Analysis of Floral Scent Component of Three Iris Species at Different Stages
Abstract
1. Introduction
2. Methods and Materials
2.1. Overview of the Test Field and Test Materials
2.2. Research Methodology
2.2.1. Electronic Nose
2.2.2. HS-SPME-GC-MS Analysis
2.2.3. Identification and Analysis Methods
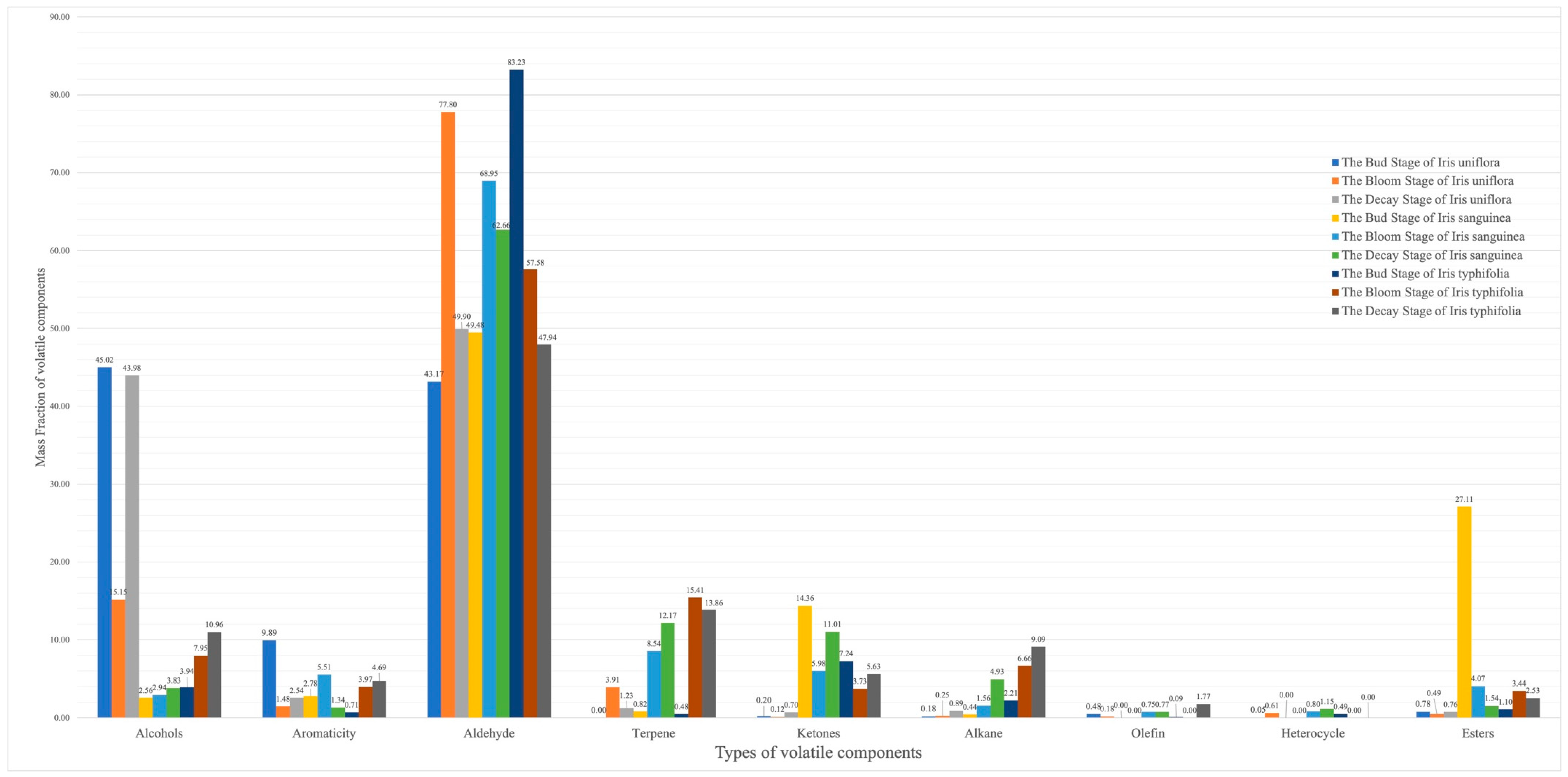
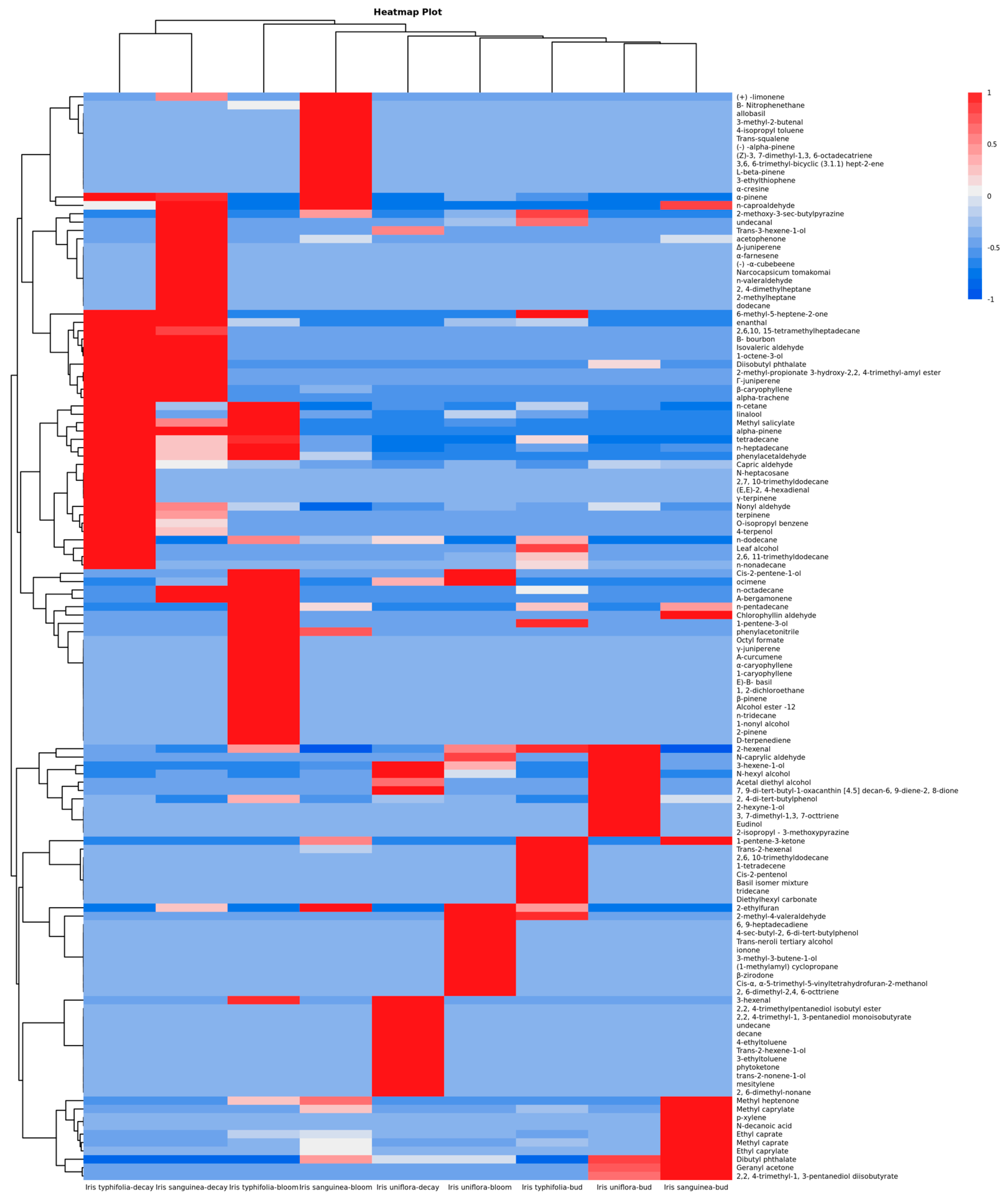
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, L.L.; Cao, X.D.; Xu, Z.R.; Zhang, J.F.; Wu, Y.H. Studies on Floral Syndrome and Mating System of Iris halophila. Acta Agrestia Sinica 2021, 29, 2731–2741. [Google Scholar]
- Knudsen, J.T.; Eriksson, R.; Gershenzon, J.; Stahl, B. Diversity and distribution of floral scent. Bot. Rev. 2006, 72, 1. [Google Scholar] [CrossRef]
- Patel, S.; Shibamoto, T. Flavor compounds in wines produced from Chardonnay grapes fermented with fruit juices. Food Sci. Technol. Res. 2003, 9, 84–86. [Google Scholar] [CrossRef]
- Pichersky, E.; Raguso, R.A.; Lewinsohn, E.; Croteau, R. Floral scent production in Clarkia (Onagraceae) (I. Localization and developmental modulation of monoterpene emission and linalool synthase activity). Plant Physiol. 1994, 106, 1533–1540. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Sun, Y.; Zhao, Y.; Liu, C.; Chen, X.; Li, F.; Bao, J. Identification of Floral Scent Profiles in Bearded Irises. Molecules 2019, 24, 1773. [Google Scholar] [CrossRef] [PubMed]
- Shalit, M.; Guterman, I.; Volpin, H.; Bar, E.; Tamari, T.; Menda, N.; Adam, Z.; Zamir, D.; Vainstein, A.; Weiss, D.; et al. Volatile ester formation in roses Identification of an acetyl-coenzyme A. Geraniol/Citronellol acetyltransferase in developing rose petals. Plant Physiol. 2003, 131, 1868–1876. [Google Scholar] [CrossRef] [PubMed]
- D’Auria, J.C.; Chen, F.; Pichersky, E. Characterization of an acyltransferase capable of synthesizing benzyl-benzoate and other volatile esters in flowers and damaged leaves of Clarkia breweri. Plant Physiol. 2002, 130, 466–476. [Google Scholar] [CrossRef]
- Zhang, W.; He, C.; Gong, Y. Pollinator attraction and outcrossing strategies in Iris. Plant Sci. J. 2019, 37, 672–681. [Google Scholar]
- Li, C.; Gao, Y.; Liu, R.; Cao, Y.; Fan, Z.; Guo, L.; Zhang, Q. Analysis on the barriers of interspecific hybridization in beardless Irises. J. Beijing For. Univ. 2018, 40, 96–101. [Google Scholar]
- Qi, J.; Zhai, Y.; Zhao, C. The Chemical Constituents of Genus Irisand Their Biological Activitie. Nat. Prod. Res. Dev. 2006, 18, 165–170. [Google Scholar]
- Wang, W.; Wang, P.; Qiao, Q.M. Research on the Classification and Application Value of Iris Plant. J. Anhui Agric. Sci. 2008, 36, 1001–1002. (In Chinese) [Google Scholar]
- Cai, K.; Feng, C.; Xu, S.; Sun, Y.; Lou, Q.; Sun, J.; Chen, H. The volatile components in different flowering stages of Iris uniflora. J. Northeast. For. Univ. 2023, 51, 53–58. [Google Scholar]
- Cai, K.; Tian, K.; Ban, Z.; Xu, H.; Jia, W.; Zhu, Y.; Chen, H. Analysis of Floral Fragrance Components in Different Parts of Iris typhifolia. Horticulturae 2023, 9, 1268. [Google Scholar] [CrossRef]
- Başer, K.H.; Demirci, B.; Orhan, I.E.; Kartal, M.; Sekeroglu, N.; Sener, B. Composition of Volatiles from Three Iris Species of Turkey. J. Essent. Oil Res. 2018, 23, 66–71. [Google Scholar] [CrossRef]
- Zito, P.; Rosselli, S.; Bruno, M.; Maggio, A.; Sajeva, M. Floral scent in Iris planifolia (Iridaceae) suggests food reward. Phytochemistry 2019, 158, 76–90. [Google Scholar] [CrossRef]
- Jia, X.; Deng, Q.; Yang, Y.; Xiang, X.; Zhou, X.; Tan, C.; Zhou, Q.; Huang, F. Unraveling of the Aroma-Active Compounds in Virgin Camellia Oil (Camellia oleifera Abel) Using Gas Chromatography–Mass Spectrometry–Olfactometry, Aroma Recombination, and Omission Studies. J. Agric. Food Chem. 2021, 69, 9043–9055. [Google Scholar] [CrossRef]
- Zhang, T.; Bao, F.; Yang, Y.; Hu, L.; Ding, A.; Ding, A.; Ding, A.; Wang, J.; Cheng, T.; Zhang, Q. A Comparative Analysis of Floral Scent Compounds in Intraspecific Cultivars of Prunus mume with Different Corolla Colours. Molecules 2020, 25, 145. [Google Scholar] [CrossRef]
- Lu, X.L.; Hai, Z.; Wang, J. Detection of Cola beverage by electronic nose. J. Zhejiang Univ. Agric. Life Sci. 2006, 32, 677–682. [Google Scholar]
- Liu, X.; Wang, W.; Fang, S. Thermodynamic studies on NO radical with 2,3-dimethylpentantal. Petrochem. Ind. Appl. 2011, 30, 4. [Google Scholar]
- SJaeger, R.; Pineau, B.; Bava, C.M.; Atkinson, K.R.; McRae, J.F.; Axten, L.G.; Chheang, S.L.; Beresford, M.K.; Peng, M.; Paisley, A.G.; et al. Investigation of the impact of sensitivity to cis-3-hexenol (green/grassy) on food acceptability and selection. Food Qual. Prefer. 2012, 24, 230–242. [Google Scholar] [CrossRef]
- León, D.C.S.; Ortíz, D.K.R.; González, D.F.J. Sensory approach and chiral analysis for determination of odour active compounds from feijoa (Acca sellowiana). Food Chem. 2020, 317, 126383. [Google Scholar] [CrossRef]
- Jin, K.S.; Bak, M.J.; Jun, M.; Lim, H.J.; Jo, W.K.; Jeong, W.S. α-Pinene Triggers Oxidative Stress and Related Signaling Pathways in A549 and HepG2 Cells. Food Sci. Biotechnol. 2010, 19, 1325–1332. [Google Scholar] [CrossRef]
- Hyun, J.; Lee, J.G.; Yang, K.Y.; Lim, S.; Lee, E.J. Postharvest Fumigation of (E)-2-Hexenal on Kiwifruit (Actinidia chinensis cv. ‘Haegeum’) Enhances Resistance to Botrytis cinerea. Postharvest Biol. Technol. 2022, 187, 111854. [Google Scholar] [CrossRef]
- Garcia, L.; Perrin, C.; Nolleau, V.; Godet, T.; Farines, V.; Garcia, F.; Caillé, S.; Saucier, C. Impact of Acetaldehyde Addition on the Sensory Perception of Syrah Red Wines. Foods 2022, 11, 1693. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Shen, H.; Zhao, M.; Sun, W. Flavour binding mechanism between a typical meat flavour compound (nonanal) and porcine myofibrillar proteins with consideration of conformational changes. Int. J. Food Sci. Technol. 2018, 53, 1954–1961. [Google Scholar] [CrossRef]
- Iyer, M.M.; Sacks, G.L.; Padilla-Zakour, O.I. Impact of Harvesting and Processing Conditions on Green Leaf Volatile Development and Phenolics in Concord Grape Juice. J. Food Sci. 2010, 75, C297–C304. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, N.; Lee, I.S.; Gupta, M.P.; Soejarto, D.D.; Kinghorn, A.D. (+)-4β-Hydroxyhernandulcin, A New Sweet Sesquiterpene from the Leaves and Flowers of Lippia dulcis. J. Nat. Prod. 1992, 55, 1136–1141. [Google Scholar] [CrossRef]
- Neelam Singh-Sangwan, N.S.S.; Sangwan, R.S.; Rajesh Luthra, R.L.; Thakur, R.S. Geraniol Dehydrogenase: A Determinant of Essential Oil Quality in Lemongrass. Planta Medica 1993, 59, 168–170. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi, A.; Shamspur, T.; Afazali, D. Chemical Composition of the Essential Oil of Ducrosia assadii Alava. from Kerman Province in Iran. J. Essent. Oil Res. 2010, 22, 300–302. [Google Scholar] [CrossRef]
- Van Aardt, M.; Duncan, S.E.; Marcy, J.E.; Long, T.E.; Nielsen-Sims, S.R. Aroma Analysis of Light-Exposed Milk Stored with and Without Natural and Synthetic Antioxidants. J. Dairy Sci. 2005, 88, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Gao, H.; Sun, Q.; Wu, F.; Ge, T.; Sui, K.; Wang, Z.; Song, L.; Huang, X.; Yu, Q. Puerarin, an efficient natural stabilizer for both polyethylene and polypropylene. J. Appl. Polym. Sci. 2020, 137, e49599. [Google Scholar] [CrossRef]
- Satoh, M.; Kusumoto, N.; Matsui, N.; Makino, R.; Hashida, K.; Arai, D.; Iiduka, Y.; Ashitani, T. Antitermitic and antifungal properties of enantiopure linalool and furanoid linalool oxide confirmed in Lindera umbellata var. membranacea. J. Wood Chem. Technol. 2021, 42, 37–45. [Google Scholar] [CrossRef]
- Castellar, A.; Oliveira, D.R.; Leitão, S.G.; Bizzo, H.R.; Soares MD, L.C.; Kinupp, V.F.; Veiga-Junior, V.F. Essential oil from Philodendron fragrantissimum, an aromatic Araceae from Amazonia, Brazil. J. Essent. Oil Res. 2013, 25, 194–197. [Google Scholar] [CrossRef]
- Wolken, W.A.M.; Tramper, J.; van der Werf, M.J. Amino acid-catalyzed retroaldol condensation: The production of natural benzaldehyde and other flavour compounds. Flavour Fragr. J. 2004, 19, 115–120. [Google Scholar] [CrossRef]
- Dong, Y.; Li, X.; Zhao, Y.; Ren, X.; Zheng, Y.; Song, R.; Zhong, X.; Shan, D.; Lv, F.; Deng, Q.; et al. Biotransformation and metabolism of three methyl salicylate glycosides by gut microbiota in vitro. J. Pharm. Biomed. Anal. 2023, 233, 115474. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Yuasa, Y. Practical Synthesis of 3-Methylnonane-2,4-dione, an Intense Strawlike and Fruity Flavored Compound. J. Agric. Food Chem. 2001, 49, 3864–3866. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Chen, F.; Wu, C.; Wang, X.; Chung, H.Y.; Jin, Z. Evaluation of Antioxidant Activity of Australian Tea Tree (Melaleuca alternifolia) Oil and Its Components. J. Agric. Food Chem. 2004, 52, 2849–2854. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Zhang, X.; Zhao, T.; Zhou, L. Effects of Origanum vulgare essential oil and its two main components, carvacrol and thymol, on the plant pathogen Botrytis cinerea. PeerJ 2020, 8, e9626. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.G.; Lee, H.S. Growth-inhibiting Effects and Chemical Composition of Essential Oils Extracted from Platycladus orientalis Leaves and Stemstoward Human Intestinal Bacteria. Food Sci. Biotechnol. 2015, 24, 427–431. [Google Scholar] [CrossRef]
- Raice, R.T.; Sjoholm, I.; Wang, H.L.; Bergenståhl, B. Characterization of volatile components extracted from Vangueria infausta (African medlar) by using GC–MS. J. Essent. Oil Res. 2015, 27, 76–81. [Google Scholar] [CrossRef]
- Sun, J.; Chin, J.H.; Zhou, W.; Yu, B.; Curran, P.; Liu, S.Q. Biocatalytic Conversion of Coconut Oil to Natural Flavor Esters Optimized with Response Surface Methodology. J. Am. Oil Chem. Soc. 2012, 89, 1991–1998. [Google Scholar] [CrossRef]
- Wang, L.; Dou, G.; Guo, H.; Zhang, Q.; Qin, X.; Yu, W.; Xiao, H. Volatile organic compounds of Hanseniaspora uvarum increase strawberry fruit flavor and defense during cold storage. Food Sci. Nutr. 2019, 7, 2625–2635. [Google Scholar] [CrossRef] [PubMed]
- Kafkas, E.; Cabaroglu, T.; Selli, S.; Bozdoğan, A.; Kürkçüoğlu, M.; Paydaş, S.; Başer, K.H.C. Identification of volatile aroma compounds of strawberry wine using solid-phase microextraction techniques coupled with gas chromatography–mass spectrometry. Flavour Fragr. J. 2006, 21, 68–71. [Google Scholar] [CrossRef]
- Plesage, L.; Candy, J.P.; Hirigoyen, C.; Humblot, F.; Basset, J.M. Selective dehydrogenation of dipentene (R-(+)-limonene) into paracymene on silica supported palladium assisted by α-olefins as hydrogen acceptor. J. Mol. Catal. A Chem. 1996, 112, 431–435. [Google Scholar] [CrossRef]
- Procida, G.; Lagazio, C.; Cateni, F.; Zacchigna, M.; Cichelli, A. Characterization of arabica and robusta volatile coffees composition by reverse carrier gas headspace gas chromatography–mass spectrometry based on a statistical approach. Food Sci. Biotechnol. 2020, 29, 1319–1330. [Google Scholar] [CrossRef]
- Effimia, E.; Karabagias, I.K.; Sofia, M.; Dionysios, K.; Nikolaos, K. Geographical origin discrimination of “Ntopia” olive oil cultivar from ionian islands using volatile compounds analysis and computational statistics. Eur. Food Res. Technol. Z. Lebensm. Unters. Forschung. A 2021, 247, 3083–3098. [Google Scholar]
- Gupta, S.; Gaurav, B.; Dwivedi, D.G.; Srivastava, K. Antimycobacterial activity of fractions and isolated compounds from Vetiveria zizanioides. Med. Chem. Res. 2012, 21, 1283–1289. [Google Scholar] [CrossRef]


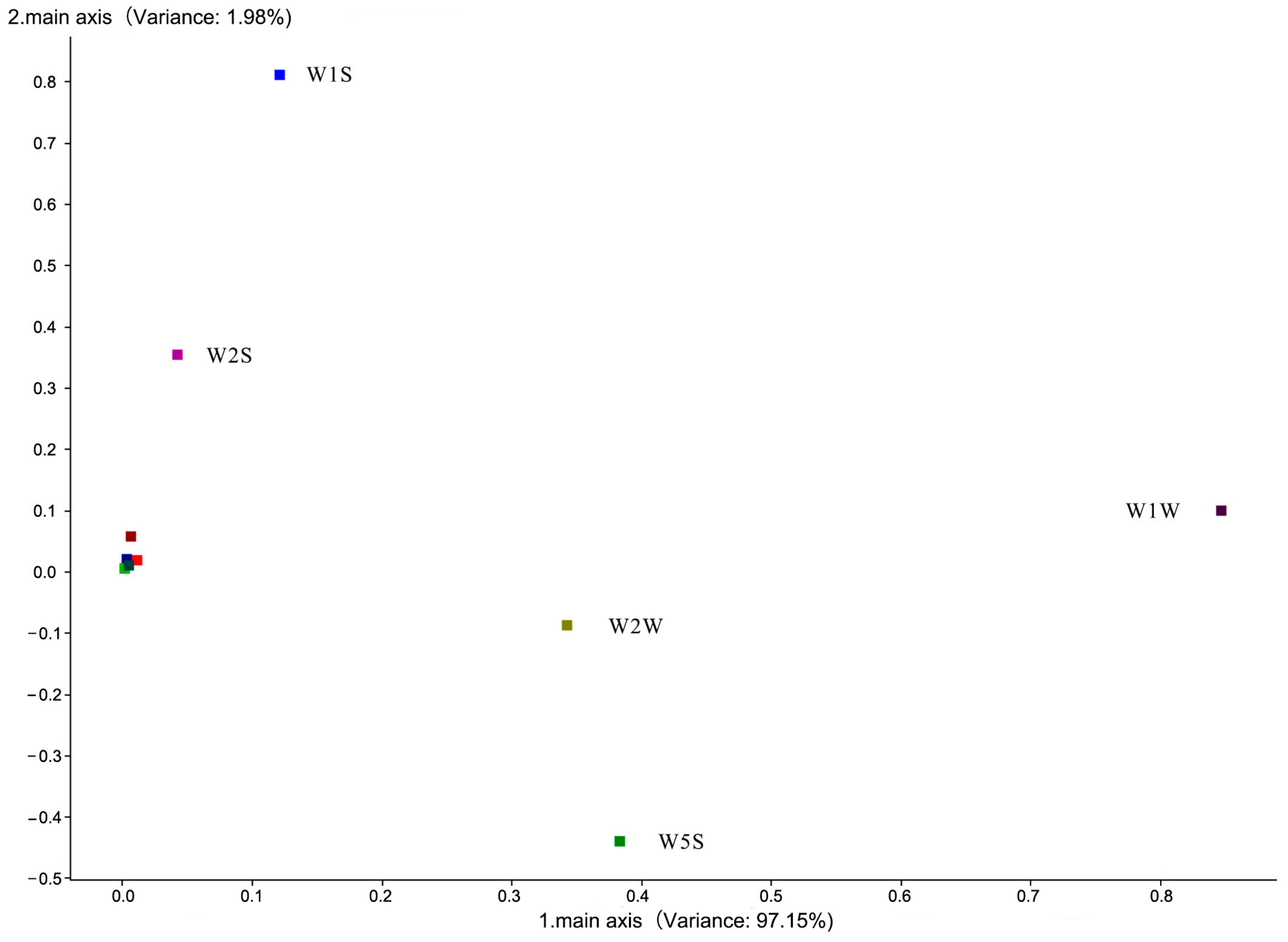
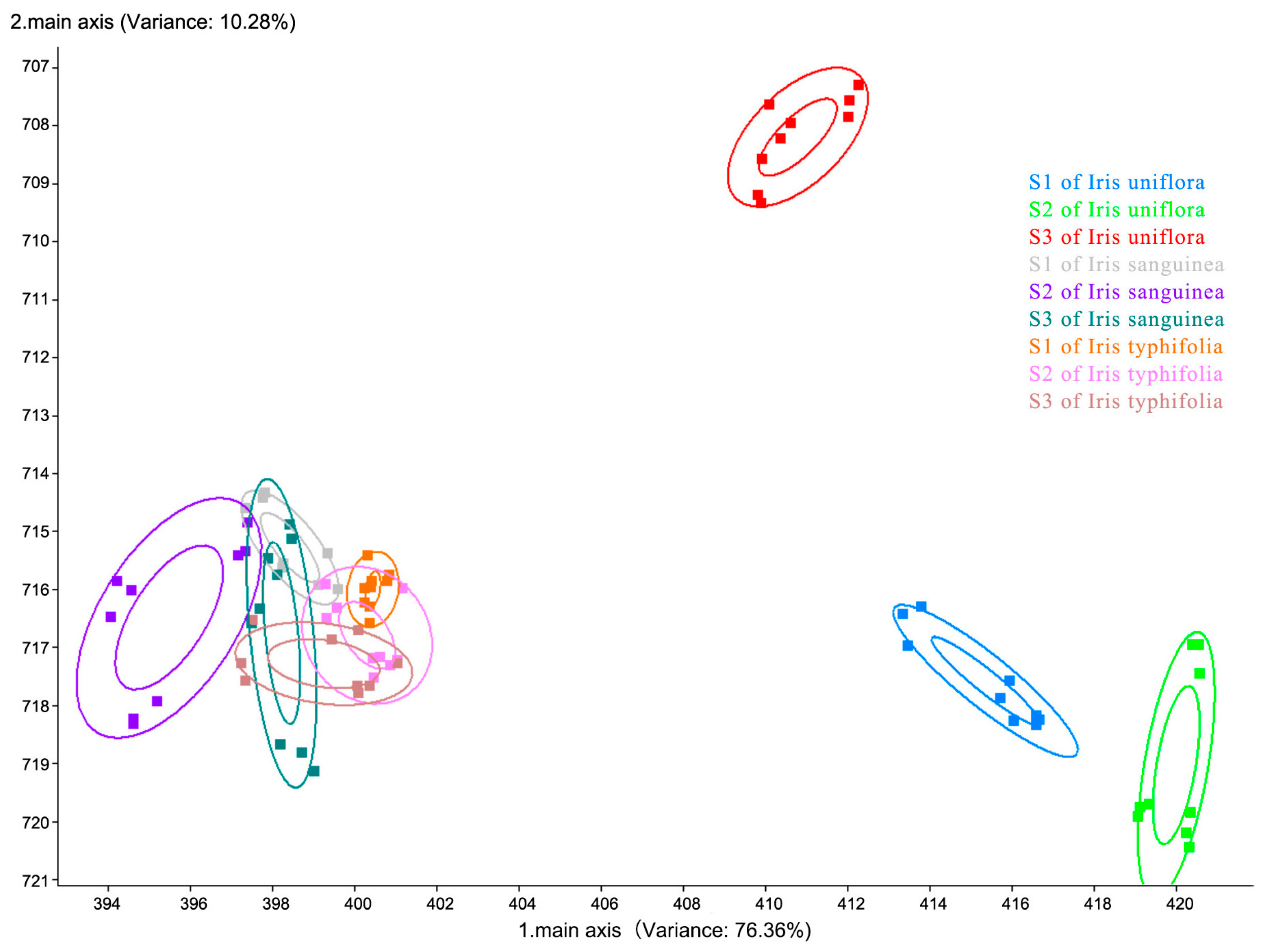
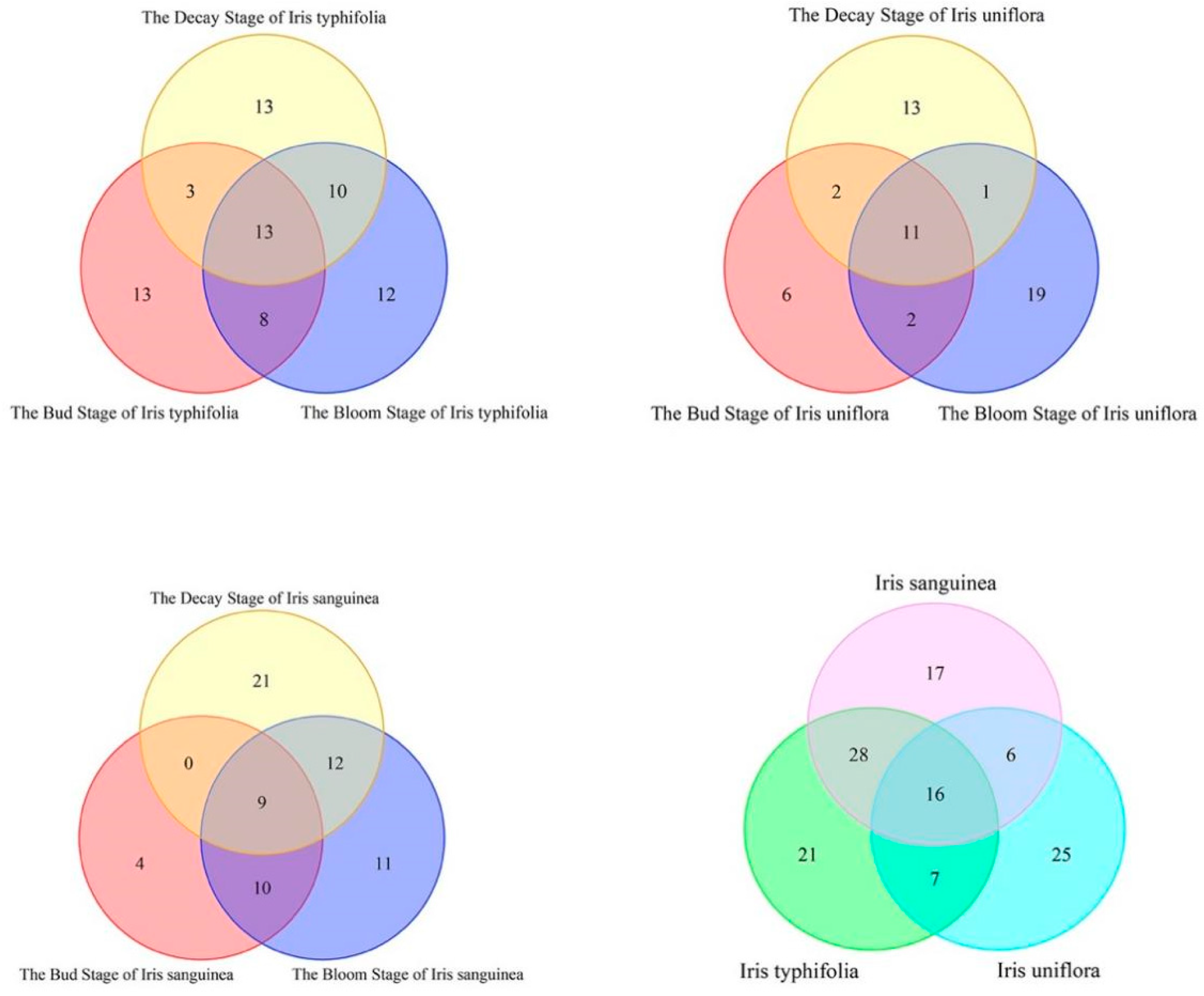
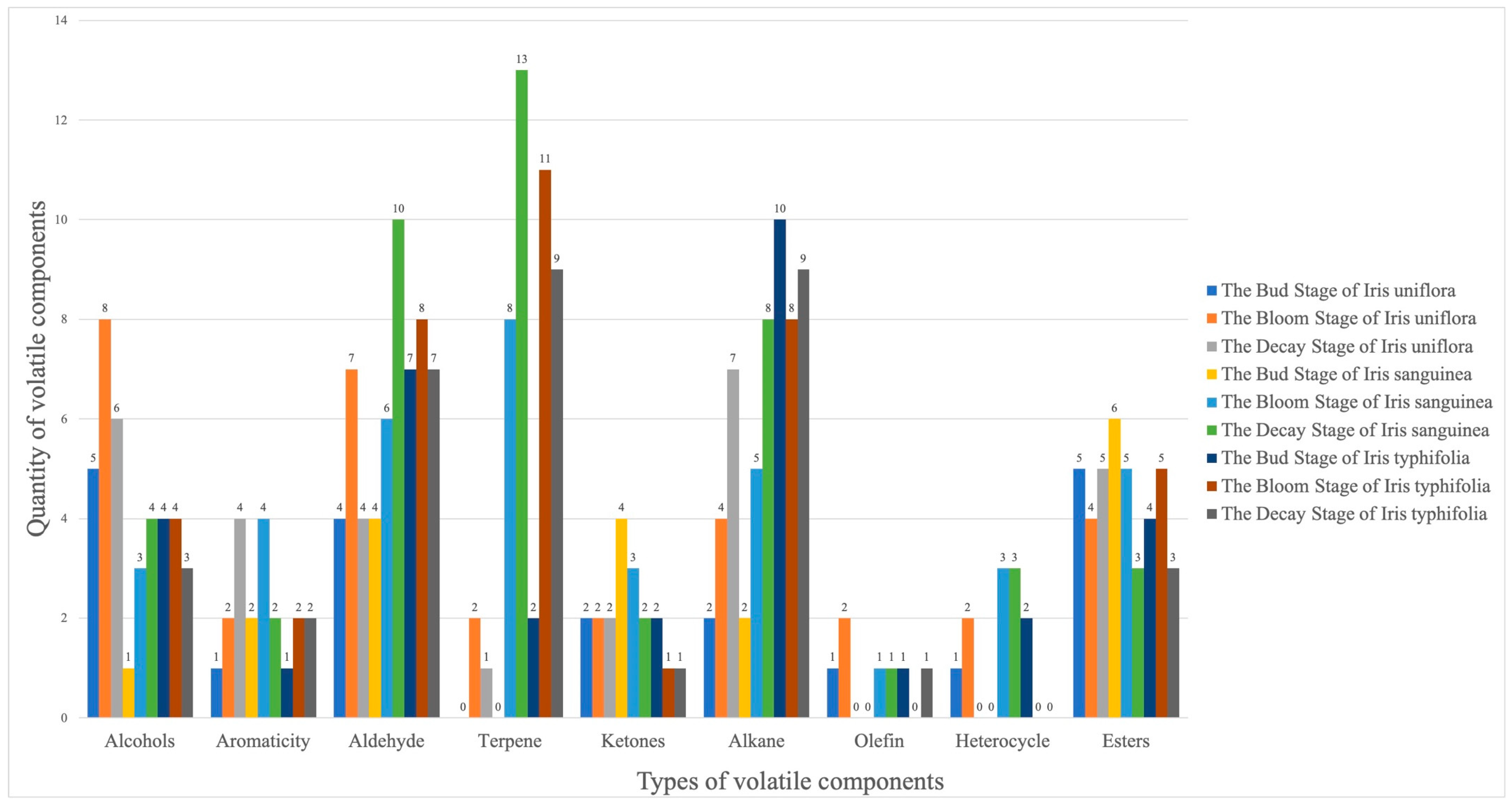
| Array Serial Number | Sensor Name | Performance Description |
|---|---|---|
| 1 | W1C | aromatic |
| 2 | W5S | broadrange |
| 3 | W3C | aromatic |
| 4 | W6S | hydrogen |
| 5 | W5C | arom-aliph |
| 6 | W1S | broad-methane |
| 7 | W1W | sulphur-organic |
| 8 | W2S | broad-alcohol |
| 9 | W2W | sulph-chlor |
| 10 | W3S | methane-aliph |
| Compound | Case# | The Bud Stage of Iris uniflora | The Bloom Stage of Iris uniflora | The Decay Stage of Iris uniflora | The Bud Stage of Iris typhifolia | The Bloom Stage of Iris typhifolia | The Decay Stage of Iris typhifolia | The Bud Stage of Iris sanguinea | The Bloom Stage of Iris sanguinea | The Decay Stage of Iris sanguinea |
|---|---|---|---|---|---|---|---|---|---|---|
| acetal diethyl alcohol | 105-57-7 | 0.20 | 0.08 | |||||||
| 2-hexyne-1-ol | 764-60-3 | 8.28 | ||||||||
| 2-hexenal | 505-57-7 | 39.02 | 22.38 | 7.30 | 27.06 | 21.02 | 9.11 | 6.09 | ||
| 3-hexenol | 544-12-7 | 20.00 | 9.43 | 23.00 | 2.56 | 1.65 | ||||
| N-hexyl alcohol | 111-27-3 | 16.07 | 3.66 | 17.23 | 0.97 | 1.15 | ||||
| N-caprylic aldehyde | 124-13-0 | 0.83 | 0.39 | |||||||
| Eudinol | 470-82-6 | 0.47 | ||||||||
| 3,7-dimethyl-1,3,7-octtriene | 502-99-8 | 0.48 | ||||||||
| 2-isopropyl-3-methoxypyrazine | 25773-40-4 | 0.05 | ||||||||
| nonyl aldehyde | 124-19-6 | 2.36 | 1.70 | 1.33 | 1.56 | 2.14 | 8.29 | 1.16 | 0.44 | 3.95 |
| capric aldehyde | 112-31-2 | 0.95 | 0.42 | 0.16 | 0.10 | 0.65 | 6.83 | 0.75 | 0.24 | 1.31 |
| methyl caprate | 110-42-9 | 0.22 | 0.06 | 0.53 | 0.40 | 9.68 | 1.46 | |||
| ethyl caprate | 110-38-3 | 0.22 | 0.32 | 0.58 | 0.14 | 0.96 | 11.60 | 1.54 | ||
| geranyl acetone | 3796-70-1 | 0.14 | 0.34 | |||||||
| 2,4-di-tert-butylphenol | 96-76-4 | 9.89 | 1.43 | 1.92 | 0.71 | 3.41 | 1.42 | 2.33 | 1.03 | 0.70 |
| 2,2,4-trimethyl-1,3-pentanediol diisobutyrate | 6846-50-0 | 0.11 | 0.28 | |||||||
| n-cetane | 544-76-3 | 0.09 | 0.05 | 0.09 | 0.25 | 1.04 | 0.96 | 0.21 | ||
| n-heptadecane | 629-78-7 | 0.09 | 0.10 | 0.05 | 0.16 | 0.72 | 1.01 | 0.14 | 0.17 | 0.40 |
| diisobutyl phthalate | 84-69-5 | 0.07 | 0.30 | 0.18 | ||||||
| 7,9-di-tert-butyl-1-oxacanthin[4,5]decan-6,9-diene-2,8-dione | 82304-66-3 | 0.05 | 0.03 | |||||||
| Dibutyl phthalate | 84-74-2 | 0.16 | 0.07 | 0.07 | 0.27 | 0.12 | ||||
| 3-methyl-3-butenol | 763-32-6 | 0.30 | ||||||||
| Cis-2-pentenol | 1576-95-0 | 0.42 | 0.61 | |||||||
| 2-methyl-4-valeraldehyde | 5187-71-3 | 52.68 | 27.06 | |||||||
| (1-methylamyl) cyclopropane | 6976-28-9 | 0.06 | ||||||||
| enanthal | 111-71-7 | 0.21 | 0.29 | 0.26 | 1.56 | 0.98 | ||||
| 2-ethylfuran | 3208-16-0 | 0.57 | 0.28 | 0.54 | 0.25 | |||||
| α-pinene | 80-56-8 | 0.36 | 0.19 | 1.76 | 2.26 | 1.50 | ||||
| ocimene | 13877-91-3 | 3.55 | 1.23 | 2.07 | 0.35 | |||||
| cis-α,α-5-trimethyl-5-vinyltetrahydrofuran-2-methanol | 5989-33-3 | 0.13 | ||||||||
| linalool | 78-70-6 | 1.05 | 0.25 | 6.10 | 4.06 | 0.32 | 0.23 | |||
| 2,6-dimethyl-2,4,6-octtriene | 3016-19-1 | 0.07 | ||||||||
| 2-methoxy-3-sec-butylpyrazine | 24168-70-5 | 0.04 | 0.20 | 0.15 | 0.41 | |||||
| undecanal | 112-44-7 | 0.02 | 0.12 | 0.30 | ||||||
| ionone | 127-41-3 | 0.06 | ||||||||
| β-zirodone | 79-77-6 | 0.07 | ||||||||
| 2,6,11-trimethyldodecane | 31295-56-4 | 0.04 | 0.24 | 1.14 | ||||||
| trans-neroli tertiary alcohol | 40716-66-3 | 0.09 | ||||||||
| 4-sec-butyl-2,6-di-tert-butylphenol | 17540-75-9 | 0.05 | ||||||||
| 6,9-heptadecadiene | 81265-03-4 | 0.11 | ||||||||
| 3-hexenal | 4440-65-7 | 41.10 | 21.47 | |||||||
| trans-3-hexenol | 928-97-2 | 0.75 | 2.12 | |||||||
| trans-2-hexene-1-ol | 928-95-0 | 0.94 | ||||||||
| 3-ethyltoluene | 620-14-4 | 0.13 | ||||||||
| mesitylene | 108-67-8 | 0.30 | ||||||||
| 4-ethyltoluene | 622-96-8 | 0.20 | ||||||||
| decane | 124-18-5 | 0.24 | ||||||||
| 2,6-dimethyl-nonane | 17302-28-2 | 0.18 | ||||||||
| n-dodecane | 112-40-3 | 0.22 | 0.28 | 0.31 | 0.72 | 0.14 | ||||
| undecane | 1120-21-4 | 0.11 | ||||||||
| transnon-2-enol | 31502-14-4 | 1.99 | ||||||||
| 2,2,4-trimethyl-1,3-pentanediol monoisobutyrate | 25265-77-4 | 0.07 | ||||||||
| 2,2,4-trimethylpentanediol isobutyl ester | 6846-50-0 | 0.04 | ||||||||
| phytoketone | 502-69-2 | 0.67 | ||||||||
| pentene-3-ol | 616-25-1 | 0.46 | 0.91 | |||||||
| 1-pentene-3-ketone | 1629-58-9 | 0.68 | 0.68 | 0.37 | ||||||
| cis-2-pentenol | 1576-95-0 | 0.35 | ||||||||
| trans-2-hexenal | 6728-26-3 | 27.06 | 2.20 | |||||||
| leaf alcohol | 928-96-1 | 2.88 | 6.32 | |||||||
| 6-methylhept-5-en-2-one | 110-93-0 | 6.56 | 5.63 | 7.57 | ||||||
| basil isomer mixture | 13877-91-3 | 0.29 | ||||||||
| methyl caprylate | 111-11-5 | 0.13 | 2.26 | 0.55 | ||||||
| tridecane | 629-50-5 | 0.21 | ||||||||
| 1-tetradecene | 1120-36-1 | 0.09 | ||||||||
| tetradecane | 629-59-4 | 0.31 | 0.62 | 1.03 | 0.09 | 0.38 | ||||
| n-nonadecane | 629-92-5 | 0.11 | 0.58 | |||||||
| 2,6,10-trimethyldodecane | 3891-98-3 | 0.27 | ||||||||
| n-pentadecane | 629-62-9 | 0.27 | 0.85 | 0.31 | 0.23 | |||||
| n-octadecane | 593-45-3 | 0.12 | 0.59 | 0.35 | ||||||
| diethylhexyl carbonate | 14858-73-2 | 0.30 | ||||||||
| 1,2-dichloroethane | 107-06-2 | 1.90 | ||||||||
| chlorophyllin aldehyde | 6728-26-3 | 10.60 | 6.35 | |||||||
| 2-pinene | 2437-95-8 | 5.05 | ||||||||
| β-pinene | 18172-67-3 | 0.77 | ||||||||
| methyl heptenone | 110-93-0 | 3.73 | 12.88 | 5.18 | ||||||
| D-terpenediene | 5989-27-5 | 4.87 | ||||||||
| (E)-Β-basil | 3779-61-1 | 0.24 | ||||||||
| phenylacetaldehyde | 122-78-1 | 1.13 | 1.59 | 0.30 | 0.52 | |||||
| octyl formate | 112-32-3 | 0.40 | ||||||||
| phenylacetonitrile | 140-29-4 | 0.32 | 0.13 | |||||||
| 1-nonyl alcohol | 143-08-8 | 0.32 | ||||||||
| methyl salicylate | 119-36-8 | 0.03 | 1.26 | 1.34 | 0.69 | |||||
| Β-Nitrophenethane | 6125-24-2 | 0.57 | 4.21 | |||||||
| n-tridecane | 629-50-5 | 0.63 | ||||||||
| alcohol ester-12 | 77-68-9 | 0.41 | ||||||||
| alpha-pinene | 3856-25-5 | 0.90 | 1.05 | 0.77 | ||||||
| 1-caryophyllene | 87-44-5 | 0.36 | ||||||||
| A-bergamonene | 17699-05-7 | 0.50 | 0.46 | |||||||
| α-caryophyllene | 6753-98-6 | 0.25 | ||||||||
| A-curcumene | 644-30-4 | 0.23 | ||||||||
| γ-juniperene | 39029-41-9 | 0.19 | ||||||||
| isovaleric aldehyde | 590-86-3 | 0.60 | 0.33 | |||||||
| n-caproaldehyde | 66-25-1 | 19.97 | 41.22 | 65.62 | 45.94 | |||||
| (E,E)-2,4-hexadienal | 142-83-6 | 1.77 | ||||||||
| 1-octen-3-ol | 3391-86-4 | 0.57 | 0.33 | |||||||
| terpinene | 99-86-5 | 1.82 | 0.54 | |||||||
| O-isopropyl benzene | 527-84-4 | 3.27 | 0.64 | |||||||
| γ-terpinene | 99-85-4 | 1.82 | ||||||||
| 4-terpenol | 562-74-3 | 2.84 | 0.67 | |||||||
| 2,7,10-trimethyldodecane | 74645-98-0 | 1.05 | ||||||||
| 2-methyl-propionate 3-hydroxy-2,2,4-trimethyl-amyl ester | 77-68-9 | 0.88 | 0.66 | |||||||
| Β-bourbon | 5208-59-3 | 1.32 | 0.81 | |||||||
| β-caryophyllene | 87-44-5 | 1.53 | 0.10 | 1.49 | ||||||
| alpha-trachene | 6753-98-6 | 1.00 | 0.90 | |||||||
| Γ-juniperene | 39029-41-9 | 0.74 | 0.54 | |||||||
| 2,6,10,15-tetramethylheptadecane | 54833-48-6 | 1.12 | 0.50 | |||||||
| N-heptacosane | 593-49-7 | 1.48 | ||||||||
| p-xylene | 106-42-3 | 0.45 | ||||||||
| acetophenone | 98-86-2 | 0.47 | 0.44 | 3.44 | ||||||
| ethyl caprylate | 106-32-1 | 3.02 | 0.40 | |||||||
| N-decanoic acid | 334-48-5 | 0.82 | ||||||||
| 3-methyl-2-butenal | 107-86-8 | 0.14 | ||||||||
| 3-ethylthiophene | 1795-01-3 | 0.11 | ||||||||
| α-cresine | 99-83-2 | 0.06 | ||||||||
| L-beta-pinene | 18172-67-3 | 0.43 | ||||||||
| 4-isopropyl toluene | 99-87-6 | 0.14 | ||||||||
| (+)-limonene | 5989-27-5 | 5.09 | 1.60 | |||||||
| 3,6,6-trimethyl-bicyclic(3,1,1)hept-2-ene | 4889-83-2 | 0.13 | ||||||||
| (Z)-3,7-dimethyl-1,3,6-octadecatriene | 3338-55-4 | 0.92 | ||||||||
| allobasil | 7216-56-0 | 0.19 | ||||||||
| (-)-alpha-pinene | 3856-25-5 | 0.34 | ||||||||
| trans-squalene | 111-02-4 | 0.06 | ||||||||
| n-valeraldehyde | 110-62-3 | 0.33 | ||||||||
| 2-methylheptane | 592-27-8 | 0.61 | ||||||||
| 2,4-dimethylheptane | 2213-23-2 | 2.20 | ||||||||
| narcocapsicum tomakomai | 20697-20-5 | 0.49 | ||||||||
| dodecane | 112-40-3 | 0.29 | ||||||||
| (-)-α-cubebeene | 17699-14-8 | 0.77 | ||||||||
| e-β-farnesene | 18794-84-8 | 0.93 | ||||||||
| Δ-juniperene | 483-76-1 | 1.60 |
| Compound | Threshold (µg/kg) | The Bud Stage of Iris uniflora | The Bloom Stage of Iris uniflora | The Decay Stage of Iris uniflora | The Bud Stage of Iris typhifolia | The Bloom Stage of Iris typhifolia | The Decay Stage of Iris typhifolia | The Bud Stage of Iris sanguinea | The Bloom Stage of Iris sanguinea | The Decay Stage of Iris sanguinea |
|---|---|---|---|---|---|---|---|---|---|---|
| Acetal diethyl alcohol | 0.1 | 7.78 | 5.28 | |||||||
| 2-hexenal | 0.15 | 118.30 | 394.26 | 325.83 | 87.62 | 64.47 | 18.57 | |||
| N-hexyl alcohol | 8 | 7.86 | 1.28 | 14.39 | 0.11 | 0.66 | ||||
| N-caprylic aldehyde | 0.001 | 3266.84 | 126.92 | |||||||
| Eudinol | 0.01 | 185.90 | ||||||||
| Nonyl aldehyde | 0.0035 | 2638.26 | 1284.40 | 2547.50 | 217.11 | 281.66 | 475.72 | 298.44 | 117.33 | 516.43 |
| Capric aldehyde | 0.005 | 745.33 | 223.73 | 218.13 | 9.80 | 6.57 | 274.42 | 135.65 | 44.11 | 119.63 |
| Ethyl caprate | 0.2 | 4.29 | 4.28 | 19.32 | 0.35 | 2.21 | 52.34 | 7.94 | ||
| enanthal | 0.031 | 17.99 | 4.47 | 3.97 | 1.18 | 14.48 | ||||
| linalool | 0.0015 | 185.36 | 8.53 | 1872.43 | 544.47 | 197.87 | 71.36 | |||
| undecanal | 0.014 | 3.42 | 4.43 | 9.84 | ||||||
| Chlorophyllin aldehyde | 0.15 | 32.51 | 38.30 | |||||||
| 2-pinene | 0.12 | 19.34 | ||||||||
| Methyl heptenone | 0.1 | 17.15 | 116.18 | 47.82 | ||||||
| D-terpenediene | 0.22 | 1.18 | ||||||||
| phenylacetaldehyde | 0.009 | 57.53 | 35.58 | 31.76 | 26.63 | |||||
| Methyl salicylate | 0.06 | 9.65 | 4.53 | 5.27 | ||||||
| alpha-pinene | 0.12 | 3.47 | 1.77 | 2.93 | ||||||
| n-caproaldehyde | 0.0075 | 535.11 | 4958.52 | 878.13 | 28.71 | |||||
| alpha-trachene | 0.16 | 1.25 | 2.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, K.; Ban, Z.; Xu, H.; Chen, W.; Jia, W.; Zhu, Y.; Chen, H. Analysis of Floral Scent Component of Three Iris Species at Different Stages. Horticulturae 2024, 10, 153. https://doi.org/10.3390/horticulturae10020153
Cai K, Ban Z, Xu H, Chen W, Jia W, Zhu Y, Chen H. Analysis of Floral Scent Component of Three Iris Species at Different Stages. Horticulturae. 2024; 10(2):153. https://doi.org/10.3390/horticulturae10020153
Chicago/Turabian StyleCai, Keyu, Zhengjie Ban, Haowen Xu, Wanlin Chen, Wenxu Jia, Ying Zhu, and Hongwu Chen. 2024. "Analysis of Floral Scent Component of Three Iris Species at Different Stages" Horticulturae 10, no. 2: 153. https://doi.org/10.3390/horticulturae10020153
APA StyleCai, K., Ban, Z., Xu, H., Chen, W., Jia, W., Zhu, Y., & Chen, H. (2024). Analysis of Floral Scent Component of Three Iris Species at Different Stages. Horticulturae, 10(2), 153. https://doi.org/10.3390/horticulturae10020153






