Abstract
Coconut cultivation faces serious challenges caused by pests and diseases, whose targets are often not reached by conventional application methods such as spraying and soil application. New control strategies, such as vegetative endotherapy, have emerged, but knowledge gaps persist regarding many aspects, especially in pesticide translocation within palm trees, which is crucial for an efficient practical field application. This study investigated the translocation of a mixture of commercial insecticides and fungicides—difenoconazole, imidacloprid, thiabendazole, cyproconazole, thiamethoxam, spirodiclofen, and carbosulfan—applied via pressurized and nonpressurized endotherapeutic methods to coconut stems. This assessment aimed to quantify the concentrations of pesticide translocation through the stem, from the application site to the plant canopy. Due to the difficulty of applying the solution to the instrument used for pressurized endotherapy, the solution had to be diluted and used at a lower volume. In experimental field conditions, stem samples were assessed at 50 and 100 cm above the application point following endotherapy treatments conducted over a period ranging from 2 to 45 days. The analyses were performed using LC-MS/MS. In the pressurized method, the highest concentrations were observed for difenoconazole (1684 µg kg−1), imidacloprid (1278 µg kg−1), and thiabendazole (781 µg kg−1). Conversely, in the nonpressurized method, the highest concentrations were recorded for imidacloprid (5803 µg kg−1), followed by difenoconazole (3660 µg kg−1) and thiabendazole (2598 µg kg−1). To address the issue with formulation conditions in the pressurized method and to allow a comparison between the two application methods, we simulated extrapolated results for comparison with the nonpressurized method. This evaluation aimed to evaluate both methods under similar formulation conditions (volume and concentration). The results predicted that if the solution had not been diluted, the pressurized method would present the best translocations, mainly near the plant canopy, except for carbofuran. All pesticides were translocated independently of their physical–chemical properties or formulation. No pesticide residues were detected in the coconut water and pulp up to 120 days after the endotherapy application.
1. Introduction
Vegetative endotherapy is used to treat perennial trees, control diseases and pests, or apply fertilizers, growth inductors, and other inputs affecting their growth and development. This technique consists of applying chemical solutions into the trunks of trees via perforations in the trunk/stem and distributing them throughout the plant via the photosynthetic cycle [1,2,3,4]. There are two ways of applying solutions inside the trees: (1) the nonpressurized method and (2) the pressurized method. The nonpressurized method is performed with the aid of a syringe used to dose and introduce the solution without pressure into a hole made in the trunk/stem. However, the uneven distribution of the solution in the trees may occur due to the potential for part of the solution to become stuck or degrade at the application port. In the pressurized method, the chemical solution is applied under pressure into the tree trunk/stem, with specific instruments that force the solution’s entry into the plant trunk/stem [4]. Currently, the vegetative endotherapy market offers some models of pressurized equipment for the application of products such as ENDOterapia Vegetal®, Chemjet®, Mauget®, and Arborjet®, among others. The pressurized equipment uses everything from spring-loaded syringes, pressure applicators, and air cylinders/tanks to capsules in its systems that forces products into the tree trunks [2,3,4].
The vegetative endotherapy technique has several advantages over conventional pest control techniques, mainly foliar pesticide application, which may affect food quality and cause adverse environmental impacts. On the other hand, the use of vegetative endotherapy techniques requires qualified professionals to carry out the treatment and presents challenges and limitations that still must be considered. One of the main challenges is the need for more standardization of the application methods and chemicals used, which can affect the effectiveness of the treatment [2,5,6,7,8]. To evaluate the efficiency of the endotherapeutic treatment, several factors must be considered, such as physiological and morphological plant structure, accurate identification of the specific problem affecting the plant, control measures adopted, plant nutritional status, disease or pest incidence and severity, and local climatic conditions. However, some questions remain about the translocation efficiency of products in vegetative endotherapy using pressurized or nonpressurized methods. These knowledge gaps make it difficult for professionals to recommend because there is not much information about the product’s translocation and concentration at different plant parts or knowledge about the correct dosage and appropriate application techniques beyond the risk of the applied product reaching the fruit [2,9,10].
The coconut palm tree is a crop that has faced serious sanitary problems in several regions worldwide, including viral and fungal infections and insect attacks [11]. According to the phytosanitary pesticide system of the Ministry of Agriculture and Livestock (Phytosanitary Pesticide System 12 May 2023), in Brazil, several insects and mites have been causing damage to the coconut palm tree, affecting all its leaves, trunk, and fruits, such as coconut sucking insects (Aspidiotus destructor, Cerataphis lataniae, Aleurodicus pseudugesii), coconut caterpillars (Automeris cinctistriga, Brassolis astyra astyra, Brassolis sophorae, Opsiphanes invirae), leaf beetles (Coraliomela brunnea, Delocrania cossyphoides), coconut mites (Eriophyes guerreronis (Acari: Eriophyidae), Raoiella indica, Amrineus cocopholius), oil palm borers (Eupalamides dedalus), black coconut bunch weevil (Homalinotus coriaceus), and black coconut weevil (Rhynchophorus palmarum). Similarly, there are several diseases caused by pathogens such as fungi or nematodes that can affect coconut palm trees, namely: coconut palm leaf spot (Bipolaris incurvata), red ring nematode (Bursaphelenchus cocophilus), stem-bleeding (Ceratocystis paradoxa), leaf spots (Drechslera halodes, Helminthosporium spp.), spiral nematode (Helicotylenchus dihystera), leaf blight disease (Lasiodiplodia theobromae), small-verrucosis (Phyllachora torrendiella (Batista) Subileau), and bud rot (Phytophthora palmivora). To control these diseases and pests, it is important to adopt integrated control measures that involve appropriate cultural practices, biological control, the use of pesticides, and other management strategies. It is always important to stay up-to-date on local regulations regarding pesticide use and farming practices, ensuring that they comply with established standards and regulations [3,9,12].
Some endotherapeutic studies have been carried out on coconut palm trees using pesticides, such as the evaluation of the two methods, pressurized and nonpressurized, with no detection of residues in fruits using ultra-high-performance liquid chromatography coupled to mass spectrometry (UHPLC-MS/MS) [4]. The application of cyproconazole via nonpressurized endotherapy was also reported by Moura et al. [13], and treated plants exhibited an average increase in the number of functional leaves and fruits. Based on physicochemical characteristics and the pesticide stem-sap partition coefficient, Paraiba and collaborators [14] recently published a study that modeled pesticide translocation in the coconut palm. The authors used LC-MS/MS to quantify pesticide residues in the fruit and sap after nonpressurized endotherapy with commercial SOS Palm® equipment.
A study evaluating doses of tebuconazole applied to coconut palms through root feeding was reported [15]. That study involved administering tebuconazole solution at both the recommended dose (5 mL/100 mL water/tree) and double the recommended dose (10 mL/100 mL water/tree) to ten coconut palms per treatment. The application process entailed inserting the tebuconazole solution into a polythene bag containing fresh and live roots, fixed tightly with a cotton thread. The absorption of the solution was assessed eight hours later, with two rounds of root feeding administered at a 30-day interval. Monitoring of tebuconazole residues across various coconut plant parts and concentrations was conducted over several days. Leaf samples were collected at intervals of 1, 5, and 10 days following the second application for both treatments. Residue analysis revealed detectable leaf levels after 1 and 3 days of root feeding, ranging from 0.008 to 0.02 μg g−1. These residues decreased to 0.0126 μg g−1 in the plants subjected to the double-dose treatment after five days and were below the quantification limits in those subjected to the recommended dose treatment. Coconut kernel and water samples showed levels below quantifiable limits in the 1st and 3rd-day samples and remained so afterward. Despite not employing endotherapy as an application method, the study comprehensively addresses translocation in coconut palms. It utilized LC-MS/MS analysis for tebuconazole residue assessment in roots, leaves, and fruits.
In the present study, we propose to carry out a more comprehensive field assessment on the translocation of pesticides through coconut palms by expanding the range of products used (a mixture of difenoconazole, imidacloprid, thiabendazole, cyproconazole, thiamethoxam, spirodiclofen, and carbosulfan) and extending the sampling period to collect stem samples for up to 45 days and fruit samples for up to 120 days after the treatment application. The analyses of the stem and fruit samples were conducted using LC-MS/MS. The main objectives of this work were to compare the translocation of different commercial fungicides and insecticides in a mixture applied to the stems of coconut palm trees through two endotherapeutic application methods, pressurized and nonpressurized. Additionally, this study aimed to determine the extent of pesticide contamination in the fruits. The pesticides used in this study are potential options for the regulation and treatment of the aforementioned pests and diseases.
2. Materials and Methods
2.1. Field Trials and Experimental Design
The field experiment was conducted in a coconut hybrid plantation at the Sococo S/A Agroindustry located in the municipality of Mojú/PA in the northern region of Brazil (11°07′ S and 37°11′ W) from September 2015 (spring) to February 2016 (summer), corresponding to a drought period in the region. This study was implemented using a completely randomized design (CRD) with two treatments and twelve replications. The coconut cultivar used was PB 121 (Port Bouet 121—Malaysian yellow dwarf × West African tall), which was 8 years of age. During the experimental period, no phytosanitary problems were detected, and the plants had an excellent phytosanitary appearance and high productivity.
2.2. Pesticide Mixture for Endotherapeutic Applications
The mixture utilized in this field experiment consisted of seven commercial insecticides, fungicides, and an adjuvant (Table 1) [16].

Table 1.
Commercial pesticides and the adjuvant used in the mixed solution.
2.3. Endotherapeutic Application Methods
Conducting endotherapeutic investigations in the field is a costly endeavor that requires setting up the experiment, employing expert personnel, obtaining a sufficient number of healthy plants, and conducting expensive chemical analyses. In this study, the selected fungicides and insecticides were simultaneously mixed in a single solution, which was then applied to the stem of the coconut palm tree. Two methods were used for applying the mixed pesticide solution (Table 1) into the trunk/stem of selected plants: (a) nonpressurized: holes were made with a drill machine at a 45° angle on two opposite sides of the coconut palm stem, followed by the injection of a mixed solution of seven pesticides, with the aid of a polyethylene syringe (20 mL) as a dispenser, and; (b) pressurized: the application under pressure on the stem was made using the Bite Infusion® (Padova, Italy) equipment Di Palma version. This equipment has a sliding hammer that allows the blade to be introduced into the trunk, with little plant damage, as well as the entrance of pesticide solution into the plant tissues. The blade is hollow and has small holes on both sides, facilitating the introduction of the pesticide solution as soon as pressure is exerted on the syringe plunger.
In both procedures, the solutions containing a mixture of insecticides and fungicides (Table 2) diluted in Break-thru were applied 80 cm above the ground.

Table 2.
Characteristics of the solutions used in the nonpressurized and pressurized endotherapeutic treatments applied to the coconut palms in the field experiment located in Mojú, PA, North Brazil.
A pure concentration of Break-thru® was added to the solution to increase the mobility of the pesticides. For both treatments, the solutions were prepared with dilutions in the 1:1 (v/v) proportion of the commercial pesticide mixture and Break-thru®. A volume of 20 mL of the final mixed solution was applied to each coconut palm in the nonpressurized treatment, 10 mL being applied at two opposite points. However, the resulting solution was very viscous for application using the Bite Infusion® equipment and needed to be more diluted to facilitate solution penetration in the plant due to the limitations of the commercial instrument used. The solution in the pressurized treatment was diluted to a ratio of 1:2 (v/v) in distilled water. The injection methods, pressurized and nonpressurized, were studied and compared. Figure 1 illustrates how the endotherapeutic methods used in these field trials were conducted.

Figure 1.
Treatments were applied to coconut palms via vegetative endotherapy. (A) Nonpressurized endotherapy involving the use of a syringe to measure the volume after drilling a hole; (B) Pressurized endotherapy utilizing commercial Bite Infusion® equipment. The field experiment was conducted in Mojú, PA, in Northern Brazil.
2.4. Field Sampling and Analysis of Pesticide Residues in the Coconut Trunk/Stem
Twenty-four coconut palms were randomly selected for treatment via endotherapy techniques and sampled at different intervals. The samples were collected at intervals of 2, 15, 30, and 45 days and at heights of 50 and 100 cm above the application point, which was set at 80 cm above the ground. This design characterizes a longitudinal (repeated measures) study. At each collection interval, three new treated plants were sampled and subsequently discarded. This was carried out to prevent disruptions in sap flow during subsequent sample collections caused by injuries from the drill machine.
Stem tissue and fruit samples were collected according to Ferreira et al. [11]. Briefly, the study standardized the application point on coconut palm trunks to 80 cm above the ground. For nonpressurized treatment, a drill machine was used to create holes at a 45-degree downward slope, approximately 15 cm deep and 2 cm wide, which were then sealed with wood after the product application. Pressurized treatment involved the insertion of commercial Bite Infusion blades into the trunk and subsequent product application. The experimental design included three plants per treatment, with sample collection at intervals of 2, 15, 30, and 45 days post-application. Samples were collected at 50 and 100 cm above the application point and in four or five holes around the trunk, disrupting vascular bundles. The samples were refrigerated in sterile plastic bags and sent to the laboratory for freezing. The coconut fruit samples were collected from two different coconut bunches at two maturity stages: one intended for coconut water consumption (bunch 19) and the other for industrial purposes (bunch coconut dry). These samples were collected 45, 90, and 120 days after pesticide application.
The stem samples were analyzed by using the modified QuEChERS for sample preparation and the LC-MS/MS to determine the concentration of the pesticides at the times and heights sampled. Briefly, after sample collection, drying, and grinding, 10 g of powdered sawdust stem was mixed with 30 mL of purified and homogenized water to form a slurry. Modified QuEChERS was performed in three steps: (1) Extraction: 10 g of slurry was mixed in 10 mL of acetonitrile with 1% (v/v) acetic acid in a 50 mL tube for 1 min. (2) Partition: 1.7 g of sodium acetate and 4 g of anhydrous magnesium sulfate were added, mixed for 1 min, and centrifuged (3400 rpm) for eight min. (3) Clean-up: Four mL of the supernatant was transferred to another tube containing 100 mg of PSA, 500 mg of C18, and 600 mg of MgSO4, and the tube was vortexed for 1 min and then centrifuged again (3400 rpm) for 8 min. The extract was filtered through a 0.22 mm PTFE filter, transferred into a vial, and then diluted 1:4 (v/v) with ultrapure water to inject in LC-MS/MS.
2.5. Field Sampling and Analysis of Pesticide Residues in Coconut Water and Pulp
For fruit analysis, twelve other coconut palm trees were chosen, and the pesticide solutions were applied using the same endotherapeutic techniques. At 45, 90, and 120 days post-treatment application, three coconut fruits were collected from each of the two bunches of plants treated with the two endotherapeutic methods. The samples included fruits from the bunch on leaf number 19, consisting of green fruits intended for coconut water, and the bunch with mature coconut fruits intended for industrial use. Subsequently, coconut water and coconut kernel (white meat/pulp) samples were prepared in the field, frozen, and then sent to the laboratory for pesticide residue analysis, totaling 18 samples for coconut water and 18 samples for coconut pulp. Samples from the fruit tissues collected from both coconut water and pulp were analyzed separately. Three fruits were harvested at each stage from each bunch and then homogenized. Coconut water was extracted through a hole in the fruit and transferred to sterilized polyethylene bottles, while the pulp was collected with a spatula and placed in sterile plastic bags.
All samples were carefully identified in the field, placed in sterile plastic bags or bottles, frozen at a temperature of −17 °C, then prepared for shipment in thermal boxes and sent to the Institute of Chemistry at the University of Campinas, Campinas-SP, Brazil, and kept in freezer conditions until the moment of the analysis in LC-MS/MS. Pesticide concentrations in the coconut water and coconut pulp were evaluated separately using these techniques. All instrumental and methodological conditions are thoroughly discussed in the references [11].
Pesticide residue from coconut water and pulp was analyzed using modified QuEChERS and the LC-MS/MS as proposed by Ferreira et al. [11]. The procedural steps of QuEChERS extraction conducted on the procedure for the coconut stem described in item Section 2.4 were the same as those for the coconut fruit. However, the methodology for the coconut fruit involved separate analyses for each sample due to the divergent physical properties of coconut water, characterized by its liquid consistency, and coconut pulp, which exhibits a more viscous texture. The distinctive aspect of the procedure for the coconut stem was the inclusion of a freezing-out step before the clean-up process. In this step, 8 mL of the supernatant was transferred to a separate tube, cooled in dry ice for 5 min, and subsequently centrifuged for 1 min.
2.6. LC-MS/MS
Pesticide determination was performed on ultra-performance liquid chromatography (UPLC) coupled to a sequential mass spectrometer, triple quadrupole (QqQ), model sequential mass spectrometer (Model TQ Quattro Micro API) by Waters (Milford, MA, USA) using an electrospray ionization source operated in positive mode (ESI+) and a mass analyzer in selective reaction monitoring mode (SRM). The ESI parameters were as follows: capillary voltage of 2.5 kV, source temperature of 150 °C, desolvation temperature of 500 °C, and nitrogen flow rates of 600 and 80 L h−1 for the cone and desolvation gases, respectively. Collision-induced dissociation was carried out using argon as the collision gas at a pressure of 4 bar with a flow rate of 0.15 mL min−1.
Pesticide separation was performed on an ACQUITY UPLC BEH C18 analytical chromatographic column (2.1 mm × 50 mm × 1.7 μm of particle diameter) from Waters. The mobile phase consisted of eluent A: water/methanol (98:2, v/v) and eluent B: methanol, with 0.1% formic acid and 5 mmol L−1 ammonium formate. A linear gradient program was used, with eluent B as follows: 5% at 0 min, 100% at 8.50 min, and 5% at 8.51 min until 10.00 min. The flow rate was 0.225 mL min−1, and the injection volume was 10 µL. The data acquisition system used Masslynx software version 4.1 by Waters (Milford, MA, USA).
Analytical curves were constructed for both the coconut water and pulp matrices, covering a concentration range of 2.5 to 250.0 µg L−1. The limit of quantitation (LOQ) for the method was 10 µg kg−1. For the coconut stem, the analytical curve ranged from 10 to 1000 µg L−1, and the LOQ was 40 µg kg−1. All the details of the development and validation of the analytical methods are discussed in the articles of Ferreira et al. [11].
2.7. Statistical Methods
The mean concentrations of the pesticides measured in stem tissues at each height and sampling time, in coconut pulp and in coconut water, were analyzed using analysis of variance (ANOVA). The mean pesticide concentrations in plants subjected to pressurized endotherapy were compared to the respective ones in plants subjected to the nonpressurized method using the Snedecor F test at a significance level of 0.10. As only two methods were being compared, no multicomparison tests were required. The analysis was performed using the MIXED procedure in the statistical software SAS/STAT® [17].
The pesticide concentrations measured in the field samples obtained through the pressurized method were adjusted using a ‘correction factor’ to estimate the concentrations that would have been obtained if the solution used had the same volume and concentration as those employed in the nonpressurized method. This transformation aimed to address challenges associated with applying the solution using the equipment utilized in this study while maintaining consistent pressure and employing the solution from the nonpressurized method. Subsequently, following the extrapolation, the mean pesticide concentrations at each height and time were compared with the respective ones obtained through the nonpressurized method.
The variables, the number of bunches, leaf length, and stem thickness for all coconut palms used in both treatments are described in Table S1 (pressurized and nonpressurized).
A principal component analysis (PCA) was also conducted based on plant variables, including the number of bunches, leaves, length, stem thickness, and pesticide concentrations measured at two heights. This analysis aimed to determine whether the clustering of trees could be associated with factors such as stem height, sampling time, and the type of endotherapy. The analysis was performed using the CLUSTER and TREE Procedures of the statistical software SAS/STAT® [17].
3. Results
The translocation of different fungicides and insecticides applied to the stems of coconut palm trees through two endotherapeutic methods, pressurized and nonpressurized, was evaluated separately and are presented in Section 3.1 and Section 3.2.
3.1. Pressurized Endotherapeutic Results
Figure 2 shows the pesticide concentration data from samples that UHPLC-MS/MS analyzed. The samples were taken 50 and 100 cm above the point of application and treated with pressurized endotherapy. Differently from the other pesticides in the mixture, carbosulfan is not detected in chromatographic analyses, but only its metabolites, carbofuran and 3-OH-carbofuran, are detected.
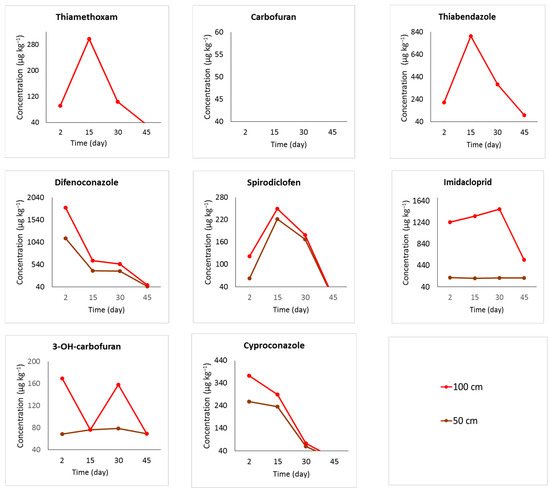
Figure 2.
Concentrations of pesticides (µg kg−1) in coconut palm stem samples determined by UHPLC-MS/MS. The samples were obtained either 50 or 100 cm above the application site (80 cm above ground). The field experiment was conducted in Mojú, PA, in Northern Brazil.
The pesticides with high concentrations found near the coconut palm canopy in the first two days were difenoconazole, imidacloprid, and cyproconazole, in decreasing order. The pesticides that showed higher concentrations 15 days after application, in decreasing order, were thiamethoxam, thiabendazole, and spirodiclofen, and at 30 days, it was imidacloprid. All pesticides studied showed a decrease in concentration 45 days after treatment. These results suggest that the apex of translocation took up to 30 days. The pesticides listed at decreasing rank of concentrations at 30 days were difenoconazole, imidacloprid, thiabendazole, cyproconazole, thiamethoxam, spirodiclofen, and 3-OH-carbofuran.
The translocation efficiency of the pesticide solution, which was diluted for pressurized endotherapy application, was determined by analyzing the concentrations primarily found near the canopy of the coconut palm. The red line in Figure 2 represents the concentration of pesticides at 100 cm, indicating that the compounds were translocated to the upper parts. Carbofuran, a metabolite of carbosulfan, was not detected, while another metabolite the 3-OH-carbofuran was quantified in the samples. The decrease in 3-OH-carbofuran at 15 days can be explained by the severe drought when the sample was collected.
3.2. Nonpressurized Endotherapeutic Results
The pesticide concentration data obtained from samples collected at 50 cm and 100 cm stem heights, above the application point, using a nonpressurized endotherapeutic treatment, are presented in Figure 3. The concentrations were determined using UHPLC-MS/MS to assess the pesticide’s translocation efficiency into the plant, as indicated in Table 2.
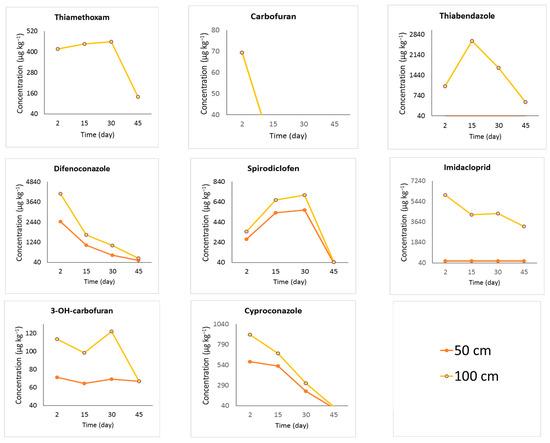
Figure 3.
Pesticide concentration (µg kg−1) evaluated by UHPLC-MS/MS through coconut palm trunk/stem samples collected at 50 or 100 cm above the application point (80 cm above ground) in palms subjected to nonpressurized endotherapy. The field experiment was conducted in Mojú, PA, in Northern Brazil.
In the nonpressurized treatment, difenoconazole, imidacloprid, cyproconazole, and carbofuran, in decreasing order, were the pesticides that moved the fastest. They were found near the plant’s canopy two days after the treatment was applied. The pesticides that showed the highest concentrations at 15 days were thiabendazole and, at 30 days, thiamethoxam, spirodiclofen, and 3-OH-carbofuran. At 45 days after treatment, all the compounds decreased in concentration, especially near the canopy of the plants (100 cm above the application point).
3.3. Analysis of Coconut Fruit Treated with a Mixture of Pesticides by Vegetative Endotherapy
In both pressurized and nonpressurized endotherapy methods, the fruits of bunches 19 and dry coconut bunches were evaluated, and no pesticide residue was detected in the coconut water or coconut kernels up to 120 days later. It is important to highlight that two types of coconut bunches were harvested: bunch 19, intended for the consumption of coconut water, and dry coconut bunches, used for the consumption of pulp and coconut water. During the endotherapeutic treatments, the type 19 bunches harvested for analysis at 45 days corresponded to bunch 17, those harvested at 90 days corresponded to bunch 15, and those harvested at 120 days were from bunch 14. In the case of dry coconut bunches, fruit samples collected at 45 days corresponded to bunch 20, those at 90 days were from bunch 18, and those at 120 days were from bunch 17.
3.4. Prediction of the Pesticide Concentration for the Pressurized Treatment Assuming the Same Volume and Concentration of the Solution Used in the Nonpressurized Method
A simulation/extrapolation of the data from the results found in the pressurized method (Figure 4) was necessary due to the following factors: the formulation viscosity, which had to be diluted; the difficulty of application by the Bite Infusion® equipment, which was overcome during treatment application; and the same volume and concentration of the solution that was applied in both methods. The concentrations obtained through the pressured approach were recalibrated using the guidelines outlined in Section 2.7 to facilitate a comparison between the different procedures. Based on this extrapolation, it was feasible to evaluate whether the pressurized endotherapy exhibited distinct translocation performance compared to the nonpressurized technique. From a chemical point of view, it is inappropriate to compare results when the applied solutions differ between the two endotherapeutic methods. This disparity arose due to the greater challenges associated with pressurized endotherapeutic applications. Nevertheless, through extrapolation and statistical adjustments based on field-obtained results, it became feasible to predict translocation if the samples were under the same volume and formulation concentration conditions.
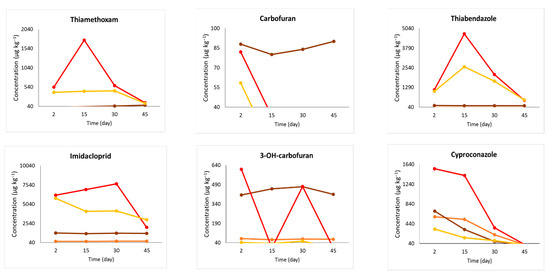
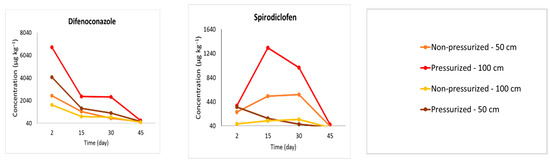
Figure 4.
Pesticide concentration (µg kg−1) analyzed by UHPLC-MS/MS in coconut palm stem samples collected 50 or 100 cm above the application point. The concentrations measured in plants treated with pressurized endotherapy were extrapolated considering that 20 mL of the mixture was still diluted in distilled water to simulate the conditions of the nonpressurized treatment. The field experiment was conducted in Mojú, PA, Brazil.
3.5. Exploratory Analysis via Principal Component Analysis (PCA)
The PCA did not separate groups for any of the factors examined (height, sampling time, or endotherapeutic treatments) (Figure S1). However, the PCA visualization is useful for revealing the different levels of global variability associated with the levels of each factor separately analyzed. For example, the dispersion of results from measurements performed at a height of 50 cm is much greater than that at 100 cm (Figure S1A). Similarly, the nonpressurized treatment led to more variability in pesticide concentration than did the pressurized treatment (Figure S1B). In terms of temporal factors, concentrations at 45 days after application exhibited less variation than those measured at 2, 15, and 30 days, all of which exhibited similar amounts of variation (Figure S1C). According to the PCA, the combined variability of both the mixture and plant variables was found to be similar for the periods of 2 and 15 days. However, this variability increased for the periods of 30 and 45 days (Figure S1D).
3.6. Comparison of Endotherapy Methods Regarding the Mean Concentration of Pesticides in the Stem
Significant effects of treatments on the mean concentration of pesticides in the coconut palm stem occurred in only a few cases: (a) Plants treated with pressurized endotherapy presented higher concentrations, approximately 3–5 times greater, compared to those treated with the nonpressurized method. This effect was observed for specific combinations of height and days following the application of carbofuran and thiamethoxam (F-test, p > 0.06), (b) In contrast, difenoconazole presented a superior concentration in the nonpressurized treatment of about 2.5 times only at 45 days and 100 cm height (F-test, p = 0.011; Tables S2 and S3).
In all cases for which the treatment effect was statistically significant (F-test, p < 0.10), the pesticide concentration was greater for the pressurized endotherapy, except for difenoconazole at 100 cm height.
3.7. Stem Damage Observed in Plants Subjected to Endotherapeutic Treatments after Five Years
A major concern for producers is the method by which entry holes are created, as they can potentially serve as entry also for pests or pathogenic microorganisms. In many cases, a substantial area near the application point or port may undergo decay, affecting multiple areas that can be rotten, compromising several vascular bundles, and thus hindering the path of translocation from the mixture, as illustrated in Figure 5.
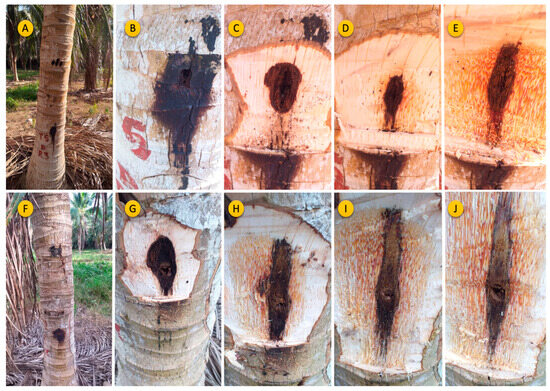
Figure 5.
The visual aspect of the coconut palm stems subjected to endotherapy over five years via the pressurized method using Bite Infusion® (A–E) and those subjected to the nonpressurized method using a drill machine (F–J).
4. Discussion
The choice of this mixture of active ingredients/pesticides was based on the simplicity and reduced cost associated with acquiring ready-made commercial formulations. Additionally, the active ingredients contained in these commercial formulations exhibit analytical patterns that allow evaluation by LC-MS/MS, serving as markers for monitoring the translocation in the stem of coconut palm trees.
The diversity of active ingredients (a.i) of insecticides and fungicides with different physicochemical properties suggests different translocation patterns through the xylem when applied to the coconut stem. The results indicated no correlation between the percentage of the active ingredient (a.i.) specified by the pesticide manufacturers in the rule and the actual amount detected in the samples collected in the stem. For example, the greatest translocation was from difenoconazole and imidacloprid, which contain 25% and 20%, respectively, of the a.i. in the formulation. Thiabendazole contained 48.5% a.i. in the formulation, but its translocation was lower than that of the previously mentioned products. In addition to the pesticide manufacturers’ formula, an organosilicon adjuvant was added to the endotherapeutic solution. This made the movement of all pesticides much more effective, as suggested by Ferreira et al. [4]. Bromilow’s model predicts the mobility of pesticides in plants based on the physicochemical properties of the compounds. Lipophilicity (logP) and dissociation constant (pKa) determine its solubility in the plant, absorption, and translocation. Their model describes mobility inside the plant, plotting logP and pKa for predicting pesticide translocation in plants. Therefore, the best systematicity (translocation potential) is related to higher pKa values and lower logP. Most of the compounds selected in this study, such as difenoconazole, thiabendazole, cyproconazole, and thiamethoxam, have moderate water solubility and a relatively high affinity for organic compounds. Imidacloprid is water-soluble, while spirodiclofen has a limited ability to dissolve in water due to its lipophilicity. Additionally, spirodiclofen is the only nonsystemic pesticide that has been tested. The solubility and stability of pesticide mixtures can vary depending on the pH of the environment [16]. Overall, Bromilow’s model may not serve to explain the results found in endotherapy since the translocation by using this technique is related to other factors, such as formulations, the impulse of the products through the raw sap in the xylem, and the endotherapy application methods employed.
The products are boosted through raw sap when applied to the trunk tree. This can be explained by the theory proposed by Joly and Dixon in 1804, in which water is sucked by the plant’s xylem vessels due to the combined forces of cohesion, tension, and adhesion which normally occur inside the coconut palm. Numerous experimental studies have supported this theory and are widely accepted as the most accurate explanation of plant water transport [4,18,19,20,21].
A study by Paraiba and collaborators [14] used physicochemical properties and the pesticide stem-sap partition coefficient to model how pesticides move through the coconut palm. The authors used LC-MS/MS to quantify pesticide residues in the fruit and sap after application of nonpressurized endotherapy with commercial SOS Palm® equipment (Castellón, Spain). Simulations showed that (i) the pesticides dimethoate, metalaxyl, and thiamethoxam were the active ingredients showing the greatest potential for translocation in the sap of the coconut tree stem; and (ii) the pesticides imidacloprid and metalaxyl translocated upward in the stem and more rapidly than did the pesticides abamectin and cyproconazole. In our work, after taking samples from the trunk for up to 45 days, we found that imidacloprid continued at a high concentration for up to 30 days and decayed after 45 days. As Paraiba’s model also predicted, cyproconazole had a very low concentration close to the canopy after 30 days, as shown in Figure 3; pressurized endotherapy can solve this issue for the majority of compounds. Thiamethoxam did not exhibit optimal translocation performance through nonpressurized endotherapy. In contrast, imidacloprid demonstrated superior behavior in this regard, with the product being translocated at high concentrations near the canopy for a duration of up to 30 days. Carbofuran and 3-OH-carbofuran are carbosulfan metabolites in our study, resulting in their low concentrations.
Mathematical models are useful tools for predicting the translocation of products within the plants, but depending on model hypotheses, some factors cannot be included, such as (1) product/formulation characteristics; (2) plant characteristics such as species, age, and nutritional status; and (3) mode of application, such as method of application, dosage, and viscosity of the formulation. Moreover, the distribution of products throughout the plant may not be uniform, and the rate at which products translocate may fluctuate over time. Interactions with other substances in the environment or conditions such as temperature and humidity can influence the translocation of products. Understanding the complexities of pesticide translocation within plants, including the significant influence of these environmental factors, is essential for optimizing pesticide application strategies.
The fruits of the coconut palms treated with the two endotherapeutic methods were analyzed and evaluated, and no pesticide residues were detected in the coconut water or pulp (white meat). Paraiba et al. [14], using a simulation model, also did not predict or quantify pesticide residues in water and coconut fruit pulp from palm trees treated with pesticides, after the evaluated intervals. However, this monitoring must be continued.
The efficacy of commercial pesticides—thiamethoxam, emamectin benzoate, and imidacloprid—was investigated for controlling red palm weevil (RPW) infestation in Phoenix canariensis using pressurized endotherapy applied by the commercial equipment ENDOterapia Vegetal S.L. (Castelló d’Empúries, Spain). Emamectin benzoate exhibited superior systemicity and persistence in palm tissues compared to thiamethoxam and imidacloprid, with residues detected for up to 5 months and offering protection against RPW infestation for as long as 9 months. Thiamethoxam demonstrated limited efficacy, as residues were only detected once and proved insufficient to prevent the collapse of infested palm trees. Imidacloprid residues persisted for up to 3 months but exhibited varying levels of efficacy. LC-MS/MS was employed to analyze pesticide residues in palm tissues following the QuEChERS extraction method. Leaf bases were selected for analysis due to their vulnerability to RPW larvae. Field trials confirmed the efficacy of emamectin benzoate, highlighting its systemic nature and prolonged protection against RPW infestation, thus presenting a promising long-term solution for pest control in these palm trees [22].
In a different study, using the pesticides abamectin and imidacloprid in palm trees through endotherapy was very successful at lowering the number of Rhynchophorus ferrugineus in Phoenix canariensis. The results indicated that endotherapy provided better distribution and persistence of the pesticides compared to other application methods, such as frond injection and crown spraying. The lethal concentrations of these pesticides were able to effectively protect the palm trees for up to 3 months after treatment. ENDOterapia Vegetal S.L. was used to apply the pesticides, mixed with Endomix Palm®, which has been proven effective in stabilizing the mixture and enhancing pesticide movement within the palm tree. Abamectin was detected at lethal concentrations in frond tissues for up to 5 months after endotherapy, while imidacloprid was detected at lethal concentrations for up to 2–3 months after treatment. The QuEChERS method with European variation EN 15 662 was employed for pesticide residue determination, and LC-MS/MS was utilized for analysis [23].
The results obtained from both studies demonstrated that treatment using pressurized endotherapy with imidacloprid persisted in controlling RPW for approximately 3 months. These findings align with our results, as we detected imidacloprid in coconut stem tissues even after 45 days. Additionally, residue analyses by LC-MS/MS corroborated these results, further confirming the efficacy of imidacloprid in controlling this pest. The study revealed unsatisfactory outcomes regarding the efficacy of thiamethoxam in controlling pest infestation. Our findings indicated the presence of thiamethoxam residues up to 45 days post-application. These results suggest that further investigation could explore the utilization of alternative commercial formulations, potentially yielding varied outcomes in RPW control.
In recent years, published studies on endotherapy have revealed that the insecticides imidacloprid and thiamethoxam, evaluated in the list of this study, were among the most widely used products. Fungicides and insecticides were the two most frequently used substances in endotherapeutic techniques. Additionally, pressurized endotherapy has been predominant in published works [4].
In their review, Sharma and Dubey [24] critically assessed the efficacy of endotherapy in managing various challenges faced by coconut palm trees. A multi-study approach for controlling insect pests, diseases, and nutritional deficiencies involving applying different volumes and formulations was described. The authors also emphasized the benefits of endotherapy in reducing the use of water and chemicals, in addition to targeted delivery due to the systemic action of active ingredients, reduced environmental impact, long-lasting effects, and labor efficiency. However, despite these efforts, there is still a need for greater endotherapeutic technological developments specific to palm trees, as well as for the development of formulations aimed at palm farming.
5. Conclusions
In general, the pressurized endotherapy method resulted in higher concentrations of pesticides near the plant canopy when compared with the nonpressurized method. It was feasible to extrapolate pesticide concentrations that would be obtained in the pressurized method if the volume and concentration of the pesticide solution were the same as the ones used in the nonpressurized endotherapy. In addition, the nonpressurized technique led to more extensive damage to the stem, including necrosis of nearby tissues and bundles. The pressurized technique caused a lesser extent of injuries; nevertheless, there is room for improvement in the application device. Further research on palm trees is essential for advancing their technological capabilities and assisting specialists in formulating recommendations. This includes developing new formulations targeted at the endotherapy market. Through these efforts, it becomes feasible to recommend adequate dosages and intervals for the safe application of insecticides and fungicides to benefit both coconut growers and consumers.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae10040386/s1, Table S1: The variables, the number of bunches, leaf length, and stem thickness for all coconut palms for pressurized treatment, Table S2: The variables, the number of bunches, leaf length, and stem thickness for all coconut palms nonpressurized treatment, Table S3: Nominal significance level (p-value) of the F-tests used to compare the concentration means of the studied pesticides between the treatments pressurized and nonpressurized application at 50 and 100 cm above the application point and the average of the concentrations in both heights for each measurement time, Table S4: The mean concentration of the studied pesticides and respective standard errors in the treatments pressurized (P) and nonpressurized (NP) at 50 and 100 cm above the application point for each measurement time. Figure S1: Results of PCA based on sets of variables related to the pesticide mixture applied to coconut palm trees via pressurized or nonpressurized endotherapy.
Author Contributions
Conceptualization, J.A.F., C.B.G.B., J.M.S.F. and P.M.P.L.; methodology, J.A.F. and C.B.G.B.; software, J.A.F. and A.d.H.N.M.; validation, A.d.H.N.M., J.M.S.F. and C.B.G.B.; formal analysis, J.A.F. and A.d.H.N.M.; investigation, J.A.F., J.M.S.F. and P.M.P.L.; resources, J.A.F., C.B.G.B. and P.M.P.L.; data curation, J.A.F. and A.d.H.N.M.; writing—original draft preparation, J.A.F., J.M.S.F. and A.d.H.N.M.; writing—review and editing, J.M.S.F. and A.d.H.N.M.; visualization, C.B.G.B. and P.M.P.L.; supervision, C.B.G.B.; project administration, J.A.F., C.B.G.B., J.M.S.F. and P.M.P.L.; funding acquisition, J.A.F., C.B.G.B. and P.M.P.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FAPESP 2012/18318-4. This work was also financially supported by the National Institutes of Science and Technology (INCT) [grant numbers: FAPESP/INCT 2014/50867-3, CNPq/INCT 465389/2014-7]. C. B. G. Bottoli acknowledges CNPq for a research fellowship [CNPq 313185/2021-2].
Data Availability Statement
Data are contained within the article or Supplementary Materials.
Acknowledgments
The authors thank Gerson Moraes, Edivaldo Costa, Ronaldo Aranha, and Samuel C. Cohen Farias for all their support with field trials at Sococo Farm.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- VanWoerkom, A.H.; Aćimović, S.G.; Sundin, G.W.; Cregg, B.M.; Mota-Sanchez, D.; Vandervoort, C.; Wise, J.C. Trunk Injection: An Alternative Technique for Pesticide Delivery in Apples. Crop Prot. 2014, 65, 173–185. [Google Scholar] [CrossRef]
- Wise, J.C.; VanWoerkom, A.H.; Aćimović, S.G.; Sundin, G.W.; Cregg, B.M.; Vandervoort, C. Trunk Injection: A Discriminating Delivering System for Horticulture Crop IPM. Entomol. Ornithol. Herpetol. 2014, 3, 1–7. [Google Scholar] [CrossRef]
- Berger, C.; Laurent, F. Trunk Injection of Plant Protection Products to Protect Trees from Pests and Diseases. Crop Prot. 2019, 124, 1–9. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Esparraguera, L.B.; Queiroz, S.C.N.; Bottoli, C.B.G. Vegetative Endotherapy—Advances, Perspectives, and Challenges. Agriculture 2023, 13, 1465. [Google Scholar] [CrossRef]
- Mota-Sanchez, D.; Cregg, B.M.; McCullough, D.G.; Poland, T.M.; Hollingworth, R.M. Distribution of Trunk-Injected 14C-Imidacloprid in Ash Trees and Effects on Emerald Ash Borer (Coleoptera: Buprestidae) Adults. Crop Prot. 2009, 28, 655–661. [Google Scholar] [CrossRef]
- Byrne, F.J.; Krieger, R.I.; Doccola, J.; Morse, J.G. Seasonal Timing of Neonicotinoid and Organophosphate Trunk Injections to Optimize the Management of Avocado Thrips in California Avocado Groves. Crop Prot. 2014, 57, 20–26. [Google Scholar] [CrossRef]
- Ferry, M.; Gomez, S. Assessment of Risks and Potential of Injection Techniques in Integrated Programs to Eradicate the Red Palm Weevil: Review and New Perspectives. Fruits 2014, 69, 143–157. [Google Scholar] [CrossRef]
- Coslor, C.C.; Vandervoort, C.; Wise, J.C. Control of Insect Pests Using Trunk Injection in a Newly Established Apple Orchard. Int. J. Fruit. Sci. 2019, 19, 151–164. [Google Scholar] [CrossRef]
- Montecchio, L. A Venturi Effect Can Help Cure Our Trees. J. Vis. Exp. 2013, 80, e51199. [Google Scholar] [CrossRef]
- Wise, J.C. Advances in Insect Control and Resistance Management, 1st ed.; Horowitz, A.R., Ishaaya, I., Eds.; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-31798-4. [Google Scholar]
- Ferreira, J.A.; Ferreira, J.M.S.; Talamini, V.; Facco, J.d.F.; Rizzetti, T.M.; Prestes, O.D.; Adaime, M.B.; Zanella, R.; Bottoli, C.B.G. Determination of Pesticides in Coconut (Cocos nucifera Linn.) Water and Pulp Using Modified QuEChERS and LC–MS/MS. Food Chem. 2016, 213, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Doccola, J.J.; Wild, P.M. Tree Injection as an Alternative Method of Insecticide Application. In Insecticides-Basic and Other Applications; Soloneski, S., Ed.; InTech: Vienna, Austria, 2004; p. 268. [Google Scholar]
- Moura, J.I.L.; Warwick, D.R.N.; Luz, E.D.M.N.; Filho, L.P.d.S.; Valle, R.R. Effect of Ciproconazole Injection on Leaf Disease Control and Yield of Coconut Plants. Summa Phytopathol. 2019, 45, 186–190. [Google Scholar] [CrossRef]
- Paraiba, L.C.; Ferreira, J.M.S.; Ferracini, V.L.; Ramos, S.R.R.; Cerdeira, A.L.; Assalin, M.R.; Pazianotto, R.A.A.; Santos, A.J.; Paraiba, C.C.M. Modeling Pesticide Translocation Injected by Endotherapy into the Stem of Coconut Tree (Cocos nucifera L.). Span. J. Agric. Res. 2022, 20, e1002. [Google Scholar] [CrossRef]
- Suganthi, A.; Rajeswari, E.; Sivakumar, V.; Bhuvaneswari, K.; Madhu Sudhanan, E.; Sathiah, N.; Prabakaran, K. Analysis of Tebuconazole Residues in Coconut Water, Kernel and Leaves Using LC–MS/MS. Food Chem. 2021, 359, 129920. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.; Tzilivakis, J. Development of a Data Set of Pesticide Dissipation Rates in/on Various Plant Matrices for the Pesticide Properties Database (PPDB). Data 2017, 2, 28. [Google Scholar] [CrossRef]
- SAS Institute. SAS/STAT® User’s Guide; SAS Institute Inc.: Cary, NY, USA, 2020. [Google Scholar]
- You, X.; Lu, Q.; Guan, X.; Xu, Z.; Zenobi, R. Pesticide Uptake and Translocation in Plants Monitored In Situ via Laser Ablation Dielectric Barrier Discharge Ionization Mass Spectrometry Imaging. Sens. Actuators B Chem. 2024, 409, 135532. [Google Scholar] [CrossRef]
- Perron, N.; Baltzer, J.L.; Sonnentag, O. Spatial and Temporal Variation in Forest Transpiration across a Forested Boreal Peatland Complex. In Proceedings of the Hydrological Processes; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2023; Volume 37. [Google Scholar]
- Bentrup, F.W. Water Ascent in Trees and Lianas: The Cohesion-Tension Theory Revisited in the Wake of Otto Renner. Protoplasma 2017, 254, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Steudle, E. The Cohesion-Tension Mechanism and the Acquisition of Water by Plant Roots. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 847–875. [Google Scholar] [CrossRef] [PubMed]
- Chihaoui-Meridja, S.; Harbi, A.; Abbes, K.; Chaabane, H.; La Pergola, A.; Chermiti, B.; Suma, P. Systematicity, Persistence and Efficacy of Selected Insecticides Used in Endotherapy to Control the Red Palm Weevil Rhynchophorus Ferrugineus (Olivier, 1790) on Phoenix Canariensis. Phytoparasitica 2020, 48, 75–85. [Google Scholar] [CrossRef]
- Dembilio, Ó.; Riba, J.M.; Gamón, M.; Jacas, J.A. Mobility and Efficacy of Abamectin and Imidacloprid against Rhynchophorus Ferrugineus in Phoenix Canariensis by Different Application Methods. Pest. Manag. Sci. 2015, 71, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Dubey, S.C. Tree Injection Method to Manage Coconut Pests with Special Reference to Blackheaded Caterpillar, Opisina Arenosella and Mite, Aceria Guerreronis—A Review. Pest. Manag. Hortic. Ecosyst. 2023, 29, 1–12. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).