Sensitivity and Regulation of Diel Photosynthesis in Red-Fleshed Pitaya (Hylocereus polyrhizus) Micropropagules under Mannitol-Induced Water Stress/Rehydration Cycle In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants and Pre-Experimental Conditions
2.2. Photosynthetic Responses after Application of Different Flow Rates
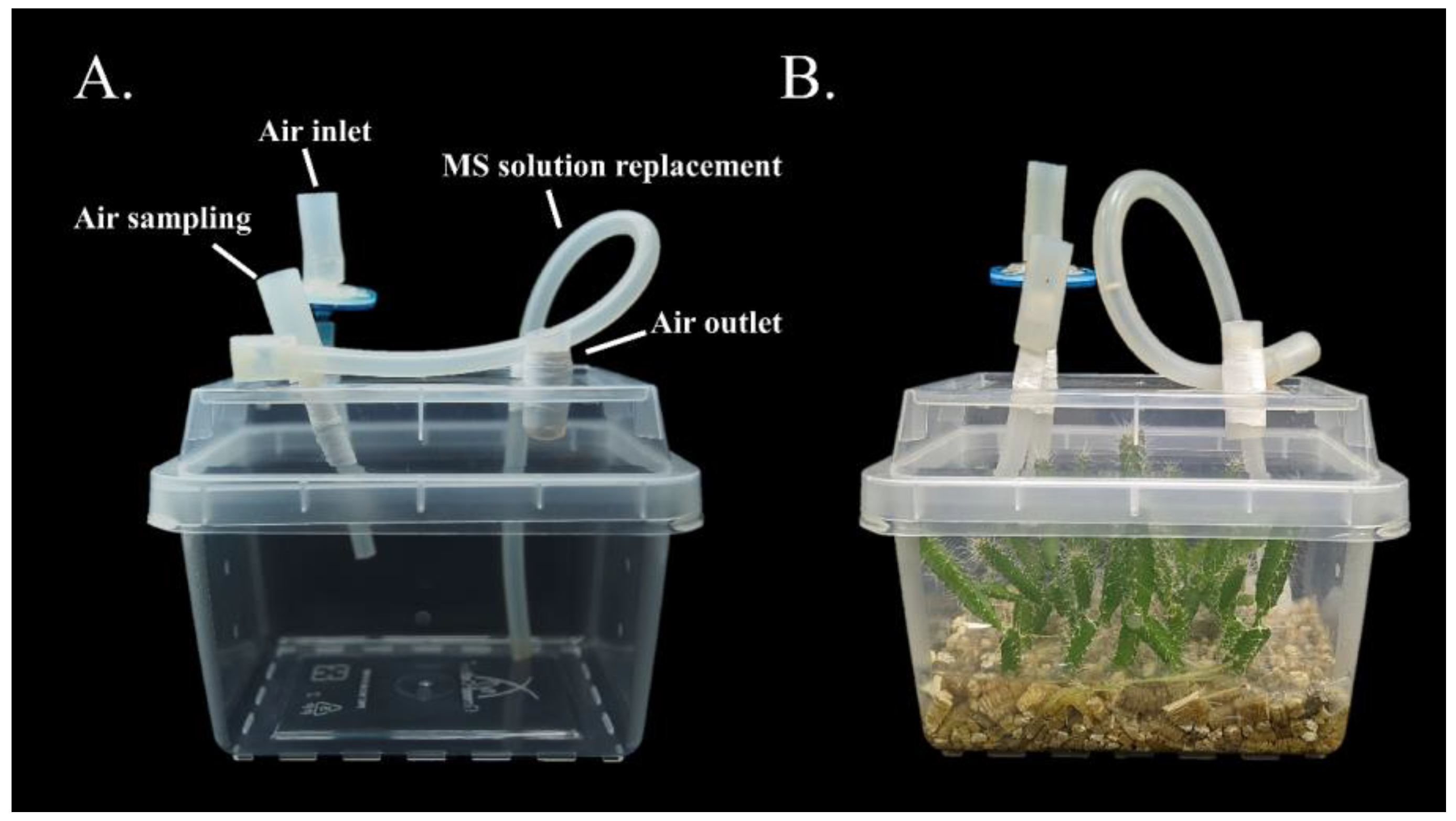
2.3. A System with Lid-Modified and Vermiculite-Supplemented Growth Vessels
2.4. Mannitol-Induced Dynamic Water Stress Modulation for Micropropagule Photosynthesis Evaluation
2.5. Dynamic Photosynthesis Analysis
2.6. Data and Statistical Analysis
3. Results
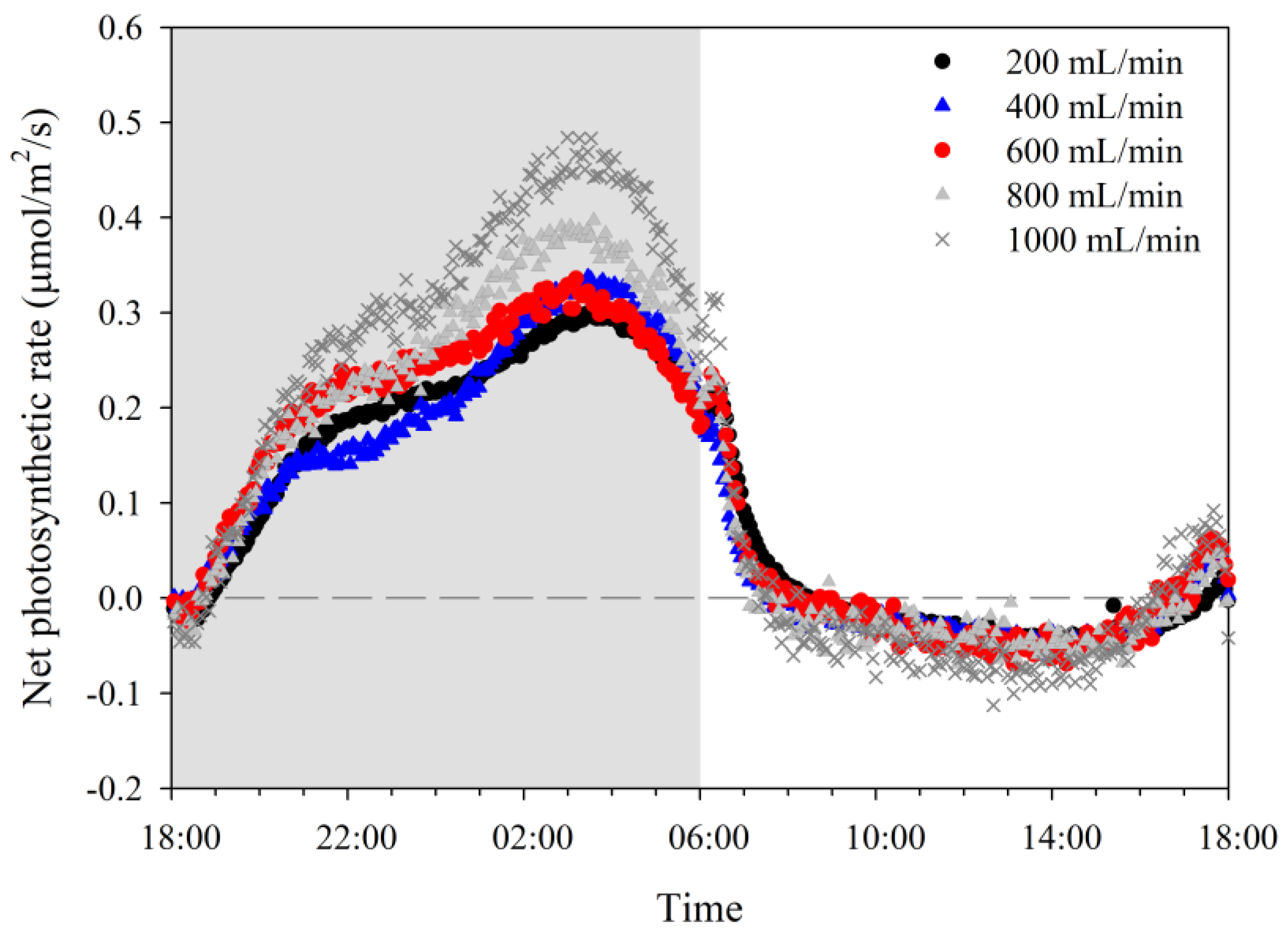
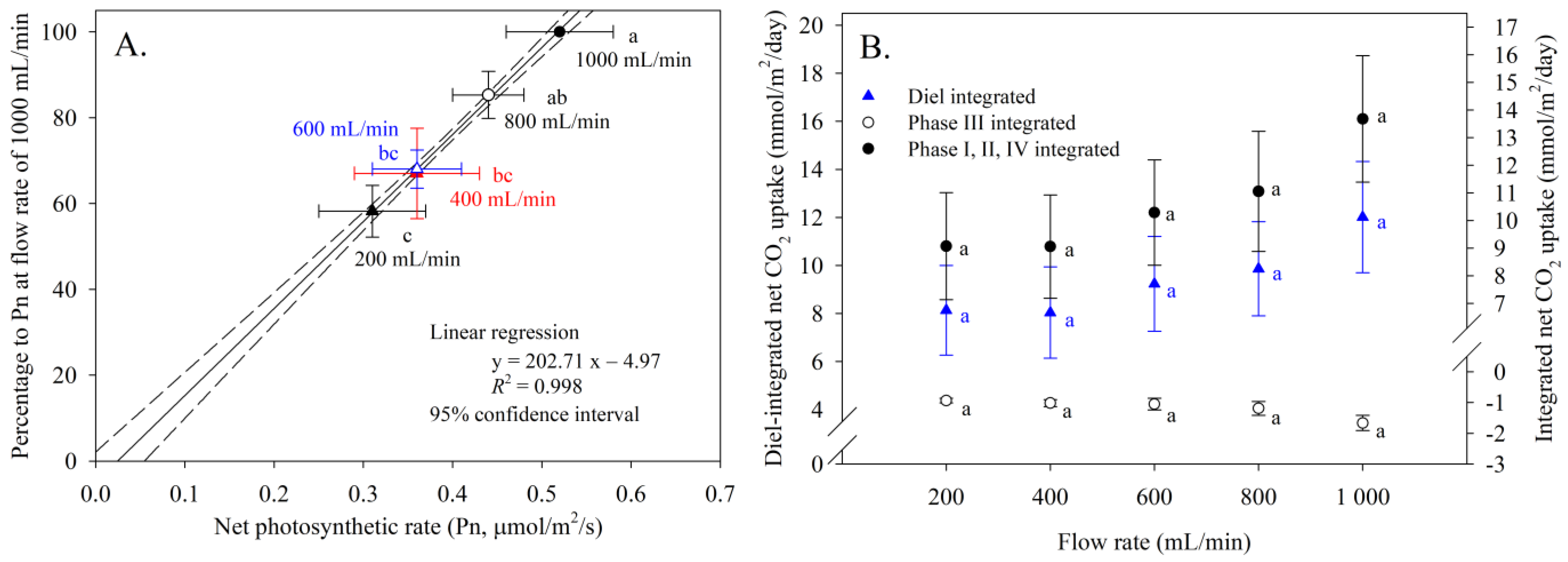
3.1. Photosynthetic Response Determination after Application of Different Flow Rates
3.2. Mannitol-Induced Dynamic Water Stress Fluctuated Diel Photosynthetic Pattern and Value
3.3. Relative Integrated Net CO2 Uptake after Water Stress Application
3.4. Photosynthetic Difference Intensity to Water Stress Application
4. Discussion
4.1. Photosynthesis Expression Response to Airflow Rates
4.2. Response of Photosynthesis to Dynamic Water Stress
4.3. Regulated Photosynthetic Pattern to Dynamic Water Stress
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bartlett, M.S.; Vico, G.; Porporato, A. Coupled carbon and water fluxes in CAM photosynthesis: Modeling quantification of water use efficiency and productivity. Plant Soil 2014, 383, 111–138. [Google Scholar] [CrossRef]
- Levin, A.; Nackley, L. Principles and practices of plant-based irrigation management. HortTechnology 2021, 31, 650–660. [Google Scholar] [CrossRef]
- Flore, J.A.; Lakso, A.N. Environmental and physiological regulation of photosynthesis in fruit crops. Hortic. Rev. 1989, 11, 111–157. [Google Scholar] [CrossRef]
- IPCC. Summary for policymakers. In Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Team, C.W., Lee, H., Romero, J., Eds.; IPCC Press: Geneva, Switzerland, 2023; pp. 1–34. [Google Scholar]
- Kapoor, D.; Bhardwaj, S.; Landi, M.; Sharma, A.; Ramakrishnan, M.; Sharma, A. The impact of drought in plant metabolism: How to exploit tolerance mechanisms to increase crop production. Appl. Sci. 2020, 10, 5692. [Google Scholar] [CrossRef]
- Calzadilla, P.I.; Carvalho, F.E.L.; Gomez, R.; Lima Neto, M.C.; Signorelli, S. Assessing photosynthesis in plant systems: A cornerstone to aid in the selection of resistant and productive crops. Environ. Exp. Bot. 2022, 201, 104950. [Google Scholar] [CrossRef]
- Ozturk, M.; Turkyilmaz Unal, B.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and its actions during the drought stress in plants. Physiol. Plant. 2021, 172, 1321–1335. [Google Scholar] [CrossRef]
- CWB. Weather Analysis of Taiwan in 2022; Central Weather Bureau: Taipei, Taiwan, 2022; pp. 1–5.
- Nobel, P.S. Parenchyma-chlorenchyma water movement during drought for the hemiepiphytic cactus Hylocereus undatus. Ann. Bot. 2006, 97, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Hartsock, T.L.; Nobel, P.S. Watering converts a CAM plant to daytime CO2 uptake. Nature 1976, 262, 574–576. [Google Scholar] [CrossRef]
- Szarek, S.R.; Ting, I.P. Physiological responses to rainfall in Opuntia basilaris (Cactaceae). Am. J. Bot. 1975, 62, 602–609. [Google Scholar] [CrossRef]
- Winter, K. Ecophysiology of constitutive and facultative CAM photosynthesis. J. Exp. Bot. 2019, 70, 6495–6508. [Google Scholar] [CrossRef]
- Nerd, A.; Neumann, P.M. Phloem water transport maintains stem growth in a drought-stressed crop cactus (Hylocereus undatus). J. Am. Soc. Hortic. Sci. 2004, 129, 486–490. [Google Scholar] [CrossRef]
- Graham, E.A.; Nobel, P.S. Daily changes in stem thickness and related gas exchange patterns for the hemiepiphytic cactus Hylocereus undatus. Int. J. Plant Sci. 2005, 166, 13–20. [Google Scholar] [CrossRef]
- Lüttge, U. Ability of crassulacean acid metabolism plants to overcome interacting stresses in tropical environments. AoB Plants 2010, 2010, plq005. [Google Scholar] [CrossRef] [PubMed]
- Silvera, K.; Neubig, K.M.; Whitten, W.M.; Williams, N.H.; Winter, K.; Cushman, J.C. Evolution along the crassulacean acid metabolism continuum. Funct. Plant Biol. 2010, 37, 995–1010. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Ma, Y.; Qing, Y.; Wang, H.; Huang, X. The highly drought-tolerant pitaya (Hylocereus undatus) is a non-facultative CAM plant under both well-watered and drought conditions. J. Hortic. Sci. Biotechnol. 2019, 94, 643–652. [Google Scholar] [CrossRef]
- Nobel, P.S.; De la Barrera, E. Stem water relations and net CO2 uptake for a hemiepiphytic cactus during short-term drought. Environ. Exp. Bot. 2002, 48, 129–137. [Google Scholar] [CrossRef]
- Winter, K.; Garcia, M.; Holtum, J.A.M. On the nature of facultative and constitutive CAM: Environmental and developmental control of CAM expression during early growth of Clusia, Kalanchoë, and Opuntia. J. Exp. Bot. 2008, 59, 1829–1840. [Google Scholar] [CrossRef]
- Ma, Y.; Dias, M.C.; Freitas, H. Drought and salinity stress responses and microbe-induced tolerance in plants. Front. Plant Sci. 2020, 11, 591911. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Singh Sidhu, G.P.; Bali, A.S.; Handa, N.; Kapoor, D.; Yadav, P.; Khanna, K.; et al. Photosynthetic response of plants under different abiotic stresses: A review. J. Plant Growth Regul. 2020, 39, 509–531. [Google Scholar] [CrossRef]
- Yan, W.; Zhong, Y.; Shangguan, Z. Responses of different physiological parameter thresholds to soil water availability in four plant species during prolonged drought. Agric. For. Meteorol. 2017, 247, 311–319. [Google Scholar] [CrossRef]
- Osmond, C.B. Crassulacean acid metabolism: A curiosity in context. Annu. Rev. Plant Physiol. 1978, 29, 379–414. [Google Scholar] [CrossRef]
- Holtum, J.A.M.; Smith, J.A.C.; Neuhaus, H.E. Intracellular transport and pathways of carbon flow in plants with crassulacean acid metabolism. Funct. Plant Biol. 2005, 32, 429–449. [Google Scholar] [CrossRef] [PubMed]
- Kluge, M.; Ting, I.P. Crassulacean Acid Metabolism: Analysis of An Ecological Adaptation; Springer Press: Berlin/Heidelberg, Germany, 1978. [Google Scholar]
- Roberts, A.; Borland, A.M.; Griffiths, H. Discrimination processes and shifts in carboxylation during the phases of crassulacean acid metabolism. Plant Physiol. 1997, 113, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Borland, A.M.; Griffiths, H. Variations in the phases of crassulacean acid metabolism and regulation of carboxylation patterns determined by carbon-isotope-discrimination techniques. In Crassulacean Acid Metabolism; Winter, K., Smith, J.A.C., Eds.; Springer Press: Berlin/Heidelberg, Germany, 1996; pp. 230–249. [Google Scholar] [CrossRef]
- Hunt, S. Measurements of photosynthesis and respiration in plants. Physiol. Plant. 2003, 117, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Nobel, P.S.; De la Barrera, E. CO2 uptake by the cultivated hemiepiphytic cactus, Hylocereus undatus. Ann. Appl. Biol. 2004, 144, 1–8. [Google Scholar] [CrossRef]
- Raveh, E.; Gersani, M.; Nobel, P.S. CO2 uptake and fluorescence responses for a shade-tolerant cactus Hylocereus undatus under current and doubled CO2 concentrations. Physiol. Plant. 1995, 93, 505–511. [Google Scholar] [CrossRef]
- Tisarum, R.; Samphumphung, T.; Theerawitaya, C.; Cha-Um, S. Free proline, total soluble sugar enrichment, photosynthetic abilities and growth performances in dragon fruit (Hylocereus undatus (Haw) Britt. & Rose) grown under mannitol-induced water deficit stress. Acta Hortic. 2018, 1206, 113–120. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Ho, M.-C.; Chang, J.-C. Re-identification of non-facultative crassulacean acid metabolism behavior for red-fleshed pitaya (Hylocereus polyrhizus) micropropagules in vitro using an Arduino-based open system. Sci. Hortic. 2023, 309, 111646. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, W.; Li, X.; Shu, C.; Jiang, W.; Cao, J. Nutrition, phytochemical profile, bioactivities and applications in food industry of pitaya (Hylocereus spp.) peels: A comprehensive review. Trends Food Sci. Technol. 2021, 116, 199–217. [Google Scholar] [CrossRef]
- Anderson, E.F. The cacti. In The Cactus Family, 1st ed.; Anderson, E.F., Ed.; Timber Press Press: Portland, OR, USA, 2001; pp. 105–681. [Google Scholar]
- Mauseth, J.D. Structure-function relationships in highly modified shoots of Cactaceae. Ann. Bot. 2006, 98, 901–926. [Google Scholar] [CrossRef]
- Lim, T.K. Cactaceae. In Edible Medicinal and Non-Medicinal Plants; Lim, T.K., Ed.; Springer Press: New York, NY, USA, 2012; Volume 1, pp. 636–692. [Google Scholar] [CrossRef]
- Weiss, J.; Nerd, A.; Mizrahi, Y. Flowering behavior and pollination requirements in climbing cacti with fruit crop potential. HortScience 1994, 29, 1487–1492. [Google Scholar] [CrossRef]
- Chu, Y.-C.; Chang, J.-C. Regulation of floral bud development and emergence by ambient temperature under a long-day photoperiod in white-fleshed pitaya (Hylocereus undatus). Sci. Hortic. 2020, 271, 109479. [Google Scholar] [CrossRef]
- Mizrahi, Y. Vine-cacti pitayas: The new crops of the world. Rev. Bras. Frutic. 2014, 36, 124–138. [Google Scholar] [CrossRef]
- Jiang, Y.-L.; Yang, W.-J. Development of integrated crop management systems for pitaya in Taiwan. In Improving Pitaya Production and Marketing; Jiang, Y.-L., Liu, P.-C., Huang, P.H., Eds.; Food and Fertilizer Technology Center: Taipei, Taiwan, 2015; pp. 73–78. [Google Scholar]
- Chu, Y.-C.; Chang, J.-C. High temperature suppresses fruit/seed set and weight, and cladode regreening in red-fleshed ‘Da Hong’ pitaya (Hylocereus polyrhizus) under controlled conditions. HortScience 2020, 55, 1259–1264. [Google Scholar] [CrossRef]
- Long, S.P.; Farage, P.K.; Garcia, R.L. Measurement of leaf and canopy photosynthetic CO2 exchange in the field. J. Exp. Bot. 1996, 47, 1629–1642. [Google Scholar] [CrossRef]
- Tusi, A.; Shimazu, T. The essential factor of ventilation rate in prediction of photosynthetic rate using the CO2 balance method. Rev. Agric. Sci. 2020, 8, 279–299. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-C.; Chang, J.-C. Development of an improved micropropagation protocol for red-fleshed pitaya ‘Da Hong’ with and without activated charcoal and plant growth regulator combinations. Horticulturae 2022, 8, 104. [Google Scholar] [CrossRef]
- Bugbee, B. Steady-state canopy gas exchange: System design and operation. HortScience 1992, 27, 770–776. [Google Scholar] [CrossRef]
- Garcia, R.L.; Norman, J.M.; McDermitt, D.K. Measurements of canopy gas exchange using an open chamber system. Remote Sens. Rev. 1990, 5, 141–162. [Google Scholar] [CrossRef]
- Millan-Almaraz, J.R.; Guevara-Gonzalez, R.G.; Romero-Troncoso, R.; Osornio-Rios, R.A.; Torres-Pacheco, I. Advantages and disadvantages on photosynthesis measurement techniques: A review. Afr. J. Biotechnol. 2009, 8, 7340–7349. [Google Scholar]
- De Oliveira, M.M.T.; Shuhua, L.; Kumbha, D.S.; Zurgil, U.; Raveh, E.; Tel-Zur, N. Performance of Hylocereus (Cactaceae) species and interspecific hybrids under high-temperature stress. Plant Physiol. Biochem. 2020, 153, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, K.J.; Day, W.; Leach, J.E. A portable system for measuring the photosynthesis and transpiration of Graminaceous leaves. J. Exp. Bot. 1980, 31, 1441–1453. [Google Scholar] [CrossRef]
- Nobel, P.S. Environmental Biology of Agaves and Cacti; Cambridge University Press: Cambridge, UK, 1988. [Google Scholar]
- Chiu, Y.C.; Lin, C.P.; Hsu, M.C.; Liu, C.P.; Chen, D.Y.; Liu, P.C. Cultivation and Management of Pitaya; Taiwan Agricultural Research Insititute: Tainan, Taiwan, 2015; pp. 1–93.
- Yamori, W.; Hikosaka, K.; Way, D. Temperature response of photosynthesis in C3, C4, and CAM plants: Temperature acclimation and temperature adaptation. Photosynth. Res. 2014, 119, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Pharr, D.M.; Stoop, J.M.H.; Williamson, J.D.; Feusi, M.E.S.; Massel, M.O.; Conkling, M.A. The dual role of mannitol as osmoprotectant and photoassimilate in celery. HortScience 1995, 30, 1182–1188. [Google Scholar] [CrossRef]
- Yaseen, M.; Ahmad, T.; Sablok, G.; Standardi, A.; Hafiz, I.A. Review: Role of carbon sources for in vitro plant growth and development. Mol. Biol. Rep. 2013, 40, 2837–2849. [Google Scholar] [CrossRef] [PubMed]
- Tholakalabavi, A.; Zwiazek, J.J.; Thorpe, T.A. Effect of mannitol and glucose-induced osmotic stress on growth, water relations, and solute composition of cell suspension cultures of poplar (Populus deltoides var. Occidentalis) in relation to anthocyanin accumulation. Vitr. Cell. Dev. Biol. Plant 1994, 30, 164–170. [Google Scholar] [CrossRef]
- Ortiz, T.A.; Gomes, G.R.; Takahashi, L.S.A.; Urbano, M.R.; Strapasson, E. Water and salt stress in germinating seeds of pitaya genotypes (Hylocereus spp.). Afr. J. Agric. Res. 2014, 9, 3610–3619. [Google Scholar]
- Pérez-Molphe-Balch, E.; Del Socorro Santos-Díaz, M.; Ramírez-Malagón, R.; Ochoa-Alejo, N. Tissue culture of ornamental cacti. Sci. Agric. 2015, 72, 540–561. [Google Scholar] [CrossRef]
- Nelson, E.A.; Sage, R.F. Functional constraints of CAM leaf anatomy: Tight cell packing is associated with increased CAM function across a gradient of CAM expression. J. Exp. Bot. 2008, 59, 1841–1850. [Google Scholar] [CrossRef]
- Van Tongerlo, E.; Trouwborst, G.; Hogewoning, S.W.; van Ieperen, W.; Dieleman, J.A.; Marcelis, L.F.M. Crassulacean acid metabolism species differ in the contribution of C3 and C4 carboxylation to end of day CO2 fixation. Physiol. Plant. 2021, 172, 134–145. [Google Scholar] [CrossRef]
- Liu, P.-C.; Yu, C.-M. Industrial development. In Handbook of Export Process Technical to Pitaya; Wu, C.-T., Chuang, K.-E., Lin, Y.-C., Chiang, H.-E., Eds.; Taiwan Agricultural Research Insititute, Council of Agriculture, Executive Yuan Press: Taichung, Taiwan, 2021; pp. 5–20. [Google Scholar]
- Raveh, E.; Nerd, A.; Mizrahi, Y. Responses of two hemiepiphytic fruit crop cacti to different degrees of shade. Sci. Hortic. 1998, 73, 151–164. [Google Scholar] [CrossRef]
- Guo, W.-J.; Lin, Y.-Z.; Lee, N. Photosynthetic light requirements and effects of low irradiance and daylength on Phalaenopsis amabilis. J. Am. Soc. Hortic. Sci. 2012, 137, 465–472. [Google Scholar] [CrossRef]
- Corelli-Grappadelli, L.; Magnanini, E. A whole-tree system for gas-exchange studies. HortScience 1993, 28, 41–45. [Google Scholar] [CrossRef]
- Burkart, S.; Manderscheid, R.; Weigel, H.J. Design and performance of a portable gas exchange chamber system for CO2- and H2O-flux measurements in crop canopies. Environ. Exp. Bot. 2007, 61, 25–34. [Google Scholar] [CrossRef]
- Wünsche, J.N.; Palmer, J.W. Portable through-flow cuvette system for measuring whole-canopy gas exchange of apple trees in the field. HortScience 1997, 32, 653–658. [Google Scholar] [CrossRef]
- Whiting, M.D.; Lang, G.A. Canopy architecture and cuvette flow patterns influence whole-canopy net CO2 exchange and temperature in sweet cherry. HortScience 2001, 36, 691–698. [Google Scholar] [CrossRef]
- Zobayed, S.M.A.; Afreen-Zobayed, F.; Kubota, C.; Kozai, T. Mass propagation of Eucalyptus camaldulensis in a scaled-up vessel under in vitro photoautotrophic condition. Ann. Bot. 2000, 85, 587–592. [Google Scholar] [CrossRef]
- Dodd, A.N.; Borland, A.M.; Haslam, R.P.; Griffiths, H.; Maxwell, K. Crassulacean acid metabolism: Plastic, fantastic. J. Exp. Bot. 2002, 53, 569–580. [Google Scholar] [CrossRef]
- Ceusters, J.; Borland, A.M.; Taybi, T.; Frans, M.; Godts, C.; De Proft, M.P. Light quality modulates metabolic synchronization over the diel phases of crassulacean acid metabolism. J. Exp. Bot. 2014, 65, 3705–3714. [Google Scholar] [CrossRef]
- Borland, A.M.; Hartwell, J.; Jenkins, G.I.; Wilkins, M.B.; Nimmo, H.G. Metabolite control overrides circadian regulation of phosphoenolpyruvate carboxylase kinase and CO2 fixation in crassulacean acid metabolism. Plant Physiol. 1999, 121, 889–896. [Google Scholar] [CrossRef]
- Ortiz-Hernández, Y.D.; Livera-Muñoz, M.; Carrillo-Salazar, J.A.; Valencia-Botin, A.J.; Castillo-Martínez, R. Agronomical, physiological, and cultural contributions of pitahaya (Hylocereus spp.) in Mexico. Isr. J. Plant Sci. 2012, 60, 359–370. [Google Scholar]
- Griffiths, H.; Males, J. Succulent plants. Curr. Biol. 2017, 27, R890–R896. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhou, G.; He, Q. Critical leaf water content for maize photosynthesis under drought stress and its response to rewatering. Sustainability 2021, 13, 7218. [Google Scholar] [CrossRef]
- Ramírez, D.A.; Yactayo, W.; Rens, L.R.; Rolando, J.L.; Palacios, S.; De Mendiburu, F.; Mares, V.; Barreda, C.; Loayza, H.; Monneveux, P.; et al. Defining biological thresholds associated to plant water status for monitoring water restriction effects: Stomatal conductance and photosynthesis recovery as key indicators in potato. Agric. Water Manag. 2016, 177, 369–378. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 153–188. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Xiao, Y.; Niu, G.; Kozai, T. Development and application of photoautotrophic micropropagation plant system. Plant Cell Tissue Organ Cult. 2011, 105, 149–158. [Google Scholar] [CrossRef]
- Boxall, S.F.; Kadu, N.; Dever, L.V.; Kneřová, J.; Waller, J.L.; Gould, P.J.D.; Hartwell, J. Kalanchoë PPC1 is essential for crassulacean acid metabolism and the regulation of core circadian clock and guard cell signaling genes. Plant Cell 2020, 32, 1136–1160. [Google Scholar] [CrossRef]
- Ceusters, J.; Borland, A.M.; Londers, E.; Verdoodt, V.; Godts, C.; De Proft, M.P. Differential usage of storage carbohydrates in the CAM bromeliad Aechmea ‘Maya’ during acclimation to drought and recovery from dehydration. Physiol. Plant. 2009, 135, 174–184. [Google Scholar] [CrossRef]
- Ahluwalia, O.; Singh, P.C.; Bhatia, R. A review on drought stress in plants: Implications, mitigation and the role of plant growth promoting rhizobacteria. Resour. Environ. Sustain. 2021, 5, 100032. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, G.; Shimizu, H. Plant responses to drought and rewatering. Plant Signal. Behav. 2010, 5, 649–654. [Google Scholar] [CrossRef]
- Pinheiro, C.; Chaves, M.M. Photosynthesis and drought: Can we make metabolic connections from available data? J. Exp. Bot. 2011, 62, 869–882. [Google Scholar] [CrossRef]
- De Mattos, E.A.; Herzog, B.; Lüttge, U. Chlorophyll fluorescence during CAM-phases in Clusia minor L. under drought stress. J. Exp. Bot. 1999, 50, 253–561. [Google Scholar] [CrossRef]



| Treatments 1 | Water Potential of MS Solution (MPa) | |||||
|---|---|---|---|---|---|---|
| Pre-Treatment for Adaptation 2 | Weeks after Treatment | |||||
| 1 | 2 | 3 | 4 | 5 | ||
| Control | −0.5 | −0.5 | −0.5 | −0.5 | −0.5 | −0.5 |
| Moderate water stress | −0.5 | −0.5 | −1.0 | −0.5 | −0.5 | −0.5 |
| Intensified water stress | −0.5 | −0.5 | −1.0 | −1.5 | −0.5 | −0.5 |
| Treatments 1 | Relative Integrated Net CO2 Uptake to Week 1 (%) | ||||||
|---|---|---|---|---|---|---|---|
| Weeks after Treatment | Mean | ||||||
| 1 | 2 | 3 | 4 | 5 | |||
| Control | 100 ± 0 | 99.80 ± 35.13 | 87.55 ± 41.38 | 120.43 ± 39.44 | 158.21 ± 96.73 | 113.20 ± 12.43 | A 2 |
| Moderate water stress | 100 ± 0 | 143.21 ± 16.60 | 69.14 ± 23.49 | 71.76 ± 38.26 | 88.51 ± 21.61 | 94.52 ± 13.41 | A |
| Intensified water stress | 100 ± 0 | 46.03 ± 21.82 | 59.92 ± 18.29 | 39.34 ± 7.04 * | 32.27 ± 3.16 * | 55.51 ± 12.02 | B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-C.; Chang, J.-C. Sensitivity and Regulation of Diel Photosynthesis in Red-Fleshed Pitaya (Hylocereus polyrhizus) Micropropagules under Mannitol-Induced Water Stress/Rehydration Cycle In Vitro. Horticulturae 2024, 10, 235. https://doi.org/10.3390/horticulturae10030235
Lee Y-C, Chang J-C. Sensitivity and Regulation of Diel Photosynthesis in Red-Fleshed Pitaya (Hylocereus polyrhizus) Micropropagules under Mannitol-Induced Water Stress/Rehydration Cycle In Vitro. Horticulturae. 2024; 10(3):235. https://doi.org/10.3390/horticulturae10030235
Chicago/Turabian StyleLee, Yu-Chi, and Jer-Chia Chang. 2024. "Sensitivity and Regulation of Diel Photosynthesis in Red-Fleshed Pitaya (Hylocereus polyrhizus) Micropropagules under Mannitol-Induced Water Stress/Rehydration Cycle In Vitro" Horticulturae 10, no. 3: 235. https://doi.org/10.3390/horticulturae10030235
APA StyleLee, Y.-C., & Chang, J.-C. (2024). Sensitivity and Regulation of Diel Photosynthesis in Red-Fleshed Pitaya (Hylocereus polyrhizus) Micropropagules under Mannitol-Induced Water Stress/Rehydration Cycle In Vitro. Horticulturae, 10(3), 235. https://doi.org/10.3390/horticulturae10030235







