Abstract
Banana is a typical tropical fruit that is widely cultivated in tropical and subtropical regions, and consumed daily because of its superior nutritional profile. This study aimed to investigate the changes in bananas’ physicochemical properties and nutritional composition from ripening stages 1 to 9. The analysis included color (peel and flesh), texture, total starch content, resistant starch content, free sugar content, minerals (calcium, magnesium, potassium, sodium, iron, phosphorus), and vitamin content. At the same time, the browning enzyme activities of polyphenol oxidase (PPO), peroxidase, oxalic acid, and tannin were also measured. In this study, the banana peel gradually changed from dark green to light green to yellow during the ripening process, eventually appearing with many black spots, while the pulp color changed from white to light yellow and decreased in brightness. The pulp became softer with a prolonged ripening period, which was inconsistent with the time point of peel color change (ripening period 4–5). However, total starch content decreased from 74% to 31%, resistant starch originally at 32% gradually decreased to 5%, and free sugar content gradually increased with increasing ripening period. Vitamin C content and PPO activity decreased significantly during the ripening period, while other vitamins and minerals did not change significantly. In contrast, oxalic acid, tannin content, and peroxidase activity were negatively correlated with the ripening period. Therefore, this study reveals the changes in the nutrient content of bananas at different ripening stages, which may provide helpful information for researchers and producers to identify suitable raw materials for producing stable and high-nutrition-value banana-related foods during the peak raw material abundance seasons while reducing business risk.
1. Introduction
Bananas are an important tropical fruit, with 1000 varieties cultivated in many tropical and subtropical countries, regardless of world economic development, with high consumption rates [1,2,3,4,5,6,7,8]. Approximately 37% of the production is in Asia and the Pacific and is classified into commercial and non-commercial varieties (also known as native varieties) [1,2]. Commonly served raw as a dessert or sweet fruit, it is also used in some regions as a staple and plantain [6,9]. Unripe bananas are fried and made into thin slices, and they can also be processed into various other forms, such as juice, puree, and flour, so that they can be stored longer without adding preservatives. Ripe bananas are sliced and used as ingredients in baked goods, beverages, dairy products, desserts, and many other processed foods [9,10]. However, relatively few have been cultivated on a large scale [3]. Widely known for their high nutritional value, they contain dietary fiber, pectin, high levels of minerals (potassium and phosphorus), phenolic compounds (e.g., catechins, trichothecenes, lignans, tannins, and anthocyanins), vitamins (A, B, C, and E), β-carotene, and phytosterols [1,3,4,9,10,11]. It is worth noting that contemporary consumers are increasingly health conscious (daily sufficient consumption) and prioritize ingredients that promote overall health and wellness, such as high fiber, antioxidants, and micronutrients (minerals, vitamins, etc.), apart from appearance and sensory properties [12,13,14]. This shift in consumer preference towards health-promoting ingredients has significant implications for related industries, and businesses that can effectively cater to this demand will eventually be successful. Concurrently, micronutrient fortification and crop improvement modulate the critical role of plant genetics in maintaining plant health, growth stability, and stress tolerance. Maximized yields satisfy human dietary and nutritional needs by ensuring healthy crop development. It is crucial to regulate plant genes by implementing micronutrient fortification and crop improvement strategies to maintain plant health, promote growth stability, and enhance stress tolerance [12]. This is essential to ensure healthy crop development and optimal yield, which is necessary to satisfy human dietary nutritional requirements. Therefore, the most cost-effective way to reduce micronutrient malnutrition banana is to introduce the cultivars selected and bred for increased mineral nutrition [15]. Remarkably, the differences in mineral content can be attributed not only to banana cultivars but also to soil, climate, agricultural practices (fruit development stages and conditions), irrigation water quality, and storage conditions [15,16,17,18,19,20,21]. Unfortunately, current scholarly reports on the use of genetic modification to develop bananas rich in iron, zinc, and carotenoids or breeding to enhance the mineral content of other fruits are still in their infancy and are not yet commercialized [10,15,20,22,23]. Improving the bioavailability of micronutrients and educating people about unhealthy foods through food preparation and processing techniques is currently effective in enhancing trace mineral deficits in populations [20,24]. Apart from the factors mentioned earlier, the visual quality is the defect that affects the consumer’s purchasing decision; namely, the product’s appearance, including color variations, texture, flavor, shape different from the norm, pests, and physical damage [12,25,26]. However, the ripening of bananas involves various physiological changes that impact the fruit’s texture, sugar content, and color [27]. The color of the banana peel greatly impacts consumer acceptance and purchase, with a uniform yellow color indicating optimal ripeness and quality [28]. It is imperative to clarify that the physical appearance of a banana does not necessarily reflect its gustatory appeal [28]. As such, avoiding discarding those that may appear aesthetically imperfect is recommended to mitigate economic losses and reduce food waste.
Moreover, because of the global diversity of dietary habits, food taboos, body composition, and disease conditions, standardizing dietary standards is a daunting challenge, and similar databases and standards are needed across food cultures to facilitate scientific communication, trade, and policy development [29,30]. Hence, this study aimed to investigate the relationship between micronutrient content and the ripening stages of Pei Chiao. This study provides a comprehensive understanding of shedding light on the nutrient dynamics of bananas, and it serves as a stepping stone toward promoting sustainable and efficient production practices in the related industries.
2. Materials and Methods
2.1. Materials
All bananas (AAA Cavendish Var. Pei Chiao, originating from Zhushan Township, Nantou, Taiwan) were purchased from Tai-Yi Agricultural and Livestock Products Co. (Taichung, Taiwan) between February and April 2023. The banana plants were grown in flood deposited sandy soil under 25–30 °C. Bananas harvested on the same day were delivered to Tai-Yi Co. (around 30 kg) within 2 h at room temperature, followed by pre-cooling (at 12–18 °C for 24 h), then ripening treatment with 1000 ppm ethylene at 15 °C. In this study, the bananas were divided into 9 stages of peel color according to the ripening schedule [31,32] (Figure 1), immediately placed in plastic bags (around 3 kg per stage), and returned to the laboratory for 15 min for analysis. Unless otherwise specified, all chemicals used in this study were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
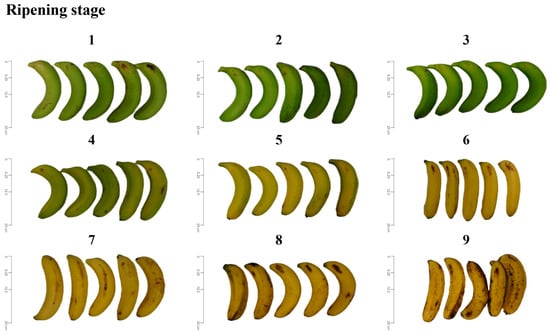
Figure 1.
Changes in the appearance and color of banana peel at ripening stages 1–9.
2.2. Color Analysis of Banana Peel and Pulp
Color was determined according to the method described by Huang et al. [33], with modifications. Color measurements were performed using a spectrophotometer (Model CM-5; Konica Minolta Co., Ltd., Tokyo, Japan) to determine the brightness (L*, larger values indicate higher brightness; conversely, darker), redness–greenness (a*; a positive value means close to red and a negative value means the color tends to be closer to green), and yellowness–blueness (b*; positive values indicate that the color is closer to yellow and negative values indicate that the color tends to be blue) of the banana peel and pulp at different ripening stages. The banana peel was tested at five random points, whereas the banana pulp was measured separately on the surface of the pulp and cut. The white index (WI) was computed using the following equation:
2.3. Banana Texture Profile Analysis
A texture analyzer (TA-XT2, Stable Micro Systems, Vienna Court, UK) was used for the analysis. The banana pulp was sliced to a thickness of 1 cm, and a P/10 diameter probe was used. The parameters were set at an initial speed of 3 mm/s, a speed of 1 mm/s during and after the test, and 50% deformation for the measurement.
A three-point-bend rig was used for the pulp-cutting analysis, and the HDP/BSK tool was used as the probe. The parameters were set to an initial speed of 3 mm/s and 1 mm/s, respectively, during and after the test, and the force required to cut a 1 cm banana slice was recorded.
2.4. Banana Flour Preparation
Pei Chiao samples ripened for 1–9 days and were washed and peeled, and then the pulps were cooled rapidly in liquid nitrogen to avoid chemical reactions during freezing. Afterward, the samples were placed in a −80 °C refrigerator for freezing, followed by freeze-drying. Finally, the powder was ground and stored in double-layer zipper bags (PET/PVDC/CPP) in a freezer (−20 °C) until use.
2.5. Basic Components Analysis
The basic components were analyzed using the American Association of Cereal Chemists (AOAC) [34] method for moisture (44-15A), crude fat (30-10), ash (08-01), crude fiber (32-10), and crude protein (46-11A) contents.
2.6. Analysis of Total Starch and Resistant Starch Contents
In this study, total starch was analyzed using the kit (AA/AMG) (K-TSTA-100A, Neogen Co., Lansing, MI, USA), whereas resistant starch was analyzed using the assay kit (rapid) (K-RAPRS, Neogen Co.) according to the standard operating procedures in the kit and calculated using the following equation:
where,
ΔA is the sample absorbance value at 510 nm minus the blank absorbance value,
D is the dilution factor.
where,
ΔA is the sample absorbance value at 510 nm minus the blank absorbance value,
W is the sample weight (mg).
2.7. Free Sugar Content Determination
Free sugar content was determined according to Nielsen [35], with minor modifications. The sample (0.2 g) was added to 10 mL 80% ethanol and extracted by refluxing in a hot water bath at 90 °C for 30 min. Centrifugation (Model CR22F, Hitachi Co., Tokyo, Japan) was performed at 6000× g for 15 min, and the residue was repeated three times. Finally, all the supernatants were collected and quantified to 100 mL, and the absorbance values were determined using the phenol-sulfuric acid method at 490 nm. A standard curve was prepared with glucose, and the free sugar content of the samples was calculated by interpolation.
2.8. Vitamin Content Determination via High-Performance Liquid Chromatography (HPCL)
2.8.1. Ascorbic Acid (Vitamin C)
The methodology for the vitamin C assay was based on the Gentili and Caretti [36] description with slight modifications. The sample (2 g) was homogenized with 30 mL of 5% HPO3 for 10 min, and the solution was quantitated to 50 mL. The solution was filtered through a 0.22 µm filter membrane, followed by a 20 µL sample for HPLC analysis. The operation should be performed at low temperatures and should be kept away from light to avoid oxidation. The mobile phase was prepared in a ratio of 0.05 M KH2PO4 (pH 4.6) to cyanomethane 30:70 (v/v). The flow rate was 1 mL/min, a UV detector was used, and the measured wavelength was 210 nm. The analytical column was a Mightysil RP-18 (4.0 × 250 mm × 5 µm, Kanto Chemical Co., Inc., Tokyo, Japan). A standard curve was prepared with L-ascorbic acid, followed by an interpolation to calculate the concentration of ascorbic acid in the samples.
2.8.2. Thiamin (Vitamin B1), Riboflavin (Vitamin B2), and Pyridoxine (Vitamin B6)
Vitamins B1, B2, and B6 were determined according to the Gentili and Caretti [36] description with slight modifications. Sample (5 g) was added to 65 mL of 0.1 M HCl and reacted in a boiling water bath for 30 min. After cooling, the pH was adjusted to 4.5 with a 2.5 M sodium acetate solution. Then, 50 mg of β-amylase and 500 mg of takadiastase were added, mixed well, and incubated in an oven at 37 °C for 18 h before quantification to 100 mL. The sample was filtered through a 0.22 µm cellulose acetate filter membrane, and 20 µL was used for HPLC analysis (parameters as described below). The standard curves were prepared with riboflavin and pyridoxine and then interpolated to calculate the samples’ vitamin B2 and B6 amounts.
In addition, 1 mL of the filtrate was added to 3 mL of potassium ferricyanide solution (consisting of 1 mL of 1% potassium ferricyanide mixed with 24 mL of 3.75 M NaOH) and left to stand for 1 min, after which 10 mL of 0.05 M sodium acetate solution was added. Finally, 20 µL was filtered through a 0.22 µm filter membrane and analyzed by HPLC. The mobile phase was methanol and 0.05 M sodium acetate solution at a ratio of 30:70 (v/v). The flow rate was 1 mL/min, the UV detector was measured at 275 nm, and the column was Mightysil RP-18 (Kanto Chemical Co., Inc.). The standard curve was prepared by oxidizing vitamin B1 to thiachrome with potassium ferricyanide solution, and then the content of vitamin B1 in the samples was calculated by interpolation.
2.9. Minerals Contents Determination
The mineral content assay was performed as described by Jorhem [37] with minor modifications. The sample (5 g) was carbonized at 250 °C for 2 h in an aching oven and then ashed at 600 °C until it appeared white or off-white. Next, 0.15% nitric acid was used to dissolve and prepare a 100 ppm sample solution. The minerals (potassium, sodium, magnesium, iron, calcium, and phosphorus) contained in the samples were analyzed using atomic absorption spectrometry (PerkinElmer Inc., Waltham, MA, USA), and standard curves of each element were prepared to calculate the content in the samples.
2.10. Determination of Brown Pigment Enzyme Activity
2.10.1. Polyphenol Oxidase (PPO) Activity
PPO activity was measured according to the method described by Sikora et al. [38], with slight modifications. The crude enzyme extract was prepared using 5 g of sample and 100 mL of 0.05 M Tris-HCl buffer solution (pH 7.0), homogenized for 60 s in a homogenizer, and then centrifuged at 6000× g for 20 min. Subsequently, 2.6 mL of 0.2 M pyrocatechol was mixed with 0.1 mL of crude enzyme extract solution, recording the absorbance value at 390 nm for each min. The enzyme activity unit (U) was defined as the amount of enzyme that increased the absorbance value by 0.001 per minute.
2.10.2. Peroxidase Activity
Peroxidase activity was determined according to the method described by Nelson and Parsonage [39], with minor modifications. The crude enzyme extract was prepared by adding 5 g of sample, adding 60 mL of 0.2 M sodium phosphate buffer solution (pH 6.0) containing 5% NaCl, homogenizing for 60 s with a homogenizer, and then filtering. Then, 2.9 mL of the substrate solution (0.2 M sodium phosphate buffer solution with 0.05 M Guaiaol and 0.02 M H2O2) was added to 0.1 mL of crude enzyme extract solution. The absorbance was measured at 420 nm, and the increase in absorbance per minute was recorded. Enzyme activity was expressed in U as an OD 420 nm/min/g sample.
2.11. Oxalic Acid and Tannin Content Analysis
The oxalic acid and tannin contents were determined according to the method described by Karamad et al. [40] and Verzele and Delahaye [41], with minor modifications. The sample (1 g) was added to 40 mL of 0.6 N HC1 and placed in a boiling water bath at 100 °C for 15 min. The sample was then filtered through a 0.22 µm filter membrane, and 20 µL of the sample was used to determine the absorbance values at 210 nm (oxalic acid) and 280 nm (tannin) by HPLC. Standard oxalic acid and tannin curves were used to calculate the samples’ oxalic acid and tannin contents. The HPLC conditions for the determination of oxalic acid were as follows: 0.01 N H2SO4 was used as the mobile phase, and the analytical column was an ICSep Coregel-87H3 7.8 × 300 mm (ICE-99-9861, Chrom Tech, Inc., Apple Valley, MN, USA). The flow rate was 0.8 mL/min. In addition, the HPLC conditions for tannin determination were consistent with those described in Section 2.8.1, except that the absorbance values differed from the mobile phase (60:40 and 10:90 (v/v) for methanol and 1% acetic acid solution, respectively).
2.12. Statistical Analysis
All measurements in this study were performed in 3 replicates, and the values in the figures and tables are expressed as mean ± standard deviation (S.D.). All data were analyzed using SAS package statistical software (V9.4, SAS Institute Inc., Cary, NC, USA), and one-way analysis of variance (ANOVA) was used to analyze the differences between samples, with significant differences indicated by p < 0.05.
3. Results and Discussion
3.1. Basic Components of Bananas
In this study, Pei Chiao samples were peeled and sliced at different ripening periods, frozen with liquid nitrogen, and placed in a −80 °C freezer until completely frozen. The moisture content ranged from 70.19 to 76.81% (Table 1) and was positively correlated with the ripening period (p < 0.05). In addition, the crude fiber (ranging from 0.58 to 0.68%), crude fat (ranging from 0.30 to 0.41%), ash (ranging from 3.04 to 3.75%), and crude protein (ranging from 5.14 to 6.24%) content (Table 1) did not change significantly during the ripening stages. However, the crude fiber and fat contents of the banana cultivar Pei Chiao used in this study showed similar results to those reported by Oyeyinka and Afolayan [42] for M. sinensis. Generally, the basic components of bananas are related to the effects of the variety, soil, agronomic practices, cultivation, and harvest season [4,18,21,43,44,45].

Table 1.
Changes in moisture, crude fiber, crude fat, crude protein, ash, total starch, resistant starch, and free sugar contents of bananas in different ripening stages.
3.2. Variation of Banana Color Analysis
3.2.1. Banana Appearance (Peel) Color Changes
Commercially, different research-developed standard color charts have been used to visually inspect and determine the ripening stages of bananas [8]. During the 9-day ripening stage, the banana appearance changed, as shown in Figure 1, in which the unripe banana peel at ripe stage 1 was dark green, then changed to light green, and golden yellow started to appear at the head and tail. At ripe stage 5, most of the green color changed to golden yellow. At ripe stage 6, only the head and tail parts were green, and ripe stage 7 was fully ripe and yellow. However, the appearance of many brown spots in ripe stage 9 also indicates that it reaches the overripe stage. In addition, color measurement showed that the L value of bananas gradually increased during ripening, but the brightness of overripe bananas decreased significantly (Table 2), mainly because of the large number of brown spots produced by overripe bananas. The a value changed from negative at the beginning to positive with ripening, which meant that the green color of the banana peel gradually disappeared. The b value gradually increased with ripening time, which means that the color of banana peel tends to be golden yellow; however, numerous brown spots of over ripening caused the b value to decrease. The WI value represents the whiteness of the sample, which increased with the ripening of the banana during the ripening stage, but the lowest whiteness was observed on the ninth day of development, which was also affected by brown spots. Thus, evidence of the ripening course is visible, in which the color of the banana peel changes from green to yellow due to chlorophyll degradation with marginal changes in carotenoids and phenols, while brown spots appear during ripening stage 9, followed by softening of the pulp, which begins spoilage, leading to serious economic losses [5,7,8,10,21,46,47].

Table 2.
Changes in the color of banana peel and pulp (surface and cutting surface) in different ripening stages.
3.2.2. Banana Pulp Color Change
The pulp color of different banana cultivars ranged from cream, light, and dark yellow to orange and was highly correlated with carotenoid content accumulation [46,48]. Interestingly, Jaiswal et al. [49] reported that a values could predict peel and flesh hardness with good correlation, but practical values may not be stable with the predicted values. Analysis of banana pulp color showed a gradual decrease in the L value during ripening, indicating a gradual reduction in pulp brightness during development (Table 2). It was assumed that the white color of the unripe banana pulp was due to the presence of more starch, and the trend of decreasing L value of the banana pulp surface and pulp truncation was consistent. However, the L values on the pulp cut-off were lower than those on the surface, which may be due to the partial presence of banana seeds on the pulp cut-off, resulting in lower L values. The a value of the pulp exhibited no significant trend at any ripening stage, whereas the highest a value was observed at ripening stage 9. The b value increased gradually with the ripening stage; in particular, that at ripening stage 9 increased significantly, which was related to the tendency of the pulp to turn yellow. Unfortunately, brown spots on banana peels affect consumers’ willingness to buy and reduce prices. Several studies have reported that the green to yellow changing of the banana peel during ripening can be attributed to chlorophyll degradation [7,16,49,50,51]. Moreover, the yellow coloration of the banana peel may be attributed to changes in the content of lutein and α- and β-carotene compounds, which may be highest at ripening stage 4 in turning yellow [7,16,45,46,49].
3.3. Banana Texture Variation
Several studies have shown that the factors affecting the banana firmness include cultivar, cultivation method, climate, supply chain, and ripening stage [7,8,15,16,21,49]. In this study, the results of the change in banana pulp texture showed that firmness decreased significantly during the ripening stage (Table 3). During ripening stage 1, the firmness of bananas was 6059 N. In ripening stage 4, half of the banana peel turned yellow; the firmness decreased to 1343 N, followed by a continuous decrease. This study revealed a sharp decrease in banana firmness during peel color transitions. It is well known that pectin methyl esterase (PME), polygalacturonate (PG), and pectin lyases (PLs) play an essential role in the fruit softening process [52]. In addition, other textural-related indicators, such as adhesiveness, gumminess, and chewiness, showed significant changes, among which adhesiveness increased with the ripening period of the banana [7,49,53]. This was attributed to starch and undegraded pectin at the initial ripening stage and low moisture content, whereas starch was degraded to small molecules and high moisture content at the post-ripening stage of banana; thus, the adhesiveness increased. Gumminess and chewiness were positively correlated with banana pulp firmness. Therefore, physicochemical variations may be responsible for the decrease in banana firmness, including the hydrolysis of starch into sugar by amylase and the increased solubility of pectin substances, leading to a decline in banana texture adhesion and the migration of water from the peel to the pulp resulting from increased osmotic pressure [6,16,45,53,54,55].

Table 3.
Changes in the texture profile of banana pulp slices at different ripening stages.
3.4. Variation of Total Starch and Resistant Starch Content during Ripening
The total starch content of bananas depends on the cultivar and cultivation conditions, whereas the resistant starch of bananas is RS2 naturally resistant starch with a compact structure and crystalline form [19,21,45]. In this study, the results of the total starch content of bananas showed that total starch content was as high as 74% in ripening stage 1 and gradually decreased with the ripening stage, which was reduced to 31% in ripening stage 9 (Table 1). In particular, a rapid decrease in the total starch content was achieved at ripening stages 3–4, namely, at the point where the yellow color of the peel was evident. According to a previous report, green Pei Chiao bananas (without postharvest treatment) have an extremely high total starch content of 95.7% [21]. Several studies have reported that amylase, β-amylase, α-1,4-, α-1,6-glucosidase, and amyloglucosidase are involved in the ripening stage of bananas, which hydrolyze starch into tiny molecules of sugars [45,56,57].
The variation in resistant starch content showed the same trend as the total starch content variation, which also decreased gradually with the ripening stage, where resistant starch decreased from a maximum of 35 to 5% (Table 1). It is worth mentioning that the resistant starch content in this study was slightly higher in ripening stage 3 than in stages 1–2, probably due to the difference in sampling. The resistant starch content decreased significantly from ripening stage 4, while the banana peel exhibited a largely yellow color. However, several studies have also reported a positive correlation between the amylose content in banana flour and its RS content and solubility [18,19,58,59].
3.5. Free Sugar Content
Sugars, organic acids, and non-structural carbohydrates are the main components of ripe banana pulp dry mass [53]. Moreover, the starch content in bananas decreased from 15–25% to less than 5% during ripening, mainly because of the degradation of sucrose by sucrose phosphate synthase in the initial stage of ripening, followed by conversion to glucose and fructose in the post-ripening stage, while the total sugar content exhibited a positive correlation with the ripening stages [3]. In this study, the free sugar content of bananas varied from 19.30 mg glucose/g at ripening stage 1 and increased gradually as the ripening time increased (Table 1). The same trend was observed for the total starch content, implying that enzymes hydrolyze the starch in bananas, whereas the changes in monosaccharide concentration during ripening involve the sweetness of the fruit [16,45].
3.6. Changes in Vitamins Content during Banana Ripening
Like most other fruits, banana pulp is acidic (pH below 4.5) and contains significant ascorbic, citric, malic, and oxalic acids, which increase as the fruit ripens [3]. In this study, changes in vitamin C content during banana ripening showed a slight increase with increasing ripening period (Table 4). The vitamin C content of bananas at ripening stage 1 was 277 µg/g and reached 346 µg/g at ripening stage 8, which unfortunately showed a significant decrease at ripening stage 9. It was assumed that the overripe yellow banana tissues became soft and rotten, causing vitamin C degradation by reacting with other substances. Moreover, there were no significant changes in the content of vitamin B1 (between 0.55 µg/g sample and 0.87 µg/g sample), B2 (between 3.57 µg/g sample and 4.20 µg/g sample), and B6 (between 43.57 µg/g sample and 50.94 µg/g sample) during ripening. However, no significant changes were observed during ripening (Table 4).

Table 4.
Changes in the content of vitamins (C, B1, B2, and B6), oxalic acid, tannic acid, polyphenol oxidase activity, and peroxidase activity of bananas in different ripening stages.
3.7. Changes in the Minerals Content of Bananas during Ripening
Mineral elements are essential nutritional components, and critical minerals are primarily provided in a balanced diet with physiologically functional effects related to the body’s structural, physiological, and metabolic processes [42,60]. Potassium (recommended daily intake (RDA) of 4700 mg for adults) manages hypertension, which helps in the proper functioning of the heart and physiological processes, including water balance regulation [3,22,42,60,61]. Moreover, sodium (RDA, 1500 mg) is an important electrolyte that helps maintain intracellular and extracellular water balance, including the regulation of osmotic pressure homeostasis, and a sodium–potassium ion ratio below 1 contributes to the reduction and control of hypertension risk, including blood pressure and related conditions [42,60,62,63]. Magnesium (RDA, 450 mg) is physiologically active and essential for heart function, curbing early diabetes, nerve impulse transmission, detoxification, and the structural strength of bones and teeth [42,60]. Iron (RDA of 14–18 mg) is essential for the immune system, hemoglobin synthesis, oxygen production, and electronic transport [17,18,42]. Calcium (RDA, 1000 mg) is essential for optimal bone development and growth with proper functioning of the heart, muscular, and nervous systems [18,42,60]. Phosphorus (RDA, 200–1000 mg) improves calcium absorption and strengthens bones and teeth, especially in minors and pregnant women [42,60]. However, the diversity of diets may cause nutritional issues regarding trace elements among people in different countries and regions [17].
The minerals during the banana ripening period (potassium, copper, magnesium, iron, calcium, and phosphorus) showed no significant trend (Table 5). Notably, the most abundant mineral in Pei Chiao was potassium, 946.69 mg/100 g sample, followed by magnesium, phosphorus, and calcium, whereas copper and iron were less abundant. It is worth mentioning that the Fe content was the lowest in this study, and this difference could be due to the low amount of Fe present in the cultivated soil, which is consistent with the results reported by Watharkar et al. [16]. In addition, the mineral content slightly increases at ripening stages 3–5, with these minor changes presumably due to osmotic pressure from the peel to the pulp and vice versa [16]. Oyeyinka and Afolayan [42] reported that two banana cultivars (M. sinensis and M. paradisiaca) have high potassium content, particularly in peel extracts. In addition, do Prado Ferreira and Teixeira Tarley [18] reported significant differences between the mineral contents of different brands of green banana (M. paradisiaca) flour, with the following ranking of the mineral elements in order of abundance: Mg > Ca > Fe ≥ Mn > Zn > Cu. However, the high potassium content may be attributed to the use of potassium fertilizer in artificial fertilization, whereas differences in the mineral contents of plants might be due to factors such as soil mineral composition availability, pH, oxidation–reduction conditions, cation exchange capacity, and plant root structure, whereas the diversity of bacterial and mycorrhizal fungi may also affect mineral content. Cultivars, agricultural practices, agronomic methods, and climate change may have similar effects [3,15,16,17,18,19,23,44,64]. However, Forster and Díaz Romero [65] reported no effects of cultivar on chemical compositions in bananas harvested in Tenerife and in bananas harvested in Ecuador, except for insoluble fiber contents.

Table 5.
Changes in mineral contents of banana pulp at different ripening stages.
3.8. Changes in Oxalic Acid and Tannin Content of Bananas during Ripening
From a fruit-consuming point of view, tannins are probably the most critical phenolic substances, but they provide berries with an unpleasant astringent taste [3]. Antinutrients (such as tannins, phytates, oxalates, phosphates, carbonates, and fibers) are naturally present in fruits, grains, and vegetables and have the potential to reduce the absorption of minerals, proteins, vitamins, and minerals in the gastrointestinal tract because of their chelating effect [17,18,66]. As we all know, oxalic acid interacts with iron, calcium, and magnesium ions during metabolism in the body, affecting mineral assimilation. One possible approach for preventing the recurrence of calcium oxalate kidney stones is to reduce the consumption of oxalic acid-rich foods. Although the long-term effectiveness of dietary restriction is uncertain, it has been reported to reduce stone recurrence [67,68,69]. In recent years, a positive effect on patients with calcium oxalate kidney stones has been reported by maintaining the balance of the human gut microbiota while increasing populations of oxalate-degrading bacteria [70,71].
In this study, the analysis of oxalic acid content in the banana pulp at different ripening stages revealed significant changes (Table 4). During ripening stage 1, the oxalic acid content was as high as 22,000 µg/g. In comparison, it decreased to 13,260 µg/g in ripening stage 2 and 320 µg/g in ripening stage 4, reaching as low as 13 µg/g in ripening stage 9. Moreover, Oyeyinka and Afolayan [42] reported a relatively high oxalic acid content in two banana cultivars (M. sinensis and M. paradisiaca), which was similar to that observed in this study at ripening stages 1–3. Sourness is an essential aspect of banana sensory quality, which is related to the perception of sweetness and sourness based on the sugar–acid ratio, which is an indicator that influences consumer acceptability [53]. Regarding the changes in tannic content, it also decreased gradually with the ripening process of the banana (Table 4), with approximately 578 µg/g sample at ripening stage 2 and a significant decrease at ripening stages 3–4, with a steady reduction to ripening stage 9. In addition, the astringent taste of the pulp decreases as ripening advances, which Pareek [3] reported appears to be related to changes in the tannin structure to form polymers rather than a decrease in their content. Interestingly, Agama-Acevedo et al. [43] reported tannins (31.0 mg GAEs/g) and hydrolyzable tannins (20.1 mg GAEs/g) in banana peels with antioxidant capacity. However, the peel contains high levels of bioactive substances (e.g., polyphenols and tannins), which are associated with the natural defense system of plant tissues [43].
3.9. Changes in PPO and Peroxidase Activities during Banana Ripening
PPO plays a vital role in the browning reaction of fruits [72]. However, the disintegration of cell wall tissues during ripening causes enzymes in the cells to be released and interact with phenolic compounds to produce o-quinones, leading to an electronic oxidation mechanism [73]. Denaxa et al. [72] reported that pre-harvest use of glycine betaine in bananas might reduce PPO activity and darkening of fruit, while it reduced chlorophyll degradation, prevented the premature rise of ethylene, and reduced the evolution of ethylene.
In this study, the changes in PPO activity during the banana ripening period gradually increased (Table 4), reaching its highest at ripening stage 8 but slightly decreasing at ripening stage 9. This implies that banana pulp is more prone to browning during ripening. Moreover, peroxidase activity gradually decreased with increasing ripening period (Table 4), with the highest activity (3.90 U) at ripening stage 1.
4. Conclusions
This study reveals the changes in the nutrient content of bananas at different ripening stages, which may provide helpful information for researchers and producers to identify suitable raw materials for producing stable and high-nutrition-value banana-related foods. Following the change in the color of the Pei Chiao peel from green to yellow, the firmness of the pulp softened significantly, and the content of total starch and resistant starch also showed a rapid decrease while the free sugar content increased. However, vitamins B1, B2, B6, and minerals (potassium, magnesium, calcium, and phosphorus) showed no significant trend during the ripening period of the bananas. A slight increase in vitamin C was observed but decreased in the overripe bananas. Notably, tannic acid and oxalic acid, both sources of astringency and nutrient antagonists, decreased during banana ripening, whereas oxalic acid rapidly decreased at the beginning of ripening. The browning enzyme PPO gradually increased activity during ripening but slightly decreased after over-ripening, while peroxidase showed the opposite trend during ripening. Therefore, the results of this study may provide a reference for the selection of raw materials for banana processing in the future.
Author Contributions
Conceptualization, P.-H.H., C.-C.W. and P.-H.L.; methodology, P.-H.H., W.-C.L., J.-L.Y., C.-C.W. and P.-H.L.; software, W.-C.L., J.-L.Y. and P.-H.L.; validation, W.-C.L., J.-L.Y., C.-C.W. and P.-H.L.; formal analysis, W.-C.L., J.-L.Y. and P.-H.L.; investigation, W.-C.L., J.-L.Y., C.-C.W., Y.-S.L. and P.-H.L.; resources, Y.-T.C., P.-Y.C., Y.-S.L., P.-Y.C. and P.-H.L.; data curation, J.-L.Y., C.-C.W., Y.-S.L. and P.-H.L.; writing—original draft preparation, P.-H.H. and P.-H.L.; writing—review and editing, P.-Y.C. and P.-H.L.; visualization, P.-Y.C. and P.-H.L.; supervision, P.-H.L.; project administration, Y.-T.C. and P.-H.L.; funding acquisition, Y.-T.C. and P.-H.L. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by Taichung Veterans General Hospital.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this study.
References
- Keivani Rad, N.; Mohri, M.; Seifi, H.A.; Haghparast, A. Supplementation of overripe pulp extract and green peel extract or powder of banana fruit peel (musa. Cavendish) to diets of neonatal dairy calves: Effects on haematological, immunological and performance characteristics. Vet. Med. Sci. 2021, 7, 876–887. [Google Scholar] [CrossRef] [PubMed]
- Anyasi, T.A.; Jideani, A.I.O.; Mchau, G.A. Morphological, physicochemical, and antioxidant profile of noncommercial banana cultivars. Food Sci. Nutr. 2015, 3, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Pareek, S. Chapter 3—Nutritional and biochemical composition of banana (musa spp.) cultivars. In Nutritional Composition of Fruit Cultivars; Simmonds, M.S.J., Preedy, V.R., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 49–81. [Google Scholar]
- Lopes, S.; Moresco, R.; Peruch, L.A.M.; Rocha, M.; Maraschin, M. UV-Vis spectrophotometry and chemometrics as tools for recognition of the biochemical profiles of organic banana peels (musa sp.) according to the seasonality in southern Brazil. In Proceedings of the 11th International Conference on Practical Applications of Computational Biology & Bioinformatics, Porto, Portugal, 21–23 June 2017; Springer International Publishing: Cham, Switzerland, 2017; pp. 289–296. [Google Scholar]
- Mukherjee, S.; Dey, P.K.; Mitra, A. Application of anantmul (Hemidesmus indicus) root extract improves postharvest shelf-life of dessert banana. Postharvest Biol. Technol. 2023, 199, 112290. [Google Scholar] [CrossRef]
- Kumar, P.S.; Thayumanavan, S.; Pushpavalli, S.; Saraswathi, M.S.; Backiyarani, S.; Mohanasundaram, A.; Uma, S. Comparing physico-chemical characteristics, antioxidant properties, glycemic response, and volatile profiles of eleven banana varieties. Int. J. Food Sci. Technol. 2023, 58, 2893–2908. [Google Scholar] [CrossRef]
- Owoeye, O.R.; Oluwole, A.M.; Jolayemi, O.S.; Oluwalana, I.B. Linear and nonlinear regression modeling of the chemical, physical and quality variations in cardaba banana (Musa acuminata x balbisiana—ABB) during ripening. J. Food Meas. Charact. 2023, 17, 12–23. [Google Scholar] [CrossRef]
- Saha, K.K.; Zude-Sasse, M. Estimation of chlorophyll content in banana during shelf life using LiDAR laser scanner. Postharvest Biol. Technol. 2022, 192, 112011. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Bioactive compounds in banana and their associated health benefits—A review. Food Chem. 2016, 206, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sheng, O.; Yin, Z.; Huang, W.; Chen, M.; Du, M.; Kong, Q.; Fernie, A.R.; Yi, G.; Yan, S. Metabolic profiling reveals genotype-associated alterations in carotenoid content during banana postharvest ripening. Food Chem. 2023, 403, 134380. [Google Scholar] [CrossRef]
- Arora, A.; Choudhary, D.; Agarwal, G.; Singh, V.P. Compositional variation in β-carotene content, carbohydrate and antioxidant enzymes in selected banana cultivars. Int. J. Food Sci. Technol. 2008, 43, 1913–1921. [Google Scholar] [CrossRef]
- Ahmed, N.; Zhang, B.; Chachar, Z.; Li, J.; Xiao, G.; Wang, Q.; Hayat, F.; Deng, L.; Narejo, M.-U.-N.; Bozdar, B.; et al. Micronutrients and their effects on horticultural crop quality, productivity and sustainability. Sci. Hortic. 2024, 323, 112512. [Google Scholar] [CrossRef]
- Assunção, A.G.L.; Cakmak, I.; Clemens, S.; González-Guerrero, M.; Nawrocki, A.; Thomine, S. Micronutrient homeostasis in plants for more sustainable agriculture and healthier human nutrition. J. Exp. Bot. 2022, 73, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- Melse-Boonstra, A. Bioavailability of micronutrients from nutrient-dense whole foods: Zooming in on dairy, vegetables, and fruits. Front. Nutr. 2020, 7, 101. [Google Scholar] [CrossRef]
- Devarajan, R.; Jayaraman, J.K.; Somasundaram, S.M.; Ragupathy, S.; Raman, P.; Sathiamoorthy, K.; Subbaraya, U. Genetic diversity in fresh fruit pulp mineral profile of 100 Indian Musa accessions. Food Chem. 2021, 361, 130080. [Google Scholar] [CrossRef] [PubMed]
- Watharkar, R.B.; Pu, Y.; Ismail, B.B.; Srivastava, B.; Srivastav, P.P.; Liu, D. Change in physicochemical characteristics and volatile compounds during different stage of banana (Musa nana lour vs. Dwarf cavendish) ripening. J. Food Meas. Charact. 2020, 14, 2040–2050. [Google Scholar] [CrossRef]
- Freeland-Graves, J.H.; Sachdev, P.K.; Binderberger, A.Z.; Sosanya, M.E. Global diversity of dietary intakes and standards for zinc, iron, and copper. J. Trace Elem. Med. Biol. 2020, 61, 126515. [Google Scholar] [CrossRef] [PubMed]
- do Prado Ferreira, M.; Teixeira Tarley, C.R. Assessment of in vitro bioacessibility of macrominerals and trace elements in green banana flour. J. Food Compos. Anal. 2020, 92, 103586. [Google Scholar] [CrossRef]
- Khoza, M.; Kayitesi, E.; Dlamini, B.C. Physicochemical characteristics, microstructure and health promoting properties of green banana flour. Foods 2021, 10, 2894. [Google Scholar] [CrossRef] [PubMed]
- Ofori, K.F.; Antoniello, S.; English, M.M.; Aryee, A.N.A. Improving nutrition through biofortification–A systematic review. Front. Nutr. 2022, 9, 1043655. [Google Scholar] [CrossRef] [PubMed]
- Samanros, A.; Lin, J. Physicochemical properties and in vitro digestibility of starches from different Taiwanese banana cultivars. Int. Food Res. J. 2021, 28, 1257–1267. [Google Scholar] [CrossRef]
- Borges, C.V.; Maraschin, M.; Coelho, D.S.; Leonel, M.; Gomez, H.A.G.; Belin, M.A.F.; Diamante, M.S.; Amorim, E.P.; Gianeti, T.; Castro, G.R.; et al. Nutritional value and antioxidant compounds during the ripening and after domestic cooking of bananas and plantains. Food Res. Int. 2020, 132, 109061. [Google Scholar] [CrossRef]
- Costa, B.N.S.; Costa, I.D.J.S.; de Souza, G.A.; de Abreu, R.A.A.; de Melo, E.T.; Pio, L.A.S.; Pasqual, M. Different types of soil mulches in the leaf anatomy and physiology of ‘brs platina’ banana in a non-irrigated management. Sci. Hortic. 2022, 292, 110605. [Google Scholar] [CrossRef]
- Lu, W.-C.; Cheng, Y.-T.; Lai, C.-J.; Chiang, B.-H.; Huang, P.-H.; Li, P.-H. Mathematical modeling of modified atmosphere package/LDPE film combination and its application to design breathing cylinders for extending the shelf life of green asparagus. Chem. Biol. Technol. Agric. 2023, 10, 60. [Google Scholar] [CrossRef]
- Cheng, Y.-T.; Huang, P.-H.; Chan, Y.-J.; Chen, S.-J.; Lu, W.-C.; Li, P.-H. A new strategy to design novel modified atmosphere packaging formulation maintains the qualities of postharvest strawberries (Fragaria ananassa) during low-temperature storage. J. Food Saf. 2023, 43, e13082. [Google Scholar] [CrossRef]
- Lu, W.-C.; Chan, Y.-J.; Chen, S.-J.; Mulio, A.T.; Wang, C.-C.R.; Huang, P.-H.; Li, P.-H. Using calcined oyster shell powder as a natural preservative for extending the quality of black king fish (Rachycentron canadum) fillets. J. Food Process Pres. 2022, 46, e17262. [Google Scholar] [CrossRef]
- Maduwanthi, S.; Marapana, R. Biochemical changes during ripening of banana: A review. Int. J. Food Sci. Nutr. 2017, 2, 166–170. [Google Scholar]
- Symmank, C.; Zahn, S.; Rohm, H. Visually suboptimal bananas: How ripeness affects consumer expectation and perception. Appetite 2018, 120, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Swinnen, J.; Maertens, M.; Colen, L. The role of food standards in trade and development. In Food Safety, Market Organization, Trade and Development; Hammoudi, A., Grazia, C., Surry, Y., Traversac, J.-B., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 133–149. [Google Scholar]
- Argumedo, A.; Song, Y.; Khoury, C.K.; Hunter, D.; Dempewolf, H.; Guarino, L.; de Haan, S. Biocultural diversity for food system transformation under global environmental change. Front. Sustain. Food Syst. 2021, 5, 685299. [Google Scholar] [CrossRef]
- PBMH-PIF. Banana musa spp.: Normas de Classificação. 2006. Available online: https://www.bdpa.cnptia.embrapa.br/consulta/busca?b=ad&id=879920&biblioteca=vazio&busca=PROGRAMA%20BRASILEIRO%20PARA%20A%20MODERNIZAÇÃO%20DA%20HORTICULTURA%20&%20PRODUÇÃO%20INTEGRADA%20DE%20FRUTAS&qFacets=PROGRAMA%20BRASILEIRO%20PARA%20A%20MODERNIZAÇÃO%20DA%20HORTICULTURA%20&%20PRODUÇÃO%20INTEGRADA%20DE%20FRUTAS&sort=&paginacao=t&paginaAtual=1 (accessed on 11 March 2023).
- Cheng, Y.; Huang, P.; Chan, Y.; Chiang, P.; Lu, W.; Hsieh, C.; Liang, Z.; Yan, B.; Wang, C.R.; Li, P. Investigate the composition and physicochemical properties attributes of banana starch and flour during ripening. Carbohydr. Polym. Technol. Appl. 2024, 7, 100446. [Google Scholar] [CrossRef]
- Huang, P.-H.; Chiu, C.-S.; Lu, W.-C.; Li, P.-H. Effect of compositions on physicochemical properties and rheological behavior of gelatinized adzuki-bean cake (yokan). LWT 2022, 168, 113870. [Google Scholar] [CrossRef]
- AACC. AACC Approved Methods of Analysis, 11th ed.; American Association of Cereal Chemists: St. Paul, MN, USA, 2010. [Google Scholar]
- Nielsen, S.S. Phenol-sulfuric acid method for total carbohydrates. In Food Analysis Laboratory Manual; Nielsen, S.S., Ed.; Springer: Boston, MA, USA, 2010; pp. 47–53. [Google Scholar]
- Gentili, A.; Caretti, F. Chapter 19—Analysis of vitamins by liquid chromatography. In Liquid Chromatography, 2nd ed.; Fanali, S., Haddad, P.R., Poole, C.F., Riekkola, M.-L., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 571–615. [Google Scholar]
- Jorhem, L. Determination of metals in foods by atomic absorption spectrometry after dry ashing: NMKL1 collaborative study. J. AOAC Int. 2019, 83, 1204–1211. [Google Scholar] [CrossRef]
- Sikora, M.; Świeca, M.; Franczyk, M.; Jakubczyk, A.; Bochnak, J.; Złotek, U. Biochemical properties of polyphenol oxidases from ready-to-eat lentil (Lens culinaris medik.) sprouts and factors affecting their activities: A search for potent tools limiting enzymatic browning. Foods 2019, 8, 154. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.J.; Parsonage, D. Measurement of peroxiredoxin activity. Curr. Protoc. Toxicol. 2011, 49, 7–10. [Google Scholar] [CrossRef]
- Karamad, D.; Khosravi-Darani, K.; Hosseini, H.; Tavasoli, S. Analytical procedures and methods validation for oxalate content estimation. Biointerface Res. Appl. Chem. 2019, 9, 4305–4310. [Google Scholar] [CrossRef] [PubMed]
- Verzele, M.; Delahaye, P. Analysis of tannic acids by high-performance liquid chromatography. J. Chromatogr. A 1983, 268, 469–476. [Google Scholar] [CrossRef]
- Oyeyinka, B.O.; Afolayan, A.J. Comparative evaluation of the nutritive, mineral, and antinutritive composition of Musa sinensis L. (banana) and Musa paradisiaca L. (plantain) fruit compartments. Plants 2019, 8, 598. [Google Scholar] [CrossRef] [PubMed]
- Agama-Acevedo, E.; Sañudo-Barajas, J.A.; Vélez De La Rocha, R.; González-Aguilar, G.A.; Bello-Peréz, L.A. Potential of plantain peels flour (Musa paradisiaca L.) as a source of dietary fiber and antioxidant compound. CyTA-J. Food 2016, 14, 117–123. [Google Scholar] [CrossRef]
- Li, Z.; Jiao, Y.; Yin, J.; Li, D.; Wang, B.; Zhang, K.; Zheng, X.; Hong, Y.; Zhang, H.; Xie, C.; et al. Productivity and quality of banana in response to chemical fertilizer reduction with bio-organic fertilizer: Insight into soil properties and microbial ecology. Agric. Ecosyst. Environ. 2021, 322, 107659. [Google Scholar] [CrossRef]
- Li, M.-C.; Chou, C.-F.; Hsu, S.-C.; Lin, J.-S. Physicochemical characteristics and resistant starch of different varieties of banana from Taiwan. Int. J. Food Prop. 2020, 23, 1168–1175. [Google Scholar] [CrossRef]
- Fu, X.; Cheng, S.; Liao, Y.; Huang, B.; Du, B.; Zeng, W.; Jiang, Y.; Duan, X.; Yang, Z. Comparative analysis of pigments in red and yellow banana fruit. Food Chem. 2018, 239, 1009–1018. [Google Scholar] [CrossRef]
- Ningsih, R.; Rafi, M.; Tjahjoleksono, A.; Bintang, M.; Megia, R. Ripe pulp metabolite profiling of ten Indonesian dessert banana cultivars using UHPLC-Q-Orbitrap HRMS. Eur. Food Res. Technol. 2021, 247, 2821–2830. [Google Scholar] [CrossRef]
- Pereira, A.; Maraschin, M. Banana (musa spp.) from peel to pulp: Ethnopharmacology, source of bioactive compounds and its relevance for human health. J. Ethnopharmacol. 2015, 160, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, P.; Jha, S.N.; Kaur, P.P.; Bhardwaj, R.; Singh, A.K.; Wadhawan, V. Prediction of textural attributes using color values of banana (Musa sapientum) during ripening. J. Food Sci. Technol. 2014, 51, 1179–1184. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kudachikar, V.B.; Kulkarni, S.G.; Prakash, M.N. Effect of modified atmosphere packaging on quality and shelf life of ‘Robusta’ banana (musa sp.) stored at low temperature. J Food Sci. Technol. 2011, 48, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Seymour, G.B. Banana. In Biochemistry of Fruit Ripening; Seymour, G.B., Taylor, J.E., Tucker, G.A., Eds.; Springer: Dordrecht, The Netherlands, 1993; pp. 83–106. [Google Scholar]
- Wu, M.-C.; Jiang, C.-M.; Huang, P.-H.; Wu, M.-Y.; Wang, Y.T. Separation and utilization of pectin lyase from commercial pectic enzyme via highly methoxylated cross-linked alcohol-insoluble solid chromatography for wine methanol reduction. J. Agric. Food Chem. 2007, 55, 1557–1562. [Google Scholar] [CrossRef] [PubMed]
- Biabiany, S.; Araou, E.; Cormier, F.; Martin, G.; Carreel, F.; Hervouet, C.; Salmon, F.; Efile, J.-C.; Lopez-Lauri, F.; D’Hont, A.; et al. Detection of dynamic qtls for traits related to organoleptic quality during banana ripening. Sci. Hortic. 2022, 293, 110690. [Google Scholar] [CrossRef]
- Chiang, P.-Y.; Li, P.-H.; Huang, C.-C.; Wang, C.-C.R. Changes in functional characteristics of starch during water caltrop (Trapa Quadrispinosa Roxb.) growth. Food Chem. 2007, 1, 376–382. [Google Scholar] [CrossRef]
- Nannyonga, S.; Bakalis, S.; Andrews, J.; Mugampoza, E.; Gkatzionis, K. Mathematical modelling of color, texture kinetics and sensory attributes characterisation of ripening bananas for waste critical point determination. J. Food Eng. 2016, 190, 205–210. [Google Scholar] [CrossRef]
- Durán-Soria, S.; Pott, D.M.; Osorio, S.; Vallarino, J.G. Sugar Signaling During Fruit Ripening. Front. Plant Sci. 2020, 11, 564917. [Google Scholar] [CrossRef] [PubMed]
- Cordenunsi-Lysenko, B.R.; Nascimento, J.R.O.; Castro-Alves, V.C.; Purgatto, E.; Fabi, J.P.; Peroni-Okyta, F.H.G. The starch is (not) just another brick in the wall: The primary metabolism of sugars during banana ripening. Front. Plant Sci. 2019, 10, 452915. [Google Scholar] [CrossRef]
- Vu, H.T.; Scarlett, C.J.; Vuong, Q.V. Phenolic compounds within banana peel and their potential uses: A review. J. Funct. Foods 2018, 40, 238–248. [Google Scholar] [CrossRef]
- Passo Tsamo, C.V.; Herent, M.-F.; Tomekpe, K.; Happi Emaga, T.; Quetin-Leclercq, J.; Rogez, H.; Larondelle, Y.; Andre, C. Phenolic profiling in the pulp and peel of nine plantain cultivars (musa sp.). Food Chem. 2015, 167, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Abib, B.; Qin, Z.; Ze, X.; Ali, S.E. Dietary macrominerals: Updated review of their role and orchestration in human nutrition throughout the life cycle with sex differences. Curr. Res. Food Sci. 2023, 6, 100450. [Google Scholar] [CrossRef] [PubMed]
- Ekmekcioglu, C.; Wallner, P.; Kundi, M.; Weisz, U.; Haas, W.; Hutter, H.-P. Red meat, diseases, and healthy alternatives: A critical review. Crit. Rev. Food Sci. Nutr. 2018, 58, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kwock, C.K.; Yang, Y.J. The effect of the sodium to potassium ratio on hypertension prevalence: A propensity score matching approach. Nutrients 2016, 8, 482. [Google Scholar] [CrossRef] [PubMed]
- Perez, V.; Chang, E.T. Sodium-to-potassium ratio and blood pressure, hypertension, and related factors. Adv. Nutr. 2014, 5, 712–741. [Google Scholar] [CrossRef] [PubMed]
- Omidvari, M.; Abbaszadeh-Dahaji, P.; Hatami, M.; Kariman, K. Chapter 2—Biocontrol: A novel eco-friendly mitigation strategy to manage plant diseases. In Plant Stress Mitigators; Ghorbanpour, M., Shahid, M.A., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 27–56. [Google Scholar]
- Forster, M.P.; Rodríguez Rodríguez, E.; Díaz Romero, C. Differential characteristics in the chemical composition of bananas from tenerife (canary islands) and ecuador. J. Agric. Food Chem. 2002, 50, 7586–7592. [Google Scholar] [CrossRef] [PubMed]
- Nguyễn, H.V.H.; Savage, G.P. Oxalate content of New Zealand grown and imported fruits. J. Food Compos. Anal. 2013, 31, 180–184. [Google Scholar] [CrossRef]
- Ivanovski, O.; Drüeke, T.B. A new era in the treatment of calcium oxalate stones? Kidney Int. 2013, 83, 998–1000. [Google Scholar] [CrossRef] [PubMed]
- Huynh, N.K.; Nguyen, D.H.M.; Nguyen, H.V.H. Effects of processing on oxalate contents in plant foods: A review. J. Food Compos. Anal. 2022, 112, 104685. [Google Scholar] [CrossRef]
- Durdaği, S.; Al-Jalawee, A.H.H.; Yalçin, P.; Bozkurt, A.S.; Salcan, S. Morphological characterization and phase determination of kidney stones using X-ray diffractometer and scanning electron microscopy. Chin. J. Phys. 2023, 83, 379–388. [Google Scholar] [CrossRef]
- Wu, F.; Cheng, Y.; Zhou, J.; Liu, X.; Lin, R.; Xiang, S.; Liu, Z.; Wang, C. Zn2+ regulates human oxalate metabolism by manipulating oxalate decarboxylase to treat calcium oxalate stones. Int. J. Biol. Macromol. 2023, 234, 123320. [Google Scholar] [CrossRef] [PubMed]
- Zampini, A.; Nguyen, A.H.; Rose, E.; Monga, M.; Miller, A.W. Defining dysbiosis in patients with urolithiasis. Sci. Rep. 2019, 9, 5425. [Google Scholar] [CrossRef] [PubMed]
- Denaxa, N.-K.; Tsafouros, A.; Ntanos, E.; Roussos, P.A. Chapter 8—Role of glycine betaine in the protection of plants against environmental stresses. In Plant Stress Mitigators; Ghorbanpour, M., Adnan Shahid, M., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 127–158. [Google Scholar]
- Bolton, J.L.; Dunlap, T.L.; Dietz, B.M. Formation and biological targets of botanical o-quinones. Food Chem. Toxicol. 2018, 120, 700–707. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).