar, A.N.; Gururani, M.A. Exogenous Application of Gamma Aminobutyric Acid Improves the Morpho-Physiological and Biochemical Attributes in Lavandula dentata L. under Salinity Stress. Horticulturae 2024, 10, 410. https://doi.org/10.3390/horticulturae10040410
Shala AY, Aboukamar AN, Gururani MA. Exogenous Application of Gamma Aminobutyric Acid Improves the Morpho-Physiological and Biochemical Attributes in Lavandula dentata L. under Salinity Stress. Horticulturae. 2024; 10(4):410. https://doi.org/10.3390/horticulturae10040410
Chicago/Turabian StyleShala, Awad Y., Amira N. Aboukamar, and Mayank A. Gururani. 2024. "Exogenous Application of Gamma Aminobutyric Acid Improves the Morpho-Physiological and Biochemical Attributes in Lavandula dentata L. under Salinity Stress" Horticulturae 10, no. 4: 410. https://doi.org/10.3390/horticulturae10040410
APA StyleShala, A. Y., Aboukamar, A. N., & Gururani, M. A. (2024). Exogenous Application of Gamma Aminobutyric Acid Improves the Morpho-Physiological and Biochemical Attributes in Lavandula dentata L. under Salinity Stress. Horticulturae, 10(4), 410. https://doi.org/10.3390/horticulturae10040410








