Effect of the Intensity and Spectral Quality of LED Light on Growth and Quality of Spinach Indoors
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Growing Conditions, and Light Treatments
2.2. Plant Growth Parameters
2.3. Chlorophyll Fluorescence Parameters and SPAD
2.4. Mineral Analysis
2.5. Antioxidant Capacity, and Chlorophyll and Carotenoid Concentration
2.6. Primary Metabolites
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Light Treatment | Antioxidant Capacity (%) | Chlorophyll A | Chlorophyll B | Total Chlorophyll | Carotenoids |
|---|---|---|---|---|---|
| Intensity | |||||
| LI | 37.8 | 22.9 | 10.6 | 33.5 | 8.74 |
| MI | 34.3 | 21.6 | 10.0 | 31.7 | 8.43 |
| HI | 37.8 | 21.2 | 9.44 | 30.6 | 8.61 |
| ANOVA | ns | ns | ns | ns | ns |
| Quality | |||||
| NS12 (2 R/B) | 23.2 | 22.2 | 10.3 ab | 32.6 | 8.30 |
| AP67 (5 R/B) | 24.9 | 22.2 | 11.5 b | 33.8 | 8.43 |
| AP673L (6 R/B) | 24.0 | 19.2 | 8.98 a | 28.1 | 7.32 |
| G2 (10 R/B) | 23.2 | 21.2 | 10.1 a | 31.3 | 8.09 |
| ANOVA | ns | ns | * | ns | ns |
References
- FAOSTAT. Food and Agriculture Organization of the United Nations Database. 2020. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 22 June 2022).
- Saini, R.K.; Nile, S.H.; Park, S.W. Carotenoids from fruits and vegetables: Chemistry, analysis, occurrence, bioavailability and biological activities. Food Res. Int. 2015, 76, 735–750. [Google Scholar] [CrossRef]
- Qin, J.; Shi, A.; Mou, B.; Grusak, M.A.; Weng, Y.; Ravelombola, W.; Yang, W. Genetic diversity and association mapping of mineral element concentrations in spinach leaves. BMC Genom. 2017, 18, 941. [Google Scholar] [CrossRef]
- Tai, C.; Sawada, Y.; Masuda, J.; Daimon, H.; Fukao, Y. Cultivation of spinach in hot seasons using a micro-mist-based temperature-control system. Sci. Hortic. 2020, 273, 109603. [Google Scholar] [CrossRef]
- Adeyeye, S.A.O.; Babu, A.S. Vegetables as a Functional Food for Health. In Functional Foods; CRC Press: Boca Raton, FL, USA, 2024; pp. 102–118. [Google Scholar]
- Qureshi, M.U.N.; Nadeem, M.U. Comparative study of Hydroponic and Geoponic Systems. Doctoral Dissertation, Department of Civil Engineering, Capital University of Science & Technology Islamabad, Islamabad, Pakistan, 2017. [Google Scholar]
- Ogli, A.Y.I.; Esirgapovich, J.A. Hydroponic Technology and its Advantages. Span. J. Innov. Integr. 2022, 6, 507–510. [Google Scholar]
- Khan, F.A.; Kurklu, A.; Ghafoor, A.; Ali, Q.; Umair, M.S. A review on hydroponic greenhouse cultivation for sustainable agriculture. Int. J. Agric. Environ. Food Sci. 2019, 2, 59–66. [Google Scholar] [CrossRef]
- Mir, M.S.; Naikoo, N.B.; Kanth, R.H.; Bahar, F.A.; Bhat, M.A.; Nazir, A.; Ahngar, T.A. Vertical farming: The future of agriculture: A review. Pharma Innov. J. 2022, 11, 1175–1195. [Google Scholar]
- Jan, S.; Rashid, Z.; Ahngar, T.A.; Iqbal, S.; Naikoo, M.A.; Majeed, S.; Nazir, I. Hydroponics—A review. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 1779–1787. [Google Scholar] [CrossRef]
- Utasi, L.; Monostori, I.; Végh, B.; Pék, Z.; Éva, D. Effects of light intensity and spectral composition on the growth and metabolism of spinach (Spinacia oleracea L.). Acta Biol. Plantarum. Agriensis 2019, 7, 3–18. [Google Scholar]
- Rehman, M.; Ullah, S.; Bao, Y.; Wang, B.; Peng, D.; Liu, L. Light-emitting diodes: Whether an efficient source of light for indoor plants? Environ. Sci. Pollut. Res. 2017, 24, 24743–24752. [Google Scholar] [CrossRef]
- Flores, M.; Urrestarazu, M.; Amors, A.; Escalona, V. High intensity and red enriched LED lights increased growth of lettuce and endive. Ital. J. Agron. 2022, 25, 354–562. [Google Scholar] [CrossRef]
- Fan, X.; Zang, J.; Xu ZGuo, S.; Jiao, X.; Liu, Y.; Gao, Y. Effects of different light quality on growth, chlorophyll concentration and chlorophyll biosynthesis precursors of non-heading Chinese cabbage (Brassica campestris L.). Acta Physiol. Plant. 2013, 35, 2721–2726. [Google Scholar] [CrossRef]
- Nguyen, P.; Ngoc, T.; Huyen, T.; Quang, T.; Phi, B.; Il Seop, K.; Dong, J. Growth and Quality of Hydroponic Cultivated Spinach (Spinacia oleracea L.) Affected by the Light Intensity of Red and Blue LEDs. Sains Malays. 2022, 51, 473–483. [Google Scholar] [CrossRef]
- Najera, C.; Urrestarazu, M. Effect of the intensity and spectral quality of LED light on yield and nitrate accumulation in vegetables. HortScience 2019, 54, 1745–1750. [Google Scholar] [CrossRef]
- Harun, A.N.; Ahmad, R.; Mohamed, N. Plant growth optimization using variable intensity and Far Red LED treatment in indoor farming. In Proceedings of the International Conference on Smart Sensors and Application (ICSSA), Kuala Lumpur, Malaysia, 26–28 May 2015; pp. 92–97. [Google Scholar]
- Pennisi, G.; Pistillo, A.; Orsini, F.; Cellini, A.; Spinelli, F.; Nicola, S.; Marcelis, L.F. Optimal light intensity for sustainable water and energy use in indoor cultivation of lettuce and basil under red and blue LEDs. Sci. Hortic. 2020, 272, 109508. [Google Scholar] [CrossRef]
- Marcos, L.; Mai, K.V. Light spectra optimization in indoor plant growth for internet of things. In Proceedings of the IEEE International IOT, Electronics and Mechatronics Conference (IEMTRONICS), Vancouver, BC, Canada, 9–12 September 2020; pp. 1–6. [Google Scholar]
- Terashima, I.; Fujita, T.; Inoue, T.; Chow WSOguchi, R. Green light drives leaf photosynthesis more efficiently than red light in strong white light: Revisiting the enigmatic question of why leaves are green. Plant Cell Physiol. 2009, 50, 684–697. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Zheng, J.M.; Little, C.; Khosla, S. LED inter-lighting in year-round greenhouse mini cucumber production. Acta Hortic. 2012, 956, 335–340. [Google Scholar] [CrossRef]
- Sabzalian, M.R.; Heydarizadeh, P.; Zahedi, M.; Boroomand, A.; Agharokh, M.; Sahba, M.R.; Schoefs, B. High performance of vegetables, flowers, and medicinal plants in a red-blue LED incubator for indoor plant production. Agron. Sustain. Dev. 2014, 34, 879–886. [Google Scholar] [CrossRef]
- Choi, H.G.; Moon, B.Y.; Kang, N.J. Effects of LED light on the production of strawberry during cultivation in a plastic greenhouse and in a growth chamber. Sci Hortic. 2015, 189, 22–31. [Google Scholar] [CrossRef]
- Bartucca, M.L.; Guiducci, M.; Falcinelli, B.; Del Buono, D.; Benincasa, P. Blue:Red LED light proportion affects vegetative parameters, pigment content, and oxidative status of einkorn (Triticum monococcum L. ssp. monococcum) wheatgrass. J. Agric. Food Chem. 2020, 68, 8757–8763. [Google Scholar] [CrossRef] [PubMed]
- Tosti, G.; Benincasa, P.; Cortona, R.; Falcinelli, B.; Farneselli, M.; Guiducci, M.; Onofri, A.; Pannacci, E.; Tei, F.; Giulietti, M. Growing lettuce under multispectral light-emitting diodes lamps with adjustable light intensity. Ital. J. Agron. 2018, 13, 57–62. [Google Scholar] [CrossRef]
- Yao, X.; Liu, X.; Xu, Z.; Jiao, X. Effects of light intensity on leaf microstructure and growth of rape seedlings cultivated under a combination of red and blue LEDs. J. Integr. Agric. 2017, 16, 97–105. [Google Scholar] [CrossRef]
- Koleva, I.I.; Van Beek, T.A.; Linssen, J.P.H.; De Groot, A.; Evstatieva, L.N. Screening of plant extracts for antioxidant activity: A comparative study on three testing methods. Phytochem. Anal. 2002, 13, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Frutos-Totosa, A.; Hernández-Adasme, C.; Martínez, V.; Mestre, T.; Díaz-Mula, H.M.; Botella, M.A.; Flores, P.; Martínez-Moreno, A. Light spectrum effects on rocket and lamb’s lettuce cultivated in a vertical indoor farming system. Sci. Hortic. 2023, 321, 112221. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C.; Knapp, M. How to correctly determine the different chlorophyll fluorescence parameters and the chlorophyll fluorescence decrease ratio R Fd of leaves with the PAM fluorometer. Photosynthetica 2005, 43, 379–393. [Google Scholar] [CrossRef]
- Moing, A.; Maucourt, M.; Renaud, C.; Gaudillère, M.; Brouquisse, R.; Lebouteiller, B.; Gousset-Dupont, A.; Vidal, J.; Granot, D.; Denoyes-Rothan, B.; et al. Quantitative metabolic profiling by 1-dimensional 1H-NMR analyses: Application to plant genetics and functional genomics. Funct. Plant Biol. 2004, 31, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, K.; Oka, N.; Shinano, T.; Osaki, M.; Takebe, M. Differences in the Metabolite Profiles of Spinach (Spinacia oleracea L.) Leaf in Different Concentrations of Nitrate in the Culture Solution. Plant Cell Physiol. 2008, 49, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Lea, P.J.; Sodek, L.; Parry, M.A.; Shewry, P.R.; Halford, N.G. Asparagine in Plants. Ann. Appl. Biol. 2007, 150, 1–26. [Google Scholar] [CrossRef]
- Viršilė, A.; Brazaitytė, A.; Vaštakaitė-Kairienė, V.; Jankauskienė, J.; Miliauskienė, J.; Samuolienė, G.; Duchovskis, P. Nitrate, nitrite, protein, amino acid contents, and photosynthetic and growth characteristics of tatsoi cultivated under various photon flux densities and spectral light compositions. Sci. Hortic. 2019, 258, 108781. [Google Scholar] [CrossRef]
- Fan, X.X.; Xue, F.; Song, B.; Chen, L.Z.; Xu, G.; Xu, H. Effects of Blue and Red Light on Growth and Nitrate Metabolism in Pakchoi. Open Chem. 2019, 17, 456–464. [Google Scholar] [CrossRef]
- Li, J.; Wu, T.; Huang, K.; Liu, Y.; Liu, M.; Wang, J. Effect of LED Spectrum on the Quality and Nitrogen Metabolism of Lettuce Under Recycled Hydroponics. Front. Plant Sci. 2021, 12, 678197. [Google Scholar] [CrossRef] [PubMed]
- Huché-Thélir, L.; Laurent, C.; José Le, G.; Philippe, M.; Soulaiman, S.; Nathalie, L. Light Signaling and Plant Responses to Blue and UV Radiations-Perspectives for Applications in Horticulture. Environ. Exp. Bot. 2016, 121, 22–38. [Google Scholar] [CrossRef]
- Voutsinos, O.; Maria, M.; Georgia, N.; Georgios, L.; Dimitrios, S. Comparative Assessment of Hydroponic Lettuce Production Either under Artificial Lighting, or in a Mediterranean Greenhouse during Wintertime. Agriculture 2021, 11, 503. [Google Scholar] [CrossRef]
- Zou, T.; Huang, C.; Wu, P.; Ge, L.; Xu, Y. Optimization of Artificial Light for Spinach Growth in Plant Factory Based on Orthogonal Test. Plants 2020, 9, 490. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.E.; Teo, Z.W.N.; Shen, L.; Yu, H. Seeing the Lights for Leafy Greens in Indoor Vertical Farming. Trends Food Sci. Technol. 2020, 106, 48–63. [Google Scholar] [CrossRef]
- Schenkels, L.; Saeys, W.; Lauwers, A.; Maurice, P. Green light induces shade avoidance to alter plant morphology and increases biomass production in Ocimum basilicum L. Sci. Hortic. 2020, 261, 109002. [Google Scholar] [CrossRef]
- Grygoray, E.E.; Tabalenkova, G.N.; Dalke, I.V.; Golovko, T.K. Mineral nutrition and productivity of the greenhouse cucumber crop depending on lighting. Agrokhimiya 2015, 4, 74–79. [Google Scholar]
- Joniyas, A.; Surif, M.; Dehgahi, R. Effect of Nutrient and Light Intensity on Nutrient Uptakes of Gracilaria manilaensis. Int. J. Sci. Res. Environ. Sci. 2016, 4, 173–185. [Google Scholar] [CrossRef]
- Xu, J.; Guo, Z.; Jiang, X.; Ahammed, G.J.; Zhou, Y. Light regulation of horticultural crop nutrient uptake and utilization. Hortic. Plant J. 2021, 7, 367–379. [Google Scholar] [CrossRef]
- Demotes-Mainard, S.; Péron, T.; Corot, A.; Bertheloot, J.; Le Gourrierec, J.; Pelleschi-Travier, S.; Sakr, S. Plant responses to red and far-red lights, applications in horticulture. Environ. Exp. Bot. 2016, 121, 4–21. [Google Scholar] [CrossRef]
- Signore, A.; Bell, L.; Santamaria, P.; Wagstaff, C.; Van Labeke, M.C. Red Light Is Effective in Reducing Nitrate Concentration in Rocket by Increasing Nitrate Reductase Activity, and Contributes to Increased Total glucosinolate content. Front. Plant Sci. 2020, 11, 532456. [Google Scholar] [CrossRef]
- Bantis, F.; Fotelli, M.; Ilić, Z.S.; Koukounaras, A. Physiological and Phytochemical Responses of Spinach Baby Leaves Grown in a PFAL System with Leds and Saline Nutrient Solution. Agriculture 2020, 10, 574. [Google Scholar] [CrossRef]
- Dos Santos, C.M.; Verissimo, V.; de Lins Wanderley Filho, H.C.; Ferreira, V.M.; da Silva Cavalcante, P.G.; Rolim, E.V.; Endres, L. Seasonal variations of photosynthesis, gas exchange, quantum efficiency of photosystem II and biochemical responses of Jatropha curcas L. grown in semi-humid and semi-arid areas subject to water stress. Ind. Crops Prod. 2013, 41, 203–213. [Google Scholar] [CrossRef]
- Di Mola, I.; Cozzolino, E.; Ottaiano, L.; Nocerino, S.; Rouphael, Y.; Colla, G.; Mori, M. Nitrogen use and uptake efficiency and crop performance of baby spinach (Spinacia oleracea L.) and Lamb’s Lettuce (Valerianella locusta L.) grown under variable sub-optimal N regimes combined with plant-based biostimulant application. Agronomy 2020, 10, 278. [Google Scholar] [CrossRef]
- Zhen, S.; Haidekker, M.; van Iersel, M.W. Far-red light enhances photochemical efficiency in a wavelength-dependent manner. Physiol. Plant. 2019, 167, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Lisiewska, Z.; Kmiecik, W.; Gębczyński, P.; Sobczyńska, L. Amino Acid Profile of Raw and As-Eaten Products of Spinach (Spinacia oleracea L.). Food Chem. 2011, 126, 460–465. [Google Scholar] [CrossRef]
- Carrillo, P.; Giuseppe, C.; Giovanna, M.F.; Emilia, D.; Christophe, E.N.; Maria, G.; Antonio, P.; Eugenio, C.; Mauro, M.; Hélène, R.; et al. Morphological and Physiological Responses Induced by Protein Hydrolysate-Based Biostimulant and Nitrogen Rates in Greenhouse Spinach. Agronomy 2019, 9, 450. [Google Scholar] [CrossRef]
- Nguyen, P.D.; Cheol, J.; Thi Thanh, H.; Quang Thach, N.; Il Seop, K.; Thi Lan Huong, H.; Ngoc, T. Influence of Green Light Added with Red and Blue Leds on the Growth, Leaf Microstructure and Quality of Spinach (Spinacia oleracea L.). Agronomy 2021, 11, 1724. [Google Scholar] [CrossRef]
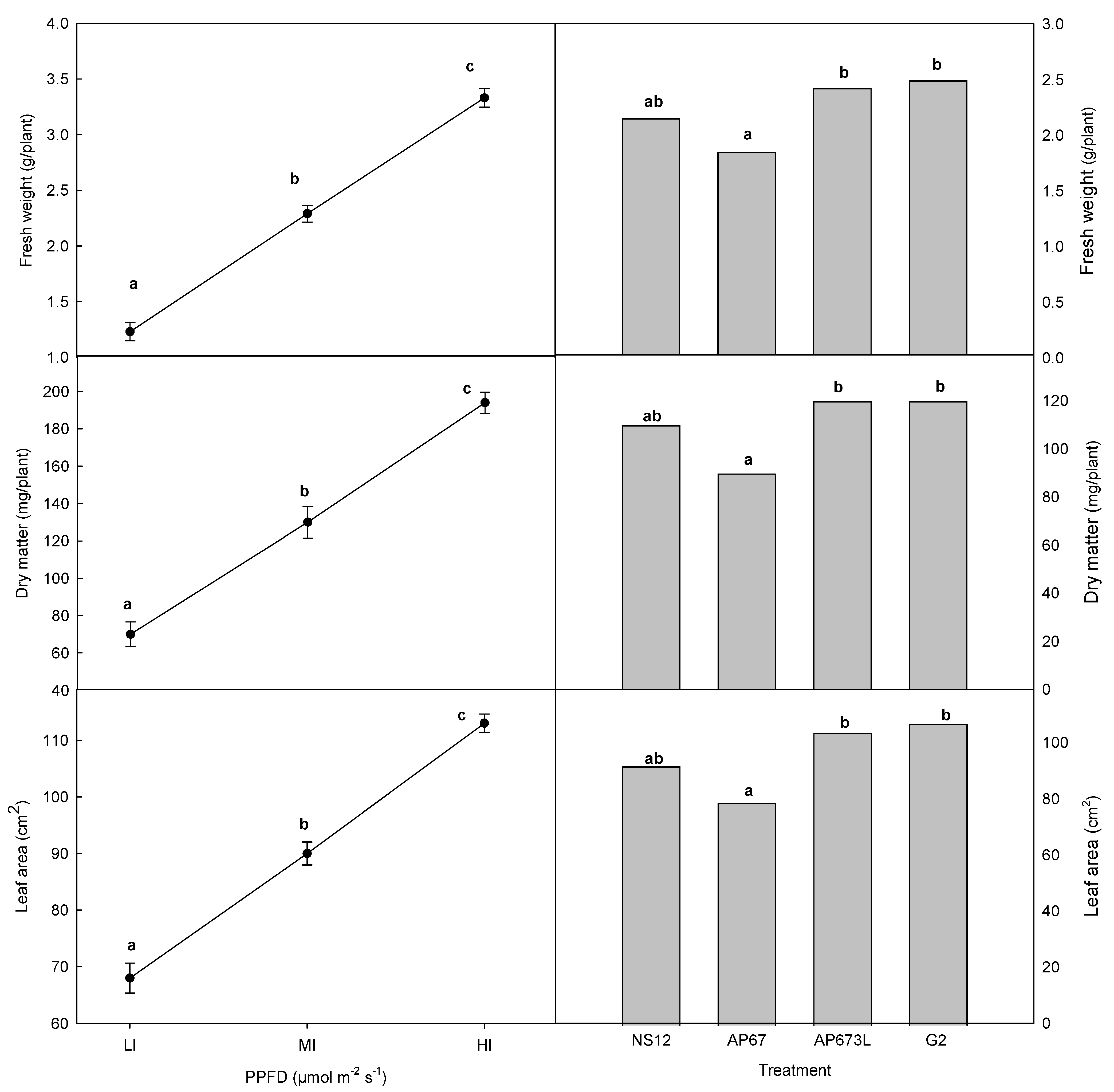
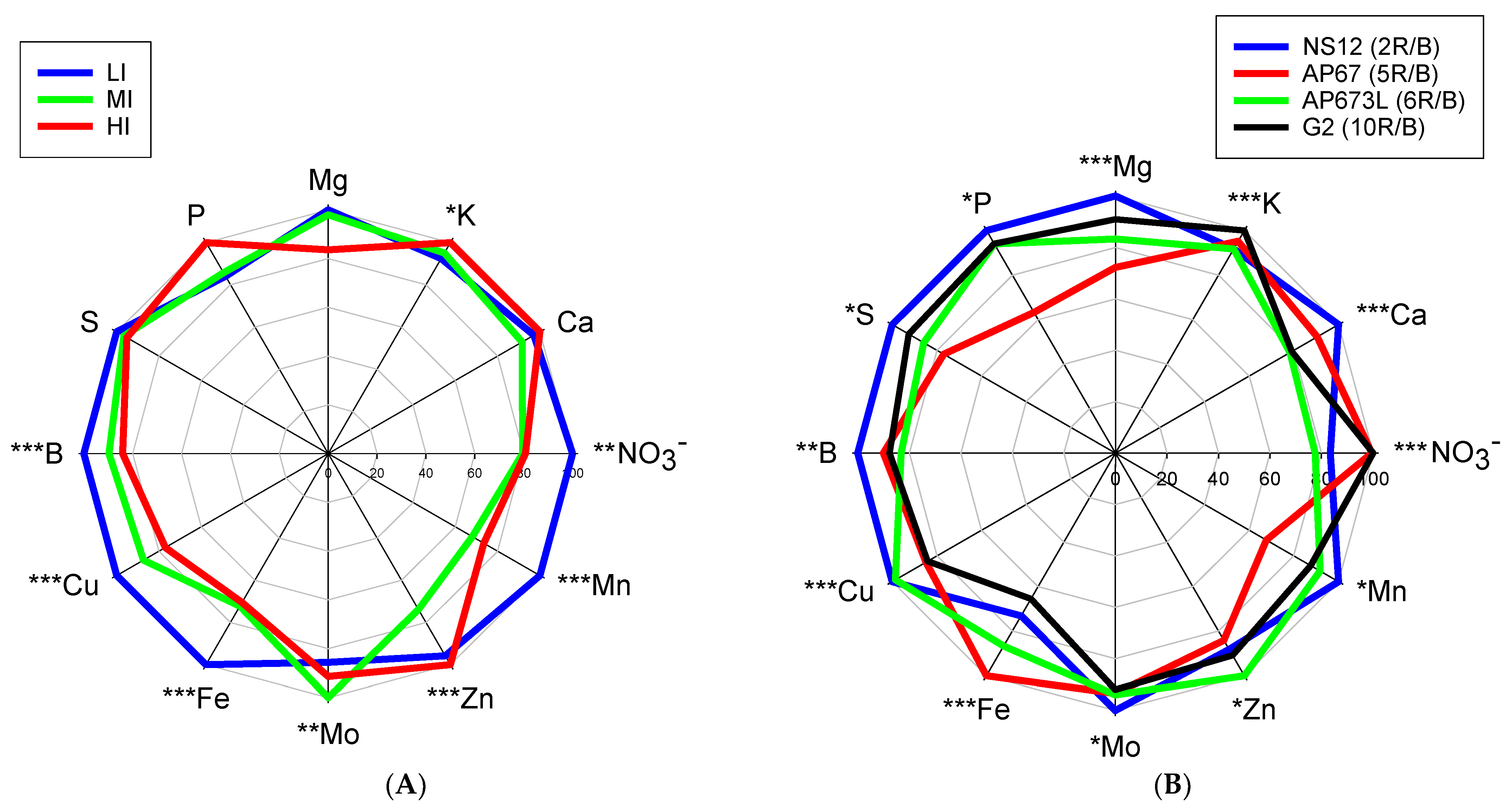
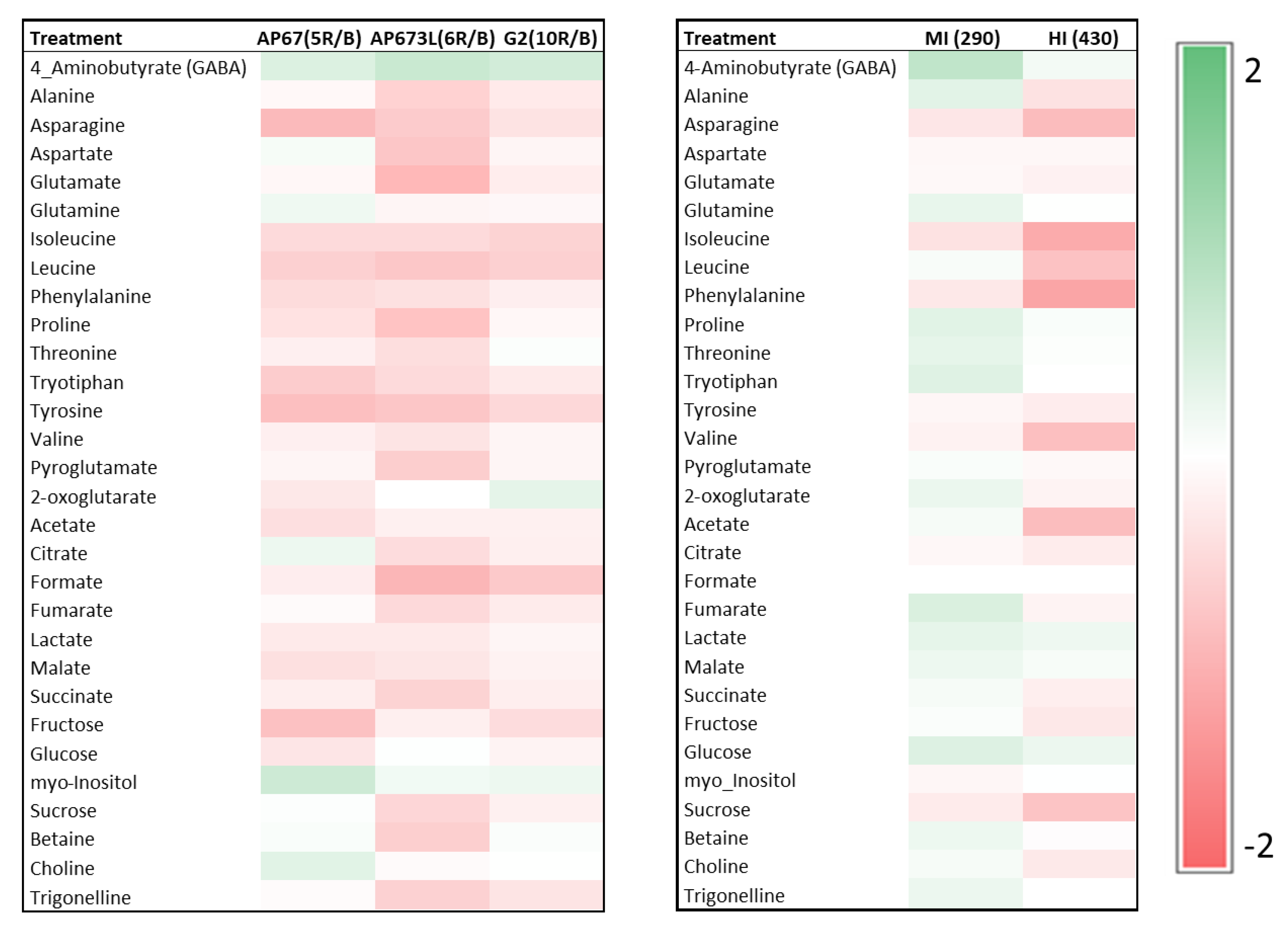

| Treatment | Intensity PPFD (µmol m−2 s−1) | LED Grow Lights | UV <400 | Blue 400–500 | Green 500–600 | Red 600–700 | Far-Red 700–800 | |
|---|---|---|---|---|---|---|---|---|
| Light Intensity | LI | 150 | AP673L (%) | 0 | 10 | 19 | 63 | 8 |
| MI | 290 | AP673L (%) | 0 | 10 | 19 | 63 | 8 | |
| HI | 430 | AP673L (%) | 0 | 10 | 19 | 63 | 8 | |
| Light Quality | 2 R/B | 150 | NS12 (%) | 1 | 20 | 36 | 38 | 5 |
| 5 R/B | 150 | AP67 (%) | 0 | 12 | 16 | 56 | 16 | |
| 6 R/B | 150 | AP673L (%) | 0 | 10 | 19 | 63 | 8 | |
| 10 R/B | 150 | G2 (%) | 0 | 7 | 2 | 70 | 21 |
| Light Treatment | ФPSII | Fv′/Fm′ | qP | Chlorophyll (SPAD) |
|---|---|---|---|---|
| Intensity | ||||
| LI | 0.64 a | 0.77 | 0.83 a | 15.4 a |
| MI | 0.71 b | 0.79 | 0.90 b | 15.9 a |
| HI | 0.71 b | 0.77 | 0.91 b | 22.5 b |
| ANOVA | * | ns | *** | *** |
| Quality | ||||
| NS12 (2 R/B) | 0.59 b | 0.73 b | 0.80 | 9.70 |
| AP67 (5 R/B) | 0.50 a | 0.69 a | 0.72 | 9.67 |
| AP673L (6 R/B) | 0.60 b | 0.74 b | 0.80 | 10.83 |
| G2 (10 R/B) | 0.48 a | 0.69 a | 0.69 | 9.81 |
| ANOVA | * | * | ns | ns |
| Treatment | Glutamate | Glutamine | Aspartate | Asparagine | Alanine | 4_Aminobutyrate (GABA) | Phenylalanine | Tryptophan |
|---|---|---|---|---|---|---|---|---|
| Intensity | ||||||||
| LI | 2.98 | 1.18 a | 1.37 | 0.35 c | 0.30 b | 0.47 a | 0.16 c | 0.06 a |
| MI | 2.81 | 1.45 b | 1.28 | 0.28 b | 0.39 c | 0.74 b | 0.13 b | 0.08 b |
| HI | 2.64 | 1.20 a | 1.28 | 0.19 a | 0.23 a | 0.42 a | 0.07 a | 0.05 a |
| ANOVA | ns | * | ns | *** | *** | *** | *** | * |
| Quality | ||||||||
| NS12 (2 R/B) | 5.36 b | 1.43 | 2.47 b | 0.98 c | 0.56 | 0.24 a | 0.29 b | 0.35 c |
| AP67 (5 R/B) | 5.15 b | 1.65 | 2.27 b | 0.52 a | 0.54 | 0.33 bc | 0.21 a | 0.22 a |
| AP673L (6 R/B) | 3.66 a | 1.31 | 1.35 a | 0.61 ab | 0.44 | 0.41 c | 0.22 a | 0.25 ab |
| G2 (10 R/B) | 4.87 ab | 1.34 | 2.07 ab | 0.76 b | 0.50 | 0.36 bc | 0.25 ab | 0.29 b |
| ANOVA | ** | ns | *** | *** | ns | * | ** | *** |
| Organic Acids | Non-Structural Carbohydrates | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Malate | Citrate | Ascorbate | Succinate | Acetate | Fumarate | 2-Oxoglutarate | Sucrose | Betaine | Glucose | Fructose | Myo-Inositol | Total Carbohydrates |
| Intensity | |||||||||||||
| LI | 4.54 | 2.94 | 1.30 b | 0.56 ab | 0.11 b | 0.10 a | 0.39 a | 9.69 c | 4.88 a | 3.16 a | 2.71 b | 1.03 | 22.5 b |
| MI | 5.34 | 3.16 | 1.44 b | 0.61 b | 0.12 b | 0.14 b | 0.47 b | 8.08 b | 5.74 b | 4.28 b | 2.59 b | 0.95 | 22.8 b |
| HI | 4.90 | 2.66 | 0.65 a | 0.48 a | 0.06 a | 0.09 a | 0.35 a | 5.63 a | 4.79 a | 3.77 ab | 2.10 a | 1.04 | 18.2 a |
| ANOVA | ns | ns | *** | * | *** | *** | *** | *** | ** | * | * | ns | ** |
| Quality | |||||||||||||
| NS12 (2 R/B) | 4.37 | 3.29 c | 0.21 a | 0.57 b | 0.08 | 0.24 b | 0.37 a | 10.85 b | 8.17 b | 5.72 | 3.87 c | 0.83 a | 29.9 b |
| AP67 (5 R/B) | 3.29 | 2.80 bc | 0.19 a | 0.49 ab | 0.06 | 0.23 b | 0.30 a | 10.56 b | 8.20 b | 4.53 | 2.19 a | 1.30 b | 28.6 b |
| AP673L (6 R/B) | 3.48 | 2.04 a | 0.58 b | 0.38 a | 0.07 | 0.17 a | 0.37 a | 7.29 a | 4.99 a | 5.83 | 3.36 bc | 0.94 a | 23.5a |
| G2 (10 R/B) | 3.89 | 2.42 ab | 0.26 a | 0.49 ab | 0.07 | 0.20 ab | 0.47 b | 9.25 b | 7.77 b | 5.12 | 2.82 ab | 0.98 a | 27.6 b |
| ANOVA | ns | *** | ** | * | ns | ** | *** | *** | *** | ns | *** | ** | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Moreno, A.; Frutos-Tortosa, A.; Diaz-Mula, H.; Mestre, T.C.; Martínez, V. Effect of the Intensity and Spectral Quality of LED Light on Growth and Quality of Spinach Indoors. Horticulturae 2024, 10, 411. https://doi.org/10.3390/horticulturae10040411
Martínez-Moreno A, Frutos-Tortosa A, Diaz-Mula H, Mestre TC, Martínez V. Effect of the Intensity and Spectral Quality of LED Light on Growth and Quality of Spinach Indoors. Horticulturae. 2024; 10(4):411. https://doi.org/10.3390/horticulturae10040411
Chicago/Turabian StyleMartínez-Moreno, Alejandro, Antonio Frutos-Tortosa, Huertas Diaz-Mula, Teresa Carmen Mestre, and Vicente Martínez. 2024. "Effect of the Intensity and Spectral Quality of LED Light on Growth and Quality of Spinach Indoors" Horticulturae 10, no. 4: 411. https://doi.org/10.3390/horticulturae10040411
APA StyleMartínez-Moreno, A., Frutos-Tortosa, A., Diaz-Mula, H., Mestre, T. C., & Martínez, V. (2024). Effect of the Intensity and Spectral Quality of LED Light on Growth and Quality of Spinach Indoors. Horticulturae, 10(4), 411. https://doi.org/10.3390/horticulturae10040411






