Sugar Receding in Aril Benefits the Recalcitrant Seeds of Litchi (Litchi chinensis) and Longan (Dimocarpus longan) to Cope with Dry Spells after Maturation
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
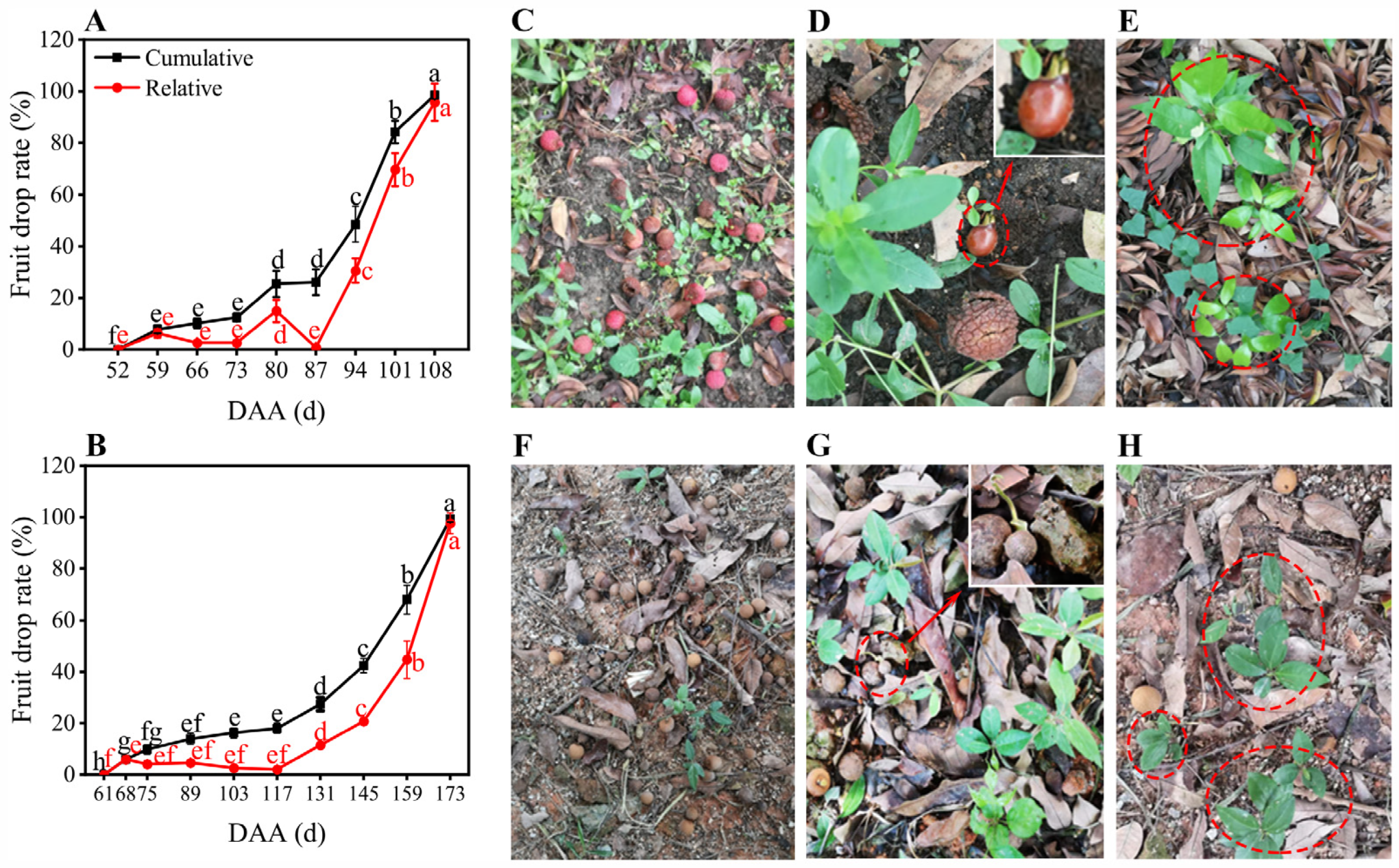
2.2. Fruit Drop Rate Evaluation
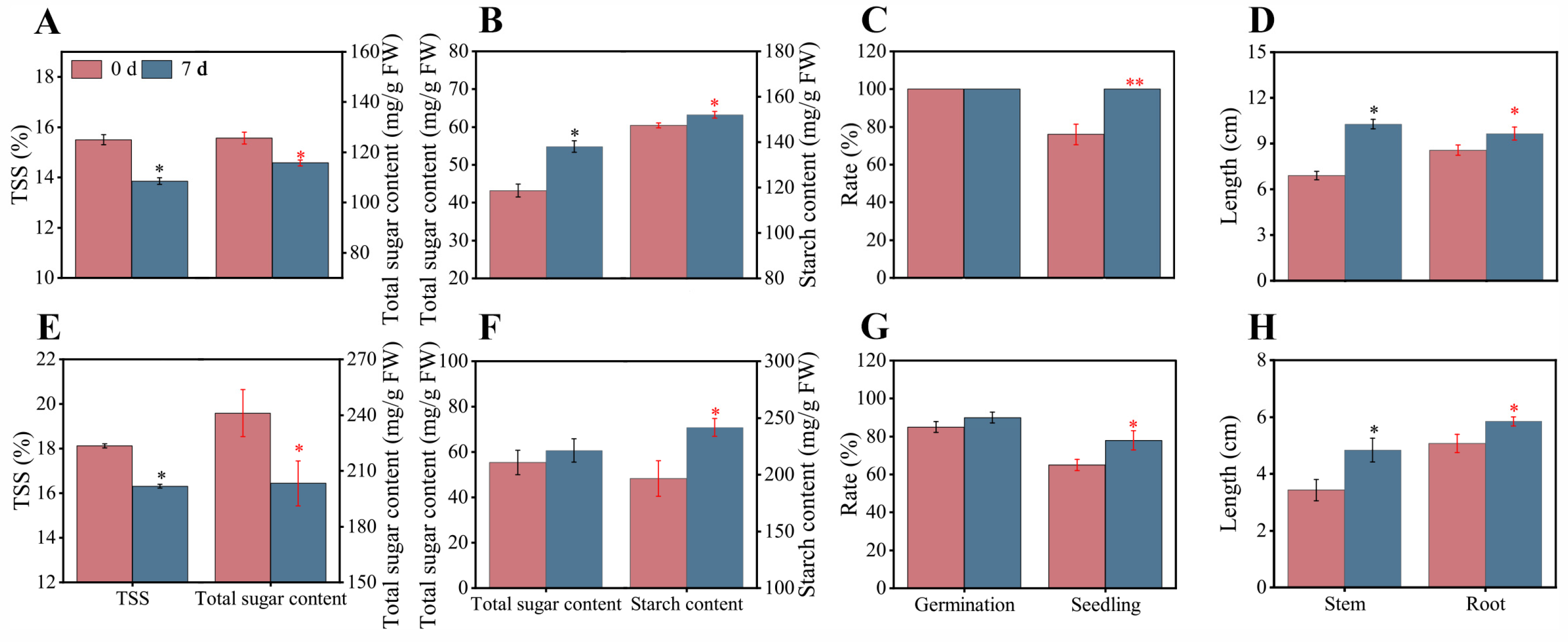
2.3. Fruit Storage Treatment after the Fruit Detachment from the Trees
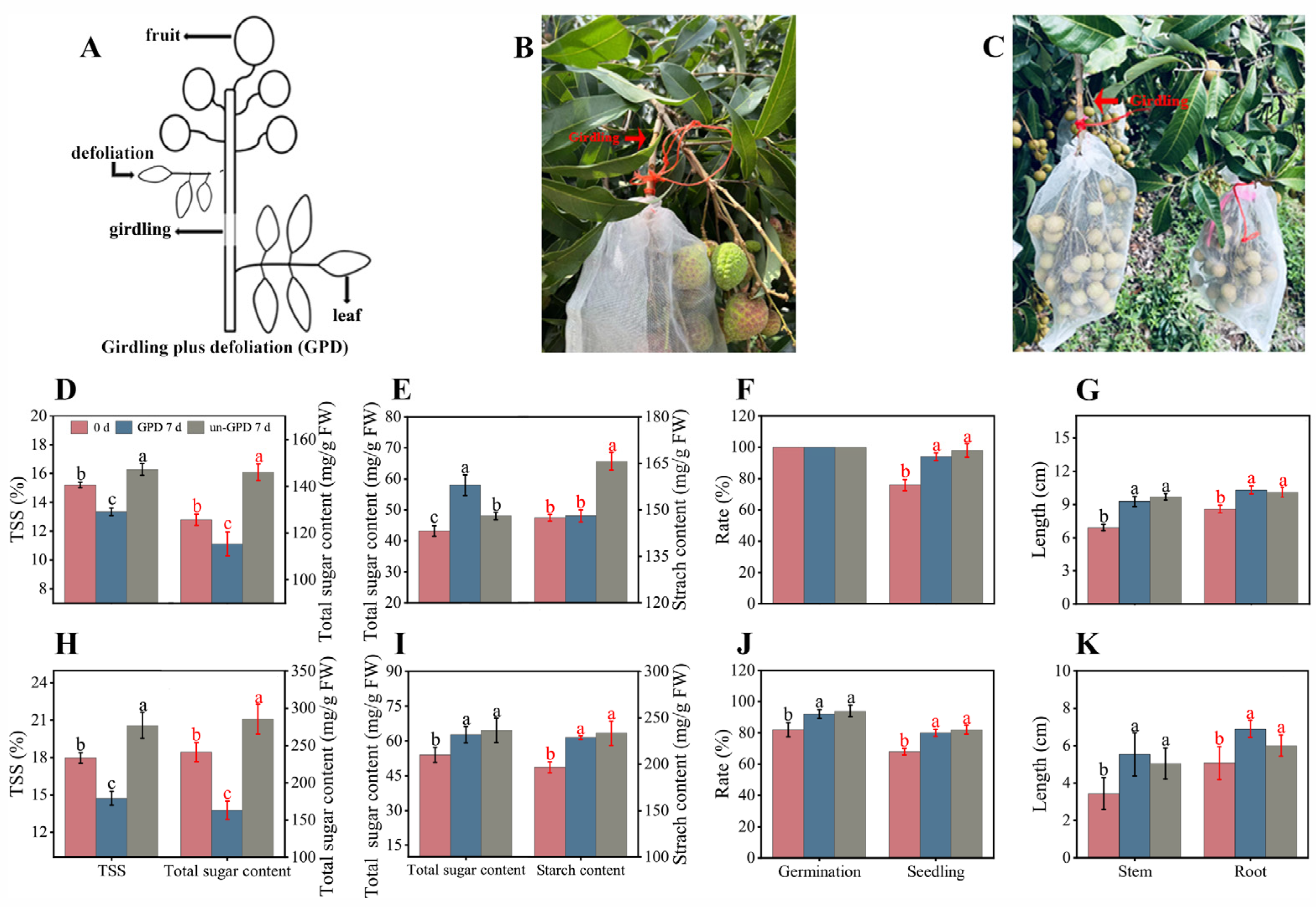
2.4. Girdling Plus Defoliation (GPD) Treatment on Fruit Clusters
2.5. Fruit Weight
2.6. Ripe Seed Definition, Seed Germination, and Seedling Establishment Rate
2.7. Determination of the Total Soluble Solid Contents
2.8. Determination of the Total Soluble Sugars
2.9. Determination of the Starch Contents
2.10. Data Statistical Analysis
3. Results
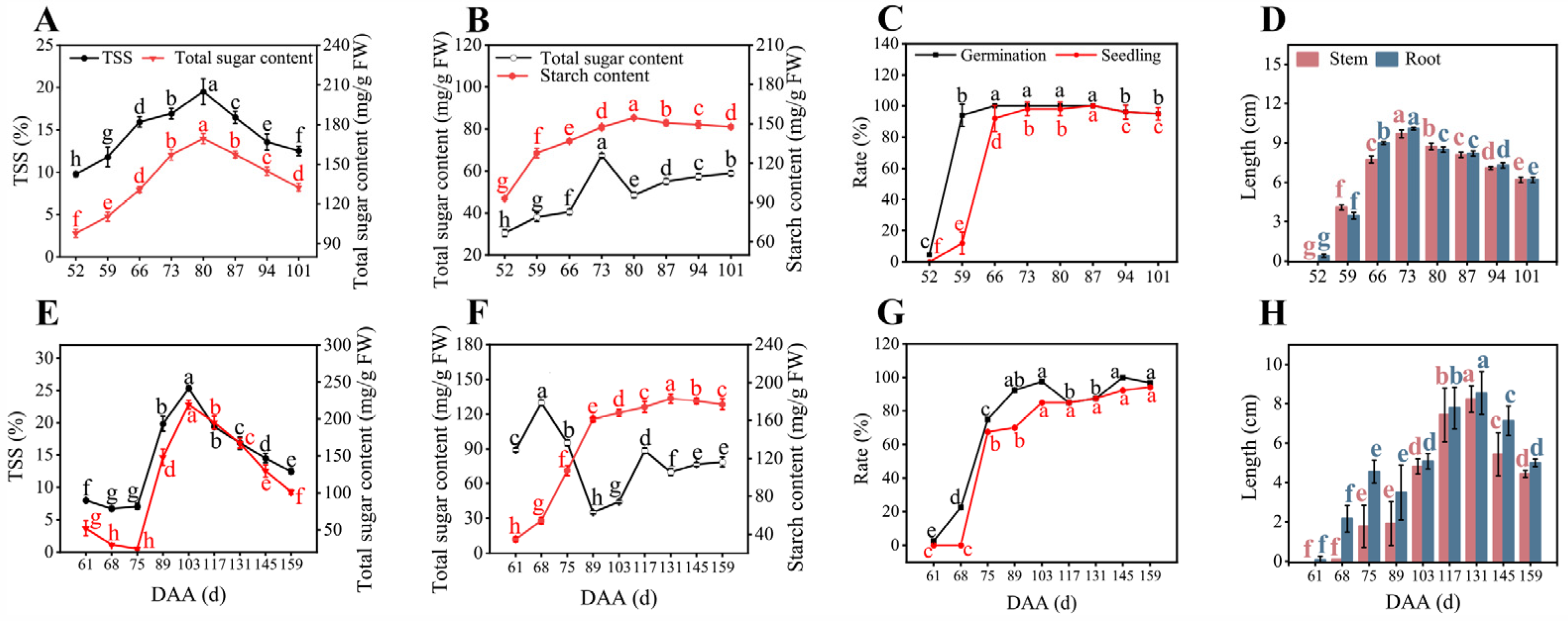
3.1. The Growth Profiles of the Arils of Litchi and Longan Fruits in the Developmental Season with Fluctuating Weather
3.2. The Relationship between Seed Vigor and Sugar/Starch Contents in the Arils and Seeds throughout the Development of Litchi and Longan Fruits
3.3. Arils Benefit Seed Vigor Maintenance/Enhancement after Photosynthate Blockage of the Fruits on-Tree
3.4. Fruit Drop Profiles of the Litchi and Longan Trees at the Developmental Stages after Aril Initiation under Fluctuating Weather
3.5. Arils Benefit Seed Vigor Enhancement after Fruit Detachment from Trees
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tindall, H.D. Sapindaceous Fruits: Botany and Horticulture: Horticultural Reviews; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1994. [Google Scholar]
- Menzel, C.M.; Waite, G.K. Litchi and Longan: Botany, Production and Uses; Cabi Publishing: Wallingford, UK, 2005. [Google Scholar]
- Pham, V.T.; Herrero, M.; Hormaza, J.I. Phenological growth stages of longan (Dimocarpus longan) according to the BBCH scale. Sci. Hortic. 2015, 189, 201–207. [Google Scholar] [CrossRef]
- Trong, L.V.; Khanh, N.N.; Huyen, L.T.; Phuong, H.T.; Hien, V.T.T. Physiological changes during the growth and development of litchi fruit (litchi chinensis sonn.) Grown in vietnam. Rev. Bras. De Frutic. 2020, 43, e658. [Google Scholar] [CrossRef]
- Fan, S.; Wang, D.; Xie, H.; Wang, H.; Qin, Y.; Hu, G.; Zhao, J. Sugar transport, metabolism and signaling in fruit development of litchi chinensis sonn: A review. Int. J. Mol. Sci. 2021, 22, 11231. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, Z.; Joyce, D.C.; Ketsa, S. Postharvest biology and handling of longan fruit (Dimocarpus longan Lour.). Postharvest Biol. Technol. 2002, 26, 241–252. [Google Scholar] [CrossRef]
- Jiang, Y.M.; Wang, Y.; Song, L.; Liu, H.; Lichter, A.; Kerdchoechuen, O.; Joyce, D.C.; Shi, J. Postharvest characteristics and handling of litchi fruit—An overview. Aust. J. Exp. Agric. 2006, 46, 1541. [Google Scholar] [CrossRef]
- Luo, T.; Shuai, L.; Liao, L.; Li, J.; Duan, Z.; Guo, X.; Xue, X.; Han, D.; Wu, Z. Soluble acid invertases act as key factors influencing the sucrose/hexose ratio and sugar receding in longan (Dimocarpus longan Lour.). Pulp. J. Agric. Food Chem. 2019, 67, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Shuai, L.; Lai, T.; Liao, L.; Li, J.; Duan, Z.; Xue, X.; Han, D.; Wu, Z. Up-regulated glycolysis, tca, fermentation and energy metabolism promoted the sugar receding in ‘shixia’ longan (Dimocarpus longan Lour.) Pulp. Sci. Hortic. 2021, 281, 109998. [Google Scholar] [CrossRef]
- Luo, T.; Lin, X.; Lai, T.; Long, L.; Lai, Z.; Du, X.; Guo, X.; Shuai, L.; Han, D.; Wu, Z. GA3 treatment delays the deterioration of ‘shixia’ longan during the on-tree preservation and room-temperature storage and up-regulates antioxidants. Foods 2023, 12, 2032. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Du, J.; Ma, W.; Chen, T.; Shui, X.; Liao, H.; Lin, X.; Zhou, K. Transcriptome analysis on the underlying physiological mechanism of calcium and magnesium resolving “sugar receding” in ‘feizixiao’ litchi pulp. Horticulturae 2022, 8, 1197. [Google Scholar] [CrossRef]
- Peng, J.; Du, J.; Wuqiang, M.; Chen, T.; Shui, X.; Liao, H.; Lin, X.; Zhou, K. Transcriptomics-based analysis of the causes of sugar receding in feizixiao litchi (Litchi chinensis Sonn.). Pulp. Front. Plant Sci. 2022, 13, 1083753. [Google Scholar] [CrossRef]
- Shuai, L.; Li, J.; Niu, J.J.; Qian, P.H.; Liu, W.H.; Xue, X.Q.; Han, D.M.; Wu, Z.X. Sucrose-metabolizing enzymes and their genes in the arils of two dimocarpus longan cultivars. Biol. Plant. 2016, 60, 741–748. [Google Scholar] [CrossRef]
- Piazzolla, F.; Pati, S.; Amodio, M.L.; Colelli, G. Effect of harvest time on table grape quality during on-vine storage. J. Sci. Food Agric. 2016, 96, 131–139. [Google Scholar] [CrossRef]
- Zhao, M.; Li, J. Molecular events involved in fruitlet abscission in litchi. Plants 2020, 9, 151. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology and Development; Sinauer Associates: Sunderland, MA, USA, 2015. [Google Scholar]
- Sobral, M. All traits are functional: An evolutionary viewpoint. Trends Plant Sci. 2021, 26, 674–676. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.K.; Sharma, S.B. Growth, maturity, germination and storage of litchi seeds. Sci. Hortic. 1987, 33, 213–221. [Google Scholar] [CrossRef]
- Song, S.Q.; Patricia, B.; Pammenter, N.; Ntuli, T.M.; Fu, J.R. Seed recalcitrance: A current assessment. Acta Bot. Sin. 2003, 45, 638–643. [Google Scholar]
- Liu, B.; Xue, W.; Guo, Z.; Liu, S.; Zhu, Q.; Pang, X.; Zhang, Z.; Fang, F. Water loss and pericarp browning of litchi (Litchi chinensis) and longan (Dimocarpus longan) fruit maintain seed vigor. Sci. Hortic. 2021, 290, 110519. [Google Scholar] [CrossRef]
- Roberts, E.H. Predicting the storage life of seeds. Seed Sci. Technol. 1973, 1, 499–514. [Google Scholar]
- Berjak, P.; Pammenter, N.W. From avicennia to zizania: Seed recalcitrance in perspective. Ann. Bot. 2008, 101, 213–228. [Google Scholar] [CrossRef]
- Marques, A.; Buijs, G.; Ligterink, W.; Hilhorst, H. Evolutionary ecophysiology of seed desiccation sensitivity. Funct. Plant Biol. FPB 2018, 45, 1083–1095. [Google Scholar] [CrossRef]
- Daws, M.I.; Bolton, S.; Burslem DF, R.P.; Garwood, N.C.; Mullins, C.E. Loss of desiccation tolerance during germination in neo-tropical pioneer seeds: Implications for seed mortality and germination characteristics. Seed Sci. Res. 2007, 17, 273–281. [Google Scholar] [CrossRef]
- Silveira, S.R.; Dornelas, M.C.; Martinelli, A.P. Perspectives for a framework to understand aril initiation and development. Front. Plant Sci. 2016, 7, 1919. [Google Scholar] [CrossRef] [PubMed]
- Huang, H. Towards a better insight into the development of the arillate fruit of litchi and longan. Acta Hortic. 2021, 558, 185–192. [Google Scholar] [CrossRef]
- Herrera, C.M. Seed dispersal by animals: A role in angiosperm diversification? Am. Nat. 1989, 133, 309–322. [Google Scholar] [CrossRef]
- Willson, M.F.; Irvine, A.K.; Walsh, N.G. Vertebrate dispersal syndromes in some australian and new zealand plant communities, with geographic comparisons. Biotropica 1989, 21, 133–147. [Google Scholar] [CrossRef]
- Evrard, Q.; Hardy, O.J.; Tagg, N.; Doucet, J. Removal and predation of aril-covered seeds: The case of afzelia bipindensis (fabaceae—Detarioidae). Plant Ecol. Evol. 2019, 152, 460–469. [Google Scholar] [CrossRef]
- Vaz TA, A.; Rodrigues Junior, A.G.; Davide, A.C.; Nakamura, A.T.; Toorop, P.E. A role for fruit structure in seed survival and germination of swartzia langsdorffii Raddi beyond dispersal. Plant Biol. 2018, 20, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Y.; Huang, X.; Li, J.; Wang, H.; Li, J. An improved fruit transcriptome and the identification of the candidate genes involved in fruit abscission induced by carbohydrate stress in litchi. Front. Plant Sci. 2015, 6, 439. [Google Scholar] [CrossRef]
- Buysse, J.; Smolders, E.; Merckx, R. The role of free sugars and amino acids in the regulation of biomass partitioning and plant growth. Plant Soil 1993, 155, 191–194. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Wang, Q.; Liu, J.; Sun, Y. Diurnal regulation of leaf photosynthesis is related to leaf-age-dependent changes in assimilate accumulation in camellia oleifera. Plants 2023, 12, 2161. [Google Scholar] [CrossRef]
- Huang, H.; Xu, J. The developmental patterns of fruit tissue and their correlative relationships in litchi chinensis sonn. Sci. Hortic. 1983, 19, 335–342. [Google Scholar] [CrossRef]
- Tweddle, J.C.; Dickie, J.B.; Baskin, C.C.; Baskin, J.M. Ecological aspects of seed desiccation sensitivity. J. Ecol. 2003, 91, 294–304. [Google Scholar] [CrossRef]
- Pritchard, H.W.; Daws, M.I.; Fletcher, B.J.; Gaméné, C.S.; Msanga, H.P.; Omondi, W. Ecological correlates of seed desiccation tolerance in tropical african dryland trees. Am. J. Bot. 2004, 91, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Daws, M.I.; Garwood, N.C.; Pritchard, H.W. Traits of recalcitrant seeds in a semi-deciduous tropical forest in panamá: Some ecological implications. Funct. Ecol. 2005, 19, 874–885. [Google Scholar] [CrossRef]
- Yu, Y.; Baskin, J.M.; Baskin, C.C.; Tang, Y.; Cao, M. Ecology of seed germination of eight non-pioneer tree species from a tropical seasonal rain forest in southwest China. Plant Ecol. 2008, 197, 1–16. [Google Scholar] [CrossRef]
- Hay, F.R.; Probert, R.J. Advances in seed conservation of wild plant species: A review of recent research. Conserv. Physiol. 2013, 1, cot030. [Google Scholar] [CrossRef] [PubMed]
- Gautier-Hion, A.; Duplantier, J.; Quris, R.; Feer, F.; Sourd, C.; Decoux, J.; Dubost, G.; Emmons, L.; Erard, C.; Hecketsweiler, P.; et al. Fruit characters as a basis of fruit choice and seed dispersal in a tropical forest vertebrate community. Oecologia 1985, 65, 324–337. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Toledo, L.; Perez-Decelis, A.; Macedo-Santana, F.; Cuevas, E.; Endress, B.A.; Doucet, D. Chronic leaf harvesting reduces reproductive success of a tropical dry forest palm in northern mexico. PLoS ONE 2018, 13, e0205178. [Google Scholar] [CrossRef]
- Bohn, A.; Bortolin, G.S.; Job, R.B.; Pedroso, C.E.; Galviz, Y.C.; Dorneles, A.O.; Pereira, A.S.; Do Amarante, L.; Mittelmann, A.; Deuner, S. Leaf stage as a defoliation criterion for the production of high-vigour annual ryegrass seeds. Crop Pasture Sci. 2021, 72, 575–588. [Google Scholar] [CrossRef]
- Malcolm, P.J.; Holford, P.; McGlasson, W.B.; Newman, S. Temperature and seed weight affect the germination of peach rootstock seeds and the growth of rootstock seedlings. Sci. Hortic. 2003, 98, 247–256. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Z.; He, M.; Yang, C.; Liu, B.; Fang, F.; Pang, X.; Zhang, Z. Sugar Receding in Aril Benefits the Recalcitrant Seeds of Litchi (Litchi chinensis) and Longan (Dimocarpus longan) to Cope with Dry Spells after Maturation. Horticulturae 2024, 10, 319. https://doi.org/10.3390/horticulturae10040319
Guo Z, He M, Yang C, Liu B, Fang F, Pang X, Zhang Z. Sugar Receding in Aril Benefits the Recalcitrant Seeds of Litchi (Litchi chinensis) and Longan (Dimocarpus longan) to Cope with Dry Spells after Maturation. Horticulturae. 2024; 10(4):319. https://doi.org/10.3390/horticulturae10040319
Chicago/Turabian StyleGuo, Zeli, Maoxin He, Chunping Yang, Bin Liu, Fang Fang, Xuequn Pang, and Zhaoqi Zhang. 2024. "Sugar Receding in Aril Benefits the Recalcitrant Seeds of Litchi (Litchi chinensis) and Longan (Dimocarpus longan) to Cope with Dry Spells after Maturation" Horticulturae 10, no. 4: 319. https://doi.org/10.3390/horticulturae10040319
APA StyleGuo, Z., He, M., Yang, C., Liu, B., Fang, F., Pang, X., & Zhang, Z. (2024). Sugar Receding in Aril Benefits the Recalcitrant Seeds of Litchi (Litchi chinensis) and Longan (Dimocarpus longan) to Cope with Dry Spells after Maturation. Horticulturae, 10(4), 319. https://doi.org/10.3390/horticulturae10040319





