Egyptian Citrus Essential Oils Recovered from Lemon, Orange, and Mandarin Peels: Phytochemical and Biological Value
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Extraction of Essential Oils
2.3. Characterization of Essential Oils through GC/MS
2.4. GC/MS Molecular Networking (GNPS-MN)
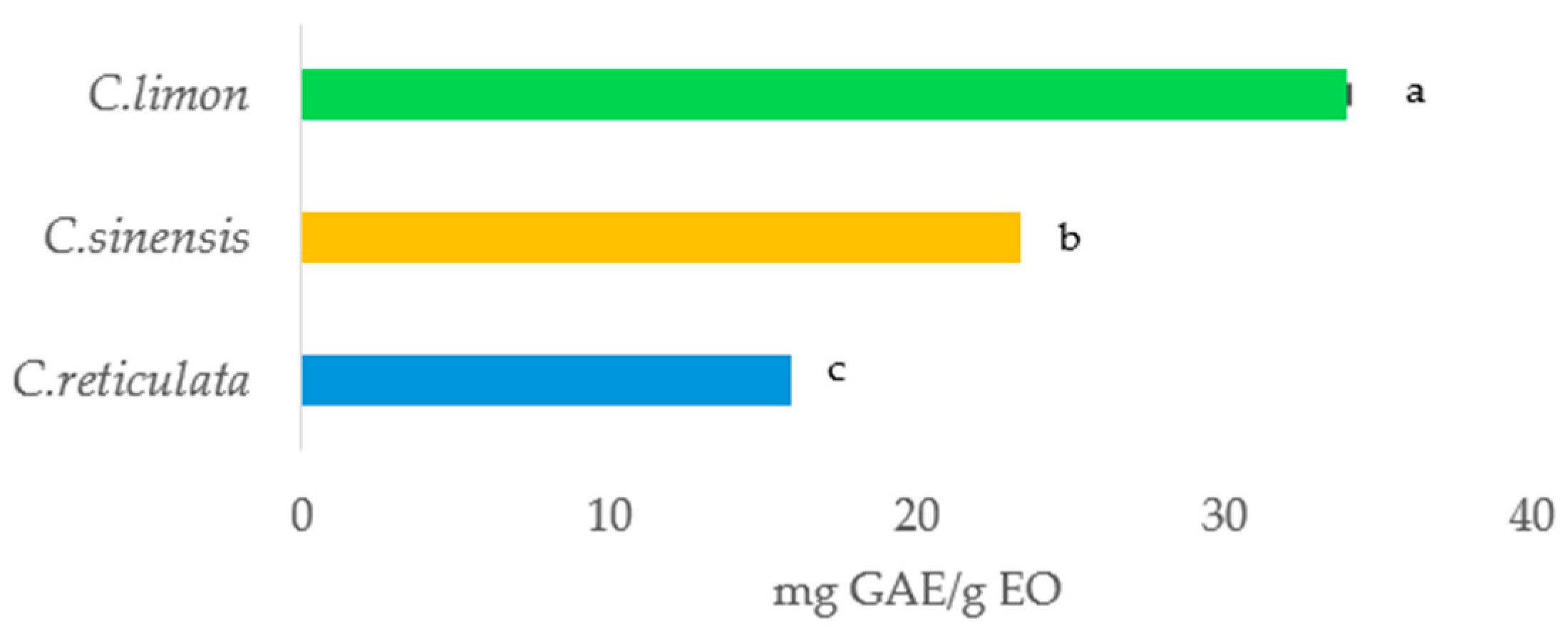
2.5. Total Phenolic Content (TPC) Determination
2.6. Biological Activities
2.6.1. Antioxidant Activity
DPPH Radical Scavenging Assay
Nitric Oxide (NO) Radical Scavenging Assay
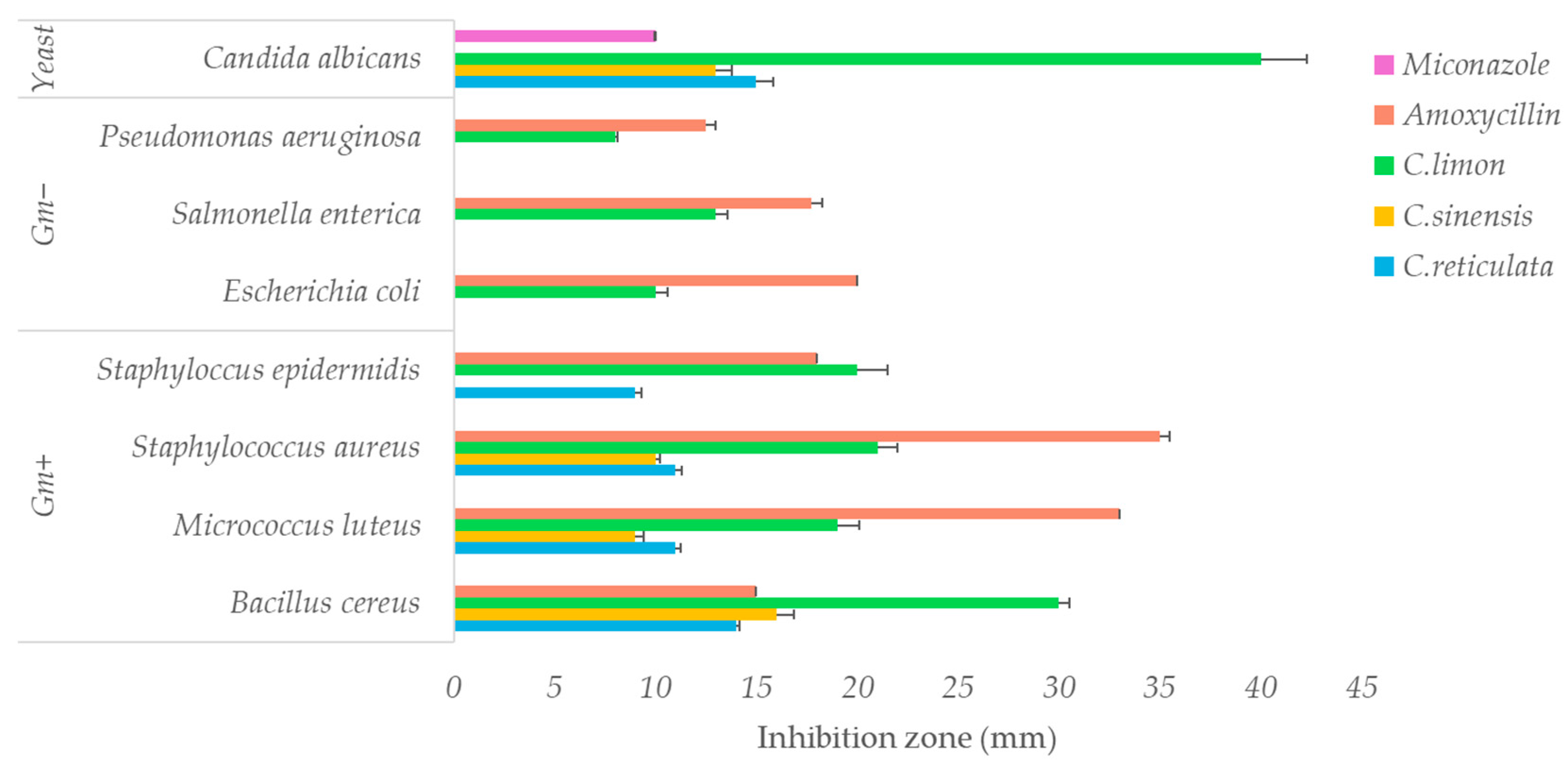
2.6.2. Antimicrobial Activity
Microbial Cultures
Disk Diffusion Assay
2.6.3. Anti-Inflammatory Activity
2.7. Statistical Analysis
3. Results and Discussion
3.1. Phytochemical Analysis
3.1.1. Chemical Composition of Essential Oils from Citrus Peels
3.1.2. Total Phenolic Content (TPC)
3.2. Biological Studies
3.2.1. Antioxidant Activity
DPPH Assay
Nitric Oxide (NO) Assay
3.2.2. Antimicrobial Activity
3.2.3. Anti-Inflammatory Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. FAO Citrus Fruit Statistical Compendium 2020; FAO: Rome, Italy, 2021. [Google Scholar]
- Suri, S.; Singh, A.; Nema, P.K. Current Applications of Citrus Fruit Processing Waste: A Scientific Outlook. Appl. Food Res. 2022, 2, 100050. [Google Scholar] [CrossRef]
- Magalhães, D.; Vilas-Boas, A.A.; Teixeira, P.; Pintado, M. Functional Ingredients and Additives from Lemon By-Products and Their Applications in Food Preservation: A Review. Foods 2023, 12, 1095. [Google Scholar] [CrossRef]
- Vilas-boas, A.A.; Magalhães, D.; Campos, D.A.; Porretta, S.; Dellapina, G.; Poli, G.; Istanbullu, Y.; Demir, S.; Mart, Á.; Mart, S.; et al. Innovative Processing Technologies to Develop a New Segment of Functional Citrus-Based Beverages: Current and Future Trends. Foods 2022, 11, 3859. [Google Scholar] [CrossRef]
- Maqbool, Z.; Khalid, W.; Atiq, H.T.; Koraqi, H.; Javaid, Z.; Alhag, S.K.; Al-Shuraym, L.A.; Bader, D.M.D.; Almarzuq, M.; Afifi, M.; et al. Citrus Waste as Source of Bioactive Compounds: Extraction and Utilization in Health and Food Industry. Molecules 2023, 28, 1636. [Google Scholar] [CrossRef]
- Shehata, M.G.; Awad, T.S.; Asker, D.; El Sohaimy, S.A.; Abd El- Aziz, N.M.; Youssef, M.M. Antioxidant and Antimicrobial Activities and UPLC-ESI-MS/MS Polyphenolic Profile of Sweet Orange Peel Extracts. Curr. Res. Food Sci. 2021, 4, 326–335. [Google Scholar] [CrossRef]
- Sorrenti, V.; Burò, I.; Consoli, V.; Vanella, L. Recent Advances in Health Benefits of Bioactive Compounds from Food Wastes and By-Products: Biochemical Aspects. Int. J. Mol. Sci. 2023, 24, 2019. [Google Scholar] [CrossRef]
- Wedamulla, N.E.; Fan, M.; Choi, Y.J.; Kim, E.K. Citrus Peel as a Renewable Bioresource: Transforming Waste to Food Additives. J. Funct. Foods 2022, 95, 105163. [Google Scholar] [CrossRef]
- Visakh, N.U.; Pathrose, B.; Chellappan, M.; Ranjith, M.T.; Sindhu, P.V.; Mathew, D. Chemical Characterisation, Insecticidal and Antioxidant Activities of Essential Oils from Four Citrus Spp. Fruit Peel Waste. Food Biosci. 2022, 50, 102163. [Google Scholar] [CrossRef]
- Agarwal, P.; Sebghatollahi, Z.; Kamal, M.; Dhyani, A.; Shrivastava, A.; Singh, K.K.; Sinha, M.; Mahato, N.; Mishra, A.K.; Baek, K.H. Citrus Essential Oils in Aromatherapy: Therapeutic Effects and Mechanisms. Antioxidants 2022, 11, 2374. [Google Scholar] [CrossRef] [PubMed]
- FDA. Substances Generally Recognized as Safe. Fed. Regist. 2016, 81, 54960–55055. [Google Scholar]
- Espina, L.; Somolinos, M.; Lorán, S.; Conchello, P.; García, D.; Pagán, R. Chemical Composition of Commercial Citrus Fruit Essential Oils and Evaluation of Their Antimicrobial Activity Acting Alone or in Combined Processes. Food Control 2011, 22, 896–902. [Google Scholar] [CrossRef]
- Fisher, K.; Phillips, C. Potential Antimicrobial Uses of Essential Oils in Food: Is Citrus the Answer? Trends Food Sci. Technol. 2008, 19, 156–164. [Google Scholar] [CrossRef]
- Ben Hsouna, A.; Ben Halima, N.; Smaoui, S.; Hamdi, N. Citrus Lemon Essential Oil: Chemical Composition, Antioxidant and Antimicrobial Activities with Its Preservative Effect against Listeria Monocytogenes Inoculated in Minced Beef Meat. Lipids Health Dis. 2017, 16, 146. [Google Scholar] [CrossRef]
- González-Mas, M.C.; Rambla, J.L.; López-Gresa, M.P.; Amparo Blázquez, M.; Granell, A. Volatile Compounds in Citrus Essential Oils: A Comprehensive Review. Front. Plant Sci. 2019, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Li, X.; Zhao, P.; Zhang, X.; Qiao, O.; Huang, L.; Guo, L. A Review of Chemical Constituents and Health-Promoting Effects of Citrus Peels. Food Chem. 2021, 365, 130585. [Google Scholar] [CrossRef] [PubMed]
- Putnik, P.; Barba, F.J.; Lorenzo, J.M.; Gabrić, D.; Shpigelman, A.; Cravotto, G.; Bursać Kovačević, D. An Integrated Approach to Mandarin Processing: Food Safety and Nutritional Quality, Consumer Preference, and Nutrient Bioaccessibility. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1345–1358. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, A.; Bekhit, A.E.D.; Jambrak, A.R.; Regenstein, J.M.; Chemat, F.; Morton, J.D.; Gudjónsdóttir, M.; Carpena, M.; Prieto, M.A.; Varela, P.; et al. The Fourth Industrial Revolution in the Food Industry—Part II: Emerging Food Trends. Crit. Rev. Food Sci. Nutr. 2024, 64, 407–437. [Google Scholar] [CrossRef] [PubMed]
- Khamsaw, P.; Sangta, J.; Chaiwan, P.; Rachtanapun, P.; Sirilun, S.; Sringarm, K.; Thanakkasaranee, S.; Sommano, S.R. Bio-Circular Perspective of Citrus Fruit Loss Caused by Pathogens: Occurrences, Active Ingredient Recovery and Applications. Horticulturae 2022, 8, 748. [Google Scholar] [CrossRef]
- Egyptian Pharmacopoeia, General Organization for Governmental. Print. Off. Minist. Health Cairo Egypt 1984, 31–33.
- Ibrahim, F.M.; EL-Hallouty, S.; Hendawy, S.F.; Omer, E.A.; SMohammed, R. Egyptian Myrtus Communis L. Essential Oil Potential Role as in Vitro Antioxidant, Cytotoxic and α-Amylase Inhibitor. Egypt. J. Chem. 2021, 64, 3005–3017. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing: Carol Stream, IL, USA, 2017. [Google Scholar]
- Aksenov, A.A.; Laponogov, I.; Zhang, Z.; Doran, S.L.; Belluomo, I.; Veselkov, D.; Veselkov, K. Auto-Deconvolution and Molecular Networking of Gas Chromatography–Mass Spectrometry Data. Nat. Biotechnol. 2021, 39, 169–173. [Google Scholar] [CrossRef]
- Smoot, M.E.; Ono, K.; Ruscheinski, J.; Wang, P.-L.; Ideker, T. Systems Biology Cytoscape 2.8: New Features for Data Integration and Network Visualization. Bioinforma 2011, 27, 431–432. [Google Scholar] [CrossRef]
- Farid, M.M.; Ibrahim, F.M.; Ragheb, A.Y.; Mohammed, R.S.; Hegazi, N.M.; Shabrawy, M.O.E.; Kawashty, S.A.; Marzouk, M.M. Comprehensive Phytochemical Characterization of Raphanus Raphanistrum L.: In Vitro Antioxidant and Antihyperglycemic Evaluation. Sci. Afr. 2022, 16, e01154. [Google Scholar] [CrossRef]
- Marcocci, L.; Maguire, J.J.; Droylefaix, M.T.; Parcker, L. The Nitric Oxide-Scavenging Properties of Ginkgo Biloba Extract EGb 761. Biochem. Biophys. Res. Commun. 1994, 201, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, F.A.; Abd El Aty, A.A.; Hamed, E.R.; Eid, B.M.; Ibrahim, N.A. Enzymatic, Kinetic and Anti-Microbial Studies on Aspergillus Terreus Culture Filtrate and Allium Cepa Seeds Extract and Their Potent Applications. Biocatal. Agric. Biotechnol. 2016, 5, 116–122. [Google Scholar] [CrossRef]
- El-serwy, W.S.; Mohamed, N.A.; El-serwy, W.S.; Kassem, E.M.M.; Aty, A.A.A. El Synthesis of New Benzofuran Derivatives and Evaluation of Their Antimicrobial Activities. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 213. [Google Scholar]
- Mcfarland, J. The Nephelometer: An Instrument for Estimating the Number of Bacteria in Suspensions Used for Calculating the Opsonic Index and for Vaccines. J. Am. Med. Assoc 1907, 49, 1176–1178. [Google Scholar] [CrossRef]
- Ricciotti, E.; Fitzgerald, G.A. Prostaglandins and Inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- Blobaum, A.L.; Marnett, L.J. Structural and Functional Basis of Cyclooxygenase Inhibition. J. Med. Chem. 2007, 50, 1425–1441. [Google Scholar] [CrossRef] [PubMed]
- Alejandro Raspo, M.; Belén Vignola, M.; Ester Andreatta, A.; Rodolfo Juliani, H. Antioxidant and Antimicrobial Activities of Citrus Essential Oils from Argentina and the United States. Food Biosci. 2020, 36, 651. [Google Scholar] [CrossRef]
- Kim, Y.W.; Kim, M.J.; Chung, B.Y.; Bang, D.Y.; Lim, S.K.; Choi, S.M.; Lim, D.S.; Cho, M.C.; Yoon, K.; Kim, H.S.; et al. Safety Evaluation and Risk Assessment Of D-Limonene. J. Toxicol. Environ. Health Part B 2013, 16, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lin, Z.X.; Xiang, W.L.; Huang, M.; Tang, J.; Lu, Y.; Zhao, Q.H.; Zhang, Q.; Rao, Y.; Liu, L. Antifungal Activity and Mechanism of D-Limonene against Foodborne Opportunistic Pathogen Candida Tropicalis. LWT 2022, 159, 113144. [Google Scholar] [CrossRef]
- Essadik, F.Z.; Haida, S.; Kribii, A.; Kribii, A.R.; Ounine, K. Antioxidant Activity of Citrus Aurantium L. Var. Amara Peel from Western of Morocco, Identification of Volatile Compounds of Its Essential Oil by GC-MS and a Preliminary Study of Their Antibacterial Activity. Int. J. Innov. Sci. Res. 2015, 16, 425–432. [Google Scholar]
- Sanei-Dehkordi, A.; Sedaghat, M.M.; Vatandoost, H.; Abai, M.R. Chemical Compositions of the Peel Essential Oil of Citrus Aurantium and Its Natural Larvicidal Activity against the Malaria Vector Anopheles Stephensi (Diptera: Culicidae) in Comparison with Citrus Paradisi. J. Arthropod. Borne. Dis. 2016, 10, 577–585. [Google Scholar]
- Costa, C.A.; Cury, T.C.; Cassettari, B.O.; Takahira, R.K.; Flório, J.C.; Costa, M. Citrus Aurantium L. Essential Oil Exhibits Anxiolytic- like Activity Mediated by 5-HT 1A -Receptors and Reduces Cholesterol after Repeated Oral Treatment. BMC Complement. Altern. Med. 2013, 13, 42. [Google Scholar] [CrossRef]
- Moosavy, M.H.; Hassanzadeh, P.; Mohammadzadeh, E.; Mahmoudi, R.; Khatibi, S.A.; Mardani, K. Antioxidant and Antimicrobial Activities of Essential Oil of Lemon (Citrus Limon) Peel in Vitro and in a Food Model. J. Food Qual. Hazards Control 2017, 4, 42–48. [Google Scholar]
- Lota, M.-L.; de Rocca Serra, D.; Lix Tomi, F.; Casanova, J. Chemical Variability of Peel and Leaf Essential Oils of 15 Species of Mandarins. Biochem. Syst. Ecol. 2001, 29, 77–104. [Google Scholar] [CrossRef]
- Djenane, D. Chemical Profile, Antibacterial and Antioxidant Activity of Algerian Citrus Essential Oils and Their Application in Sardina Pilchardus. Foods 2015, 4, 208–228. [Google Scholar] [CrossRef]
- Benayad, O.; Bouhrim, M.; Tiji, S.; Kharchoufa, L.; Addi, M.; Mimouni, M. Inhibition Potential and Toxicity Evaluation of Extracts from Citrus Aurantium (L) Peel, a Valuable By-Product from Northeastern Morocco. Biomolecules 2021, 11, 1555. [Google Scholar] [CrossRef]
- Matera, R.; Lucchi, E.; Valgimigli, L. Plant Essential Oils as Healthy Functional Ingredients of Nutraceuticals and Diet Supplements: A Review. Molecules 2023, 28, 901. [Google Scholar] [CrossRef]
- Durmus, M.; Özogul, Y.; Ozyurt, G.; Ucar, Y.; Kosker, A.R.; Yazgan, H.; Ibrahim, S.A.; Özogul, F. Effects of Citrus Essential Oils on the Oxidative Stability of Microencapsulated Fish Oil by Spray-Drying. Front. Nutr. 2023, 9, 978130. [Google Scholar] [CrossRef]
- Ghasemi, K.; Ghasemi, Y.; Ebrahimzadeh, M.A. Antioxidant Activity, Phenol and Flavonoid Contents of 13 Citrus Species Peels and Tissues. Pak. J. Pharm. Sci. 2009, 22, 277–281. [Google Scholar]
- Guimarães, R.; Barros, L.; Barreira, J.C.M.; Sousa, M.J.; Carvalho, A.M.; Ferreira, I.C.F.R. Targeting Excessive Free Radicals with Peels and Juices of Citrus Fruits: Grapefruit, Lemon, Lime and Orange. Food Chem. Toxicol. 2010, 48, 99–106. [Google Scholar] [CrossRef]
- Kiselova, Y.; Ivanova, D.; Chervenkov, T.; Gerova, D.; Galunska, B.; Yankova, T. Correlation between the in Vitro Antioxidant Activity and Polyphenol Content of Aqueous Extracts from Bulgarian Herbs. Phytother. Res. 2006, 20, 961–965. [Google Scholar] [CrossRef] [PubMed]
- Klimczak, Ã.I.; Małecka, M.; Szlachta, M.; Gliszczyńska-Świgło, A. Effect of Storage on the Content of Polyphenols, Vitamin C and the Antioxidant Activity of Orange Juices. J. Food Compos. Anal. 2007, 20, 313–322. [Google Scholar] [CrossRef]
- Loganayaki, N.; Siddhuraju, P.; Manian, S. Antioxidant Activity and Free Radical Scavenging Capacity of Phenolic Extracts from Helicteres Isora L. and Ceiba Pentandra L. J. Food Sci Technol. 2013, 50, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Al-Saman, M.A.; Abdella, A.; Mazrou, K.E.; Tayel, A.A.; Irmak, S. Antimicrobial and Antioxidant Activities of Different Extracts of the Peel of Kumquat (Citrus Japonica Thunb). J. Food Meas. Charact. 2019, 13, 3221–3229. [Google Scholar] [CrossRef]
- Basavegowda, N.; Baek, K. Synergistic Antioxidant and Antibacterial Advantages of Essential Oils for Food Packaging Applications. Biomolecules 2021, 11, 1267. [Google Scholar] [CrossRef] [PubMed]
- Baliyan, S.; Mukherjee, R.; Priyadarshini, A.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Chang, C.M. Determination of Antioxidants by DPPH Radical Scavenging Activity and Quantitative Phytochemical Analysis of Ficus Religiosa. Molecules 2022, 27. [Google Scholar] [CrossRef] [PubMed]
- Sousa, D.P.; Damasceno, R.O.S.; Amorati, R.; Elshabrawy, H.A.; de Castro, R.D.; Bezerra, D.P.; Nunes, V.R.V.; Gomes, R.C.; Lima, T.C. Essential Oils: Chemistry and Pharmacological Activities. Biomolecules 2023, 13, 1144. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shang, S.; Yan, F.; Jiang, H.; Zhao, G.; Tian, S.; Chen, R.; Chen, D.; Dang, Y. Antioxidant Activities of Essential Oils and Their Major Components in Scavenging Free Radicals, Inhibiting Lipid Oxidation and Reducing Cellular Oxidative Stress. Molecules 2023, 28, 4559. [Google Scholar] [CrossRef]
- Meryem, S.; Mohamed, D.; Nour-eddine, C.; Faouzi, E. Chemical Composition, Antibacterial and Antioxidant Properties of Three Moroccan Citrus Peel Essential Oils. Sci. Afr. 2023, 20, e01592. [Google Scholar] [CrossRef]
- Denkova-Kostova, R.; Teneva, D.; Tomova, T.; Goranov, B.; Denkova, Z.; Shopska, V.; Slavchev, A.; Hristova-Ivanova, Y. Chemical Composition, Antioxidant and Antimicrobial Activity of Essential Oils from Tangerine (Citrus Reticulata L.), Grapefruit (Citrus Paradisi L.), Lemon (Citrus Lemon L.) and Cinnamon (Cinnamomum Zeylanicum Blume). Z. Naturforsch. Sect. C J. Biosci. 2021, 76, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Sarrou, E.; Chatzopoulou, P.; Dimassi-Theriou, K.; Therios, I. Volatile Constituents and Antioxidant Activity of Peel, Flowers and Leaf Oils of Citrus Aurantium L. Growing in Greece. Molecules 2013, 18, 10639–10647. [Google Scholar] [CrossRef]
- Manzur, M.; Luciardi, M.C.; Blázquez, M.A.; Alberto, M.R.; Cartagena, E.; Arena, M.E. Citrus Sinensis Essential Oils an Innovative Antioxidant and Antipathogenic Dual Strategy in Food Preservation against Spoliage Bacteria. Antioxidants 2023, 12, 246. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.I.; Grácio, M.; Silva, M.C.; Pedroso, L.; Lima, A. One Health Perspectives on Food Safety in Minimally Processed Vegetables and Fruits: From Farm to Fork. Microorganisms 2023, 11, 2990. [Google Scholar] [CrossRef] [PubMed]
- Simons, A.; Alhanout, K.; Duval, R.E. Bacteriocins, Antimicrobial Peptides from Bacterial Origin: Overview of Their Biology and Their Impact against Multidrug-Resistant Bacteria. Microorganisms 2020, 8, 639. [Google Scholar] [CrossRef] [PubMed]
- Rivera, J.; Crandall, P.G.; Bryan, C.A.O.; Ricke, S.C. Essential Oils as Antimicrobials in Food Systems: A Review. Food Control 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Bozkurt, T.; Gülnaz, O.; Kaçar, Y.A. Chemical Composition of the Essential Oils from Some Citrus Species and Evaluation of the Antimicrobial Activity. J. Environ. Sci. Toxicol. Food Technol. 2017, 11, 29–33. [Google Scholar] [CrossRef]
- Fancello, F.; Luigi, G.; Zara, S.; Lina, M.; Addis, R.; Maldini, M.; Foddai, M.; Rourke, J.P.; Chessa, M.; Pintore, G. Chemical Characterization, Antioxidant Capacity and Antimicrobial Activity against Food Related Microorganisms of Citrus Limon Var. Pompia Leaf Essential Oil. LWT Food Sci. Technol. 2016, 69, 579–585. [Google Scholar] [CrossRef]
- Aggarwal, K.K.; Khanuja, S.P.S.; Ahmad, A.; Gupta, V.K.; Kumar, S. Antimicrobial Activity Profiles of the Two Enantiomers of Limonene and Carvone Isolated from the Oils of Mentha Spicata and Anethum Sowa. Flavour Fragr. J. 2002, 17, 59–63. [Google Scholar] [CrossRef]
- Hawkey, C.J. COX-1 and COX-2 Inhibitors. Best Pract. Res. Clin. Gastroenterol. 2001, 15, 801–820. [Google Scholar] [CrossRef] [PubMed]
- Saad, J.; Pellegrini, M.V. Nonsteroidal Anti-Inflammatory Drugs Toxicity; StatPearls: Orlando, FL, USA, 2022. [Google Scholar]
- Quintans, J.S.S.; Shanmugam, S.; Heimfarth, L.; Araújo, A.A.S.; Almeida, J.R.G.d.S.; Picot, L.; Quintans-Júnior, L.J. Monoterpenes Modulating Cytokines-A Review. Food Chem. Toxicol. 2018, 123, 233–257. [Google Scholar] [CrossRef] [PubMed]
- de Cássia da Silveira e Sá, R.; Andrade, L.N.; De Sousa, D.P. A Review on Anti-Inflammatory Activity of Monoterpenes. Molecules 2013, 18, 1227–1254. [Google Scholar] [CrossRef] [PubMed]
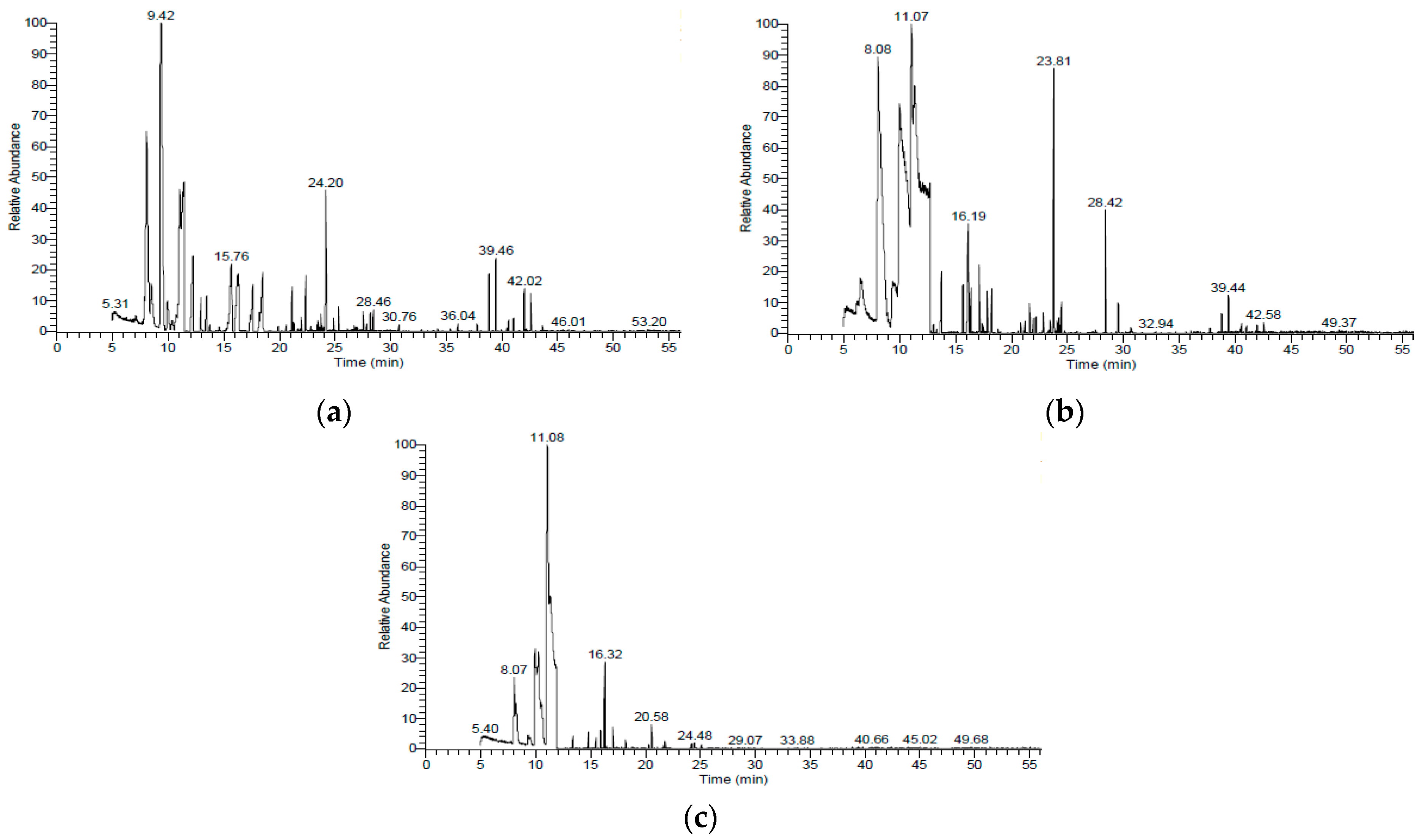

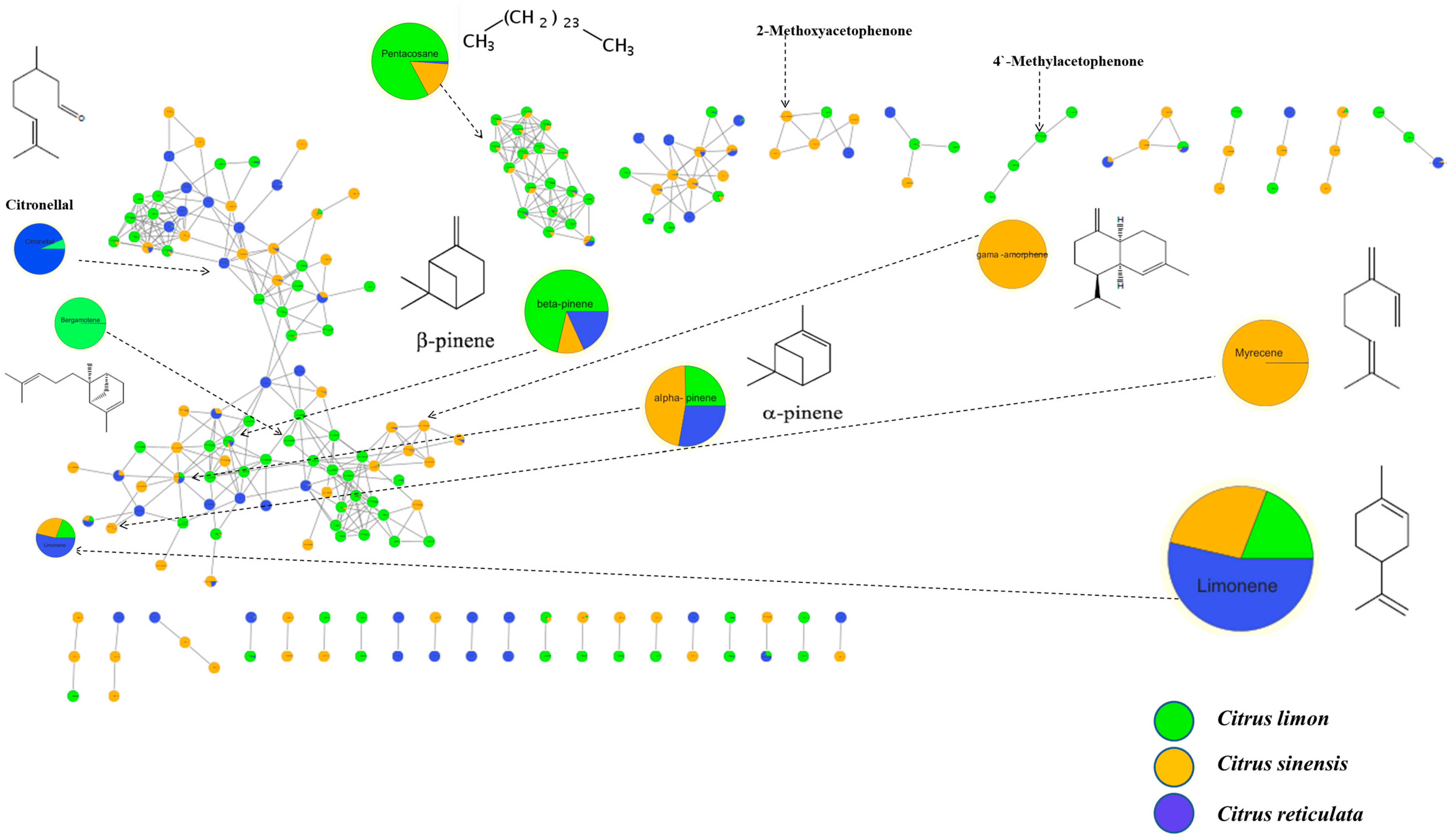
| No | Retention Time (Rt) | Base Peak (BP) | Molecular ion (M+) | RI * | RI ** | Molecular Formula | Compounds | Area (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Lemon (C. limon) | Orange (C. sinensis) | Mandarin (C. reticulata) | ||||||||
| 1 | 7.73 | 85 | 172 | 830 | 827 | C10 H20O2 | Isovaleric acid | - | 0.10 | 0.03 |
| 2 | 7.88 | 93 | 136 | 926 | 921 | C10H16 | Tricyclene | 0.21 | - | - |
| 3 | 7.89 | 93 | 136 | 931 | 924 | C10H16 | α-Thujene | - | 0.20 | 0.06 |
| 4 | 8.07 | 93 | 136 | 932 | 932 | C10H16 | α-Pinene | 9.22 | 11.32 | 6.61 |
| 5 | 8.51 | 93 | 136 | 946 | 946 | C10H16 | Camphene | 2.00 | - | 5.95 |
| 6 | 9.35 | 136 | 93 | 966 | 969 | C10H16 | Sabinene | - | 1.89 | 2.55 |
| 7 | 9.41 | 93 | 136 | 980 | 974 | C10H16 | β-Pinene | 31.38 | 4.48 | 8.97 |
| 8 | 9.99 | 69 | 136 | 987 | 988 | C10H16 | Myrcene | - | 13.52 | - |
| 9 | 10.02 | 41 | 130 | 991 | 988 | C8H18O | Octanol | - | 0.25 | - |
| 10 | 10.50 7.72 | 128 | 41 | 998 | 998 | C8H16O | Octanal | - | - | 2.93 |
| 11 | 10.60 | 93 | 136 | 1000 | 1001 | C10H16 | Mentha-1(7),8-diene | 1.51 | - | - |
| 12 | 10.62 | 93 | 136 | 1002 | 1002 | C10 H16 | δ-carene 2 | - | 0.06 | - |
| 13 | 12.63 | 93 | 136 | 1003 | 1003 | C10 H16 | β-Phellandrene | 0.04 | 0.20 | - |
| 14 | 10.64 | 93 | 136 | 1007 | 1008 | C10 H16 | δ-carene 3 | - | - | 0.05 |
| 15 | 10.69 | 93 | 136 | 1014 | 1014 | C10H16 | α-Terpinene | 0.54 | - | - |
| 16 | 11.04 | 67/79 | 136 | 1025 | 1024 | C10H16 | D-Limonene | 14.57 | 17.76 | 43.60 |
| 17 | 12.04 | 135 | 150 | - | GNPS | C9H10O2 | 2-Methoxy acetophenone | - | 0.03 | - |
| 18 | 12.70 | 93 | 136 | 1054 | 1054 | C10H16 | ɣ-Terpinene | 3.97 | 11.82 | 6.19 |
| 19 | 12.98 | 93 | 136 | 1086 | 1086 | C10H16 | Terpinolene | 0.88 | - | - |
| 20 | 13.74 | 71 | 154 | 1090 | 1095 | C10H18O | Linalool | 1.57 | 2.39 | 0.98 |
| 21 | 13.75 | 81 | 154 | 1113 | 1114 | C10H18O | α-Fenchol | 0.13 | - | - |
| 22 | 15.69 | 71 | 156 | 1136 | 1134 | C10 H20O | Terpineol <trans-dihydro-β-> | 4.28 | - | - |
| 23 | 16.18 | 71 | 154 | 1140 | 1140 | C10H18O | β-Terpineol | 1.46 | 6.59 | 0.87 |
| 24 | 16.82 | 69 | 154 | 1152 | 1148 | C10H18O | Citronellal | 0.12 | - | 0.72 |
| 25 | 16.86 | 121 | 136 | 1155 | 1155 | C8 H8O2 | 2′-Hydroxyacetophenone | - | 0.03 | 0.01 |
| 26 | 16.88 | 154 | 71 | 1168 | 1174 | C10H18O | Terpinen-4-ol | - | 1.49 | 0.84 |
| 27 | 16.90 | 93 | 135 | - | GNPS | C8H9NO | N-phenyl Acetamide | 4.64 | - | - |
| 28 | 16.95 | 156 | 41 | 1201 | 1201 | C10H20O | Decanal | - | 1.38 | 5.47 |
| 29 | 18.22 | 109 | 152 | 1215 | 1215 | C10 H16O | trans-Carveol | 0.22 | - | - |
| 30 | 18.25 | 69 | 156 | 1223 | 1223 | C10 H20O | β–Citronellol | 0.21 | 2.09 | 1.01 |
| 31 | 18.27 | 69 | 152 | 1236 | 1235 | C10H16O | Neral (z-citral) | 1.63 | 0.19 | - |
| 32 | 18.49 | 69 | 150 | 1241 | 1239 | C10H14O | Carvone | - | 0.16 | - |
| 33 | 18.85 | 69 | 154 | 1250 | 1249 | C10H18O | Geraniol | - | 1.06 | - |
| 34 | 18.89 18.62 | 69 | 152 | 1266 | 1264 | C10H16O | Geranial (E citral) | 3.35 | 0.25 | - |
| 35 | 18.90 | 55 | 158 | 1267 | 1266 | C10H22O | 1-Decano | - | 1.05 | - |
| 36 | 18.92 | 69 | 196 | 1288 | 1288 | C12H20O2 | Lavandulyl acetate | 0.04 | - | - |
| 37 | 20.28 22.41 | 81/95 | 198 | 1349 | 1348 | C12H22O2 | Citronelly acetate | - | 0.01 | 0.22 |
| 38 | 20.85 | 105/161 | 204 | 1350 | 1350 | C15H24 | α- Cubebene | - | 0.24 | - |
| 39 | 20.59 | 196 | 41 | 1360 | 1359 | C12H20O2 | Neryl acetate (Geranyl) acetate) | 1.17 | - | 1.14 |
| 40 | 23.50 23.43 | 105/119 | 204 | 1373 | 1373 | C15H24 | α-Ylangene | 0.10 | - | - |
| 41 | 23.57 | 93 | 204 | 1388 | 1389 | C15H24 | β-Elemenene | 0.16 | 0.34 | - |
| 42 | 23.60 | 161 | 204 | 1406 | 1407 | C15H24 | Longifolene | - | 0.03 | - |
| 43 | 23.64 | 41 | 184 | 1408 | 1408 | C12H24O | Dodecanal | - | 0.59 | 0.12 |
| 44 | 23.66 24.95 | 93 | 204 | 1410 | 1410 | C15H24 | Trans-Caryophyllene | 0.27 | 0.29 | - |
| 46 | 23.70 | 161 | 204 | 1418 | 1419 | C15H24 | β-Cedrene | - | 0.36 | - |
| 47 | 23.72 | 93 | 204 | 1432 | 1432 | C15H24 | α-Bergamotene | 2.13 | - | - |
| 48 | 23.73 | 69 | 204 | 1440 | 1440 | C15H24 | β-Farnesene | - | 0.45 | - |
| 49 | 23.75 26.16 | 93 | 204 | 1455 | 1452 | C15H24 | α–Humulene | 0.14 | - | - |
| 50 | 23.76 | 161/105 | 204 | 1477 | 1478 | C15H24 | D-Germacrene | 0.22 | 0.30 | |
| 51 | 23.77 | 161 | 204 | 1480 | 1480 | C15H24 | γ-muurolene | - | 0.02 | - |
| 52 | 24.24 | 220 | 205 | 1485 | 1489 | C15H24O | Butylated hydroxytoluene | - | 0.32 | 0.25 |
| 53 | 24.76 | 204 | 204 | 1491 | 1495 | C15H24 | β-Selinene | 0.34 | - | - |
| 54 | 24.80 | 105 | 204 | 1506 | 1505 | C15H24 | γ-Amorphene | - | 7.11 | - |
| 55 | 24.90 29.03 | 69 | 204 | 1514 | 1514 | C15H24 | E-α-farnesene | 4.48 | 0.26 | - |
| 56 | 24.94 | 161 | 204 | 1524 | 1522 | C15H24 | δ- cadinene | - | 0.73 | 0.43 |
| 57 | 28.19 | 109/119 | 204 | 1531 | 1528 | C15H24 | Iso- γ-Bisabolene | 0.06 | - | - |
| 58 | 28.20 | 222 | 59/93 | 1548 | 1548 | C15H26O | Elemol | 0.01 | 0.01 | 0.26 |
| 59 | 28.46 34.08 | 189 | 222 | 1630 | 1630 | C15H26O | γ-selinenol | 0.05 | - | - |
| 60 | 28.66 | 161 | 222 | 1643 | 1645 | C15H26 O | Cubenol | 0.16 | - | - |
| 61 | 28.84 | 204/161 | 222 | 1653 | 1652 | C15H26O | α-Cadinol | 0.14 | 0.01 | - |
| 62 | 30.75 | 55 | 214 | 1672 | 1671 | C14H30O | Tetradecanol | 0.17 | - | - |
| 63 | 30.80 36.17 | 69 | 222 | 1681 | 1685 | C15 H26O | α-Bisabolol | 0.41 | - | - |
| 64 | 30.81 | 93 | 218 | 1699 | 1699 | C15H22O | β-Sinensal | - | 2.70 | - |
| 65 | 30.84 | 95/204 | 222 | - | GNPS | C15H26O | Selina-6-en-4-ol | 0.45 | - | - |
| 66 | 30.86 | 93 | 218 | 1755 | 1755 | C15H22O | α –Sinensal | - | 0.66 | - |
| 67 | 30.92 | 41 | 218 | 1807 | 1806 | C15H22O | Nootkatone | - | 0.14 | - |
| 68 | 36.04 | 57 | 254 | - | GNPS | C18H38 | 2,6,11-Trimethyl dodecane | 0.19 | - | - |
| 69 | 38.83 | 57 | 282 | 2000 | 2000 | C20H42 | Eicosane | 0.10 | 0.46 | - |
| 70 | 38.84 | 57 | 310 | 2200 | 2200 | C22H46 | Docosane | 0.15 | - | - |
| 71 | 39.44 | 57 | 324 | 2300 | 2300 | C23H48 | Tricosane | 2.75 | 0.88 | 0.09 |
| 72 | 40.60 | 57 | 366 | - | GNPS | C26H54 | 5-Butyl docosane | - | 0.19 | - |
| 73 | 42.00 | 57 | 338 | 2400 | 2400 | C24H50 | Tetracosane | 1.18 | 0.18 | - |
| 74 | 42.58 | 57 | 352 | 2500 | 2500 | C25H52 | Pentacosane | 0.78 | 0.29 | 0.07 |
| Total identified (%) | 97.55 | 94.22 | 89.65 | |||||||
| Concentration (μg/mL) | Vitamin C | Lemon (C. limon) | Orange (C. sinensis) | Mandarin (C. reticulata) |
|---|---|---|---|---|
| 250 | 23.73 e ± 0.53 | 26.90 e ± 1.21 | 21.50 f ± 1.27 | 27.30 e ± 0.40 |
| 500 | 41.08 d ± 0.52 | 38.20 d ± 0.75 | 32.35 d ± 0.38 | 39.86 d ± 0.06 |
| 1000 | 64.57 c ± 0.59 | 51.30 c ± 0.75 | 45.45 c ± 0.26 | 56.83 c ± 0.28 |
| 1500 | 81.85 b ± 0.46 | 61.20 b ± 0.57 | 70.20 b ± 0.46 | 68.67 b ± 0.22 |
| 2000 | 96.09 a ± 0.14 | 86.35 a ± 0.25 | 76.15 a ± 0.32 | 81.33 a ± 0.32 |
| IC50 | 735 ± 0.89 | 947 ± 1.78 | 1073 ± 2.45 | 878 ± 1.36 |
| Concentration (µg/mL) | Vitamin C | Lemon (C. limon) | Orange (C. sinensis) | Mandarin (C. reticulata) |
|---|---|---|---|---|
| 250 | 43.32 e ± 0.32 | 13.84 e ± 0.87 | 14.97 e ± 0.55 | 16.10 e ± 0.21 |
| 500 | 63.61 d ± 0.42 | 37.32 d ± 0.65 | 25.30 d ± 0.35 | 32.53 d ± 0.69 |
| 1000 | 77.83 c ± 0.51 | 63.81 c ± 0.25 | 50.27 c ± 0.20 | 51.12 c ± 0.26 |
| 1500 | 85.25 b ± 0.32 | 74.21 b ± 0.35 | 61.70 b ± 0.32 | 62.40 b ± 0.42 |
| 2000 | 92.94 a ± 0.28 | 83.98 a ± 0.41 | 79.43 a ± 0.75 | 83.91 a ± 0.35 |
| IC50 | 263 ± 1.85 | 914 ± 0.98 | 999 ± 2.05 | 995 ± 1.24 |
| IC50 (µg/mL) | COX-1 | COX-2 |
|---|---|---|
| Celecoxib | 97.50 a ± 0.10 | 0.31 c ± 0.01 |
| Indomethacin | 6.25 e ± 0.00 | 0.52 b ± 0.01 |
| Lemon (C. limon) | 12.50 d ± 0.20 | 0.09 d ± 0.00 |
| Orange (C. sinensis) | 40.00 b ± 1.00 | 0.63 a ± 0.00 |
| Mandarin (C. reticulata) | 24.33 c ± 0.85 | 0.31 c ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, F.M.; Mohammed, R.S.; Abdelsalam, E.; Ashour, W.E.-S.; Magalhães, D.; Pintado, M.; El Habbasha, E.S. Egyptian Citrus Essential Oils Recovered from Lemon, Orange, and Mandarin Peels: Phytochemical and Biological Value. Horticulturae 2024, 10, 180. https://doi.org/10.3390/horticulturae10020180
Ibrahim FM, Mohammed RS, Abdelsalam E, Ashour WE-S, Magalhães D, Pintado M, El Habbasha ES. Egyptian Citrus Essential Oils Recovered from Lemon, Orange, and Mandarin Peels: Phytochemical and Biological Value. Horticulturae. 2024; 10(2):180. https://doi.org/10.3390/horticulturae10020180
Chicago/Turabian StyleIbrahim, Faten Mohamed, Reda Sayed Mohammed, Eman Abdelsalam, Wedian El-Sayed Ashour, Daniela Magalhães, Manuela Pintado, and El Sayed El Habbasha. 2024. "Egyptian Citrus Essential Oils Recovered from Lemon, Orange, and Mandarin Peels: Phytochemical and Biological Value" Horticulturae 10, no. 2: 180. https://doi.org/10.3390/horticulturae10020180
APA StyleIbrahim, F. M., Mohammed, R. S., Abdelsalam, E., Ashour, W. E.-S., Magalhães, D., Pintado, M., & El Habbasha, E. S. (2024). Egyptian Citrus Essential Oils Recovered from Lemon, Orange, and Mandarin Peels: Phytochemical and Biological Value. Horticulturae, 10(2), 180. https://doi.org/10.3390/horticulturae10020180









