Impacts of Yield, Nutritional Value, and Amino Acid Contents during Short-Term Composting for the Substrate for Agrocybe aegerita
Abstract
1. Introduction
2. Materials and Methods
2.1. Inoculum Source and Spawn Preparation
2.2. Composting Process and Sampling
2.3. Substrate Preparation
2.4. Cultivation Methods of A. aegerita
2.5. Physicochemical Properties of Composted Substrates
2.6. Composition Analysis
2.7. Assay of Amino Acid Composition
2.8. Statistical Analysis
3. Results
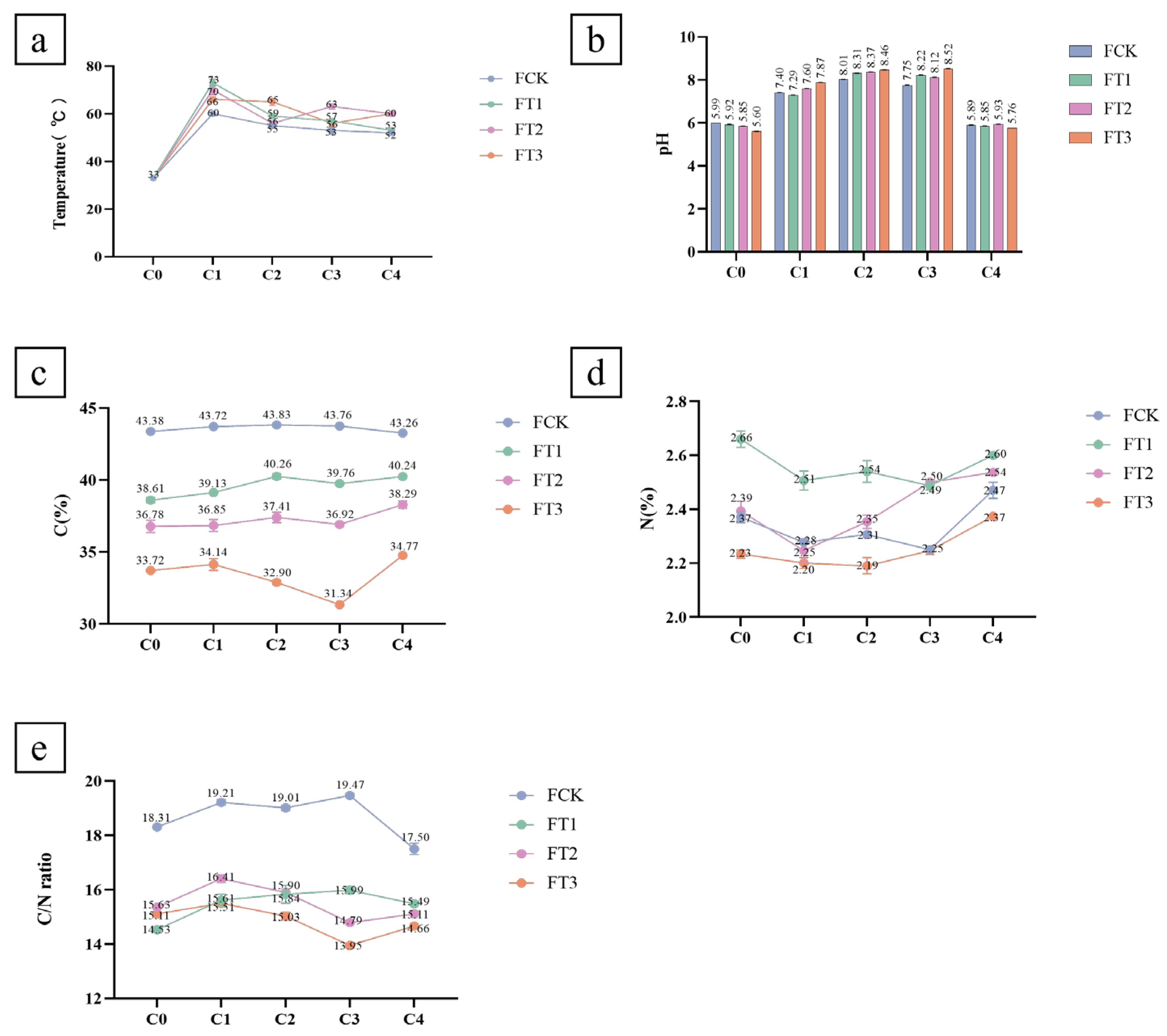
3.1. Physicochemical Properties of the Substrate Materials
3.2. Physicochemical Properties during Composting
3.3. The Fruiting Characteristics of A. aegerita
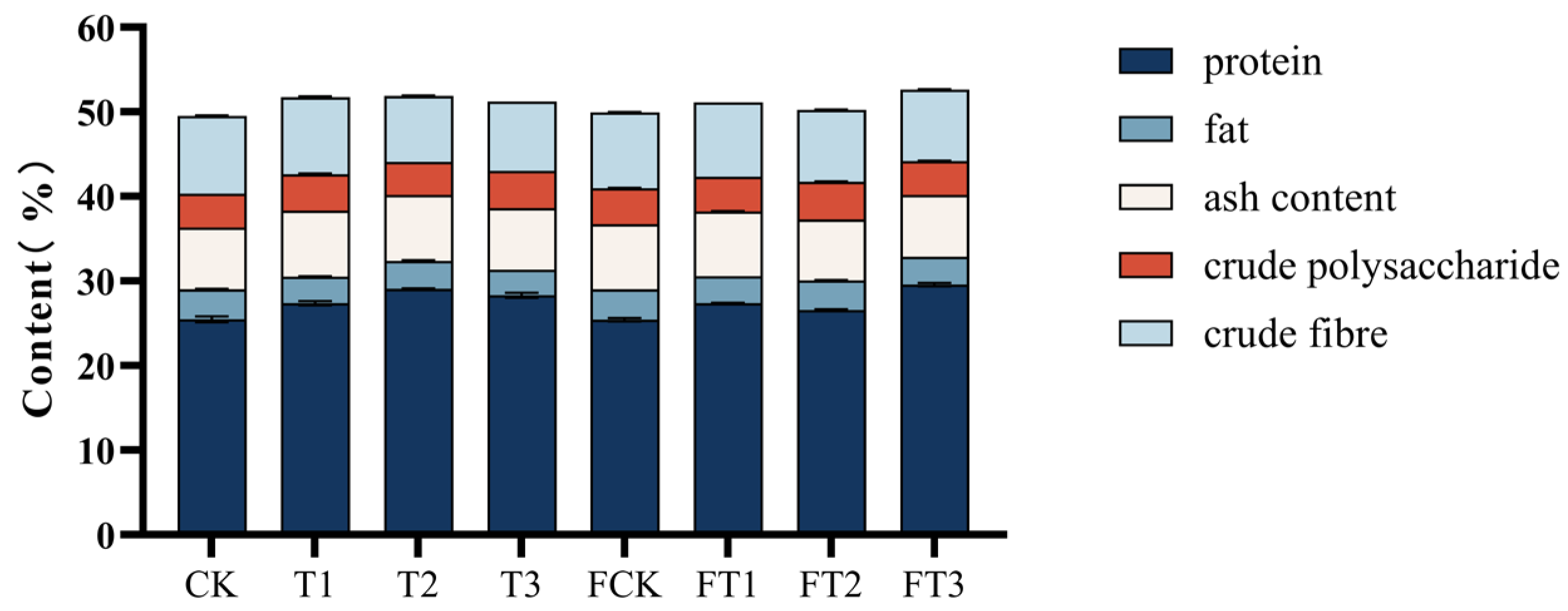
3.4. The Nutrient Contents of the Mushrooms
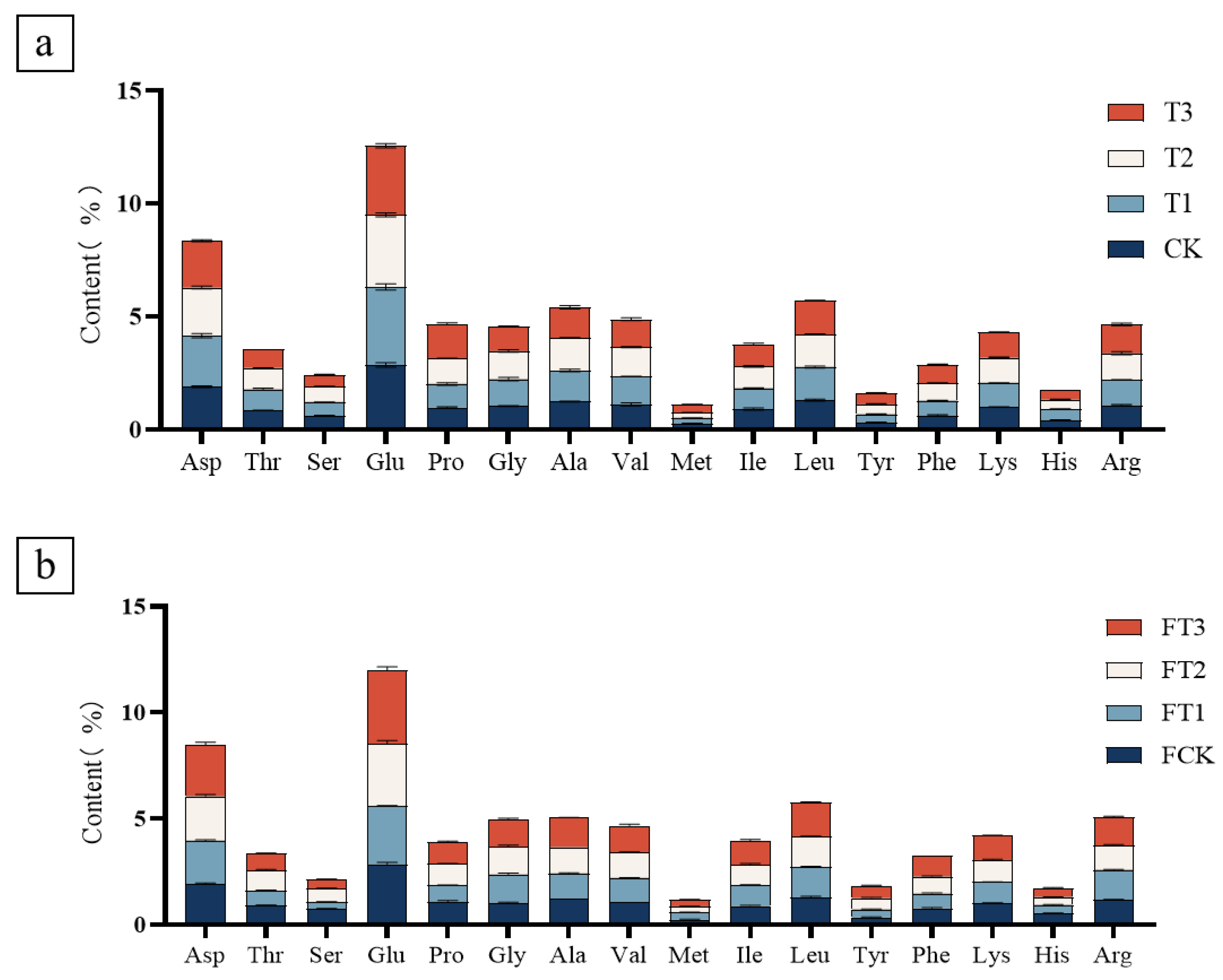
3.5. The Amino Acid Composition of Mushrooms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Valverde, M.E.; Hernandez-Perez, T.; Paredes-Lopez, O. Edible mushrooms: Improving human health and promoting quality life. Int. J. Microbiol. 2015, 2015, 376387. [Google Scholar] [CrossRef]
- Cheng, L.X.; Yang, M.Y.; Juan, J.F.; Qing, G.Z.; Ming, W.Y.; Liang, W.W. Research Progress on Nutrition, Health Value and Product Development of Agrocybe aegerita. Edible Fungi China 2019, 38, 1–4. [Google Scholar]
- Herzog, R.; Solovyeva, I.; Bolker, M.; Lugones, L.G.; Hennicke, F. Exploring molecular tools for transformation and gene expression in the cultivated edible mushroom Agrocybe aegerita. Mol. Genet. Genom. 2019, 294, 663–677. [Google Scholar] [CrossRef]
- Du, Q. Analysis of Nutritional Value of Agrocybe aegerita and its Anti-aging Effect. Edible Fungi China 2019, 38, 131–134. [Google Scholar]
- Zheng, X.; Yin, W.; Zhang, S.; Lan, Y. Effects of Different Fermentation Conditions on Extracellular Polysaccharides Produced by Mycelia of Agrocybe aegerita. Guangdong Agric. Sci. 2019, 46, 25–32. [Google Scholar]
- Philippoussis, A.; Zervakis, G.; Diamantopoulou, P. Bioconversion of agricultural lignocellulosic wastes through the cultivation of the edible mushrooms Agrocybe aegerita, Volvariella volvacea and Pleurotus spp. World J. Microb. Biot. 2001, 17, 191–200. [Google Scholar] [CrossRef]
- Isikhuemhen, O.S.; Mikiashvili, N.A.; Adenipekun, C.O.; Ohimain, E.I.; Shahbazi, G. The tropical white rot fungus, Lentinus squarrosulus Mont.: Lignocellulolytic enzymes activities and sugar release from cornstalks under solid state fermentation. World J. Microb. Biot. 2012, 28, 1961–1966. [Google Scholar] [CrossRef] [PubMed]
- Levin, L.; Diorio, L.; Grassi, E.; Forchiassin, F. Grape stalks as substrate for white rot fungi, lignocellulolytic enzyme production and dye decolorization. Rev. Argent. Microbiol. 2012, 44, 105–112. [Google Scholar]
- Varnai, A.; Makela, M.R.; Djajadi, D.T.; Rahikainen, J.; Hatakka, A.; Viikari, L. Carbohydrate-binding modules of fungal cellulases: Occurrence in nature, function, and relevance in industrial biomass conversion. Adv. Appl. Microbiol. 2014, 88, 103–165. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yuan, T.; Cui, B.; Dai, Y. Investigating lignin and hemicellulose in white rot fungus-pretreated wood that affect enzymatic hydrolysis. Bioresour. Technol. 2013, 134, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Jie, C.; Jibin, W.; Jimin, C.; Zongkuan, L. Spatial-Temporal Variability of Caragana korshinskii Vegetation Growth in the Loess Plateau. Sci. Silvae Sin. 2013, 49, 14–20. [Google Scholar]
- Jia, X.; Shao, M.; Yu, D.; Zhang, Y.; Binley, A. Spatial variations in soil-water carrying capacity of three typical revegetation species on the Loess Plateau, China. Agric. Ecosyst. Environ. 2019, 273, 25–35. [Google Scholar] [CrossRef]
- Zou, Y.; Du, F.; Zhang, H.; Hu, Q. Evaluation of korshinsk Peashrub (Caragana korshinskii Kom.) as a Substrate for the Cultivation of Pleurotus eryngii. Waste Biomass Valorization 2019, 10, 2879–2885. [Google Scholar] [CrossRef]
- Du, F.; Qu, J.; Hu, Q.; Yuan, X.; Yin, G.; Wang, L.; Zou, Y. Maximizing the value of korshinsk peashrub branches by the integration of Pleurotus tuoliensis cultivation and anaerobic digestion of spent mushroom substrate. Renew. Energ. 2021, 179, 679–686. [Google Scholar] [CrossRef]
- Hernandez, D.; Sanchez, J.E.; Yamasaki, K. A simple procedure for preparing substrate for Pleurotus ostreatus cultivation. Bioresour. Technol. 2003, 90, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.Y.; Choi, J.N.; Sharma, P.K.; Lee, W.H. Isolation and Identification of Mushroom Pathogens from Agrocybe aegerita. Mycobiology 2010, 38, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jia, Y.; Zhang, X.; Feng, X.; Wu, J.; Wang, L.; Chen, G. Wheat straw: An inefficient substrate for rapid natural lignocellulosic composting. Bioresour. Technol. 2016, 209, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Zeyang, J. Effects of Different Culture Materials on Mycelium Growth, Yield and Nutrients of Lentinous edodes. Master’s Thesis, Anhui Agricultural University, Hefei, China, 2018. [Google Scholar]
- Zhou, Y.; Liu, P.; Liu, B.; Su, R.; Zhou, J. Effects of Different Cultivation Formulas on Growth and Extracellular Enzyme Activity of Cultured Agrocybe cylindracea. North. Hortic. 2023, 19, 114–121. [Google Scholar]
- Liu, M.; Xiao, Z.; Qiu, Y.; Qiu, G.; He, H. Optimization and Application on Liquid Spawns Cultivation Medium of Agrocybe cylindracea. Edible Fungi China 2016, 35, 39–42. [Google Scholar]
- Carrasco-Cabrera, C.P.; Bell, T.L.; Kertesz, M.A. Caffeine metabolism during cultivation of oyster mushroom (Pleurotus ostreatus) with spent coffee grounds. Appl. Microbiol. Biot. 2019, 103, 5831–5841. [Google Scholar] [CrossRef] [PubMed]
- Xiaobei, L.; Feng, Z.; Yan, Y.; Jie, F.; Yanfang, L.; Tao, F. Effect of Culture Media on Nutritional Value of Proteins in Pleurotus eryngii Fruit Bodies. Food Chem. 2015, 36, 262–267. [Google Scholar]
- Chen, X.; Tan, Y.; Chu, W. Analysis on Agrocybe aegerita-Ws Sporophore Nutritional Components. J. Anhui Agri. Sci. 2013, 41, 11851–11880. [Google Scholar]
- Muthu, N.; Shanmugasundaram, K. Effect of five different culture media on mycelial growth of Agrocybe aegerita. Int. J. Pharm. Sci. Res. 2015, 12, 5193–5197. [Google Scholar]
- Feng, B.; Xu, Y.; Fu, W.; Li, H.; Li, G.; Li, J.; Wang, W.; Tao, L.; Chen, T.; Fu, G. RGA1 Negatively Regulates Thermo-tolerance by Affecting Carbohydrate Metabolism and the Energy Supply in Rice. Rice 2023, 16, 32. [Google Scholar] [CrossRef] [PubMed]
- Lechner, B.E.; Alberto, E. Search for new naturally occurring strains of Pleurotus to improve yields: Pleurotus albidus as a novel proposed species for mushroom production. Rev. Iberoam. Micol. 2011, 28, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Guo, X.; Zhou, L.; Yao, Q.; Zhu, H. Study of Biochemical and Microbiological Properties During Co-composting of Spent Mushroom Substrates and Chicken Feather. Waste Biomass Valorization 2019, 10, 23–32. [Google Scholar] [CrossRef]
- Xu, L.; Wu, X.; Chen, G.; Wu, S.; Ren, K.; Zhou, Y.; Wu, Y.; Yan, L.; Guo, W. Effects of electron beam irradiation on the properties of frozen Litopenaeus vannamei. J. Radiat. Res. Radiat. Process. 2023, 41, 57–66. [Google Scholar]
- Bao, Z.; Yi, Y.; Wan, X.; Yuan, B.; Li, C. Study on the selenium content and quality of bamboo shoots in Yichun Mingyue Mountain. Spec. Econ. Anim. Plants 2024, 27, 11–14. [Google Scholar]
- He, Q.; Chen, W.; Xie, X.; Peng, H.; Deng, B.; Wang, Y.; Chen, Y. Analysis and evaluation of nutritional components in wild “Shuaba mushroom” in Foping, Shaanxi. J. Northwest Univ. (Nat. Sci. Ed.) 2018, 46, 51–57. [Google Scholar] [CrossRef]
- Yu, W.; Lan, X.; Cai, J.; Wang, X.; Liu, X.; Ye, X.; Yang, Q.; Su, Y.; Xu, B.; Chen, T.; et al. Critical role of IL-1beta in the pathogenesis of Agrocybe aegerita galectin-induced liver injury through recruiting T cell to liver. Biochem. Biophys. Res. Commun. 2020, 521, 449–456. [Google Scholar] [CrossRef]
- Kleofas, V.; Sommer, L.; Fraatz, M.A.; Zorn, H.; Rühl, M. Fruiting body production and aroma profile analysis of Agrocybe aegerita cultivated on different substrates. Nat. Resour. 2014, 5, 233–240. [Google Scholar] [CrossRef]
- Isikhuemhen, O.S.; Mikiashvili, N.A.; Kelkar, V. Application of solid waste from anaerobic digestion of poultry litter in Agrocybe aegerita cultivation: Mushroom production, lignocellulolytic enzymes activity and substrate utilization. Biodegradation 2009, 20, 351–361. [Google Scholar] [CrossRef]
- Cunha, Z.D.; Pardo-Gimenez, A.; de Almeida, M.M.; Villas, B.R.; Alvarez-Orti, M.; Pardo-Gonzalez, J.E. Characterization, feasibility and optimization of Agaricus subrufescens growth based on chemical elements on casing layer. Saudi J. Biol. Sci. 2012, 19, 343–347. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guo, Y.X.; Chen, Q.J.; Qin, Y.; Yang, Y.R.; Yang, Q.Z.; Wang, Y.X.; Cheng, Z.A.; Cao, N.; Zhang, G.Q. Succession of the microbial communities and function prediction during short-term peach sawdust-based composting. Bioresour. Technol. 2021, 332, 125079. [Google Scholar] [CrossRef] [PubMed]
- KA, A.; TR, S.; KR, S. Cultivation of Pleurotus sajor-caju on certain agro-wastes and utilization of the residues for cellulase and D-xylanase production. Agric. Food Sci. 1990, 10, 21–26. [Google Scholar]
- Gupta, Y.; Vijay, B. Post-composting supplementation in Agaricus bisporus under seasonal growing conditions. Mushroom Res. 1992, 2, 115–117. [Google Scholar]
- Ye, D.; Hu, Q.; Bai, X.; Zhang, W.; Guo, H. Increasing the value of Phragmites australis straw in a sustainable integrated agriculture model (SIAM) comprising edible mushroom cultivation and spent mushroom substrate compost. Sci. Total Environ. 2023, 869, 161807. [Google Scholar] [CrossRef]
- Li, G.; Liu, X.; Cong, S.; Deng, Y.; Zheng, X. A novel serine protease with anticoagulant and fibrinolytic activities from the fruiting bodies of mushroom Agrocybe aegerita. Int. J. Biol. Macromol. 2021, 168, 631–639. [Google Scholar] [CrossRef]
- Cheung, P.C.K. Mini-review on edible mushrooms as source of dietary fiber: Preparation and health benefits. Food Sci. Hum. Well 2013, 2, 162–166. [Google Scholar] [CrossRef]
- Guo, D.; Lei, J.; He, C.; Peng, Z.; Liu, R.; Pan, X.; Meng, J.; Feng, C.; Xu, L.; Cheng, Y.; et al. In vitro digestion and fermentation by human fecal microbiota of polysaccharides from Clitocybe squamulose. Int. J. Biol. Macromol. 2022, 208, 343–355. [Google Scholar] [CrossRef]
- Kalac, P. A review of chemical composition and nutritional value of wild-growing and cultivated mushrooms. J. Sci. Food Agric. 2013, 93, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Murakami, H.; Shimbo, K.; Inoue, Y.; Takino, Y.; Kobayashi, H. Importance of amino acid composition to improve skin collagen protein synthesis rates in UV-irradiated mice. Amino Acids 2012, 42, 2481–2489. [Google Scholar] [CrossRef] [PubMed]
| Substrate | Cottonseed Hull (%) | Korshinsk Peashrub (%) | Wheat Bran (%) | Sugar (%) | Gypsum (%) | Lime (%) | TC (%) | TN (%) | C:N |
|---|---|---|---|---|---|---|---|---|---|
| CK | 80 | 0 | 18 | 1 | 0.5 | 0.5 | 43.59 | 2.28 | 19.16 |
| T1 | 60 | 20 | 18 | 1 | 0.5 | 0.5 | 39.80 | 2.16 | 18.40 |
| T2 | 40 | 40 | 18 | 1 | 0.5 | 0.5 | 39.00 | 2.21 | 17.65 |
| T3 | 20 | 60 | 18 | 1 | 0.5 | 0.5 | 36.71 | 2.21 | 16.58 |
| FCK | 80 | 0 | 18 | 1 | 0.5 | 0.5 | 44.17 | 2.34 | 18.87 |
| FT1 | 60 | 20 | 18 | 1 | 0.5 | 0.5 | 41.76 | 2.41 | 17.36 |
| FT2 | 40 | 40 | 18 | 1 | 0.5 | 0.5 | 40.22 | 2.48 | 16.52 |
| FT3 | 20 | 60 | 18 | 1 | 0.5 | 0.5 | 35.98 | 2.27 | 15.82 |
| Material | Cellulose (%) | Hemicellulose (%) | Lignin (%) | TC (%) | TN (%) | C:N |
|---|---|---|---|---|---|---|
| Cottonseed hull | 25.03 | 23.64 | 26.97 | 44.51 | 2.37 | 18.31 |
| Korshinsk peashrub | 21.74 | 26.36 | 12.42 | 39.40 | 2.45 | 16.08 |
| Substrate | 1st Plot Yield (g/25 Bags) | 2nd Plot Yield (g/25 Bags) | 3rd Plot Yield (g/25 Bags) | Average Yield of Plot (g/25 Bags) | Fruit Body Yield (g/Bag) | Biological Efficiency (%) |
|---|---|---|---|---|---|---|
| CK | 4749 | 5391 | 4924 | 5021.33 ± 331.88 bc | 208.05 ± 13.28 bc | 59.44 ± 3.79 bc |
| T1 | 5043.2 | 4901.8 | 5038.3 | 4994.43 ± 80.26 bc | 199.78 ± 3.21 bc | 57.08 ± 0.92 bc |
| T2 | 4523 | 4428 | 4757.1 | 4569.37 ± 169.38 c | 182.77 ± 6.78 c | 52.22 ± 1.94 c |
| T3 | 3774.6 | 3879.6 | 3958 | 3870.73 ± 92.02 d | 154.83 ± 3.68 d | 44.23 ± 1.05 d |
| FCK | 5503.6 | 5608 | 5738.4 | 5616.67 ± 117.64 ab | 224.67 ± 4.71 ab | 64.19 ± 1.35 ab |
| FT1 | 5762.2 | 5551.8 | 6475 | 5929.67 ± 483.85 a | 237.19 ± 19.35 a | 67.76 ± 5.52 a |
| FT2 | 5465.5 | 5283.6 | 5654.6 | 5467.9 ± 185.51 ab | 218.72 ± 7.42 ab | 62.49 ± 2.12ab |
| FT3 | 2168.8 | 2671 | 3645.2 | 2828.33 ± 750.67 e | 113.13 ± 30.03 e | 32.32 ± 8.58 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Zhou, Y.; Zhao, G.; Xu, C.; Pan, J.; Li, H.; Zou, Y. Impacts of Yield, Nutritional Value, and Amino Acid Contents during Short-Term Composting for the Substrate for Agrocybe aegerita. Horticulturae 2024, 10, 234. https://doi.org/10.3390/horticulturae10030234
Li Z, Zhou Y, Zhao G, Xu C, Pan J, Li H, Zou Y. Impacts of Yield, Nutritional Value, and Amino Acid Contents during Short-Term Composting for the Substrate for Agrocybe aegerita. Horticulturae. 2024; 10(3):234. https://doi.org/10.3390/horticulturae10030234
Chicago/Turabian StyleLi, Zihao, Yi Zhou, Guanghui Zhao, Congtao Xu, Jinlong Pan, Haikang Li, and Yajie Zou. 2024. "Impacts of Yield, Nutritional Value, and Amino Acid Contents during Short-Term Composting for the Substrate for Agrocybe aegerita" Horticulturae 10, no. 3: 234. https://doi.org/10.3390/horticulturae10030234
APA StyleLi, Z., Zhou, Y., Zhao, G., Xu, C., Pan, J., Li, H., & Zou, Y. (2024). Impacts of Yield, Nutritional Value, and Amino Acid Contents during Short-Term Composting for the Substrate for Agrocybe aegerita. Horticulturae, 10(3), 234. https://doi.org/10.3390/horticulturae10030234





