Abstract
The induction of variation in chrysanthemums using gamma radiation under in vitro conditions is an effective technique in ornamental plants. The purpose of this study is to obtain new mutants by isolating desirable properties from the three-colored single chrysanthemum mutant using in vitro cultures. Bud explants were cultured four times, the plantlets were acclimatized, and 520 plants were planted in outdoor conditions. Observations of the mutants were collected during flowering time, and 97 of the mutants were compared to the control group. Plants with pink, white, and chimeric flowers were obtained. Mutant plants with white flowers constituted the majority of the population followed by plants with variegated flower colors. The population is divided into six clusters, based on the plant height, plant diameter, flower number, flower diameter, number of flower colors, ray flowers’ number, leaves’ number, stem weight, and lengths and widths of leaves. The population decreased in plant height, flower number, and stem weight, while other features increased compared to the control group. Thus, a new population with similar characteristics to the parent plant was obtained from a single mutant. Chrysanthemum plants exposed to mutagens showed major changes in flower parts as well as other parts of the plant.
1. Introduction
Chrysanthemums originate from China and are considered one of the most significant cut flowers in the world [1,2]. In addition to their ornamental uses, chrysanthemum flowers offer nutritional and medicinal value due to their high antioxidant content [3,4,5,6]. Chrysanthemum morifolium Ramat (Dendranthema × grandiflora Tzvelv = Chrysanthemum × grandiflorum Ramat) is the most commercially significant ornamental species with most cultivars being autohexaploid and self-incompatible, which presents challenges for modern breeding [7,8,9].
Individuals in the genus Chrysanthemum have a wide range of flowers which are used in floriculture. They have a unique capitulum that consists of disc and ray florets, and clear distinctions can be observed between the two florets. The visual color of ray flowers is referred to as flower color and is a crucial feature of these plants, and they are available in an abundance of color and form [10,11,12,13,14].
Improving and innovating chrysanthemums by modifying their ornamental attributes such as floral color, shape, plant type, flowering time, and vase life, while also enhancing their ability to tolerate biotic and abiotic stress, is the main purpose of chrysanthemum breeding [15]. Breeders have placed a strong emphasis on manipulating floral color with a wide range of colors in the ray florets, as it is a major factor that influences customer selection [16,17]. Older colors, including yellow, pink, and white, are attributed to the presence of carotenoids and anthocyanins or the absence of these pigments. However, modern flowers have been bred to display a wider range of colors such as purplish red, orange, scarlet, and deep red by increasing the pigment content or by combining different pigments [18,19,20].
Mutation breeding in plants has proven to be a successful breeding method as mutants can also be produced directly [21,22,23]. Using novel breeding methods such as chimera management and in vitro mutation results in targeted modifications which have enhanced and changed the procedures as needed [24]. Numerous new and promising plant species, including chrysanthemums, have benefited from mutation procedures using ionizing radiation and other mutagens [25]. Chrysanthemums are the plants with the most mutant variations developed by mutation breeding among vegetatively propagated crops [26]. Chemical agents, gamma and X-rays, and other radiation sources are used as mutagens in studies carried out in chrysanthemum mutation breeding [8]. As a result of increased exposure to irradiation, more mutations in flower color have occurred in chrysanthemums, while the vegetative growth of all cultivars has only been minimally altered [27,28]. Although polyploids with significant genetic heterogeneity make up the majority of cultivated chrysanthemum varieties, mutants with related flower shapes, floral sizes, and colors are commonly observed. Radiation can easily activate associated floral colors in chimeric tissues and isolate them using in vitro techniques [29].
Mutation breeding is useful for developing new ornamental plants with visible changes in color, shape, and size. By exposure to a mutagen dose, plant height variations and significant variations in floral parts and leaves were observed on chrysanthemums. Additionally, gamma radiation is an effective mutagen when applied to in vitro bud explants for creating new types. The results of in vitro treatments with mutagenic gamma radiation show that the method of induced homogeneous mutation is an effective approach for chrysanthemum breeding. Selected mutants can be propagated vegetatively to obtain new plants with desirable traits that will be well received. Numerous experiments using tissue culture techniques for mutation breeding resulted in similar results, and diversity has been achieved with novel genotypes possessing the desired characteristics [30].
The present study aimed to create a new population from a single mutant plant with different flower types and three different colors. These three flower colors were isolated from the single mutant using tissue culture methods. The mutant had more ray flowers and was obtained by irradiating the in vitro bud explants of a white chrysanthemum variety with gamma rays. This aimed to isolate distinct colors from the single mutant and create a new population from the clones of this parent mutant plant. With this goal, rapid propagation techniques of tissue culturing were used to create mutant plants from this single mutant. In this way, morphological observations were made and compared to the control plants focused on the possibilities of obtaining new candidate varieties by selecting individuals with superior characteristics among the mutant population.
2. Materials and Methods
Materials from the single mutant plant of Chrysanthemum morifolium (Ramat.) (Dendranthema × grandiflora Tzelev.)‚ variety ‘Bacardi’, were propagated from the bud explants by tissue culture (Figure 1). The in vitro plantlets of the control plant were irradiated with 20 Gy (Gray) of cobalt 60 (Co60) gamma source at the Energy, Nuclear and Mining Research Association, Nuclear Energy Research Institute. Then, they were subcultured four times till the M1V4 (Mutation 1: Vegetation 4) growing period and then planted in outdoor conditions after acclimatization. This part was the study’s initial section, which was published in a different journal [30].
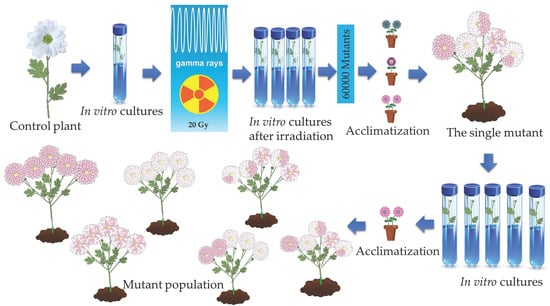
Figure 1.
Summary of the study.
The research was conducted from 2022 to 2023. One of the mutants among the 60,000 mutants showed an increase in the number of ray flower rows (daisy-eyed double flower head type) and had pink, white, and pink and white variegated (multicolored chimeric) flowers (Figure 2). This mutant was selected and propagated by tissue culture. After four subcultures were complete, rooted plantlets were transferred to outdoor conditions by acclimatization and 520 mutants were planted in an open field (Figure 1). The 97 mutants with desirable features that bloomed earlier than others in the mutant population were marked in November 2022. Morphological observations of the mutants confirming their stable status as well as control plants were recorded in November 2023 when they were in full bloom. Plant height, plant diameter, flower number, number of ray flowers, number of flower colors, number of leaves, stem weight, and lengths and widths of leaves are among the attributes that were observed.

Figure 2.
The single mutant with daisy-eyed double flower heads. (a,b) General appearance of plant; (c) white-colored flower; (d) variegated flower; (e) pink flowers of the single mutant (bars: 1 cm).
2.1. Tissue Culture
To prepare explants, the lateral buds were washed with running tap water with a few drops of dishwashing detergent for 60 min. The explants were surface sterilized in 70 percent (v/v) ethanol (EtOH) for one minute after three rounds of rinsing with distilled water. Next, three rinses were performed with a 25% hydrogen peroxide (H2O2) solution for the next 10 min, followed by autoclaved distilled water for five minutes each [30].
2.2. Planting In Vitro Explants on Nutrient Medium
The nodes of the sterilized shoot explants were planted on MS (Murashige and Skoog) [31] with 3% sucrose, 0.7% agar, and 1 mg/L BAP (benzylaminopurine) in glass tubes. The culture media with the pH adjusted to 5.8 was autoclaved at 121 °C for 15 min. The cultured explants were grown in the climate room under a 16 h photoperiod (30 mol/m2 s1). The nodes of the shoot explants were subcultured after one month [30].
2.3. Rooting and Acclimatization Stages
After four subcultures, the shoots longer than 1 cm were transferred to the plant growth regulator-free MS medium for rooting. The roots appeared 4 weeks later, and the rooted 3 cm long shoots were moved to the acclimatization stage. Rooted plantlets were removed from glass tubes and their roots were cleaned of the agar-based nutrient medium to acclimate them to the outside environment. The plants were transplanted into plastic vials containing a 3:1 mixture of peat and perlite placed in boxes and covered with transparent wrapping paper. The vials were kept for one week at 22 °C under cool white light with a 16 h photoperiod (30 mol/ m2 s1) in the climate room. Tiny holes were pierced daily on the transparent wrapping paper, as they hardened during the week. The plantlets that had hardened off were placed in the greenhouse for one week and then planted in soil in an open field in June 2022 [30].
2.4. Observations of Mutants
The observations of the mutants were collected by taking into account the plant height (cm; from the soil surface to the uppermost part of the plant), plant diameter (cm; the widest aboveground plant width) [32], number of leaves, leaf length (cm; the longest part of leaves), leaf width (cm; the widest part of leaves), number of flowers per plant, flower width (cm; the widest part of the flower head), number of flower colors each plant, number of ray florets, and stem weight in early November 2023 during anthesis. Somatic mutations of the flower color variegations chimeras were recorded as variegated or multicolored. The color variations from white to pink of ray florets were determined according to the Royal Horticultural Society (RHS) Color Chart cards [33].
2.5. Statistical Analysis
Morphological measurements were performed on 97 mutants and a control group (mean of 10 control plants). Based on these measurements, changing ratios and clusters were created according to the plant height, plant diameter, flower diameter, number of flowers, number of ray flowers, number of flower colors, flower color, number of leaves, leaf length, leaf width, and stem weight. Changing ratios of mutant genotypes according to the control group were calculated with Formula (1):
The genetic closeness in terms of morphological features was determined using the MINITAB 20 software program. A hierarchical cluster dendrogram was created to analyze the distance and similarity between mutant populations. The core algorithm was used to calculate the similarity matrix between the mutants.
3. Results
While the flower head type of the control plants was semi-double, the mutant’s flower head type was daisy-eyed double due to the increased number of ray flower rows. In the mutant group, there were six ray floret rows, compared to three in the control group, resulting in a change in flower head type. In addition to the change in flower head type, we also observed some changes in the variation in flower colors.
3.1. Color Distribution of the Mutant Population
The mutant population presented a distribution of pink, white, and pink and white variegated (multicolored) flowers despite maintaining the same type of flower heads. Ray floret color variations from white to pink were identified using the RHS Color Chart cards. While the pink color was 69A, the white one was NN155A [33]. The majority of the population consisted of white-colored mutant plants, followed by plants with variegated colored flowers. In some mutants, variegated flowers as well as pink or white flowers have been observed on the same plant. There was only one mutant plant in the population which had white, pink, and variegated flowers like the parent mutant plant (Figure 3). The number of flower colors on a plant varied from 1 to 3 and increased by 23% according to the control group (Figure 4).
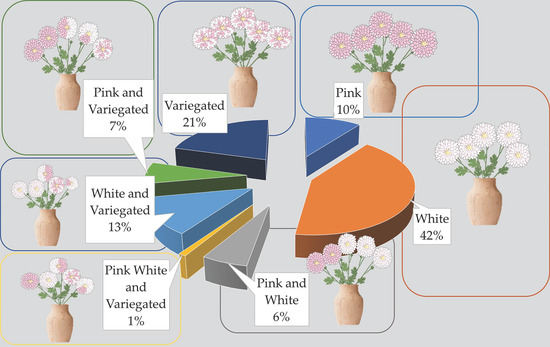
Figure 3.
Color distribution in flowers of mutant genotypes.
3.2. The Comparison between the Control Group and the Mutant Population
The population showed increasing and decreasing properties compared to the control plants in terms of plant height, plant diameter, flower diameter, number of flowers, number of ray flowers, number of flower colors, number of leaves, leaf length, leaf width, and stem weight. All characteristics of the mutant population increased except for the plant height, the number of flowers, and stem weight, which decreased compared to the control group (Figure 4).
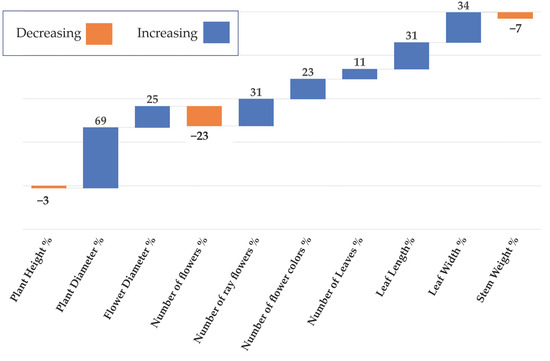

Figure 4.
Changing ratios of the population compared to the control plants.
According to the control plants, the plant height decreased by 3% (Figure 4). The tallest mutant plant was coded M31 mutant, and the shortest one was M38 (Figure 5). Plant diameter increased by 69% (Figure 4). The widest plant was M1 followed by the control group, and M32 was the narrowest plant in the population (Figure 6). Flower numbers differed from 5 to 40 flowers (Figure 7), and the flower number decreased by 23% compared to the control plants (Figure 4).
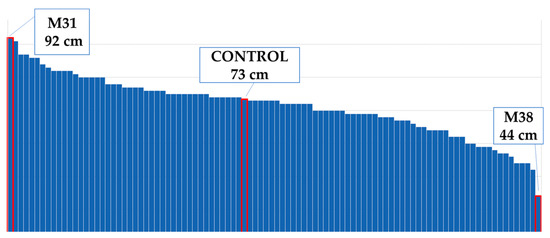
Figure 5.
Maximum and minimum plant heights of the population.
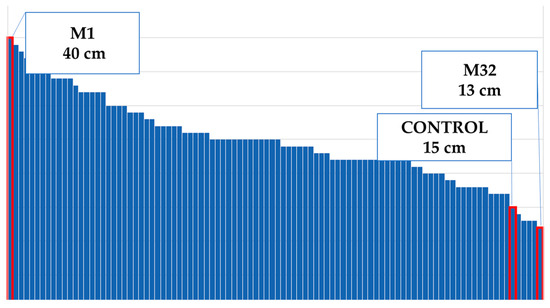
Figure 6.
Maximum and minimum plant diameters of the population.
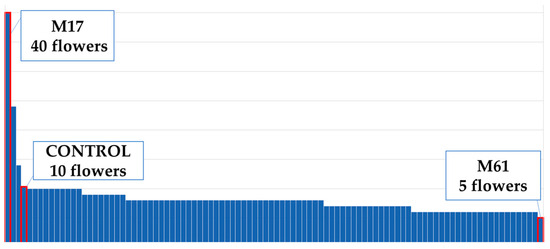
Figure 7.
Maximum and minimum flower numbers of the population.
Flower diameter increased by 25% compared to the control plants (Figure 4). The widest flower was observed on the M52 mutant; the control plants and the mutants M25–M41 had the same flower diameters (Figure 8). The M38 mutant formed the greatest amount of ray flowers, whereas the control group formed the fewest (Figure 9), and the number of ray florets increased by 31% compared to the control group (Figure 4). The number of leaves increased by 11% compared to the control group (Figure 4). M24 was the mutant with the maximum number of leaves, whereas M11 had the minimum leaf number (Figure 10). Leaf length increased by 31% compared to the control group (Figure 4). While the longest leaves were observed in the mutant plant coded M37, the mutant plant numbered M84 was the plant with the shortest leaves (Figure 11).
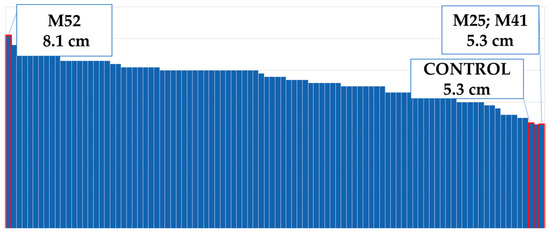
Figure 8.
Maximum and minimum flower diameters of the population.
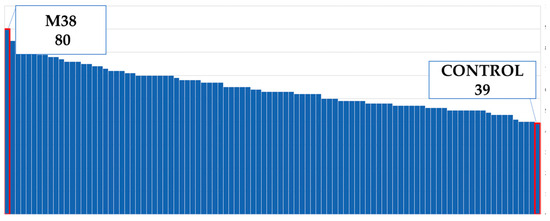
Figure 9.
Maximum and minimum ray flower numbers of the population.
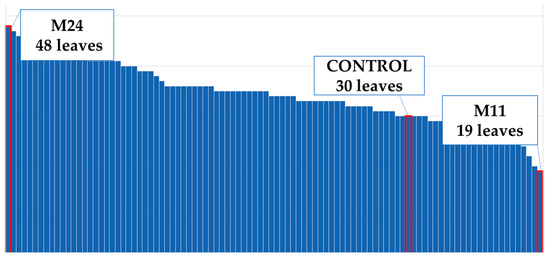
Figure 10.
Maximum and minimum leaf numbers of the population.
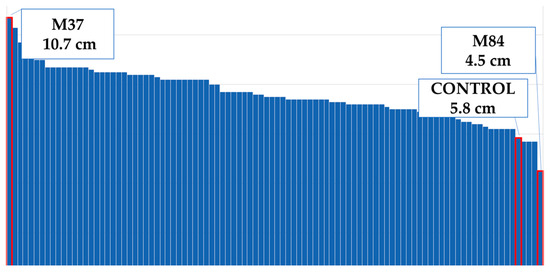
Figure 11.
Maximum and minimum leaf lengths of the population.
In comparison to the control group, the mutant plant coded M39 had the widest leaves, whereas the mutant plant coded M84 had narrowest leaves (Figure 12). The difference in leaf diameter was 34% according to the control group (Figure 4).
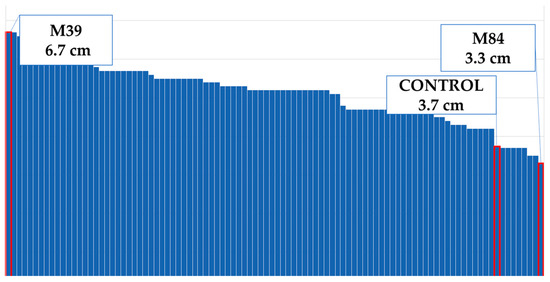
Figure 12.
Maximum and minimum leaf widths of the population.
The M74-coded mutant plant had the lightest stem weight, while the M17-coded mutant plant had a heavier stem than the control group (Figure 13). Compared to the mutant population in the control group, stem weights decreased by 7% (Figure 4).
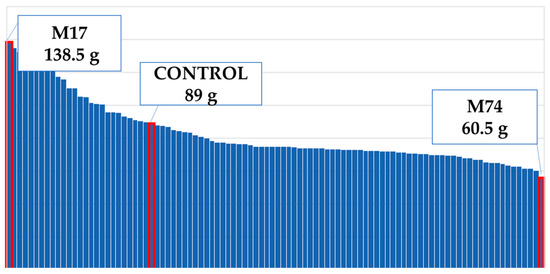
Figure 13.
Maximum and minimum stem weights of the population.
3.3. Cluster of the Mutant Population
Cluster analysis was performed on plant height, plant diameter, flower diameter, number of flowers, number of ray flowers, flower colors, number of flower colors, number of leaves, leaf length, leaf width, and stem weight. We obtained six groups; control plants and the only mutant with three colors (pink, white, and variegated) were located in the first group among 43 genotypes. There were 6 genotypes in the second group and 33 genotypes in the third group. The shortest plant (M38) was the only mutant in the fourth group, 11 genotypes were located in the fifth group, and the multi-flowered plants (M7 and M11) formed the sixth group. Both pink-flowered mutant genotypes and the pink and variegated flowered plants were observed in the first and third groups (Figure 14 and Figure 15). According to the correlation matrix, the similarity ratio was 97.2% between the closest mutants M53 and M72 (Table 1).
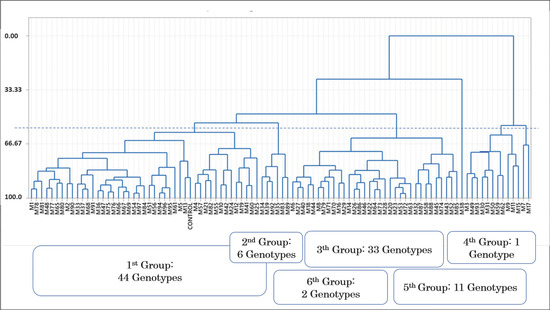
Figure 14.
Dendrogram of mutant genotypes.

Figure 15.
The mutant plants: (a) white-colored mutant—M38; (b) the mutant with pink, white, and variegated flowers; (c) white and pink–white variegated flowers (bars: 1 cm).

Table 1.
Distance between the genotypes according to the cluster groups.
In the principal component analysis, eigenvalues are shown in the scree plot. As can be seen from the figure, there are four components with eigenvalues greater than 1 (Figure 16).
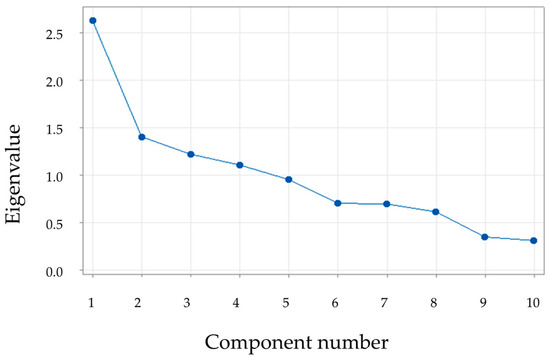
Figure 16.
Eigenvalues in the scree plot.
The first four principal components were determined to be responsible for 0.636 of the total variance in the principal component analysis (Table 2). The first group constitutes 0.263 of the total variation, and plant height, number of leaves, leaf length, and width were more effective in the formation of this group in a positive direction (Table 3).

Table 2.
Eigenvalue, variation percentage, and cumulative variation percentage values obtained as a result of principal component analysis in observed mutant genotypes.

Table 3.
PC component and factor coefficients obtained as a result of principal component analysis in the observed mutant genotypes.
In the second main component, while plant height, plant diameter, number of leaves, and stem weight had a positive effect, the number of flowers, number of flower colors, number of ray flowers, flower diameter, leaf length, and leaf width had a negative effect. Although the number of flowers, leaf length, and stem weight had a positive effect on the formation of the third group, the other traits had the opposite impact on it. The creation of the fourth group was positively impacted by flower diameter, the number of flower colors, the number of leaves, leaf length, and leaf width, whereas the other factors had the opposite effect (Table 3).
As a result of the analysis, plant height, leaf number, leaf length, and stem weight were determined as traits with high factor coefficients on the first PC component. It was determined that flower diameter and the number of ray flowers were important in the second PC component. The prominent features of the third PC component were the number of flowers and the number of flower colors. The features of the fourth PC component were the plant diameter and leaf width (Table 3).
4. Discussion
The market for ornamental plants is always in need of new commercial varieties and mutation breeding is frequently utilized to quickly achieve alterations that are significant to the market. Through this work, flowers from the semi-double white control group were used to create new daisy-eyed double white and pink mutants with more ray flowers. By using tissue culture methods, the mother mutant’s various colored flowers were separated and propagated rapidly.
Studies on the effects of mutation breeding in chrysanthemums have revealed that the results provide comparable changes in plants. The calli of chrysanthemum varieties were exposed to cobalt-60 gamma rays to modify the morphology of the original varieties. Advantageous mutant lines were obtained with the lower stem, shorter growth process, smaller leaves, changes in flower color, and larger blooms [34]. In the present study, in addition to the color changes in flowers, an increase in flower diameter and decreases in plant height were observed, while leaf size increased compared to the control group.
It was indicated that when treated with gamma rays, differences were generated in the form and color of the flowers like the yellow–white chimera, the deeper color, the pink–white color, and the dark purple color. The flower variations were the changes in the flower head diameter, ray flower number, flowering time, flower type, and flower color [35]. Our study’s findings showed a rise in the diameter of the flower head and the number of ray flowers, and we also obtained pink and white chimeric flowers which were compatible with the findings of the mentioned study. While the transformation from white to yellow was observed more depending on the carotenoid content, the transformation from white to pink was also recorded due to anthocyanin formation. Additionally, it was discovered that plants exposed to varying levels of gamma radiation showed changes in flower color, form, and shape [36]. Comparably to this research, the present research’s wide spectrum of mutants with floral patterns was observed when we compared the control plants. We have seen distinct variations in plant height, plant width, leaf number, leaf size, number of flower colors, and flower head size. With the increase in the number of ray flowers, the flower form turned into a daisy-eyed double. Among other traits, the highest increase was recorded in plant diameter compared to control group plants.
However, other types of mutagens, such as microwave radiation or synchrotron light irradiation, may also be helpful for the chrysanthemum variants. After being exposed to microwave radiation (MW), the explants of the chrysanthemum variety showed longer stems with bigger flowers [8]. It was discovered that synchrotron light irradiation caused changes in mutants that displayed a range of color patterns, including a paler and darker shade of colors than the original flowers [37]. We attained similar results in the present study such as alterations in the plant height and flower characteristics of the plants. Even if the mutagen changes, distinct variations in the flowers and other characteristics of the plant can be used in the creation of novel types. The benefit of utilizing tissue culture methods on mutants is the ability to quickly and easily develop new plants with the desired characteristics.
It was stated that treatment of gamma irradiation caused a reduction in floral size and an increase in flower yield in small-sized plants [38]. In the present study, while plant height was shortened, plant diameter and flower sizes increased compared to the control group. When we look at the findings of a different study in which a white chrysanthemum variety was irradiated by gamma radiation, the irradiated plants differed from control plants in plant height, number of leaves, leaf length and width, number of flowers, and flower diameter. The first of the selected three mutants had tubular ray florets and the others both had yellow flowers, while one of them had spoon ray florets and the other one had flat-shaped ray florets [39]. In this study, based on the control group, changes were detected in the previously mentioned characteristics such as plant height, number of leaves, leaf length and width, number of flowers, and flower diameter. In contrast to the described study, the flowers in the present study displayed pink and white variegation instead of yellow, and the ray florets did not exhibit any changes in morphology.
Consistent with the findings of previous research, it was discovered that the flower colors of mutants exposed to gamma radiation differed from the original varieties. While the original variety had pink–orange flowers, the mutants were discovered to have entirely pink ray flowers. The second mutant had fewer yellow shade ray florets than the original pink–orange variety, while the third mutant had more yellow ray florets in the center of the flower than the original pink–yellow variety. The fourth mutant had a mutation in flower morphology that resulted in multiple ray florets [40]. In addition to modifications in other parts of the mutants, we also noticed differences in color and ray floret number between the mutant group and the control group. Further support for future research is provided by the fact that the control and mutant plants produced results that were equivalent in terms of flower color, plant heights, flower head size, number of ray florets, number of leaves, leaf size, and stem weight.
These kinds of variances can be used to create distinctive and desirable properties that will appeal to producers and customers both. The reason why height and number of flowers in plants and stem weight decreased while other traits were increased compared to the control group was due to the effect of applied gamma rays and somatic variations in tissue cultures. In addition, too many flowers and extreme flower height values that are too short or too tall are already undesirable in cut flower cultivation. Considering that up to five stems are placed in bouquets, developing more flowering plants is not suitable for commercial use. On the other hand, short–tall and multi-flowered plants come to the fore in outdoor and potted use. It is an appropriate and desired feature to evaluate such plants in terms of their usage.
5. Conclusions
Our research points to great potential for expanding the understanding of breeding mutant chrysanthemums and creating new possibilities for the creation of new distinct genotypes with improved functional qualities as well as attractiveness. We can optimize the potential of these mutants and significantly impact the floriculture market by carefully choosing or breeding them in addition to continuing research on mutants and utilizing them as new varieties. These findings provide opportunities for further research into the fundamental mechanisms generating mutant populations that give rise to these variations. Results from in vitro treatments using mutagenic gamma radiation show that this method is effective in observing mutations in chrysanthemums.
Funding
This research was funded by the Republic of Türkiye Ministry of Agriculture and Forestry, General Directorate of Agricultural Research and Policies, Aegean Agricultural Research Institute.
Data Availability Statement
Data are contained within the article.
Acknowledgments
This study is a part of the project in collaborations with the Turkey Energy, Nuclear and Mining Research Association, Nuclear Energy Research Institute and Bademler Village Agricultural Development Cooperative. I would like to thank all my colleagues for their assistance with this paper. My special thanks to Yaprak Kantoglu, Burak Kunter, and Alameddin Bayav for their devoted help.
Conflicts of Interest
The author declares no conflicts of interest. The funders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Anderson, N.O. Chrysanthemum. In Flower Breeding and Genetics; Anderson, N.O., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 389–437. [Google Scholar] [CrossRef]
- Miler, N.; Jędrzejczyk, I.; Trafara, P.; Winiecki, J. Effect of high-energy ionizing radiation on the DNA content and genetic variation in chrysanthemum plants regenerated from irradiated ovaries. Acta Sci. Pol. Hortorum Cultus 2023, 22, 117–134. [Google Scholar] [CrossRef]
- Eisa, E.A.; Tilly-Mándy, A.; Honfi, P.; Shala, A.Y.; Gururani, M.A. Chrysanthemum: A comprehensive review on recent developments on in vitro regeneration. Biology 2022, 11, 1774. [Google Scholar] [CrossRef] [PubMed]
- Welch, C.W. Crysanthemums. In Breeding New Plants and Flowers; The Crowood Press: Wiltshire, UK, 2002; p. 144. [Google Scholar]
- Xu, Y.; Liao, B.; Ostevik, K.L.; Zhou, H.; Wang, F.; Wang, B.; Xia, H. The maternal donor of chrysanthemum cultivars revealed by comparative analysis of the chloroplast genome. Front. Plant Sci. 2022, 13, 923442. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.P.; Lin, K.H.; Shih, M.C.; Chen, C.L.; Lu, C.P. Optimization of aqueous extraction of antioxidants from chrysanthemum (C. morifolium Ramat and C. indicum L.) flowers and evaluation of their protection from glycoxidation damage on human αA-crystallins. Exp. Eye Res. 2023, 235, 109629. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Dongliang Chen, D.; Cheng, X.; Hua Liu, H.; Li, Y.; Huang, C. SSR analysis of genetic relationship and classification in chrysanthemum germplasm collection. Hortic. Plant J. 2018, 4, 73–82. [Google Scholar] [CrossRef]
- Miler, N.; Kulus, D. Microwave treatment can induce chrysanthemum phenotypic and genetic changes. Sci. Hortic. 2018, 227, 223–233. [Google Scholar] [CrossRef]
- Nakano, M.; Taniguchi, K.; Masuda, Y.; Kozuka, T.; Aruga, Y.; Han, J.; Motohara, K.; Nakata, M.; Sumitomo, K.; Hisamatsu, T.; et al. A Pure line derived from a self-compatible chrysanthemum seticuspe mutant as a model strain in the genus chrysanthemum. Plant Sci. 2019, 287, 110174. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wen, Z.; Meng, J.; Cheng, T.; Zhang, Q.; Sun, l. The genomics of ornamental plants: Current status and opportunities. Ornam. Plant Res. 2022, 2, 1–18. [Google Scholar] [CrossRef]
- Li, Y.; Yang, P.; Luo, Y.; Gao, B.; Sun, J.; Lu, W.; Liu, J.; Chen, P.; Zhang, Y.; Yu, L.L. Chemical compositions of chrysanthemum teas and their anti-inflammatory and antioxidant properties. Food Chem. 2019, 286, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Yang, L.; Wen, X.; Hong, Y.; Song, X.; Zhang, M.; Dai, S. Reference gene selection for RT-qPCR analysis of flower development in Chrysanthemum morifolium and Chrysanthemum lavandulifolium. Front. Plant Sci. 2016, 7, 186000. [Google Scholar] [CrossRef]
- Ryu, J.; Nam, B.; Kim, B.R.; Kim, S.H.; Jo, Y.D.; Ahn, J.W.; Kim, J.B.; Jin, C.H.; Han, A.R. Comparative Analysis of Phytochemical Composition of Gamma-Irradiated Mutant Cultivars of Chrysanthemum morifolium. Molecules 2019, 24, 3003. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Tian, Y.; Gao, K.; Li, J.; Li, Y.; Wang, J.; Deng, C.; Zhang, F.; Kong, K.; Fan, G.; et al. Genetic and QTL analysis of flower color and pigments in small-flowered chrysanthemum based on high-density genetic map. Ornam. Plant Res. 2023, 3, 17. [Google Scholar] [CrossRef]
- Mekapogu, M.; Kwon, O.K.; Song, H.Y.; Jung, J.A. Towards the improvement of ornamental attributes in chrysanthemum: Recent progress in biotechnological advances. Int. J. Mol. Sci. 2022, 23, 12284. [Google Scholar] [CrossRef] [PubMed]
- Din, A.; Qadri, Z.A.; Wani, M.A.; Rather, Z.A.; Iqbal, S.; Malik, S.A.; Hussain, P.R.; Rafiq, S.; Nazki, I.T. Congenial In Vitro γ-ray-Induced Mutagenesis Underlying the Diverse Array of Petal Colours in Chrysanthemum (Dendranthema grandiflorum kitam) cv. “Candid”. Biol. Life Sci. Forum 2021, 4, 21. [Google Scholar] [CrossRef]
- Mekapogu, M.; Vasamsetti, B.M.K.; Kwon, O.K.; Ahn, M.S.; Lim, S.H.; Jung, J.A. Anthocyanins in floral colors: Biosynthesis and regulation in chrysanthemum flowers. Int. J. Mol. Sci. 2020, 21, 6537. [Google Scholar] [CrossRef]
- Nakano, M.; Hirakawa, H.; Fukai, E.; Toyoda, A.; Kajitani, R.; Minakuchi, Y.; Itoh, T.; Higuchi, Y.; Kozuka, T.; Bono, H.; et al. A chromosome-level genome sequence of chrysanthemum seticuspe, a model species for hexaploid cultivated chrysanthemum. Commun. Biol. 2021, 4, 1167. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Li, Y.; Wang, J.; Qu, J.; Chen, Y.; Chen, X.; Huang, H.; Dai, S. Flower color classification and correlation between color space values with pigments in potted multiflora chrysanthemum. Sci. Hortic. 2021, 283, 110082. [Google Scholar] [CrossRef]
- Ohmiya, A. Molecular mechanisms underlying the diverse array of petal colors in chrysanthemum flowers. Breed. Sci. 2018, 68, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Bado, S.; Forster, B.P.; Maghuly, F. Physical and chemicals mutagenesis in plant breeding. In Mutation Breeding for Sustainable Food Production and Climate Resilience; Penna, S., Jain, S.M., Eds.; Springer: Singapore, 2023; p. 97. [Google Scholar] [CrossRef]
- Udage, A. Introduction to plant mutation breeding: Different approaches and mutagenic agents. J. Agric. Sci. Sri Lanka 2021, 16, 466. [Google Scholar] [CrossRef]
- Bezie, Y.; Tilahun, T.; Atnaf, M.; Taye, M. The potential applications of site-directed mutagenesis for crop improvement: A review. J. Crop Sci. Biotechnol. 2021, 24, 229–244. [Google Scholar] [CrossRef]
- Datta, S.K. Breeding of ornamentals: Success and technological status. Nucleus 2021, 65, 107–128. [Google Scholar] [CrossRef]
- Datta, S.K. Introduction/Review. In Induced Mutation Breeding; Springer: Singapore, 2023; p. 71. [Google Scholar] [CrossRef]
- Melsen, K.; Van De Wouw, M.; Contreras, R. Mutation Breeding in Ornamentals. Am. Soc. Hortic. Sci. Mutat. Breed. Ornam. 2021, 56, 1154–1165. [Google Scholar] [CrossRef]
- Sawada, Y.; Sato, M.; Okamoto, M.; Masuda, J.; Yamaki, S.; Tamari, M.; Tanokashira, Y.; Kishimoto, S.; Ohmiya, A.; Abe, T.; et al. Metabolome-based discrimination of chrysanthemum cultivars for the efficient generation of flower color variations in mutation breeding. Metabolomics 2019, 15, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Anne, S.; Hee Lim, J. Variability of chrysanthemum cultivars induced by gamma irradiation. Hortic. Sci. Technol. 2021, 39, 660–672. [Google Scholar] [CrossRef]
- Din, A.; Qadri, Z.A.; Wani, M.; Iqbal, S.; Malik, S.; Bhat, Z.A.; Banday, N. Developing an efficient in vitro callusing and regeneration protocol in Dendranthema × grandiflorum Kitam. J. Crop Sci. Biotechnol. 2022, 25, 393–405. [Google Scholar] [CrossRef]
- Haspolat, G.; Kunter, B.; Kantoglu, Y. Determination of mutagenic-sensitivity and induced variability in the mutant populations of ‘Bacardi’ chrysanthemum cultivar. Genetika 2022, 54, 161–172. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol. Plant. 1962, 15, 473–479. [Google Scholar] [CrossRef]
- Anderson, N.O.; Ascher, P.T. Inheritance of seed set, germination and day neutrality/heat delay insensitivity of garden chrysanthemums (Dendranthema × grandiflora) under glasshouse and field conditions. J. Am. Soc. Hortic. Sci. 2004, 129, 509–516. [Google Scholar] [CrossRef][Green Version]
- The Royal Horticultural Society, London, in Association with the Flower Council of Holland. R.H.S. Colour Chart. London: The Society. 1986. Available online: https://www.rhs.org.uk/ (accessed on 5 March 2024).
- Thao, L.; Dung, N.; Tham, N. Study on Chrysanthemum breeding by gamma (co60) irradiation on callus of 4 exotic varieties. Int. J. Agric. Technol. 2015, 11, 1813–1822. [Google Scholar]
- Wu, J.; Zhang, J.; Lan, F.; Fan, W.; Li, W. Morphological, cytological, and molecular variations induced by gamma rays in ground-grown chrysanthemum ‘Pinkling’. Can. J. Plant Sci. 2019, 100, 68–77. [Google Scholar] [CrossRef]
- Chowdhury, J.; Hoque, M.I.; Sarker, R.H. Evaluation of the Effect of Different Doses of Gamma Radiation to Induce Variation in in vitro Raised Plants of Chrysanthemum. Plant Tissue Cult. Biotech. 2023, 33, 155–165. [Google Scholar] [CrossRef]
- Sakamoto, K.; Nishi, M.; Ishiji, K.; Takatori, Y.; Chiwata, R. Induction of flower-colour mutation by synchrotron-light irradiation in spray chrysanthemum. Acta Hortic. 2019, 1237, 73–78. [Google Scholar] [CrossRef]
- Anitha, G.; Shiragur, M.; Patil, B.C.; Nishani, S.; Seetharamu, G.K.; Ramanagouda, S.H.; Naika, M.B. Mutation studies in chrysanthemum cultivar Poornima white. J. Pharmacogn. Phytochem. 2021, 10, 1235–1239. [Google Scholar]
- Soliman, T.M.; Lv, S.; Yang, H.; Hong, B.; Ma, N.; Zhao, L. Isolation of flower color and shape mutations by gamma radiation of Chrysanthemum morifolium Ramat cv. Youka. Euphytica 2014, 199, 317–324. [Google Scholar] [CrossRef]
- Puripunyavanich, V.; Piriyaphattarakit, A.; Chanchula, N.; Taychasinpitak, T. Mutation induction of in vitro Chrysanthemum by gamma irradiation. Chiang Mai J. Sci. 2019, 46, 609–617. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).