Olive Escudete (Dalmatian Disease) Caused by Botryosphaeria dothidea as a Result of Fly–Midge–Fungus Interaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Field
2.2. Temporal Evolutions of Agents
2.3. Bioassays
2.3.1. Live and Non-Live Olive Fly Oviposition Stings
2.3.2. Artificial Wounds
2.3.3. Food Behavior of Midge Larvae
2.4. Isolation of B. dothidea
2.5. Statistical Analysis
3. Results
3.1. The Dynamics of B. oleae, P. berlesiana, and B. dothidea
3.2. Role of B. olea in Disease Development
3.3. Incidence of P. berlesiana and B. dothidea in the Live and Non-Live Olive Fly Stings
3.4. Incidence of P. berlesiana and B. dothidea in Artificial Wounds
3.5. Food Behavior of Midge Larvae
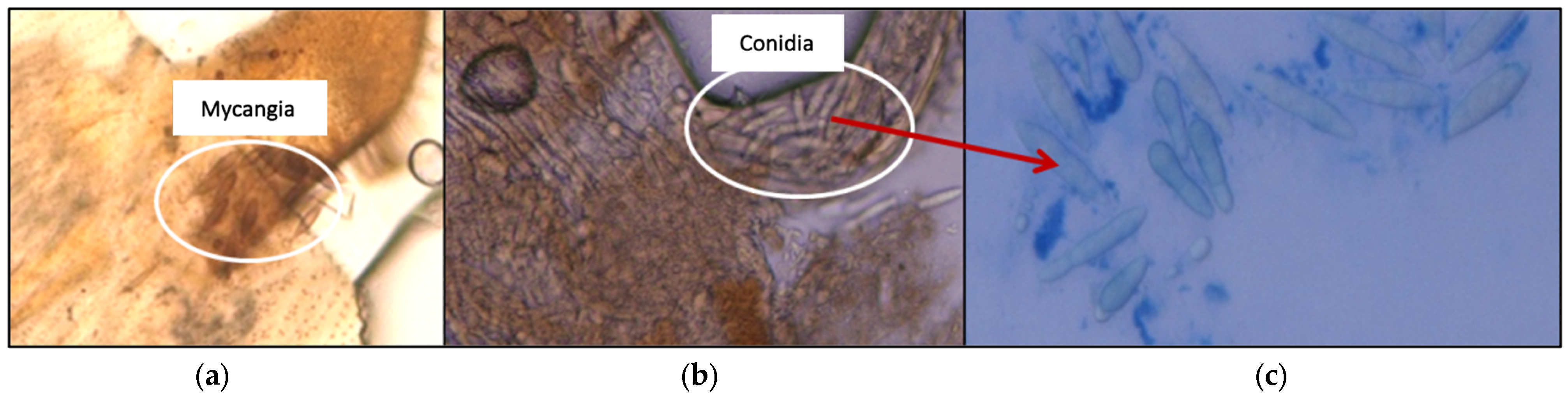
3.6. Verification of the Presence of B. dothidea in P. berlesiana
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Olive Council (IOOC). Olivae; International Olive Council: Madrid, Spain, 2010; Volume 113, Available online: www.internationalolivecouncil.org (accessed on 28 December 2022).
- Anonymous. Anuario de Estadística Agraria. Parte Segunda, Superficies y Producciones de Cultivos. Olivar. In Plataforma de Conocimiento Para el Medio Rural y Pesquero. Artículos de Revista; Ministerio de Agricultura, Alimentación y Medio Ambiente: Madrid, España, 2014. Available online: https://www.mapa.gob.es/es/estadistica/temas/publicaciones/anuario-de-estadistica/2014/default.aspx?parte=3&capitulo=13&grupo=12&seccion=1 (accessed on 28 December 2022).
- Junta de Andalucía. Boletín Oficial de La Junta de Andalucía—Histórico Del BOJA. Boletín Número 199. 2019. Available online: https://www.juntadeandalucia.es/boja/2019/199/2 (accessed on 28 December 2022).
- Trapero, A.; Blanco, M.A. Diseases. In Olive Growing; Barranco, D., Fernández-Escobar, R., Rallo, L., Eds.; Junta de Andalucía/Mundi-Prensa/RIRDC/AOA: Pendle Hill, NSW, Australia, 2010; pp. 521–578. [Google Scholar]
- Zachos, D.G.; Tzavella-Klonari, K. Recherches Sur Les Causes Des Infections Localisées Ou Géneéralisées Des Olives Attaquées Par Le Champignon Camarosporium dalmatica. I. Influence de L’humidité, de La Pression Osmotique et Du PH Des Fruits. Ann. Inst. Phytopathol. Benaki 1983, 14, 1–9. [Google Scholar]
- Iannotta, N.; Noce, M.E.; Ripa, V.; Scalercio, S.; Vizzarri, V. Assessment of Susceptibility of Olive Cultivars to the Bactrocera oleae (Gmelin, 1790) and Camarosporium dalmaticum (Thüm.) Zachos & Tzav.-Klon. Attacks in Calabria (Southern Italy). J. Environ. Sci. Health B 2007, 42, 789–793. [Google Scholar] [CrossRef]
- Moral, J.; Muñoz-Díez, C.; González, N.; Trapero, A.; Michailides, T.J. Characterization and Pathogenicity of Botryosphaeriaceae Species Collected from Olive and Other Hosts in Spain and California. Phytopathology 2010, 100, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Salgues, R. Affections Parasitaires Des Olives et Modification Physico-Chimiques de L’huile Extraite. Comptes Rendus Seances Soc. Biol. Fil. 1937, 124, 817–819. [Google Scholar]
- Gonzalez, N.; Vargas-Osuna, E.; Trapero, A. EI Escudete de La Aceituna I: Biología y Daños En Olivares de La Provincia de Sevilla. Bol. San. Veg. Plagas 2006, 32, 709–722. [Google Scholar]
- Red de Alerta e Información Fitosanitaria (RAIF): Consejería de Agricultura, Pesca, Agua y Desarrollo Rural (Junta de Andalucía) Incidencia de Escudete Durante La Campaña Del Olivar En Andalucía; Sevilla. 2022. Available online: https://www.juntadeandalucia.es/agriculturapescaaguaydesarrollorural/raif/incidencia-de-escudete-durante-la-campana-del-olivar-en-andalucia/ (accessed on 5 February 2024).
- Verona, O. Notizie Sopra Una Dannosa Micosi Delle Olive. Boll. Tec. Lst. Pat. Ueg. Pisa 1952, 5, 8. [Google Scholar]
- Ayoutantis, A.J.; Pélécassis, E.D.; Argyriou, L.C.; Mourikis, P.A.; Tsacas, L.E. Rapport Sur Le Travaux Experimentaux de Lutte Contre Le Dacus a Rovies (Eubée) Pendant l’année 1953. Ann. L’institut Phytopathol. Benaki 1954, 8, 3–75. [Google Scholar]
- Solinas, M. Osservazioni Biologiche Condotte in Puglia Sulla Prolasioptera berlesiana (Paoli), Noc Particolare Riferimento Au Rapporti Simbioti Ci Col Dacus oleae (Gmelin) a Noc La Sphaeropsis Dalmatica (Thüm.) Gigante. Entomologica 1967, 3, 129–176. [Google Scholar]
- Mateo-Sagasta, E. Daños y Enfermedades Del Olivo. In Olivicultura Moderna; FAO-INA; Agrícola Española: Madrid, Spain, 1976; pp. 213–234. [Google Scholar]
- Neuenschwander, P.; Bigler, F.; Delucchi, V.; Michelakis, S. Natural Enemies of Preimaginal Stages of Dacus oleae Gmel. (Dipt., Tephritidae) in Western Crete. I. Bionomics and Phenologies. Boll. Lab. Entomol. Agrar. “Filippo Silvestri” 1983, 40, 3–32. [Google Scholar]
- Arambourg, Y. Traité d’Entomologie Oleicole; Conseil Oleicole Internacional: Madrid, Spain, 1986; Available online: https://datos.bne.es/edicion/bimo0000004667.html (accessed on 28 December 2022).
- De Andrés Cantero, F. Enfermedades y Plagas Del Olivo; Riquelme y Vargas Ediciones, S.L.: Jaén, Spain, 2001; Available online: https://books.google.es/books/about/Enfermedades_y_plagas_del_olivo.html?id=ghVIAAAAYAAJ&redir_esc=y (accessed on 28 December 2022).
- La Greca, L.; Vrenna, G. Damages by Sphaeropsis Dalmatica in Calabria (South Italy) [Olea europaea]. Inf. Fitopatol. 1995, 45, 32–33. [Google Scholar]
- Fraval, A. Olive Fruit Midge; National Research Institute for Agriculture, Food and the Environment: Paris, France, 1997; Available online: www.inra.fr/Internet/Produits/HYPPZ (accessed on 15 November 2023).
- Longo, O.; Cavallo, C.; D’Agnano, G.; Schiavone, D.; Porcelli, E. Inusuale Cascola Di Olive per Azione Combinata Di Tre Parassiti. Inf. Agrar. 2004, 22, 57–59. [Google Scholar]
- Koronéos, J. Les Insectes de l’olivier Dans Le Pélion; Taraussopoulos Ed.: Athenas, Greece, 1946; Available online: https://icgf.myspecies.info/node/7942 (accessed on 28 December 2022).
- Silvestri, F. Contribution à La Biologie de La Petite Cécidomyie Des Olives (Prolasioptera berlesiana Paoli) En Italie. Monit. Eur. Int. Prot. Plantes 1945, 19, 73–76. [Google Scholar]
- Tominic, A. Prilog Izucavanju Maslinovih Cecidomia Lasioptera berlesiana (Paoli). Zast. Bilja 1966, 17, 221–228. (In Serbocroatian) [Google Scholar]
- Neuenschwander, P.; Michelakis, S.; Holloway, P.; Berchtol, W. Factors Affecting the Susceptibility of Fruits of Different Olive Varieties to Attack by Dacus oleae (Gmelin) (Dipt., Tephritidae). Z. Angew. Entomol. 1985, 100, 174–188. [Google Scholar] [CrossRef]
- Civantos, M.; Sánchez, M. Control Integrado En El Olivar Español y Su Influencia En La Calidad. Agricultura 1993, 735, 854–858. [Google Scholar]
- Goidànich, G. La Difesa Delle Piante Da Frutto, 5th ed.; Edagricole: Bologna, Italy, 1990; Available online: https://www.edagricole.it/wp-content/uploads/2020/03/5540-La-difesa-delle-piante-da-frutto-SFOGLIA.pdf (accessed on 28 December 2022).
- Harpaz, I.; Gerson, U. The Biocomplex of the Olive Fruit Fly Dacus Oleae (Gmelin), the Olive Fruit Midge Prolasioptera Berlesiana, and the Fungus Macrophoma dalmatica. Berl. y Vogl. in Olive Fruits in the Mediterranean Basin. Scr. Hierosolymitana 1966, 18, 81–126. [Google Scholar]
- Sasso, R.; Viggiani, G. Preliminary Notes on the Gall Midges (Diptera: Cecidomyiidae) Associated with the Olive Fly, Bactrocera Oleae (Gmelin) (Diptera: Tephritidae). Integr. Prot. Olive Crops IOBC/Wprs Bull. 2007, 30, 43–46. [Google Scholar]
- Iannotta, N.; Belfiore, T.; Noce, M.E.; Scalercio, S.; Vizzarri, V. Correlation between Bactrocera Oleae Infestation and Camarosporium Dalmaticum Infection in an Olive Area of Southern Italy. Acta Hortic. 2012, 949, 309–316. [Google Scholar] [CrossRef]
- Basher, A.; Abdelrazak, F.; Saleh, A. The Relationship between the Olive Fruit Fly Bactrocera oleae Rossi and the Predatory Fly Prolasioptera berlesiana Paoli at an Olive Orchard in Quneitra Governorate, Syria. Arab. J. Plant Prot. 2019, 37, 232–239. [Google Scholar] [CrossRef]
- Silvestri, F. Un Piccolo Insetto Amico Degli Olivicoltori. Olearia 1949, 1, 3–10. [Google Scholar]
- Silvestri, F. Nuove Notizie Sulla Cecidomia Delle Olive (Prolasioptera berlesiana Paoli). Rend. Accad. Naz. Lincei 1947, 8, 750–752. [Google Scholar]
- González, N. Aspectos Biológicos y Epidemiológicos Del Escudete de La Aceituna Causado Por Camarosporium Dalmaticum. Master’s Thesis, ETSIAM, Universidad de Córdoba, Cordoba, Spain, 2005. [Google Scholar]
- De Laurentiis, G. Attacchi Di Prolasioptera Berlesiana Sulle Olive in Abruzzo. Inf. Agrar. 1993, 49, 49–50. [Google Scholar]
- Dominici, M.; Pucci, C.; Montanan, G.E. Dacus oleae (Gmel.) Ovipositing in Olive Drupes (Diptera, Tephrytidae). J. Appl. Entomol. 1986, 101, 111–120. [Google Scholar] [CrossRef]
- Rizzo, R.; Caleca, V. Resistance to the Attack of Bactrocera oleae (Gmelin) of Some Sicilian Olive Cultivars. In Proceedings of the Olivebioteq, Mazara del Vallo, Italy, 5–10 November 2006; pp. 5–10. [Google Scholar]
- Pucci, C.; Ambrosi, G. Ovideposizione Del Dacus Oleae (Gmelin) e Dimensioni Delle Drupe. Frustala Entomol. 1981, 4, 181–194. [Google Scholar]
- Alvarado, M.; Civantos, M.; Durán, J.M. Pests. In Olive Growing; Barranco, D., Fernández-Escobar, R., Rallo, L., Eds.; Junta de Andalucía/Mundi-Prensa/RIRDC/AOA: Pendle Hill, NSW, Australia, 2010; p. 756. [Google Scholar]
- Latinović, J.; Hrnčić, S.; Perović, T.; Nedeljko Latinović, N. Botryosphaeria dothidea Causal Agent of Olive Fruit Rot, Pathogen of Wounds or Not? Integr. Prot. Olive Crops IOBC-WPRS Bull. 2014, 108, 35–38. [Google Scholar]
- Aldebis, H.K.; Trapero-Casas, A. Influencia de La Variedad y Técnicas de Cultivo Sobre Las Principales Enfermedades Del Olivar. In Proceedings of the Jornadas de Investigación y Transferencia de Tecnología al Sector Oleícola, Junta de Andalucía, Cordoba, Spain, 20–21 November 2002; pp. 178–182. [Google Scholar]
- Aldebis, H.K.; Vargas-Osuna, E. La Mosca Del Olivo, Daños y Métodos de Lucha. Vida Rural 2003, 176, 42–46. [Google Scholar]
- Sabariego-Sánchez, E.M. Evaluación de Las Poblaciones de Bactrocera Oleae (Gmelin) y Niveles de Daños En Distintas Técnicas de Cultivo, Trabajo Profesional de Fin de Carrera; Universidad de Córdoba: Cordoba, Spain, 2007. [Google Scholar]
- Viggiani, G.; Sasso, R. Lasioptera berlesiana Not a “Danger” to Olives. Inf. Agrar. 2008, 64, 54–56. [Google Scholar]
- Kobune, S.; Kajimura, H.; Masuya, H.; Kubono, T. Symbiotic Fungal Flora in Leaf Galls Induced by Illiciomyia yukawai (Diptera: Cecidomyiidae) and in Its Mycangia. Microb. Ecol. 2012, 63, 619–627. [Google Scholar] [CrossRef]
- Rohfritsch, O. Plants, Gall Midges, and Fungi: A Three-Component System. Entomol. Exp. Appl. 2008, 128, 208–216. [Google Scholar] [CrossRef]
- Heath, J.J.; Stireman, J.O. Dissecting the Association between a Gall Midge, Asteromyia carbonifera, and Its Symbiotic Fungus, Botryosphaeria dothidea. Entomol. Exp. Appl. 2010, 137, 36–49. [Google Scholar] [CrossRef]
- Lebel, T.; Peele, C.; Veenstra, A. Fungi Associated with Asphondylia (Diptera: Cecidomyiidae) Galls on Sarcocornia quinqueflora and Tecticornia arbuscula (Chenopodiaceae). Fungal Divers. 2012, 55, 143–154. [Google Scholar] [CrossRef]
- Borkent, A.; Bissett, J. Gall Midges (Diptera: Cecidomyiidae) Are Vectors for Their Fungal Symbionts. Symbiosis 1985, 1, 185–194. [Google Scholar]
- Roskam, J.C. Phylogeny of Gall Midges (Cecidomyiidae). In Biology, Ecology, and Evolution of Gall-Inducing Arthropods; Raman, A., Schaefer, C.W., Withers, T.M., Eds.; Science Publishers: Enfield, NH, USA, 2005; pp. 305–319. [Google Scholar]
- Adair, R.J.; Burgess, T.; Serdani, M.; Barber, P. Fungal Associations in Asphondylia (Diptera: Cecidomyiidae) Galls from Australia and South Africa: Implications for Biological Control of Invasive Acacias. Fungal Ecol. 2009, 2, 121–134. [Google Scholar] [CrossRef]
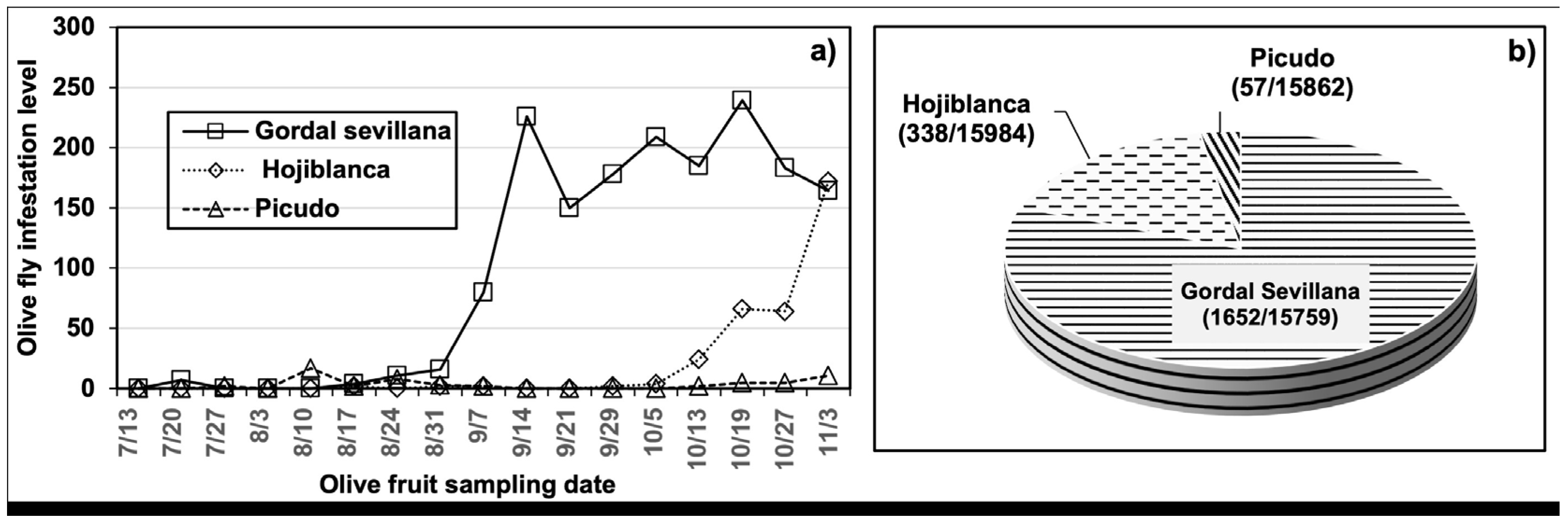
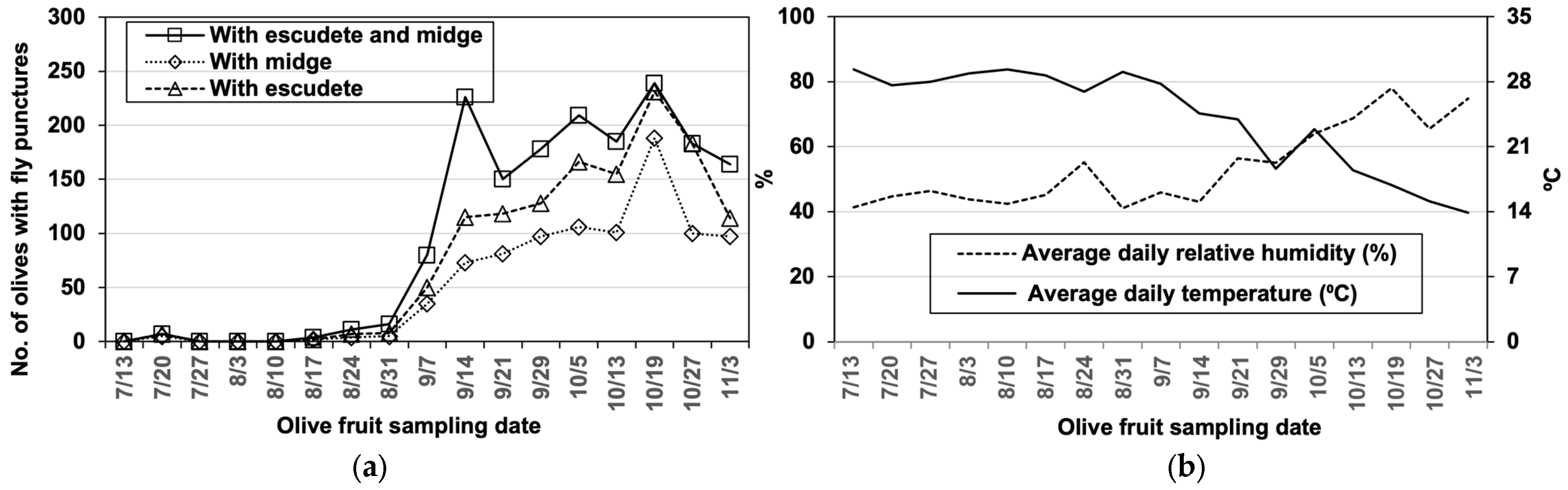
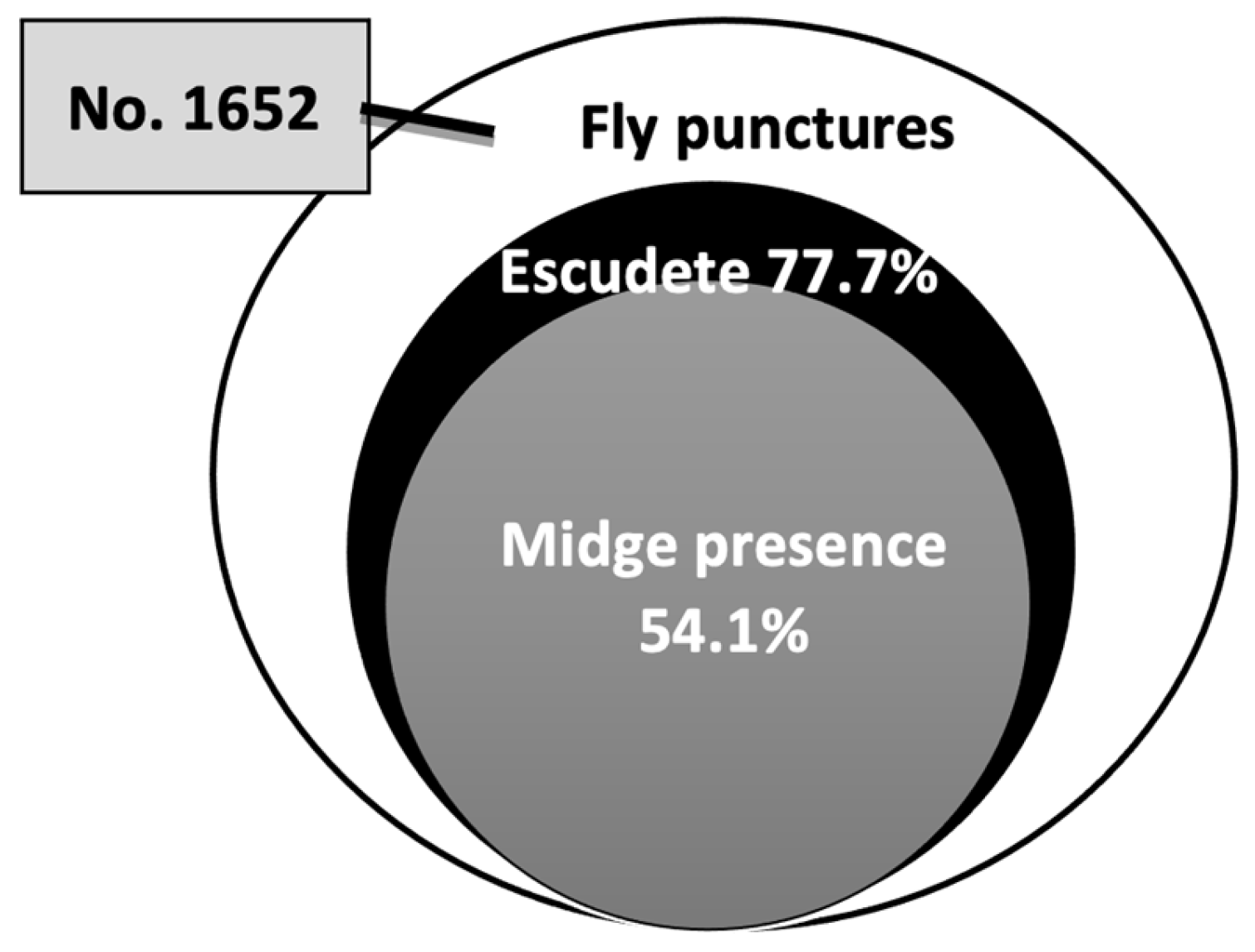
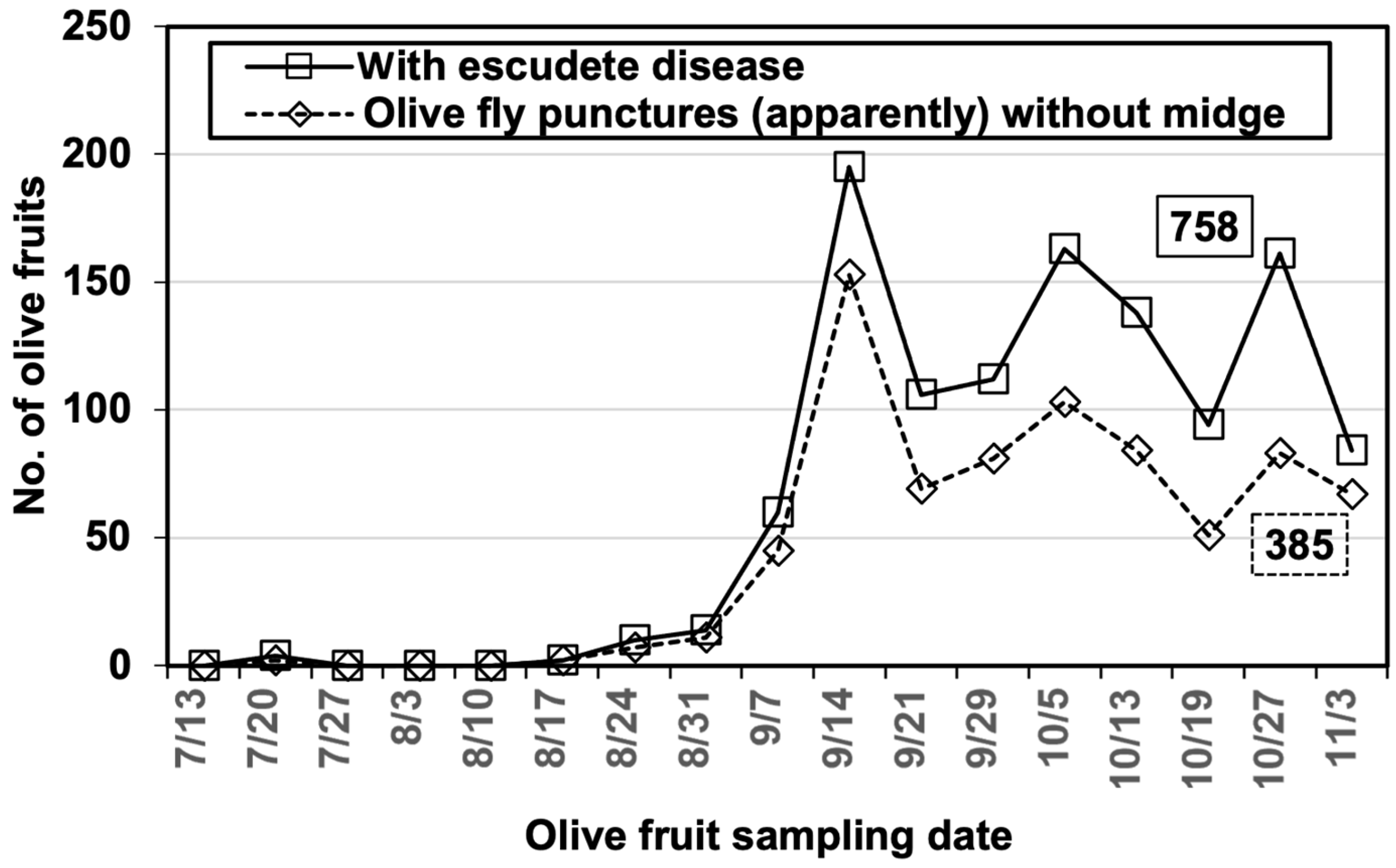
| Variety | Olives with Olive Fly Punctures | Punctures with Midges | Punctures with Escudete | With both Agents | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Gordal Sevillana | 913 | 207 | 22.7 a 1 | 376 | 41.2 b | 207 | 100 |
| Hojiblanca | 1255 | 98 | 7.8 a | 335 | 26.7 b | 98 | 100 |
| Picudo | 188 | 13 | 6.9 a | 42 | 22.3 b | 13 | 100 |
| Total | No. of Fruits Observed 1 | Punctured Fruits (N) | With B. dothidea 2 | |
|---|---|---|---|---|
| N | % | |||
| Punctured fruits | 2475 | 446 | 102 | 22.8 a |
| Healthy fruits | 1932 | - | 89 | 4.6 b |
| Stings | With Fly Stings and B. dothidea (728 Fruits) | With Fly Stings and P. berlesiana (600 Fruits) | ||
|---|---|---|---|---|
| N | % | N | % | |
| Live | 99 | 13.6 a 1 | 14 | 1.9 a |
| Non-live | 445 | 74.2 b | 268 | 44.7 b |
| Artificial SFOP Wounds (No.) | Olives with B. dothidea in Artificial SFOP Wounds | |||||
|---|---|---|---|---|---|---|
| Total | % | With P. berlesiana | Without P. berlesiana | |||
| N | % | N | % | |||
| Exp. 1 A (489) | 164 | 33.5 | 122 | 74.4 | 42 | 25.6 |
| Exp. B (554) | 330 | 59.8 | 277 | 83.9 | 53 | 16.0 |
| Exp. A + B (1043) | 494 | 47.4 | 399 | 80.8 a 2 | 95 | 19.2 b |
| Artificial Wound Types | Pricked Olives | Olives with P. berlesiana | Olives with B. dothidea 3 | ||
|---|---|---|---|---|---|
| N | N | % | N | % | |
| SFOP 1 | 400 | 161 | 40.25 | 178 | 44.50 |
| SW 2 | 406 | 0 | 0.00 | 0 | 0.00 |
| Treatment | Total Observed | With P. berlesiana | With B. dothidea | |
|---|---|---|---|---|
| N | % | |||
| Control (Olives without lesions) | 60 | - | 0 | 0.0 |
| SFOP 1 | 60 | + | 60 | 100.0 |
| 60 | - | 9 | 15.0 | |
| SW 2 | 60 | - | 0 | 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldebis, H.K.; Santos-Rufo, A.; Eldesouki-Arafat, I.; Vargas-Osuna, E.; Moral, J.; Trapero, A.; López-Escudero, F.J. Olive Escudete (Dalmatian Disease) Caused by Botryosphaeria dothidea as a Result of Fly–Midge–Fungus Interaction. Horticulturae 2024, 10, 321. https://doi.org/10.3390/horticulturae10040321
Aldebis HK, Santos-Rufo A, Eldesouki-Arafat I, Vargas-Osuna E, Moral J, Trapero A, López-Escudero FJ. Olive Escudete (Dalmatian Disease) Caused by Botryosphaeria dothidea as a Result of Fly–Midge–Fungus Interaction. Horticulturae. 2024; 10(4):321. https://doi.org/10.3390/horticulturae10040321
Chicago/Turabian StyleAldebis, Hani K., Antonio Santos-Rufo, Ibrahim Eldesouki-Arafat, Enrique Vargas-Osuna, Juan Moral, Antonio Trapero, and Francisco Javier López-Escudero. 2024. "Olive Escudete (Dalmatian Disease) Caused by Botryosphaeria dothidea as a Result of Fly–Midge–Fungus Interaction" Horticulturae 10, no. 4: 321. https://doi.org/10.3390/horticulturae10040321
APA StyleAldebis, H. K., Santos-Rufo, A., Eldesouki-Arafat, I., Vargas-Osuna, E., Moral, J., Trapero, A., & López-Escudero, F. J. (2024). Olive Escudete (Dalmatian Disease) Caused by Botryosphaeria dothidea as a Result of Fly–Midge–Fungus Interaction. Horticulturae, 10(4), 321. https://doi.org/10.3390/horticulturae10040321









