Differences in Soil Water Holding Capacity and Available Soil Water along Growing Cycle Can Explain Differences in Vigour, Yield, and Quality of Must and Wine in the DOCa Rioja
Abstract
1. Introduction
2. Materials and Methods
2.1. Area of Study
2.2. Experimental Design and Vineyard Plot Description
2.3. Soil Description and Soil Analysis
2.4. Calculation of Available Soil Water
2.5. Grapevine Nutritional Status
2.6. Grapevine Agronomic Performance
2.7. Grape Sampling and Analytical Parameters of Must
2.8. Vinification
2.9. Wine Analysis
2.10. Statistical Analysis
3. Results
3.1. Weather Conditions Recorded during the Period of the Study
3.2. Simulated Soil Water Contents and Available Soil Water for the Selected Plots and Years
3.3. Nutritional Status, Vigor, and Yield
3.4. Must Composition
3.5. Wine Composition
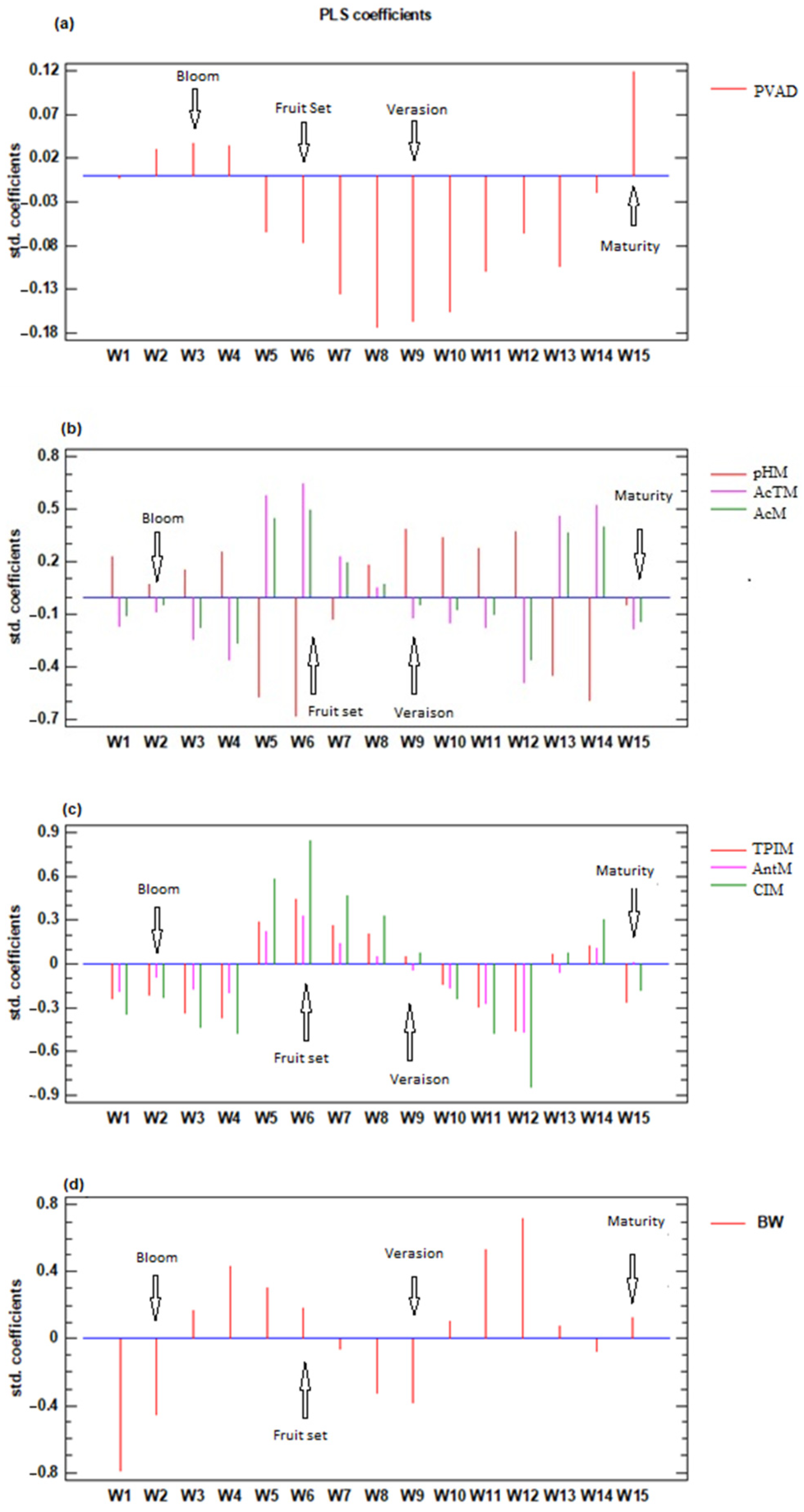
3.6. Relationship between Mean Available Soil Water and Grapevine, Must, and Wine Parameters
3.7. Relationship between Available Soil Water and Grape Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, G.; He, Y.N.; Yue, T.X.; Wang, J.; Zhang, Z.W. Effects of climatic conditions and soil properties on cabernet sauvignon berry growth and anthocyanin profiles. Molecules 2014, 19, 13683–13703. [Google Scholar] [CrossRef] [PubMed]
- Zerihun, A.; Mcclymont, L.; Lanyon, D.; Goodwin, I.; Gibberd, M. Deconvoluting effects of vine and soil properties on grape berry composition. J. Sci. Food Agric. 2015, 95, 193–203. [Google Scholar] [CrossRef]
- Lanyon, D.M.; Cass, A.; Hansen, D. The Effect of Soil Properties on Vine Performance; Land and Water Technical Report No. 34/04; CSIRO: Canberra, Australia, 2004. [Google Scholar]
- Costantini, E.A.C.; Pellegrini, S.; Vignozzi, N.; Barbetti, R. Micromorphological characterization and monitoring of internal drainage in soils of vineyards and olive groves in central Italy. Geoderma 2006, 131, 388–403. [Google Scholar] [CrossRef]
- Ramos, M.C.; Jones, G.V.; Yuste, J. Phenology and grape ripening characteristics of cv Tempranillo within the Ribera del Duero designation of origin (Spain): Influence of soil and plot characteristics. Eur. J. Agron. 2015, 70, 57–70. [Google Scholar] [CrossRef]
- Costantini, E.A.C.; Pellegrini, S.; Bucelli, P.; Barbetti, R.; Campagnolo, S.; Storchi, P.; Magini, S.; Perria, R. Mapping suitability for Sangiovese wine by means of δ13C and geophysical sensors in soils with moderate salinity. Eur. J. Agron. 2010, 33, 208–217. [Google Scholar] [CrossRef]
- Tramontini, S.; van Leeuwen, C.; Domec, J.C.; Destrac-Irvine, A.; Basteau, C.; Vitali, M.; Mosbach-Schulz, O.; Lovisolo, C. Impact of soil texture and water availability on the hydraulic control of plant and grape-berry development. Plant Soil 2013, 368, 215–230. [Google Scholar] [CrossRef]
- Keller, M. Deficit irrigation and vine mineral nutrition. Am. J. Enol. Vitic. 2005, 56, 267–283. [Google Scholar] [CrossRef]
- Conradie, W.J. Partitioning of mineral nutrients and timing of fertilizer applications for optimum efficiency. In Proceedings of the Soil Environment and Vine Mineral Nutrition Symposium, San Diego, CA, USA, 29–30 June 2004; Christensen, P., Smart, D.R., Eds.; American Society for Enology and Viticulture: Davis, CA, USA, 2005; pp. 69–81. [Google Scholar]
- Pérez-Álvarez, E.P.; Martínez-Vidaurre, J.M.; Martín, I.; García-Escudero, E.; Peregrina, F. Relationships among Soil Nitrate Nitrogen and Nitrogen Nutritional Status, Yield Components, and Must Quality in Semi-arid Vineyards from Rioja AOC, Spain. Commun. Soil Sci. Plan. 2013, 44, 232–242. [Google Scholar] [CrossRef]
- Mpelasoka, B.S.; Schachtman, D.P.; Michael, M.T.; Thomas, M.R. A review of potassium nutrition in grapevines with special emphasis on berry accumulation. Aust. J. Grape Wine Res. 2003, 9-3, 154–168. [Google Scholar] [CrossRef]
- Matthews, M.A.; Anderson, M.M.; Schultz, H.R. Phenologic and growth-responses to early and late season water deficits in Cabernet franc. Vitis 1987, 26, 147–160. [Google Scholar]
- van Leeuwen, C.; Friant, P.; Chone, X.; Tregoat, O.; Koundouras, S.; Dubourdieu, D. Influence of climate, soil, and cultivar on terroir. Am. J. Enol. Vitic. 2004, 55, 207–217. [Google Scholar] [CrossRef]
- Peacock, W.L. Fertigating drip-irrigated vineyards with macro and micronutrients. In Proceedings of the Soil Environment and Vine Mineral Nutrition Symposium, San Diego, CA, USA, 29–30 June 2004; Christensen, L.P., Smart, D.R., Eds.; American Society for Enology and Viticulture: Davis, CA, USA, 2005; pp. 129–133. [Google Scholar]
- Ramos, M.C.; Martínez-Casasnovas, J.A. Soil water balance in rainfed vineyards of the Penedès region (Northeastern Spain) affected by rainfall characteristics and land levelling: Influence on grape yield. Plant Soil 2010, 333, 375–389. [Google Scholar] [CrossRef]
- Esteban, M.A.; Villanueva, M.J.; Lissarrague, J.R. Effect of irrigation on changes in berry composition of Tempranillo during maturation. Sugars, organic acids, and mineral elements. Am. J. Enol. Vitic. 1999, 50, 418–434. [Google Scholar] [CrossRef]
- Deloire, A.; Vaudour, E.; Carey, V.; Bonnardot, V.; Van Leeuwen, C. Grapevine responses to terroir: A global approach. J. Int. Sci. Vigne Vin 2005, 39, 149–162. [Google Scholar] [CrossRef]
- Downey, M.O.; Dokoozlian, N.K.; Krstic, M.P. Cultural Practice and Environmental Impacts on the Flavonoid Composition of Grapes and Wine: A Review of Recent Research. Am. J. Enol. Vitic. 2006, 57, 257–268. [Google Scholar] [CrossRef]
- Gómez-Míguez, M.J.; Gómez-Míguez, M.; Vicario, I.M.; Heredia, F.J. Assessment of colour and aroma in white wines vinifications: Effects of grape maturity and soil type. J. Food Eng. 2007, 79, 758–764. [Google Scholar] [CrossRef]
- van Leeuwen, C.; Tregoat, O.; Choné, X.; Bois, B.; Pernet, D.; Gaudillére, J.P. Vine water status is a key factor in grape ripening and vintage quality for red Bordeaux wine. How can it be assessed for vineyard management purposes? J. Int. Sci. Vigne Vin 2009, 43, 121–134. [Google Scholar] [CrossRef]
- Ojeda, H.; Andary, C.; Kraeva, E.; Carbonneau, A.; Deloire, A. Influence of pre- and postveraison water deficit on synthesis and concentration of skin phenolic compounds during berry growth of vitis vinifera cv. shiraz. Am. J. Enol. Viticult. 2002, 53, 261–267. [Google Scholar]
- Ruby, G.; Harbertson, J.F.; Adams, D.A.; Matthews, M.A. Berry size and vine water deficits as factors in winegrape composition: Anthocyanins and tannins. Aust. J. Grape Wine Res. 2004, 10, 100–107. [Google Scholar] [CrossRef]
- Castellarin, S.D.; Matthews, M.A.; Di Gaspero, G.; Gambetta, G.A. Water deficits accelerate ripening and induce changes in gene expression regulating flavonoid biosynthesis in grape berries. Planta 2007, 227, 101–112. [Google Scholar] [CrossRef]
- Intrigliolo, D.S.; Pérez, D.; Risco, D.; Yeves, A.; Castel, J.R. Yield components and grape composition responses to seasonal water deficits in Tempranillo grapevines. Irrig. Sci. 2012, 30, 339–349. [Google Scholar] [CrossRef]
- Cooley, N.M.; Clingeleffer, P.R.; Walker, R.R. Effect of water deficits and season on berry development and composition of Cabernet Sauvignon (Vitis vinifera L.) grown in a hot climate. Aust. J. Grape Wine Res. 2017, 23, 260–272. [Google Scholar] [CrossRef]
- Bellvert, J.; Marsal, J.; Mata, M.; Girona, J. Yield, must composition, and wine quality responses to preveraison water deficits in sparkling base wines of chardonnay. Am. J. Enol. Vitic. 2016, 67, 1–12. [Google Scholar] [CrossRef]
- Torres, N.; Antolín, M.C.; Goicoechea, N. Arbuscular mycorrhizal symbiosis as a promising resource for improving berry quality in grapevines under changing environments. Front. Plant Sci. 2018, 9, 897. [Google Scholar] [CrossRef] [PubMed]
- Balint, G.; Reynolds, A.G. Effect of different irrigation strategies on vine physiology, yield, grape composition and sensory profiles of Vitis vinifera L. Cabernet-Sauvignon in a cool climate area. OENO One 2014, 48, 269–292. [Google Scholar] [CrossRef]
- Lizama, V.; Pérez-Álvarez, E.P.; Intrigliolo, D.S.; Chirivella, C.; Álvarez, I.; García-Esparza, M.J. Effects of the irrigation regimes on grapevine cv. Bobal in a Mediterranean climate: II. Wine, skins, seeds, and grape aromatic composition. Agric. Water Manag. 2021, 256, 107078. [Google Scholar] [CrossRef]
- Bucchetti, B.; Matthews, M.A.; Falginella, L.; Peterlunger, E.; Castellarin, S.D. Effect of water deficit on Merlot grape tannins and anthocyanins across four seasons. Sci. Hortic. 2011, 128, 297–305. [Google Scholar] [CrossRef]
- Martínez-Vidaurre, J.M. Influencia del Tipo de Suelo en el Estado Nutricional de la Vid, el Desarrollo Vegetativo, la Producción, la Composición de la Uva y de los Vinos de la Variedad Tempranillo Tinto (Vitis vinifera L.) en el Ámbito de la D.O.Ca Rioja. Ph.D. Thesis, Universidad de La Rioja, Logroño, Spain, 2017. Available online: https://digital.csic.es/bitstream/10261/194557/3/Influencia_tipo.pdf (accessed on 20 February 2024).
- Nelson, O.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis Part 2, 2nd ed.; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; ASA/SSSA: Madison, WI, USA, 1982; pp. 539–577. [Google Scholar]
- Orsini, L.; Remy, J.C. Utilisation du chlorure de cobaltihexammine pour la determina-tion simultanée de la capacité d’échange et des bases échangeables des sols. Bull. L’afes Sci. Sol. 1976, 4, 269–275. [Google Scholar]
- Mehlich, A. Mehlich No. 3 soil test extractant, A modification of Mehlich No. 2. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Saxton, K.E.; Rawls, W.J. Soil Water Characteristic Estimates by Texture and Organic Matter for Hydrologic Solutions. Soil Sci. Soc. Am. J. 2006, 70, 1569–1578. [Google Scholar] [CrossRef]
- Ramos, M.C.; Pérez-Álvarez, E.P.; Peregrina, F.; Martínez de Toda, F. Relationships between grape composition of Tempranillo variety and available soil water and water stress under different weather conditions. Sci. Hortic. 2020, 262, 109063. [Google Scholar] [CrossRef]
- Baillod, M.; Baggiolini, M. Les stades repères de la vigne. Rev. Suisse Vitic. Arboric. Hortic. 1993, 38, 7–9. [Google Scholar]
- Pellegrino, A.; Lebon, E.; Voltz, M.; Wery, J. Relationships between plant and soil water status in vine (Vitis vinifera L.). Plant Soil 2005, 266, 129–142. [Google Scholar] [CrossRef]
- Romero, I.; García-Escudero, E.; Martín, I. Effects of leaf position on blade and petiole mineral nutrient concentration of Tempranillo grapevine (Vitis vinifera L.). Am. J. Enol. Vitic. 2010, 61, 544–550. [Google Scholar] [CrossRef]
- Organization International de la Vigne et du Vine. Methods of Analysis of Must and Wine. Compendium of International Methods of Wine and Must Analysis; OIV: Paris, France, 2014. [Google Scholar]
- Ribereau-Gayon, J.; Ribereau-Gayon, P.; Peynaud, E.; Sudraud, P. Traité d’oEnologie-Sciences et Techniques du Vin, Tome 1: Analyse et Contrôle des Vins; Dunod: Paris, France, 1972; 671p. [Google Scholar]
- Ribéreau-Gayon, P.; Stonestreet, E. Le dosage des anthocyanes dans les vins rouges. Bull. Soc. Chim. Fr. 1965, 9, 2649–2652. [Google Scholar] [PubMed]
- EEC. European Commission Commission Regulation (EEC) No 2676/90 of 17 September 1990 determining Community methods for the analysis of wines. Off. J. Eur. Union 1990, L 272, 1–272. [Google Scholar]
- Sampaio, T.L.; Kennedy, J.A.; Vasconcelos, M.C. Use of microscale fermentations in grape and wine research. Am. J. Enol. Vitic. 2007, 58, 534–539. [Google Scholar] [CrossRef]
- Peregrina, F.; López, D.; Zaballa, O.; Villar, M.T.; González, G.; García-Escudero, E. Soil quality of vineyards in the Origin Denomination Rioja: Index of overcrusting risk (FAO-PNUMA), content of organic carbon and relation with soil fertility. Rev. Ciências Agrárias 2010, 33, 338–345. [Google Scholar] [CrossRef]
- van Leeuwen, C.; Roby, J.P.; de Rességuier, L. Soil-related terroir factors: A review. OENO One 2018, 52, 173–188. [Google Scholar] [CrossRef]
- Ramos, M.C.; Martínez-Casasnovas, J.A. Soil water variability and its influence on transpirable soil water fraction with two grape varieties under different rainfall regimes. Agric. Ecosyst. Environ. 2014, 185, 253–262. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants; Academic Press: London, UK, 1995. [Google Scholar]
- Schreiner, R.P. Mycorrhizae and mineral acquisition in grapevines. In Proceedings of the Soil Environment and Vine Mineral Nutrition Symposium, San Diego, CA, USA, 29–30 June 2004; Christensen, P., Smart, D.R., Eds.; American Society for Enology and Viticulture: Davis, CA, USA, 2005; pp. 49–60. [Google Scholar]
- Grossman, A.; Takahashi, H. Macronutrient utilization by photosynthetic eukaryotes and the fabric of interactions. Ann. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 163–210. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, D.W.; Cornic, G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ. 2002, 25, 275–294. [Google Scholar] [CrossRef] [PubMed]
- Bravdo, B.; Hepner, Y. Irrigation management and fertigation to optimise grape composition and vine performance. Acta Hortic. 1987, 206, 49–67. [Google Scholar] [CrossRef]
- Choné, X.; van Leeuwen, C.; Chéry, P.; Ribéreau-Gayon, P. Terroir influence on water status and nitrogen status of non-irrigated Cabernet-Sauvignon (Vitis vinifera). Vegetative development, must and wine composition (example of a Medoc top estate vineyard, Saint Julien area, Bordeaux, 1997). S. Afr. J. Enol. Vitic. 2001, 22, 8–15. [Google Scholar] [CrossRef][Green Version]
- Gaudillère, J.P.; van Leeuwen, C.; Choné, X.; Tregoat, O. The assessment of vine water and nitrogen uptake by means of physiological indicators influence on vine development and berry potential (Vitis vinifera L. cv Merlot, 2000, Bordeaux). J. Int. Sci. Vigne Vin 2002, 36, 133–142. [Google Scholar] [CrossRef]
- Pérez-Álvarez, E.P.; García-Escudero, E.; Peregrina, F. Soil Nutrient Availability under Cover Crops: Effects on Vines, Must, and Wine in a Tempranillo Vineyard. Am. J. Enol. Viticult. 2015, 66, 311–320. [Google Scholar] [CrossRef]
- Dundon, C.G.; Smart, R.E.; McCarthy, M.G. The Effect of Potassium Fertilizer on Must and Wine Potassium Levels of Shiraz Grapevines. Am. J. Enol. Vitic. 1984, 35, 200–205. [Google Scholar] [CrossRef]
- King, P.D.; Smart, R.E.; Mcclellan, D.J. Within-vineyard variability in vine vegetative growth, yield, and fruit and wine composition of Cabernet-Sauvignon in Hawke’s Bay, New Zealand. Aust. J. Grape Wine Res. 2014, 2, 234–246. [Google Scholar] [CrossRef]
- Zaballa, O.; García-Escudero, E. Ensayos de riego en viñedos de la D.O.Ca Rioja 1984–1994. In Reuniones del Grupo de Trabajo de Experimentación en Viticultura y Enología: La Rioja, 14, 15 y 16 de Marzo de 1995; Ministerio de Agricultura, Alimentación y Medio Ambiente: Madrid, España, 1997; pp. 56–94. ISBN 84-491-0286-3. [Google Scholar]
- Klein, I.; Strime, M.; Fanberstein, L.; Mani, Y. Irrigation and fertigation effects on phosphorus and potassium nutrition of wine grapes. Vitis 2000, 39, 55–62. [Google Scholar]
- Boyer, J.S. Water transport. Ann. Rev. Plant Physiol. 1985, 36, 473–516. [Google Scholar] [CrossRef]
- Hsiao, T.C.; Xu, L.K. Sensitivity of growth of roots versus leaves to water stress: Biophysical analysis and relation to water transport. J. Exp. Bot. 2000, 51, 1595–1616. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.E.; Dokoozlian, N.K.; Wample, R.L. Grape. In Handbook of Environmental Physiology of Fruit Crops. Vol. 1. Temperate Crops; Shaffer, B., Anderson, P.C., Eds.; CRC Press: Orlando, FL, USA, 1994; p. 83133. [Google Scholar]
- Stevens, R.M.; Harvey, G.; Aspinall, D. Grapevine growth of shoots and fruit linearly correlate with water stress indices based on root-weighted soil matric potential. Aust. J. Grape Wine Res. 1995, 1, 58–66. [Google Scholar] [CrossRef]
- Tomasi, D.; Gaiotti, F.; Bragato, G.; Mosetti, D.; Missio, A.; Battista, F. Soil Influence on Vine Performance: From Root System To Wine Quality. In Proceedings of the 33rd OIV World Congress of Vine and Wine, 8th General Assembly of the OIV, Tbilissi, Georgia, 20–27 June 2010. [Google Scholar]
- Ojeda, H.; Deloire, A.; Carbonneau, A. Influence of water deficits on grape berry growth. Vitis 2001, 40, 141–146. [Google Scholar]
- Reynard, J.S.; Zufferey, V.; Nicol, G.C.; Murisier, F. Vine water status as a parameter of the «terroir» effect under the non-irrigated conditions of the Vaud viticultural area (Switzerland). OENO One 2011, 45, 139–147. [Google Scholar] [CrossRef]
- Wenter, A.; Zanotelli, D.; Montagnani, L.; Tagliavini, M.; Andreotti, C. Effect of different timings and intensities of water stress on yield and berry composition of grapevine (cv. Sauvignon blanc) in a mountain environment. Sci. Hortic. 2018, 236, 137–145. [Google Scholar] [CrossRef]
- Ramos, M.C.; Martínez de Toda, F. Variability of Tempranillo grape composition in the Rioja DOCa (Spain) related to soil and climatic characteristics. J. Sci. Food Agric. 2019, 99, 1153–1165. [Google Scholar] [CrossRef]
- Zulini, L.; Rubinigg, M.; Zorer, R.; Bertamini, M. Effects of drought stress on chlorophyll fluorescence and photosynthetic pigments in grapevine leaves (Vitis vinifera cv.’ White Riesling’). Acta Hortic. 2007, 754, 289–294. [Google Scholar] [CrossRef]
- Lasko, A.N.; Pool, R. Efecto del estrés hídrico en el viñedo y en la calidad de los vino (climas del este). Rev. Internet Vitic. Enol. 2006, 122, 10. [Google Scholar]
- Bucelli, P.; Costantini, E.A.C.; Storchi, P. It is possible to predict Sangiovese wine quality through a limited number of variables measured on the vines? J. Int. Sci. Vigne Vine 2010, 44, 207–218. [Google Scholar] [CrossRef]
- Ubalde, J.M.; Sort, X.; Zayas, A.; Rosa, M.P. Effects of soil and climatic conditions on grape ripening and wine quality of Cabernet Sauvignon. J. Wine Res. 2010, 21, 1–17. [Google Scholar] [CrossRef]
- Jackson, D.I.; Lombard, P.B. Environmental and management practices affecting grape composition and wine quality—A review. Am. J. Enol. Vitic. 1993, 44, 409–430. [Google Scholar] [CrossRef]
- Girona, J.; Marsal, J.; Mata, M.; Del Campo, J.; Basile, B. Phenological sensitivity of berry growth and composition of tempranillo grapevines (Vitis vinifera L.) to water stress. Aust. J. Grape Wine Res. 2009, 15, 268–277. [Google Scholar] [CrossRef]
- Reynolds, A.G.; Lowrey, W.D.; Tomek, L.; Hakimi, J.; De Savigny, C. Influence of irrigation on vine performance, fruit composition, and wine quality of Chardonnay in a cool, humid climate. Am. J. Enol. Vitic. 2007, 58, 217–228. [Google Scholar] [CrossRef]
- Lopes, C.M.; Santos, T.P.; Monteiro, A.; Rodrigues, M.L.; Costa, J.M.; Chaves, M.M. Combining cover cropping with deficit irrigation in a Mediterranean low vigor vineyard. Sci. Hortic. 2011, 129, 603–612. [Google Scholar] [CrossRef]
- Peyrot des Gachons, C.P.; Van Leeuwen, C.; Tominaga, T.; Soyer, J.-P.; Gaudillère, J.-P.; Dubourdieu, D. Influence of water and nitrogen deficit on fruit ripening and aroma potential of Vitis vinifera L. cv Sauvignon blanc in field conditions. J. Sci. Food Agric. 2005, 85, 73–85. [Google Scholar] [CrossRef]
- Souza, C.R.D.; Maroco, J.; Santos, T.P.; Rodrigues, M.; Lopes, C.M.A.; Pereira, J.S.; Chaves, M.M. Grape berry metabolism in field-grown grapevines exposed to different irrigation strategies. Vitis 2005, 44, 103–109. [Google Scholar]
- dos Santos, T.P.; Lopes, C.M.; Lucília Rodrigues, M.; de Souza, C.R.; Ricardo-da-Silva, J.M.; Maroco, J.P.; Pereira, J.S.; Chaves, M.M. Effects of deficit irrigation strategies on cluster microclimate for improving fruit composition of Moscatel field-grown grapevines. Sci. Hortic. 2007, 112, 321–330. [Google Scholar] [CrossRef]
- Hochberg, U.; Batushansky, A.; Degu, A.; Rachmilevitch, S.; Fait, A. Metabolic and physiological responses of shiraz and cabernet sauvignon (Vitis vinifera L.) to near optimal temperatures of 25 and 35 °C. Int. J. Mol. Sci. 2015, 16, 24276–24294. [Google Scholar] [CrossRef]
- Cáceres-Mella, A.; Talaverano, M.I.; Villalobos-González, L.; Ribalta-Pizarro, C.; Pastenes, C. Controlled water deficit during ripening affects proanthocyanidin synthesis, concentration and composition in Cabernet Sauvignon grape skins. Plant Physiol. Biochem. 2017, 117, 34–41. [Google Scholar] [CrossRef]
- Ferrer, M.; Echeverría, G.; Carbonneau, A. Effect of berry weight and its components on the contents of sugars and anthocyanins of three varieties of Vitis vinifera L. under different water supply conditions. S. Afr. J. Enol. Vitic. 2014, 35, 103–113. [Google Scholar] [CrossRef]
- Berdeja, M.; Hilbert, G.; Lafontaine, M.; Stoll, M.; Gomes, E.; Renaud, C.; Delrot, S. Effects of drought stress and rootstock genotype on grape berry quality. Acta Hortic. 2014, 1038, 375–378. [Google Scholar] [CrossRef]
- Basile, B.; Marsal, J.; Mata, M.; Vallverdú, X.; Bellvert, J.; Girona, J. Phenological sensitivity of cabernet sauvignon to water stress: Vine physiology and berry composition. Am. J. Enol. Vitic. 2011, 62, 453–461. [Google Scholar] [CrossRef]
- Martínez de Toda, F. Biología de la Vid. Fundamentos Bilógicos de la Viticultura; Mundiprensa: Madrid, Spain, 1991; p. 346. [Google Scholar]

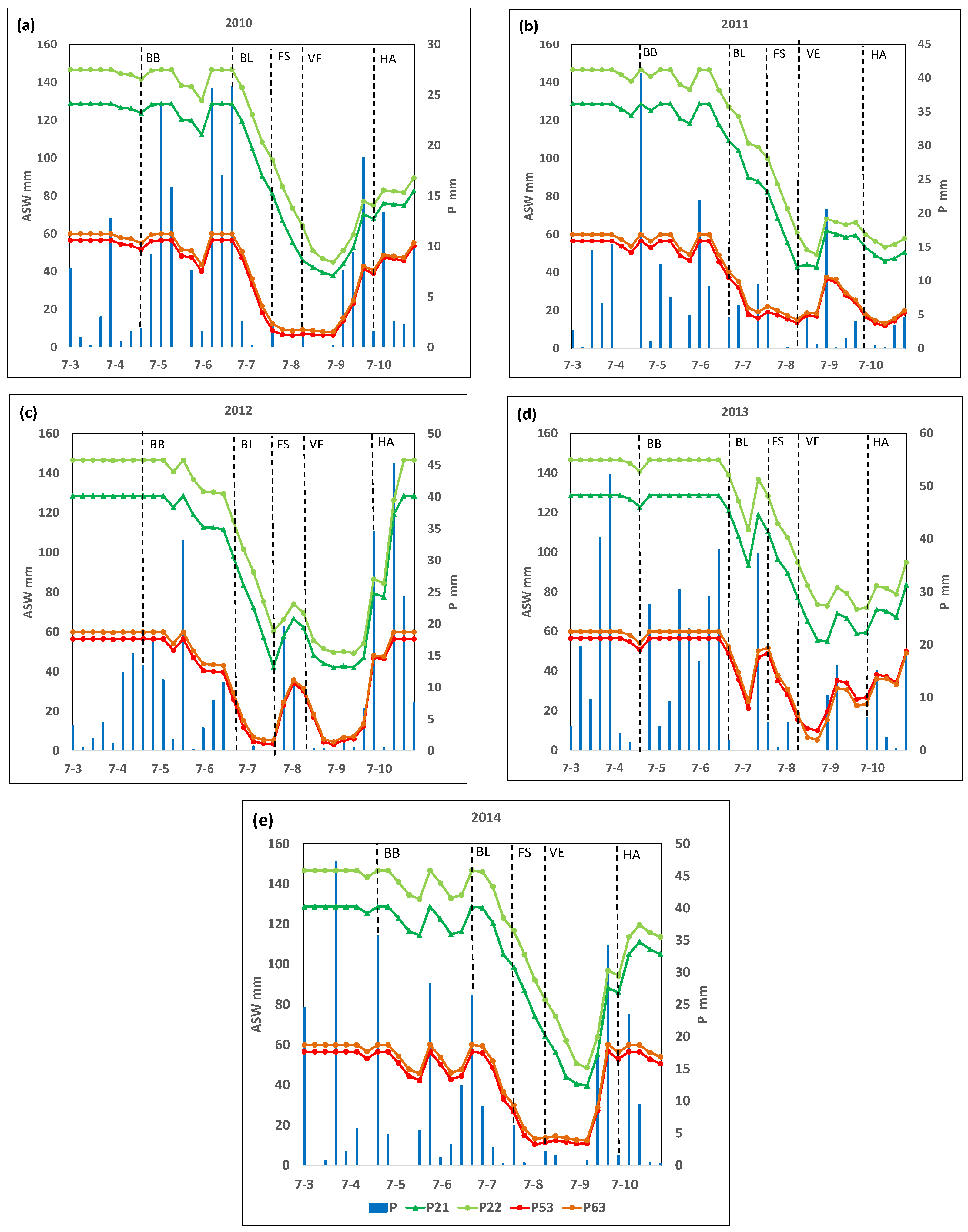

| Plot | Plant Density ha−1 | Soil Classification (USDA, 2006) | pH (H2O) | E.C. 1 dS/m | O.M. % | Clay % | Silt % | Sand % | CaCO3 % | Available Water Capacity mm |
|---|---|---|---|---|---|---|---|---|---|---|
| Ap Horizon | Section Control | |||||||||
| P21 | 3086 | Fluventic Haploxerept | 8.15 | 0.15 | 1.00 | 20.6 | 38.8 | 40.6 | 0.5 | 128.5 |
| P22 | 3086 | Fluventic Haploxerept | 8.20 | 0.13 | 1.05 | 24.1 | 28.7 | 47.2 | 1.3 | 146.5 |
| P53 | 3137 | Typic Calcixerept | 8.35 | 0.14 | 0.97 | 18.5 | 52.5 | 29.0 | 3.5 | 56.3 |
| P63 | 3086 | Petrocalcic Palexerolls | 8.40 | 0.15 | 1.87 | 22.9 | 40.0 | 37.1 | 14.7 | 59.9 |
| Petiole % N | Petiole % P | Petiole % K | Shoot Weight g | 100 Berries Weight g | Grape Weight per Vine kg vine−1 | Pruning Wood per Vine kg vine−1 | |
|---|---|---|---|---|---|---|---|
| 2010 | |||||||
| P21 | 0.59b 1 | 0.29c | 2.30c | 161.77b | 244b | 5.54c | 1.55b |
| P22 | 0.56b | 0.28c | 1.23b | 133.89b | 222ab | 4.30ab | 1.32b |
| P53 | 0.45a | 0.18b | 0.78a | 71.17a | 213a | 4.69bc | 0.71a |
| P63 | 0.45a | 0.05a | 0.81a | 75.85a | 208a | 3.55a | 0.71a |
| 2011 | |||||||
| P21 | 0.63c | 0.24c | 2.50c | 183.41c | 312b | 6.10c | 1.75c |
| P22 | 0.56b | 0.28c | 1.18b | 121.83b | 266a | 4.97b | 1.22b |
| P53 | 0.47a | 0.17b | 0.81a | 76.55a | 277ab | 3.42a | 0.75a |
| P63 | 0.45a | 0.05a | 0.78a | 70.09a | 245a | 2.89a | 0.67a |
| 2012 | |||||||
| P21 | 0.55b | 0.33c | 2.64c | 158.59c | 314d | 7.81c | 1.47c |
| P22 | 0.48a | 0.31bc | 1.66b | 129.37b | 264c | 5.69b | 1.17b |
| P53 | 0.45a | 0.22b | 1.11a | 59.50a | 183b | 2.84a | 0.59a |
| P63 | 0.49a | 0.09a | 1.11a | 48.91a | 147a | 2.02a | 0.46a |
| 2013 | |||||||
| P21 | 0.67b | 0.50c | 2.72c | 186.24b | 270a | 3.31a | 1.70c |
| P22 | 0.61b | 0.45c | 1.04b | 157.20b | 258a | 3.87a | 1.34b |
| P53 | 0.45a | 0.19b | 0.71ab | 78.46a | 269a | 4.45a | 0.80a |
| P63 | 0.41a | 0.05a | 0.47a | 74.84a | 245a | 3.29a | 0.69a |
| 2014 | |||||||
| P21 | 0.65c | 0.19b | 2.28b | 142.60c | 339c | 7.04b | 1.42c |
| P22 | 0.48b | 0.28c | 1.09a | 83.42b | 303b | 7.09b | 0.88b |
| P53 | 0.42ab | 0.25bc | 0.87a | 60.34a | 301b | 3.64a | 0.68a |
| P63 | 0.38a | 0.06a | 0.58a | 57.92a | 281a | 3.81a | 0.67a |
| Average value per year | |||||||
| 2010 | 0.51 | 0.20 | 1.28a | 110.70bc | 221.58a | 4.52 | 1.08b |
| 2011 | 0.52 | 0.18 | 1.32a | 112.97bc | 275.14b | 4.34 | 1.10b |
| 2012 | 0.49 | 0.24 | 1.63b | 99.09ab | 226.98a | 4.59 | 0.92a |
| 2013 | 0.53 | 0.30 | 1.24a | 124.18c | 260.58b | 3.73 | 1.13b |
| 2014 | 0.48 | 0.19 | 1.20a | 86.07a | 305.81c | 5.39 | 0.91a |
| Average value per plot | |||||||
| P21 | 0.62c | 0.31c | 2.49c | 166.52c | 295.83b | 5.96c | 1.58c |
| P22 | 0.54b | 0.32c | 1.24b | 125.14b | 262.67ab | 5.18bc | 1.19b |
| P53 | 0.45a | 0.20b | 0.86a | 69.21a | 248.45a | 3.81ab | 0.71a |
| P63 | 0.44a | 0.06a | 0.75a | 65.52a | 225.11a | 3.11a | 0.64a |
| p values | |||||||
| Year | 0.4088 | 0.1436 | 0.0099 | 0.0069 | 0.0063 | 0.4377 | 0.0221 |
| Soil | 0.0001 | 0.0001 | 0.0000 | 0.0000 | 0.0129 | 0.0100 | 0.0000 |
| Soil × Year | 0.0007 | 0.0000 | 0.3596 | 0.1850 | 0.000 | 0.0000 | 0.2815 |
| Probable Volumetric Alcoholic Degree | pH | Total Acidity g L−1 | Malic Acid mg L−1 | K mg L−1 | IPT | Color Intensity | Anthocyanins mg g−1 | |
|---|---|---|---|---|---|---|---|---|
| 2010 | ||||||||
| P21 | 13.3a 1 | 3.44ab | 6.52c | 3.80d | 1794b | 13.23a | 3.70a | 1.36a |
| P22 | 13.2a | 3.47b | 5.99bc | 3.09c | 1670b | 15.17b | 4.27ab | 1.62ab |
| P53 | 13.3a | 3.36a | 5.87ab | 2.56b | 1376a | 19.01c | 4.86b | 1.88b |
| P63 | 13.2a | 3.38a | 5.36a | 1.99a | 1296a | 18.79c | 4.72b | 1.83b |
| 2011 | ||||||||
| P21 | 12.8ab | 3.47a | 5.85c | 2.74c | 2005b | 13.07a | 3.32a | 1.19a |
| P22 | 12.6a | 3.49a | 4.93b | 2.32bc | 1793a | 12.38a | 2.78a | 1.11a |
| P53 | 13.5b | 3.44a | 5.11b | 2.05b | 1862ab | 18.90b | 4.44b | 1.80b |
| P63 | 13.2ab | 3.58b | 4.24a | 1.53a | 1724a | 19.22b | 4.88b | 1.64b |
| 2012 | ||||||||
| P21 | 12.8a | 3.60a | 5.47c | 2.81c | 2050b | 14.83b | 3.42a | 0.99a |
| P22 | 12.5a | 3.60a | 4.72b | 2.12b | 1837a | 12.82a | 3.30a | 1.17a |
| P53 | 13.6b | 3.53a | 4.78b | 1.71a | 1944ab | 26.36d | 7.08b | 2.08b |
| P63 | 13.6b | 3.75b | 4.04a | 1.54a | 1946ab | 22.66c | 6.27b | 2.47c |
| 2013 | ||||||||
| P21 | 12.0a | 3.32a | 9.04b | 5.01b | 2038c | 17.32a | 5.70a | 1.28a |
| P22 | 11.8a | 3.28a | 8.48b | 4.49b | 1824b | 16.15a | 6.33a | 1.61b |
| P53 | 13.1b | 3.21a | 7.11a | 3.25a | 1474a | 23.45b | 10.32b | 2.08c |
| P63 | 13.2b | 3.26a | 6.50a | 2.65a | 1373a | 23.76b | 9.93b | 1.64b |
| 2014 | ||||||||
| P21 | 12.2b | 3.41b | 5.90b | 2.90c | 1858c | 12.67a | 3.74a | 1.23a |
| P22 | 11.4a | 3.28a | 5.69b | 2.39bc | 1565b | 12.33a | 3.68a | 1.32a |
| P53 | 14.3c | 3.41b | 5.36ab | 2.22b | 1663b | 18.33b | 5.92b | 1.98b |
| P63 | 13.8c | 3.38b | 4.85a | 1.63a | 1400a | 17.67b | 5.56b | 2.05b |
| Mean value per year | ||||||||
| 2010 | 13.23 | 3.41bc | 5.94c | 2.86b | 1533.75a | 16.55ab | 4.39a | 1.67 |
| 2011 | 13.02 | 3.49c | 5.03ab | 2.16a | 1845.92b | 15.89ª | 3.85a | 1.43 |
| 2012 | 13.13 | 3.62d | 4.75a | 2.04a | 1944.42b | 19.16bc | 5.01a | 1.68 |
| 2013 | 12.52 | 3.27a | 7.78d | 3.85c | 1677.25a | 20.17c | 8.07b | 1.65 |
| 2014 | 12.92 | 3.37b | 5.45bc | 2.28a | 1621.58a | 15.25a | 4.72a | 1.65 |
| Mean value per plot | ||||||||
| P21 | 12.60a | 3.45 | 6.56c | 3.45d | 1949.07c | 14.22a | 3.98a | 1.21a |
| P22 | 12.29a | 3.42 | 5.96b | 2.88c | 1737.80b | 13.77a | 4.07a | 1.37a |
| P53 | 13.57b | 3.39 | 5.65b | 2.36b | 1663.67ab | 20.42b | 6.52b | 1.97b |
| P63 | 13.40b | 3.47 | 5.00a | 1.87a | 1547.80a | 21.21b | 6.27b | 1.93b |
| p values | ||||||||
| Year | 0.3708 | 0.0000 | 0.0000 | 0.0000 | 0.0046 | 0.0037 | 0.0001 | 0.5773 |
| Soil | 0.0046 | 0.2574 | 0.0001 | 0.0000 | 0.0027 | 0.0000 | 0.0003 | 0.0004 |
| Soil × Year | 0.0003 | 0.0011 | 0.0074 | 0.0050 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| Probable Alcoholic Degree | pH | K mg L−1 | Total Acidity g L−1 | Color Intensity | IPT 186 | Anthocyanins mg L−1 | |
|---|---|---|---|---|---|---|---|
| 2010 | |||||||
| P21 | 13.8a 1 | 3.79a | 1687b | 6.63c | 9.10a | 47.10a | 567a |
| P22 | 13.5a | 3.80a | 1540b | 6.23b | 10.44a | 49.93a | 614a |
| P53 | 13.3a | 3.70a | 1357a | 6.3bc | 15.69b | 65.83b | 836b |
| P63 | 13.1a | 3.73a | 1317a | 5.63a | 16.92b | 68.87b | 796b |
| 2011 | |||||||
| P21 | 13.5a | 3.87bc | 1713c | 6.13c | 7.89a | 51.13a | 596a |
| P22 | 13.5a | 3.88c | 1537b | 5.43a | 7.74a | 50.13a | 587a |
| P53 | 13.4a | 3.74a | 1280a | 5.83b | 13.87b | 68.47b | 845b |
| P63 | 13.5a | 3.79ab | 1377a | 5.2a | 14.63b | 68.77b | 779b |
| 2012 | |||||||
| P21 | 12.7ab | 3.81a | 1773c | 5.97c | 7.88a | 49.27a | 465a |
| P22 | 12.1a | 3.75a | 1540ab | 5.67b | 6.96a | 46.77a | 486a |
| P53 | 13.0b | 3.83a | 1423a | 5.5b | 13.45b | 80.27b | 996b |
| P63 | 13.3b | 4.02b | 1650bc | 4.37a | 15.25b | 78.57b | 1069b |
| 2013 | |||||||
| P21 | 14.0a | 3.68c | 1923c | 8.83c | 6.49a | 63.43b | 447a |
| P22 | 13.7a | 3.60bc | 1603bc | 7.9b | 7.05a | 52.97a | 508a |
| P53 | 13.5a | 3.47ab | 1263b | 7.43b | 11.82b | 60.40b | 699b |
| P63 | 13.8a | 3.43a | 882a | 6.77a | 12.80b | 60.07b | 630b |
| 2014 | |||||||
| P21 | 12.7b | 3.80b | 1522b | 5.32a | 4.35a | 31.92b | 542b |
| P22 | 11.6a | 3.68ab | 1201a | 5.54a | 3.50a | 27.19a | 439a |
| P53 | 14.9d | 3.78ab | 1437b | 5.33a | 8.81b | 50.68d | 778c |
| P63 | 14.3c | 3.61a | 1129a | 5.58a | 7.99b | 44.82c | 614b |
| Mean values per year | |||||||
| 2010 | 13.43 | 3.75bc | 1475.0 | 6.20b | 13.04c | 57.93b | 703.26ab |
| 2011 | 13.49 | 3.82cd | 1476.7 | 5.65a | 11.03bc | 59.62b | 701.79ab |
| 2012 | 12.79 | 3.85d | 1596.7 | 5.37a | 10.88bc | 63.72b | 754.08b |
| 2013 | 13.75 | 3.54a | 1418.0 | 7.73c | 9.54b | 59.21b | 571.08a |
| 2014 | 13.39 | 3.72b | 1322.3 | 5.42a | 6.16a | 38.67a | 593.17a |
| Mean values per plot | |||||||
| P21 | 13.32 | 3.79 | 1723.7b | 6.58b | 7.14a | 48.57a | 523.47ª |
| P22 | 12.90 | 3.74 | 1484.2ab | 6.15b | 7.14a | 45.40a | 526.65a |
| P53 | 13.63 | 3.70 | 1371.4a | 6.08b | 12.73b | 64.22b | 830.93b |
| P63 | 13.62 | 3.72 | 1251.6a | 5.51a | 13.52b | 65.13b | 777.63b |
| p values | |||||||
| Year | 0.4655 | 0.0037 | 0.3180 | 0.0000 | 0.0000 | 0.0012 | 0.1012 |
| Soil | 0.3728 | 0.4786 | 0.0066 | 0.0095 | 0.0000 | 0.0005 | 0.0004 |
| Soil× Year | 0.0000 | 0.0001 | 0.0000 | 0.0000 | 0.2652 | 0.0000 | 0.0000 |
| Mean ASW mm in Period | ||||
|---|---|---|---|---|
| Budbreak–Bloom | Bloom–Fruit Set | Fruit Set–Veraison | Veraison–Maturity | |
| Petiole N % | 0.7695 | 0.7282 | 0.7605 | 0.7508 |
| p value | 0.0001 | 0.0003 | 0.0001 | 0.0001 |
| Petiole P % | 0.7401 | 0.6896 | 0.7603 | 0.773 |
| p value | 0.0002 | 0.0008 | 0.0001 | 0.0001 |
| Petiole K % | 0.6191 | 0.5174 | 0.5261 | 0.5652 |
| p value | 0.0036 | 0.0195 | 0.0172 | 0.0094 |
| Shoot weight | 0.8242 | 0.7586 | 0.7618 | 0.7851 |
| p value | 0 | 0.0001 | 0.0001 | 0 |
| Berry weight | 0.4139 | 0.499 | 0.4214 | 0.4944 |
| p value | 0.0696 | 0.0251 | 0.0643 | 0.0267 |
| Bunch weight | 0.5753 | 0.5515 | 0.4427 | 0.5301 |
| p value | 0.008 | 0.0117 | 0.0506 | 0.0162 |
| Grape yield | 0.6273 | 0.6001 | 0.5044 | 0.5893 |
| p value | 0.0031 | 0.0052 | 0.0233 | 0.0063 |
| Pruning weight | 0.8306 | 0.7834 | 0.76 | 0.7872 |
| p value | 0 | 0 | 0.0001 | 0 |
| Mean ASW mm in Period | ||||
|---|---|---|---|---|
| Budbreak–Bloom | Bloom–Fruit Set | Fruit Set–Veraison | Veraison-Maturity | |
| Probable volumetric alcoholic grade | −0.7646 | −0.7687 | −0.844 | −0.8254 |
| p value | 0.0001 | 0.0001 | 0 | 0 |
| pH | −0.0338 | −0.2645 | −0.2301 | −0.1316 |
| p value | 0.8874 | 0.2597 | 0.3291 | 0.5801 |
| Acidity Total | 0.3903 | 0.498 | 0.5642 | 0.4602 |
| p value | 0.0889 | 0.0255 | 0.0096 | 0.0412 |
| Malic acid | 0.5693 | 0.6288 | 0.6801 | 0.6037 |
| p value | 0.0088 | 0.003 | 0.001 | 0.0048 |
| K | 0.4214 | 0.2451 | 0.3798 | 0.4138 |
| p value | 0.0643 | 0.2976 | 0.0986 | 0.0697 |
| TPI | −0.8148 | −0.8111 | −0.6739 | −0.801 |
| p value | 0 | 0 | 0.0011 | 0 |
| Color intensity | −0.5543 | −0.4638 | −0.3294 | −0.5098 |
| p value | 0.0112 | 0.0394 | 0.1561 | 0.0217 |
| Anthocyanins | −0.805 | −0.7325 | −0.6883 | −0.7576 |
| p value | 0 | 0.0002 | 0.0008 | 0.0001 |
| Mean ASW mm in Period | ||||
|---|---|---|---|---|
| Budbreak–Bloom | Bloom–Fruit Set | Fruit Set–Veraison | Veraison–Maturity | |
| Alcoholic degree | −0.365 | −0.2455 | −0.2869 | −0.3281 |
| p value | 0.1136 | 0.2968 | 0.22 | 0.1578 |
| pH | 0.1435 | −0.0094 | −0.0282 | 0.0674 |
| p value | 0.5462 | 0.9687 | 0.9062 | 0.7776 |
| Acidity total | 0.4061 | 0.4311 | 0.2882 | 0.3287 |
| p value | 0.0757 | 0.0577 | 0.2178 | 0.1571 |
| K | 0.5328 | 0.3928 | 0.4397 | 0.4977 |
| p value | 0.0156 | 0.0867 | 0.0524 | 0.0256 |
| Color intensity | −0.7649 | −0.8197 | −0.8071 | −0.8302 |
| p value | 0.0001 | 0 | 0 | 0 |
| Tonality | 0.3125 | 0.3651 | 0.3606 | 0.4117 |
| p value | 0.1797 | 0.1135 | 0.1183 | 0.0713 |
| IPT | −0.6578 | −0.7649 | −0.6381 | −0.689 |
| p value | 0.0016 | 0.0001 | 0.0025 | 0.0008 |
| Antochyanins | −0.8025 | −0.8387 | −0.7962 | −0.8244 |
| p value | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Vidaurre, J.M.; Pérez-Álvarez, E.P.; García-Escudero, E.; Ramos, M.C.; Peregrina, F. Differences in Soil Water Holding Capacity and Available Soil Water along Growing Cycle Can Explain Differences in Vigour, Yield, and Quality of Must and Wine in the DOCa Rioja. Horticulturae 2024, 10, 320. https://doi.org/10.3390/horticulturae10040320
Martínez-Vidaurre JM, Pérez-Álvarez EP, García-Escudero E, Ramos MC, Peregrina F. Differences in Soil Water Holding Capacity and Available Soil Water along Growing Cycle Can Explain Differences in Vigour, Yield, and Quality of Must and Wine in the DOCa Rioja. Horticulturae. 2024; 10(4):320. https://doi.org/10.3390/horticulturae10040320
Chicago/Turabian StyleMartínez-Vidaurre, José María, Eva Pilar Pérez-Álvarez, Enrique García-Escudero, María Concepción Ramos, and Fernando Peregrina. 2024. "Differences in Soil Water Holding Capacity and Available Soil Water along Growing Cycle Can Explain Differences in Vigour, Yield, and Quality of Must and Wine in the DOCa Rioja" Horticulturae 10, no. 4: 320. https://doi.org/10.3390/horticulturae10040320
APA StyleMartínez-Vidaurre, J. M., Pérez-Álvarez, E. P., García-Escudero, E., Ramos, M. C., & Peregrina, F. (2024). Differences in Soil Water Holding Capacity and Available Soil Water along Growing Cycle Can Explain Differences in Vigour, Yield, and Quality of Must and Wine in the DOCa Rioja. Horticulturae, 10(4), 320. https://doi.org/10.3390/horticulturae10040320





