Abstract
Apples are one of the most valuable fruits worldwide. ‘Honeycrisp’ is the top sales-producing cultivar in the US. Lack of red skin coloration and increased preharvest fruit drop significantly reduce the market value for cultivars such as ‘Honeycrisp’. The use of reflective groundcovers has been shown to enhance apple skin coloration. While the use of plant growth regulator AVG reduces fruit drop, it negatively affects skin coloration. Studies on the impacts of these practices in mid-Atlantic US-grown apples are limited. In this work, for two years, we compared differences in the light environment, fruit drop, internal ethylene concentration (IEC), physicochemical parameters, and skin coloration of ‘Honeycrisp’ apples in the lower third of the canopy. Apples were submitted to four treatment combinations of reflective groundcover (Extenday) and AVG (130 mg L−1). Assessments occurred throughout three ripening stages. Our results demonstrated that Extenday significantly promoted skin coloration (>75% blush) via the increased reflectance of photosynthetic photon flux density and UV radiation, and increased IEC, while also advancing fruit maturity, i.e., overripening. Conversely, AVG significantly minimized fruit drop and decreased EIC, delaying fruit maturity but drastically reducing red coloration (30–48% blush). The combined use of Extenday and AVG had a synergistic effect by decreasing fruit drop while enhancing fruit with >50% blush, without promoting overripening. Combining Extenday and AVG can boost the market value for ‘Honeycrisp’ apples in the mid-Atlantic US.
1. Introduction
Apple (Malus domestica Borkh.) red skin coloration is one of the primary determinants of consumer preference and market value [1,2]. Therefore, poor red skin coloration is a key factor that can result in downgrading apple fruit as it is associated with poor visual appearance and thus low consumer acceptance [3]. Furthermore, in recent years, standards for minimum acceptable red skin coloration have increased from 25% to 50% blush across several commercially important cultivars, such as ‘Honeycrisp’ [4,5].
Red skin coloration in apples is mainly determined by the content of anthocyanins [6]. Anthocyanin accumulation is strongly affected by environmental factors such as temperature and light [7,8,9]. The ideal conditions for red color development in apples correspond to bright, clear days with temperatures of 25 °C and cool nights (15 °C) during 3–4 weeks preharvest [10,11]. Nevertheless, due to the hot and humid environmental conditions of the mid-Atlantic region of the US, high-value cultivars such as ‘Honeycrisp’ often produce marginal red skin coloration, not meeting the market standards. Regarding light intensity and wavelength, ultraviolet radiation, intrinsic to sunlight, has shown effects on inducing fruit anthocyanin biosynthesis, thus improving apple red skin coloration [8,12]. Light penetration levels can vary widely within the canopy, with less light generally reaching the inner and lower third parts of the canopy while more sunlight tends to be intercepted by the upper and outer parts of the canopy [3]. Furthermore, considerable light is lost to red fruit color development as it is absorbed by the ground between the orchard rows [13].
Horticultural practices, such as the use of reflective groundcovers, are used to enhance the tree canopy light environment [3,14]. The deployment of reflective groundcovers improves the capacity of apple trees to harness sunlight by reflecting the light that would otherwise be absorbed at the ground surface, back into the canopy [2,4,15]. This amplifies the cumulative light reaching the apple fruit surface, particularly fruit located in the lower third and inner part of the canopy [16]. The deployment of groundcovers around 4 weeks before the anticipated harvest has been reported to increase red skin coloration in apples [3,4,11,12,15,17,18,19]. Despite its proven ability to enhance apple coloration, research on reflective groundcovers has not been widely conducted under the environmental conditions of the US mid-Atlantic region.
In addition to the lack of red skin coloration, ‘Honeycrisp’ apples are prone to preharvest fruit drop, which can begin 4 weeks before the anticipated harvest and prior to the fruit reaching horticultural maturity [20,21,22]. The practice of harvesting fruit early to avoid preharvest fruit drop is not feasible, as immature fruit will not achieve acceptable quality and particularly red skin coloration requirements, leading to poor storability and reduced marketability [23]. Therefore, reducing preharvest fruit drop is of critical importance for extending the harvest window and decreasing economic losses in the commercially important cultivar ‘Honeycrisp’ in the mid-Atlantic.
Ethylene has been widely reported as a key ripening-related hormone affecting fruit quality [24], as well as a primary driver of preharvest fruit drop in apples [25]. Fruit quality is determined by multiple irreversible physiological and biochemical modifications that take place as fruit matures [26]. These include modifications in fruit skin color (background and red surface color), texture (flesh softening), and flavor (increase in sugar contents, decrease in organic acids, and changes in aroma volatiles) [27,28,29]. In addition, regarding preharvest fruit drop, ethylene can promote the degradation of the cell wall and intercellular tissues in the abscission zone of the pedicel, resulting in fruit drop [30,31]. Furthermore, apple fruit is classified as climacteric, characterized by an upsurge in respiration rates and internal ethylene concentration (IEC) as it ripens [32,33]. Nevertheless, ethylene production in apple fruit can vary amongst cultivars, with cultivars producing a higher EIC, presenting a higher susceptibility to preharvest fruit drop [34,35], such as ‘Honeycrisp’ [21,22].
Horticultural practices to reduce preharvest fruit drop currently rely mainly on plant growth regulators such as aminoethoxyvinylglycine (AVG), which inhibit the enzyme that catalyzes the rate-limiting step in ethylene biosynthesis, 1-aminocyclopropane-1-carboxylic acid (ACC) [20,36]. AVG applications 4 weeks before the anticipated harvest have significantly reduced preharvest fruit drop in different cultivars, such as ‘Honeycrisp’, ‘Gala’, and ‘McIntosh’, grown under different environmental conditions [3,20,22,37]. In addition to reducing fruit drop, AVG has been reported to delay fruit maturity, impacting several fruit-quality-related attributes, such as reducing fruit softening, starch breakdown, and soluble solids contents; maintaining high acidity, and significantly reducing red skin coloration development in different apple cultivars [3,20,22,36,38,39,40,41]. Particularly regarding red skin coloration, endogenous ethylene has been reported to also play a critical role in regulating anthocyanin accumulation [26,42,43,44], in addition to environmental factors.
Although earlier research has documented the effects of the use of reflective groundcovers and AVG on apples, investigations of the responses to these strategies under the environmental conditions of the US mid-Atlantic region are lacking. Furthermore, to our knowledge, studies on the impacts of the combination of both horticultural practices on ‘Honeycrisp’ apples have not yet been conducted under our environmental conditions. Based on the above, the aim of the present work was three-fold: first, to evaluate the effect of the reflective groundcover Extenday in light interception and reflectance in a commercial ‘Honeycrisp’ orchard grown under US mid-Atlantic environmental conditions; secondly, to characterize and compare differences in fruit drop, internal ethylene concentration, fruit-quality-related physicochemical parameters, and skin coloration of ‘Honeycrisp’ apples submitted to reflective groundcover Extenday and AVG treatment combinations throughout ripening on the tree; and thirdly, to use multivariate data analysis to identify significant correlations amongst all assessed features.
2. Materials and Methods
2.1. Plant Material and Preharvest Orchard Treatments
A 12-year-old commercial ‘Honeycrisp’/‘M9′ apple orchard located in Aspers, PA (39.96° N, 77.28° W), was used for this study. Tree spacing was 1.5 × 4 m and trees were trained to a central leader system. Four treatments composed of different combinations of the reflective groundcover Extenday (Extenday New Zealand, Auckland, New Zealand) and the plant growth regulator AVG (ReTain, Valent Biosciences Corporation, Libertyville, IL, USA) (Table 1) were established during two consecutive production seasons (2021 and 2022). For Extenday (T1, T2), a 3.5 m wide white woven polyethylene reflective groundcover was deployed adjacent to 50 tree plots on each side of the row 4 weeks before the anticipated commercial harvest date and secured according to manufacturer recommendations. Extenday (T1, T2)- and non-Extenday (T3, T4)-treated plots were separated down the tree row by at least 30 trees and separated by 3 rows of trees on either side to mitigate potential cofounding due to altered light reflection in trees adjacent to those applied with the Extenday treatment. The AVG treatment was applied to 20 tree subplots on Extenday (T1) and non-Extenday treatments (T3) and comprised a full-rate (130 mg L−1) application, 4 weeks before the anticipated commercial harvest date. All sprays were mixed with 1.0 mL L−1 Silwet-77 organosilicone surfactant before application, which were made using a pressurized orchard sprayer. Additional trees in each plot were used as buffers to manage the potential drift of AVG treatment. A randomized complete block design with four replications was used.

Table 1.
Combination of reflective groundcover and plant growth regulator treatments established in this study.
‘Honeycrisp’ fruit maturity indices were monitored throughout the season each year to harvest fruit in the optimal commercial maturity stage using control fruit (T4) as the reference. Fruits were harvested in three different ripening stages on the tree: at optimal commercial harvest (CH) (corresponding to 3 September 2021 and 4 September 2022), 1 week after CH (CH + 1) (corresponding to 10 September 2021 and 11 September 2022), and 2 weeks after CH (CH + 2) (corresponding to 17 September 2021 and 18 September 2022). On each harvest date, for each of the four replications per treatment, a total of twenty-five fruit were harvested from the lower third of the canopy (1.5 m above the ground). Fruits with uniform size and an absence of visual blemishes, bruises, and/or diseases were chosen. After harvest, fruits were quickly transported to the laboratory. Per replication, five fruits were used for the analysis of internal ethylene concentration, while the rest of the fruits were used to assess quality-related physicochemical properties (described below).
2.2. Light Interception and Reflectance Measurements
Light interception and reflectance by the reflective groundcover Extenday (T2) and by the ground or control (T4) were quantified in the middle of the drive row (mid-row) and within the tree canopy (in-canopy), proximal to solar noon on a sunny, cloud-free day and on a cloudy day in each year of this study. Light data were collected on two mid-row positions and two trees at the center of each 20-tree-plot replication. Measurements were collected 1.5 m above the ground. Intercepted light was determined with sensors oriented towards the sun (sky), while light reflectance was quantified by inverting the sensors (facing the ground or the reflective groundcover). Photosynthetic photon flux density (PPFD; 400–700 nm waveband; µmol m−2 s−1) was evaluated using an LI-COR LI-191R Line Quantum Sensor attached to an LI-250A Light Meter (LI-COR Environmental, Lincoln, NE, USA). Two measurements of interception and reflectance were taken, with the sensor positioned perpendicular to the row, once each on the north and south side of the trunk. In-canopy measurements were carried out with the distal end of the sensor next to the trunk. Ultraviolet light (UV; 250–400 nm; µmol m−2 s−1) was measured with a portable UV meter (FieldScout model 3414F, Spectrum Technologies Inc., Aurora, IL, USA). Four measurements of interception and reflectance were collected for each tree, once at each of the four points around the trunk (north, east, south, and west). In-canopy measurements were performed with the UV meter positioned 15 cm from the trunk.
2.3. Preharvest Fruit Drop Measurements
For each treatment and replication, two weeks before CH, a total of 5 limbs (from different trees) with a total of 20 fruits each were selected and tagged from either side of the trees. Preharvest fruit drop was evaluated by counting the number of tagged fruits weekly starting from one week before CH (1WBCH) to 2 weeks after CH (CH + 2). The percentage of fruit drop was then calculated relative to the initial fruit count per limb.
2.4. Fruit Internal Ethylene Concentration
The internal ethylene concentration (IEC) of each fruit was measured on 1 mL samples of internal gas from the core cavity using a gas chromatograph (GC-2014C, Shimadzu Co., Kyoto, Japan) equipped with an activated alumina column attached to a flame ionization detector as previously described [28,45,46]. Nitrogen (N2) was used as the carrier gas at a flow rate of 30 mL min−1, while O2 and H2 were used to create the flame of the detector at a flow rate of 300 and 30 mL min−1, respectively. Injector, detector, and oven temperatures were set at 140, 150, and 80 °C, respectively.
2.5. Fruit-Quality-Related Measurements
Fruit weight, skin color, index of absorbance difference (IAD), skin red blush percentage, flesh firmness, starch pattern index (SPI), soluble solids contents (SSCs), and titratable acidity (TA) were measured. Fruit weight was quantified using an electronic balance (Sartorius, AG Gottingen, Germany). Skin color was assayed on the two opposite sides of each fruit along the equatorial axes, and the red-green (a*) and yellow-blue (b*) values were measured using a colorimeter (Konica Minolta CR400 Chroma Meter, Konica Minolta Sensing, Inc., Osaka, Japan). Hue angle (hue°), representing changes in primary colors, was calculated as h = arctan(a*/b*) [47]. The index of absorbance difference (IAD = A670 − A720; DA-Meter, TR Turoni, Forli, Italy) was measured on fruit skin by averaging the values recorded on three spots on each apple fruit [48]. Flesh firmness was measured on the two opposite peeled sides of each fruit using a TA.XT Plus Connect texture analyzer (Texture Technologies Corp., Scarsdale, NY, USA) equipped with a 50 kg loadcell and analyzed with the Exponent TE32 (v6.0, Texture Technologies Corp., Scarsdale, NY, USA) software fitted with an 11.1 mm diameter probe. The SPI of each fruit cut at the equator was assessed using the Cornell generic chart where 1 = 100%-iodine-stained starch and 8 = 0%-stained starch [49]. To determine SSC and TA, a wedge from each fruit was removed and pooled to create a composite sample of each replication. Juice was extracted from these composite samples with a hand press and filtered through cheesecloth. SSC was determined by using a digital hand-held refractometer (Atago, Tokyo, Japan) and expressed as %, whereas TA was computed by automatic titration (855 Robotic Titrosampler; Metrohm, Riverview, FL, USA) with a 0.1 N sodium hydroxide solution to an end point to pH 8.2, expressed as % malic acid [29,32].
2.6. Statistical Analysis
Response variables were modeled using generalized linear mixed models including treatments and evaluation periods as fixed factors, as well as blocks as a random factor to determine the statistical significance of the interactions and main effects (analysis of variance, ANOVA). When the analysis was statistically significant, the separation of means was carried out using Tukey’s HSD test at a significance level of 5%.
Pearson’s correlation coefficients, using mean-centered data, were calculated for each pairwise-combination of evaluated parameters. PCA, which was applied to reduce the dimensionality of the data, was visualized through a ‘biplot’ graph, thus representing the relationships among the variables (preharvest fruit drop, IEC, physicochemical measurements, and fruit skin color) and the assessed treatments and evaluation periods. The Scree test was used to select the number of principal components that captured most of the variation. The software package JMP (ver 15.2, SAS Institute) was used for all the statistical analyses.
3. Results
3.1. Effect of the Reflective Groundcover Extenday on Light Interception and Reflectance
Strong and consistent differences on mid-row and in-canopy PPFD and UV radiation were observed between the reflective groundcover Extenday-only treatment (T2) and by the ground or control treatment (T4) on both cloudy and sunny days, throughout the two evaluated years (Table 2 and Table 3). Mid-row and in-canopy-intercepted PPFD and UV radiation results showed that, for both years, values were significantly higher in sunny than in cloudy days, but there were no differences between treatments. Conversely, in the case of mid-row and in-canopy-reflected PPFD and UV radiation, differences were observed between treatments as well as between sunny and cloudy days. The Extenday-only (T2) treatment assessed on the sunny days displayed the significantly highest reflected PPFD and UV radiation values, followed by this same treatment measured on the cloudy days, and subsequently by the ground or control treatment (T4) evaluated on the sunny days. The significantly lowest values for reflected PPFD and UV radiation were for the ground or control treatment (T4) assessed on the cloudy days (Table 2 and Table 3). Mid-row measurements of PPFD and UV radiation reflected from Extenday were 5–20 times greater than those of the ground treatment, while in-canopy measurements of PPFD and UV radiation reflected from Extenday were 5–12 times greater than those of the ground treatment (Table 2 and Table 3) considering cloudy and sunny days, in both evaluated years.

Table 2.
Effect of the reflective groundcover Extenday on intercepted and reflected PPFD and UV radiation in the middle of the drive row (mid-row) and within the canopy (in-canopy) of ‘Honeycrisp’ trees on a sunny and on a cloudy day in Aspers, PA, in 2021.

Table 3.
Effect of the reflective groundcover Extenday on intercepted and reflected PPFD and UV radiation in the middle of the drive row (mid-row) and within the canopy (in-canopy) of ‘Honeycrisp’ trees on a sunny and on a cloudy day in Aspers, PA, in 2022.
3.2. Effects of Extenday and AVG Treatment Combinations on ‘Honeycrisp’ Preharvest Fruit Drop
In 2021, there was no difference in fruit drop percentage amongst Extenday and AVG treatment combinations at 1WBCH (Figure 1A), but the fruit drop percentage started to increase significantly for all treatments from this period onwards. The Extenday-only (T2) treatment and the control (T4) displayed comparable levels of fruit drop that were around two times higher than treatments where AVG was applied (T1 and T3), from CH onwards. In the last assessment period (CH + 2), the treatments that did not receive AVG, T2 and T4, displayed fruit drop values ~30%, while T1 and T3, which did receive AVG, presented fruit drop values ~15%.
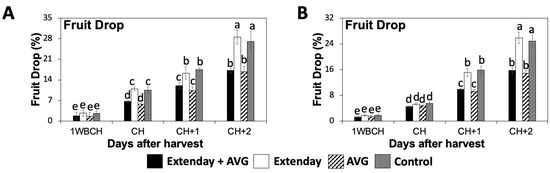
Figure 1.
Effect of Extenday and AVG treatment combinations on preharvest fruit drop of ‘Honeycrisp’ apples grown in Aspers, PA. Fruit drop percentages were assessed in (A) 2021 and (B) 2022. Apples were evaluated 1 week before optimal commercial harvest (1WBCH), at optimal commercial harvest (CH), 1 week after CH (CH + 1), and 2 weeks after CH (CH + 2). Values are means ± standard error. Different letters indicate significant differences (p ≤ 0.05) according to Tukey’s HSD test.
For 2022, differences in preharvest fruit drop amongst the four assayed treatment combinations were significant only for the evaluation periods of CH + 1 and CH + 2 (Figure 1B). Consistent with the results from 2021, fruit drop percentages started to increase significantly for all treatments from 1WBCH onwards. Furthermore, treatments lacking AVG application, T2 and T4, displayed fruit drop percentages that were ~1.5 times higher than treatments which received AVG applications, T1 and T3, from CH + 1 forward. During the last assessment period (CH + 2), T2 and T4 displayed fruit drop values ~25%, while T1 and T3, which did receive AVG, presented significantly lower fruit drop values ~15%.
3.3. Internal Ethylene Concentration and Physicochemical Properties of ‘Honeycrisp’ Fruit Submitted to Extenday and AVG Treatment Combinations
In general, Extenday and AVG treatment combinations had a consistently significant effect on IEC and most physicochemical properties of ‘Honeycrisp’ fruit across the two assessed years (Table 4 and Table 5).

Table 4.
Effects of Extenday and AVG combinations on internal ethylene concentration and physicochemical properties of ‘Honeycrisp’ fruit harvested in three different ripening stages on the tree in Aspers, PA, in 2021.

Table 5.
Effects of Extenday and AVG combinations on internal ethylene concentration and physicochemical properties of ‘Honeycrisp’ fruit harvested in three different ripening stages on the tree in Aspers, PA, in 2022.
Regarding IEC, all treatment combinations displayed significant increases as the fruit ripened on the tree (Table 4 and Table 5), and therefore, fruit assessed at CH + 2 presented the highest IEC for each treatment, with respect to the other two evaluation periods. Within each evaluation period, the highest ethylene concentrations occurred with fruits submitted to Extenday-only (T2), followed by the control (T4), Extenday + AVG (T1), and finally AVG-only (T3), which presented the lowest ethylene concentration values. The exception was for 2022, where, at CH, fruits submitted to T2 displayed no significant differences with respect to T4 (Table 5).
Throughout all the evaluation periods and treatments, fruit weight was not significantly altered, and thus no significant differences were observed.
Fruit flesh firmness decreased as fruits ripened on the tree for all treatment combinations in both years (Table 4 and Table 5). In general, flesh firmness values were highest at CH for Extenday + AVG (T1) and AVG-only (T3) (76–80 N), followed by the control (T4) and finally by Extenday-only (T2), which exhibited the significantly lowest values (61–63 N) for 2021 and 2022 assessments. These differences in treatment combinations were also observed for CH + 1, although in 2021, there was a significant difference between AVG-only (T3) and Extenday + AVG (T1) that was not observed in any other evaluation period or year (Table 4). Extenday-only (T2) assessed in CH + 1 and at CH + 2 evaluation periods presented the lowest flesh firmness values (<60 N) amongst all treatments and evaluation periods. Particularly in 2022, the control (T4) assessed at CH + 2 did not significantly differ from Extenday-only (T2) (<60 N) but did differ from Extenday + AVG (T1) and AVG-only (T3) (>60 N) (Table 5).
Extenday and AVG treatment combinations also affected the starch pattern index (Table 4 and Table 5). As the fruit ripened on the tree, the SPI values significantly increased (indicative of a lower starch content) for all treatment combinations in both years. At CH and CH + 1, SPI values were lowest for AVG-only (T3), followed by Extenday + AVG (T1) and the control (T4), and the highest SPI values were obtained for Extenday-only (T2), which displayed 1.3–2 times higher SPI values than AVG-only (T3) when considering both years. Fruits evaluated at CH + 2 presented the same trends as described for the other evaluation periods. The exception was for 2021, where Extenday-only (T2) and the control (T4) did not differ significantly (SPI ~7.8) (Table 4), and for 2022, where Extenday + AVG (T1; SPI ~7.3) exhibited no differences with any of the other assayed treatment combinations, although AVG-only (T3; SPI ~6.7) did differ from Extenday-only (T2) and the control (T4; SPI ~7.8) (Table 5).
In both years, SSC significantly increased throughout the three ripening evaluation periods for all treatment combinations, except for Extenday + AVG (T1), which still presented an increasing trend, but was not significant (Table 4 and Table 5). In 2021, within the evaluation periods, in most cases, Extenday-only (T2) and the control (T4) displayed significantly higher values than Extenday + AVG (T1) and AVG-only (T3), which, for CH + 2, corresponded to 13.2–13.5% and 14.2–14.6%, respectively (Table 4). In 2022, values for AVG-only (T3) were statistically lower than for Extenday-only (T2) and the control (T4) during CH and CH + 2, with values ranging from 13.4 to 14% for the former and 14 to 14.7% for the latter (Table 5).
Titratable acidity (TA) values significantly decreased throughout the different assayed evaluation periods of ripening on the tree for all treatment combinations (Table 4 and Table 5). During both years, at CH, AVG-only (T3) presented the significantly highest acidity values (~0.55), followed by Extenday + AVG (T1; ~0.47–0.5), the control (T4; ~0.4), and lastly Extenday-only (T2; <0.4). In 2021, these differences amongst treatments combinations were maintained through CH + 1, although at CH + 2, no significant differences were displayed between Extenday + AVG (T1) and AVG-only (T3) (~0.4) as well as between Extenday-only (T2) and the control (T4) (~0.3) (Table 4). In 2022, in the CH + 1 and CH + 2 evaluation periods, no significant differences were observed between Extenday + AVG (T1) and AVG-only (T3) nor between Extenday-only (T2) and the control (T4) (Table 5).
3.4. Effects of Extenday and AVG Treatment Combinations on ‘Honeycrisp’ Skin Coloration
Extenday and AVG treatment combinations displayed significant effects on surface red skin color as well as background skin coloration in ‘Honeycrisp’ apples throughout the two years of evaluation (Table 6 and Table 7 and Figure 2).

Table 6.
Effects of Extenday and AVG combinations on surface and background skin coloration of ‘Honeycrisp’ fruit harvested in three different ripening stages on the tree in Aspers, PA, in 2021.

Table 7.
Effects of Extenday and AVG combinations on surface and background skin coloration of ‘Honeycrisp’ fruit harvested in three different ripening stages on the tree in Aspers, PA, in 2022.

Figure 2.
Effects of Extenday and AVG treatment combinations on ‘Honeycrisp’ fruit coloration at commercial harvest in Aspers, PA, in 2022.
As the fruits ripened on the tree, the surface skin hue angle values significantly decreased (indicative of a higher red coloration) in all treatment combinations in 2021 and 2022 (Table 6 and Table 7). For all evaluation periods and in both years, surface skin hue angle values were highest for AVG-only (T3), followed by the control (T4) and Extenday + AVG (T1), and finally, the statistically lowest values were for Extenday-only (T2). In agreement with the results for surface skin hue angle, the assessment of skin blush percentage showed that from CH to CH + 2, there was a significant increase in this parameter for all treatment combinations in both assayed years (Table 6 and Table 7). Furthermore, the significantly highest skin blush percentage was consistently obtained for Extenday-only (T2; 75–90%), followed by Extenday + AVG (T1; 58–72%) and the control (T4; 40–63%), and finally, the lowest values were displayed by AVG-only (T3; 30–48%). It is important to note that the control (T4) only reached ~50% skin blush in ‘Honeycrisp’ in fruit harvested at CH + 1, while AVG-only (T3) only achieved it in fruit harvested at CH + 2 (Table 6 and Table 7).
Background skin color hue values as well as the index of absorbance difference (IAD) significantly decreased (indicative of a change in color from green to yellow, and to an increase in chlorophyll degradation, respectively), in all treatment combinations and both evaluated years, as fruit ripened on the tree (Table 6 and Table 7). Consistently for 2021 and 2022, as well as for all the assessed evaluation periods, AVG-only (T3) displayed the significantly highest values, followed by the control (T4) and Extenday + AVG (T1), which also differed amongst them. The significantly lowest values for background skin color hue and IAD were for ‘Honeycrisp’ fruit from Extenday-only (T2) in all cases, evidencing the highest rate of chlorophyll disappearance.
3.5. Relationships among Fruit Drop, Ethylene Concentration, Physicochemical Properties, and Fruit Color of ‘Honeycrisp’ Apple Fruit Submitted to Extenday and AVG Treatment Combinations
Correlation coefficients were calculated (Table 8) and a Principal Component Analysis (PCA) was performed (Figure 3) to visualize the relationships among all the parameters assessed in ‘Honeycrisp’ fruit and described above during the two assayed production seasons.

Table 8.
Pearson correlation coefficients among all assessed features in ‘Honeycrisp’ fruit including fruit drop, internal ethylene concentration (IEC), physicochemical parameters, and skin coloration (internal ethylene concentration (IEC), starch pattern index (SPI), soluble solids content (SSC), titratable acidity (TA), index of absorbance difference (IAD)).
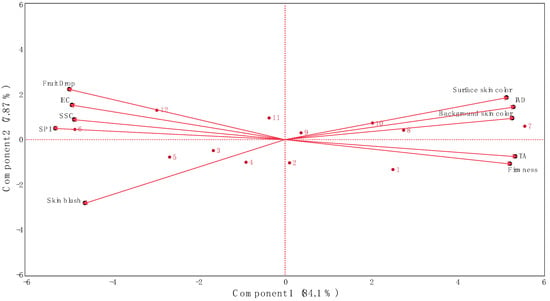
Figure 3.
Biplot from Principal Component Analysis of data obtained from fruit drop, internal ethylene concentration, physicochemical parameters, and skin coloration of ‘Honeycrisp’ apples submitted to different Extenday and AVG treatments combinations throughout ripening on the tree. Internal ethylene concentration (IEC), starch pattern index (SPI), soluble solids content (SSC), titratable acidity (TA), index of absorbance difference (IAD). Numbers correspond to the different treatments and ripening stages on the tree that were assayed (1 (T1_CH), 2 (T1_CH + 1), 3 (T1_CH + 2), 4 (T2_CH), 5 (T2_CH + 1), 6 (T2_CH + 2), 7 (T3_CH), 8 (T3_CH + 1), 9 (T3_CH + 2), 10 (T4_CH), 11 (T4_CH + 1), 12 (T4_CH + 2)). Codes for treatments are described in Table 1.
Fruit drop significantly and positively correlated with IEC (r = 0.94), SPI (r = 0.88), SSC (r = 0.78), and skin blush (r = 0.63) but was negatively associated with fruit flesh firmness (r = −0.89), titratable acidity (r = −0.89), color-related features of the surface and background skin hue angles (r = −0.69 and r = −0.82, respectively), and IAD (r = −0.77).
IEC was significantly and positively correlated with SPI (r = 0.79), SSC (r = 0.77), and skin blush (r = 0.63) (Table 8), while it negatively correlated with flesh firmness (r = −0.80), titratable acidity (r = −0.85), surface and background skin hue angles (r = −0.70 and r = −0.85, respectively), and IAD (r = −0.79).
The parameter of flesh firmness was positively associated with titratable acidity (r = 0.95), the color parameters of surface and background skin hue angles (r = 0.81 and r = 0.82, respectively), as well as IAD (r = 0.84). Furthermore, there was a significant negative correlation between flesh firmness and assessed fruit physicochemical properties such as SPI (r = −0.96) and SSC (r = −0.83), as well as with skin blush (r = −0.67).
The starch pattern index presented positive correlations with SSC (r = 0.83) and skin blush (r = 0.70) but was negatively associated with titratable acidity (r = −0.91), skin and flesh color hue (r = −0.89 and r = −0.86, respectively), and IAD (r = −0.89) (Table 8). SSC correlated positively with skin blush (r = 0.69) and negatively with titratable acidity (−0.89), surface and background skin hue angles (r = −0.75 and r = −0.73, respectively), and the index of absorbance difference (r = −0.74). Titratable acidity, on the other hand, was positively associated with the color features of surface and background skin hue angles (r = −0.83 and r = −0.85, respectively) and the index of absorbance difference (r = −0.86), but negatively associated with fruit skin blush (r = −0.75).
Amongst the color-related parameters, surface skin hue angle was positively correlated with background skin hue angle (r = 0.92) and IAD (r = 0.97), and negatively correlated with skin blush (r = −0.90). On the other hand, skin blush displayed significantly negative correlations with background skin hue angles (r = −0.86) and IAD (r = −0.89).
The PCA showed that the first and second principal components explained 84.1% (Component 1) and 7.87% (Component 2) of the observed variation (91.9% total), respectively (Figure 3). Along the first principal component, the separation of the Extenday and AVG treatments combinations was driven by preharvest fruit drop, IEC, SSC, SPI, and skin blush on the negative side of the axis (associated with treatments Extenday + AVG (T1; at CH + 2), Extenday-only (T2; all evaluation periods), and the control (T4; at CH + 1 and CH + 2)) and by surface and background skin hue values, IAD, titratable acidity, and firmness on the positive side of the axis (associated with treatments Extenday + AVG (T1; at CH and CH + 1), AVG-only (T3; all evaluation periods), and the control (T4; at CH) (Figure 3)).
4. Discussion
There are numerous factors influencing red skin coloration in apples, including environmental factors such as temperature and light, as well as plant growth regulators such as AVG, among others [1,8,9,10,12,20,43,44]. Regarding light, although there are various reports indicating that the reflective groundcover Extenday has improved red skin coloration in apples [4,11,12,15,17,18], studies evaluating its effects on fruit maturity and quality are inconsistent, and have been lacking under the environmental conditions of the mid-Atlantic of the US in ‘Honeycrisp’ apples. Regarding AVG, it has been reported that it negatively affects red skin coloration development but can effectively delay maturity and reduce preharvest fruit drop [3,20,36,37,38,41,42]. However, there is limited information about the effects of Extenday and AVG treatment combinations on fruit drop, fruit maturity and quality, and particularly the skin coloration of ‘Honeycrisp’ apples grown under the hot and humid weather of the mid-Atlantic. In the present study, there was a general trend for decreased preharvest fruit drop and increased red skin coloration (>50% blush) without the promotion of overripening in fruit harvested from the lower third of the canopy, with the combined use of the reflective groundcover Extenday and the plant growth regulator AVG, consistent throughout two consecutive years.
Ultraviolet radiation is known to improve red skin coloration in fruits, including apples [8,12,50,51]. In agreement with our results, the use of Extenday in other regions has also shown an increase in reflected UV radiation from the orchard ground back onto the trees as compared to control trees [4,12]. These results support the significantly higher red skin coloration attained by Extenday-only-treated fruit in our work, which displayed >75% skin blush in the first assayed ripening stage (CH). Red color development on apple fruits results from the accumulation of anthocyanin pigments, which have been demonstrated to be highly influenced by UV radiation, as most enzymes involved in the anthocyanin biosynthesis pathway are light-inducible [10,52]. Consistent with our results, increased reflected PPFD by the reflective groundcover Extenday was also observed in previous studies [4,11,12,17,18]. Nevertheless, it is important to mention that excessive PPFF and UV radiation in other apple-producing regions, such as the Pacific Northwest of the US (hot and dry climate), can have negative impacts on ‘Honeycrisp’ fruit, such as the development of sunburn [15]. In the current study, under our environmental conditions, we did not observe sunburn incidence with the use of the reflective groundcover Extenday in any of the treatments.
Preharvest fruit drop is a major concern in apple production in many regions, including the mid-Atlantic [20,21,22]. Plant growth regulators, such as AVG, are widely used to prevent fruit drop due to their capacity of inhibiting ethylene biosynthesis [36,40]. This is supported by the positive correlation obtained between fruit drop and IEC in this work. Consistent with our results, the application of AVG at full-rate three-to-four weeks before harvest was significantly effective at minimizing preharvest fruit drop in ‘Honeycrisp’ [22], ‘Gala’ [3,20], and ‘McIntosh’ [37]. In ‘McIntosh’ apples, it has been reported that once applied, the onset of action time for AVG to manifest and significantly reduce fruit drop is between 10 and 14 days [38]. In this study, the application of AVG consistently decreased preharvest fruit drop in ‘Honeycrisp’ apples, independent of the use of the reflective groundcover Extenday, suggesting that the use of the latter does not significantly impact apple fruit drop.
As apple fruit display a climacteric fruit ripening behavior, and thus IEC has been reported to play a key role in controlling fruit maturity [28,32,33], attempts to control fruit drop by reducing ethylene biosynthesis via AVG are expected to delay fruit maturity. In this work, AVG-treated ‘Honeycrisp’ apples displayed the significantly lowest EIC, which affected several quality-related physicochemical attributes. In agreement with previous studies [3,20,22,38,39,41], AVG delayed fruit flesh softening and starch breakdown, reduced soluble solids contents, and maintained the highest acidity values throughout ripening. Consistent with our results, AVG has been reported to have no effect on apple fruit weight [20,37,53], suggesting that this is an ethylene-independent trait. Likewise, regarding the use of the reflective groundcover Extenday, no significant effects on apple fruit weight have been reported [4,11], supporting our results. Nevertheless, the use of Extenday has shown inconsistent results in terms of its effect on fruit maturity and quality. In this study, Extenday deployment hastened fruit maturity by increasing ethylene production in ‘Honeycrisp’ apples, decreasing flesh firmness and acidity, but increasing starch degradation as well as soluble solid contents, in accordance with previous findings in peaches [54] and apples [55]. However, other studies have reported no effects on fruit-maturity-related attributes due to Extenday deployment [2,3,4,11,14,15]. This variability in findings amongst studies regarding the impact of Extenday on apple fruit maturity and physicochemical characteristics can most likely be attributed to the differences in growing conditions, apple cultivars, management practices, and the time interval in which the reflective groundcover Extenday is in place. Moreover, in this study, when combining Extenday + AVG under US mid-Atlantic environmental conditions, ‘Honeycrisp’ fruit displayed an intermediate fruit maturity, i.e., significantly advanced with respect to AVG-only-treated apples but, at the same time, significantly delayed as compared to control and Extenday-only-treated fruit. The latter is indicative of an interaction between both Extenday and AVG that is impacting fruit quality properties by advancing maturity, but not overly stimulating the ripening process, and thus avoiding overripening as the fruit is left hanging on the tree. This is of key importance as it suggests that the combined Extenday + AVG treatment could be improving subsequent fruit storability and shelf-life capacity, which has not been accounted for in this study, but is currently under investigation.
Fruit red skin coloration is of major importance for fruit quality, as it is directly tied to consumer preference and market value [1]. As discussed above, apple skin red coloration is a result of the accumulation of anthocyanins, which, in addition to environmental factors, such as UV radiation, is also partially regulated by endogenous ethylene [26,42,43,44]. This is supported by the positive correlations between IEC and skin blush, as well as the negative correlations between IEC and hue angle, obtained in this study. AVG application at full-rate three-to-four weeks before harvest in ‘Gala’, ‘Red Delicious’, ‘Jonagold’, ‘Honeycrisp’, and ‘Red Chief’ apples has been reported to significantly reduce red skin coloration [3,20,22,41], consistent with our results, where the required minimum 50% skin blush for fruit to be marketable was only attained in ‘Honeycrisp’ fruit in the last assayed ripening stage (CH + 2). On the other hand, in this work, the deployment of the reflective groundcover Extenday significantly boosted ‘Honeycrisp’ red skin coloration to >75% skin blush in the first assayed ripening stage (CH), in agreement with other studies conducted in different regions and cultivars [4,11,12,14,15,17,18,55]. Furthermore, the combined treatment of Extenday + AVG significantly enhanced the red skin coloration of ‘Honeycrisp’ apples, which displayed >50% red blush in CH under mid-Atlantic environmental conditions, suggesting an interaction between both horticultural practices. This is of crucial importance as fruit from the upper sun-exposed third of the canopy are typically redder than fruit located in the lower third of the canopy [3]. However, the combined treatment of Extenday + AVG could ensure that fruit from the lower canopy actually pack out in premium grades, while not increasing fruit drop or fruit overripening, therefore increasing total crop value and profitability, and justifying the economic investment in the reflective groundcover Extenday.
Additionally, changes from green to yellow in the background color of apples, through a decrease in the values of hue angle as well as a result of chlorophyll disappearance (IAD), have been shown to be associated with increased fruit maturity [28,48]. These results support the significantly negative correlations between IAD and IEC obtained in this work, and are consistent with other studies in apples [28,42,56] and peaches [45,48]. The significantly highest and lowest hue angle and IAD values for AVG-only- and Extenday-only-treated ‘Honeycrisp’ apples, respectively, are indicative of the delay and advancement of background color changes, respectively. Nonetheless, the combined Extenday + AVG treatment in ‘Honeycrisp’ apples presented an intermediate background color change, similar to what was observed for fruit maturity and quality-related physicochemical parameters, supporting an advancement in fruit maturity that does not translate into fruit overripening.
Particularly in this study, the distribution of each Extenday and AVG treatment combination/evaluation period along component 1 of the PCA is supported by the AVG-only-treated ‘Honeycrisp’ fruit displaying the significantly lowest IEC, most delayed fruit maturity, and reduced fruit drop but a drastically inhibited red skin coloration in all evaluation periods; followed by the Extenday + AVG treatment in ‘Honeycrisp’ fruit exhibiting an intermediate positioning in terms of IEC, fruit maturity (not leading to overripe fruit), and fruit drop, while significantly enhancing red skin coloration above the required marketable minimum (>50% blush) in all evaluation periods; by the control ‘Honeycrisp’ fruit showing a significantly increased IEC, fruit maturity (leading to overripe fruit towards the later assessed evaluation periods), and fruit drop, with a considerably hindered red skin coloration in the first evaluation periods; and finally by the Extenday-only-treated ‘Honeycrisp’ fruit, presenting the significantly highest IEC, most advanced fruit maturity (leading to overripe fruit), increased preharvest fruit drop, yet a promoted red skin coloration in all evaluation periods (>75% blush). However, the results of this study may only be applicable for fruit grown under US mid-Atlantic conditions. Thus, this work needs to be replicated in major production regions of ‘Honeycrisp’ with different environmental conditions, such as the Pacific Northwest, to assess the transferability of these outcomes between regions. Furthermore, these results are specific for ‘Honeycrisp’ fruit and future work is required to include a wide range of cultivars to assess the robustness of these results.
5. Conclusions
‘Honeycrisp’ fruit located in the lower third of the canopy and submitted to different combinations of Extenday and AVG treatments under US mid-Atlantic environmental conditions over two consecutive years revealed that Extenday deployment can significantly promote apple red skin coloration (>75% blush) via an increased reflected PPFF and UV radiation (>10 times as compared to the control) as well as via an increased IEC, while also advancing fruit maturity, i.e., overripening. The application of AVG, conversely, negatively impacted apple red skin coloration (30–48% blush), minimized fruit drop in half as compared to the control, and effectively decreased EIC, thus delaying fruit maturity in terms of fruit firmness, starch degradation, SSC, and acidity. We demonstrated that the combined use of Extenday and AVG treatments had a synergistic effect and decreased preharvest fruit drop to the same levels as AVG-only while reducing overripening, without sacrificing red skin coloration development (which reached >58% blush). Moreover, the combined use of these treatments would decrease fruit drop while increasing the uniformity of fruit maturity and the proportion of fruit with >50% blush, consequently decreasing harvest labor input and boosting total crop value and profitability. Further research is warranted for assessing the consistency of these results across contrasting environments, different cultivars, as well as after postharvest storage.
Author Contributions
Conceptualization, M.F.; methodology, M.F. and M.S.M.; validation, M.F. and M.S.M.; formal analysis, M.F. and M.S.M.; investigation, M.F. and M.S.M.; resources, M.F.; writing—original draft preparation, M.F. and M.S.M.; writing—review and editing, M.F.; visualization, M.F. and M.S.M.; supervision, M.F.; project administration, M.F.; funding acquisition, M.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Specialty Crop Block Grant Program #211501, by the State Horticultural Association of Pennsylvania, and by start-up funds from the College of Agriculture and Natural Resources and the Department of Plant Science and Landscape Architecture (UMD) to Macarena Farcuh.
Data Availability Statement
Data sharing are in article.
Acknowledgments
We are thankful to James Schupp for technical assistance for light measurements and to Joy Cline and Bear Mountain Orchards for their assistance.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Musacchi, S.; Serra, S. Apple Fruit Quality: Overview on Pre-Harvest Factors. Sci. Hortic. 2018, 234, 409–430. [Google Scholar] [CrossRef]
- Funke, K.; Blanke, M. Spatial and Temporal Enhancement of Colour Development in Apples Subjected to Reflective Material in the Southern Hemisphere. Horticulturae 2021, 7, 2. [Google Scholar] [CrossRef]
- Layne, D.R.; Jiang, Z.; Rushing, J.W. The Influence of Reflective Film and ReTain on Red Skin Coloration and Maturity OfGala’Apples. Horttechnology 2002, 12, 640–645. [Google Scholar] [CrossRef]
- Kon, T.M.; Clavet, C.D. Enhancing Red Fruit Coloration of Apples in the Southeastern US with Reflective Fabrics. Horticulturae 2023, 9, 1125. [Google Scholar] [CrossRef]
- USDA Agricultural Marketing Service Apples Grades and Standards. Available online: https://www.ams.usda.gov/grades-standards/apple-grades-standards (accessed on 13 December 2023).
- Castañeda-Ovando, A.; de Lourdes Pacheco-Hernández, M.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical Studies of Anthocyanins: A Review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Ubi, B.E.; Honda, C.; Bessho, H.; Kondo, S.; Wada, M.; Kobayashi, S.; Moriguchi, T. Expression Analysis of Anthocyanin Biosynthetic Genes in Apple Skin: Effect of UV-B and Temperature. Plant Sci. 2006, 170, 571–578. [Google Scholar] [CrossRef]
- Honda, C.; Moriya, S. Anthocyanin Biosynthesis in Apple Fruit. Hortic. J. 2018, 87, 305–314. [Google Scholar] [CrossRef]
- Lancaster, J.E. Regulation of Skin Color in Apples. CRC Crit. Rev. Plant Sci. 1992, 10, 487–502. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, L.; Liu, W.; Zhang, J.; Wang, N.; Chen, X. Research Progress of Fruit Color Development in Apple (Malus domestica Borkh.). Plant Physiol. Biochem. 2021, 162, 267–279. [Google Scholar] [CrossRef]
- Iglesias, I.; Alegre, S. The Effects of Reflective Film on Fruit Color, Quality, Canopy Light Distribution, and Profitability of “Mondial Gala” Apples. Horttechnology 2009, 19, 488–498. [Google Scholar] [CrossRef]
- Toivonen, P.; Stoochnoff, J.; Usher, K.; Lu, C.; Wiersma, P.; Zhou, C. Biochemical and Gene Expression Involved in Red Blush Color Development in ‘Ambrosia’ Apple. J. Am. Soc. Hortic. Sci. 2019, 144, 164–171. [Google Scholar] [CrossRef]
- Wünsche, J.N.; Lakso, A.N.; Robinson, T.L.; Lenz, F.; Denning, S.S. The Bases of Productivity in Apple Production Systems: The Role of Light Interception by Different Shoot Types. J. Am. Soc. Hortic. Sci. 1996, 121, 886–893. [Google Scholar] [CrossRef]
- Privé, J.-P.; Russell, L.; LeBlanc, A. Impact of Reflective Groundcover on Growth, Flowering, Yield and Fruit Quality in Gala Apples in New Brunswick. Can. J. Plant Sci. 2011, 91, 765–772. [Google Scholar] [CrossRef]
- Mupambi, G.; Valverdi, N.A.; Camargo-Alvarez, H.; Reid, M.; Kalcsits, L.; Schmidt, T.; Castillo, F.; Toye, J. Reflective Groundcover Improves Fruit Skin Color in ‘Honeycrisp’Apples Grown under Protective Netting. Horttechnology 2021, 31, 607–614. [Google Scholar] [CrossRef]
- Toye, J. Reflective Mulches—New Zealand Leads the Way. Orchardist 1995, 68, 58–60. [Google Scholar]
- Robinson, T.L.; Gonzalez, L. Effect of Different Reflective Ground Covers on Light Reflection and on the Coloring of Apples at Harvest. In Proceedings of the XXXI International Horticultural Congress (IHC2022): International Symposium on Innovative Perennial Crops Management, Angers, France, 14–20 August 2022; Volume 1366, pp. 385–392. [Google Scholar]
- Privé, J.P.; Russell, L.; Leblanc, A. Use of Extenday Reflective Groundcover in Production of ‘Gala’Apples (Malus domestica) in New Brunswick, Canada: 1. Impact on Canopy Microclimate and Leaf Gas Exchange. N. Z. J. Crop Hortic. Sci. 2008, 36, 221–231. [Google Scholar] [CrossRef]
- Miller, S.S.; Greene, G.M. The Use of Reflective Film and Ethephon to Improve Red Skin Color of Apples in the Mid-Atlantic Region of the United States. Horttechnology 2003, 13, 90–99. [Google Scholar] [CrossRef]
- Liu, J.; Islam, M.T.; Sherif, S.M. Effects of Aminoethoxyvinylglycine (AVG) and 1-Methylcyclopropene (1-MCP) on the Pre-Harvest Drop Rate, Fruit Quality, and Stem-End Splitting in ‘Gala’Apples. Horticulturae 2022, 8, 1100. [Google Scholar] [CrossRef]
- Irish-Brown, A.; Schwallier, P.; Shane, B.; Tritten, B. Why Does Apple Fruit Drop Prematurely? In Michigan State University Extension; Michigan State University: East Lansing, MI, USA, 2011. [Google Scholar]
- Arseneault, M.H.; Cline, J.A. AVG, NAA, Boron, and Magnesium Influence Preharvest Fruit Drop and Fruit Quality of ‘Honeycrisp’ Apples. Can. J. Plant Sci. 2018, 98, 741–752. [Google Scholar] [CrossRef]
- Baugher, T.A.; Schupp, J.R. Relationship Between’Honeycrisp’Crop Load and Sensory Panel Evaluations of the Fruit. J. Am. Pomol. Soc. 2010, 64, 226. [Google Scholar]
- Burg, S.P.; Burg, E.A. Ethylene Action and the Ripening of Fruits: Ethylene Influences the Growth and Development of Plants and Is the Hormone Which Initiates Fruit Ripening. Science 1965, 148, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Klee, H.J. Ethylene Signal Transduction. Moving beyond Arabidopsis. Plant Physiol. 2004, 135, 660–667. [Google Scholar] [CrossRef]
- Farcuh, M.; Tajima, H.; Lerno, L.A.; Blumwald, E. Changes in Ethylene and Sugar Metabolism Regulate Flavonoid Composition in Climacteric and Non-Climacteric Plums during Postharvest Storage. Food Chem. Mol. Sci. 2022, 4, 100075. [Google Scholar] [CrossRef] [PubMed]
- Osorio, S.; Fernie, A.R. Biochemistry of Fruit Ripening. Mol. Biol. Biochem. Fruit Ripening 2013, 1–19. [Google Scholar] [CrossRef]
- Miah, M.S.; Hinson, C.; Farcuh, M. Assessing Fruit Maturity and Quality of ‘Buckeye Gala’ Grown on a Diverse Panel of Apple (Malus domestica Borkh.) Rootstocks in Western Maryland. Agronomy 2023, 13, 2528. [Google Scholar] [CrossRef]
- Farcuh, M.; Copes, B.; Le-Navenec, G.; Marroquin, J.; Cantu, D.; Bradford, K.J.; Guinard, J.-X.; Van Deynze, A. Sensory, Physicochemical and Volatile Compound Analysis of Short and Long Shelf-Life Melon (Cucumis Melo L.) Genotypes at Harvest and after Postharvest Storage. Food Chem. X 2020, 8, 100107. [Google Scholar] [CrossRef]
- Bonghi, C.; Tonutti, P.; Ramina, A. Biochemical and Molecular Aspects of Fruitlet Abscission. Plant Growth Regul. 2000, 31, 35–42. [Google Scholar] [CrossRef]
- Roberts, J.A.; Elliott, K.A.; Gonzalez-Carranza, Z.H. Abscission, Dehiscence, and Other Cell Separation Processes. Annu. Rev. Plant Biol. 2002, 53, 131–158. [Google Scholar] [CrossRef]
- Farcuh, M.; Rivero, R.M.; Sadka, A.; Blumwald, E. Ethylene Regulation of Sugar Metabolism in Climacteric and Non-Climacteric Plums. Postharvest Biol. Technol. 2018, 139, 20–30. [Google Scholar] [CrossRef]
- Costa, F.; Stella, S.; Van de Weg, W.E.; Guerra, W.; Cecchinel, M.; Dallavia, J.; Koller, B.; Sansavini, S. Role of the Genes Md-ACO1 and Md-ACS1 in Ethylene Production and Shelf Life of Apple (Malus domestica Borkh). Euphytica 2005, 141, 181–190. [Google Scholar] [CrossRef]
- Chu, C.L. Internal Ethylene Concentration of ‘McIntosh’, ‘Northern Spy’, ‘Empire’, ‘Mutsu’, and ‘Idared’Apples during the Harvest Season. J. Am. Soc. Hortic. Sci. 1988, 113, 226–229. [Google Scholar] [CrossRef]
- Gussman, C.D.; Goffreda, J.C.; Gianfagna, T.J. Ethylene Production and Fruit-Softening Rates in Several Apple Fruit Ripening Variants. HortScience 1993, 28, 135–137. [Google Scholar] [CrossRef]
- Arseneault, M.H.; Cline, J.A. A Review of Apple Preharvest Fruit Drop and Practices for Horticultural Management. Sci. Hortic. 2016, 211, 40–52. [Google Scholar] [CrossRef]
- Schupp, J.R.; Greene, D.W. Effect of Aminoethoxyvinylglycine (AVG) on Preharvest Drop, Fruit Quality, and Maturation OfMcIntosh’Apples. I. Concentration and Timing of Dilute Applications of AVG. HortScience 2004, 39, 1030–1035. [Google Scholar] [CrossRef]
- Greene, D.W. Time of Aminoethoxyvinylglycine Application Influences Preharvest Drop and Fruit Quality of’McIntosh’apples. HortScience 2005, 40, 2056–2060. [Google Scholar] [CrossRef]
- Byers, R.E. Effects of Aminoethoxyvinylglycine (AVG) on Preharvest Fruit Drop, Maturity, and Cracking of Several Apple Cultivars. J. Tree Fruit Prod. 1997, 2, 77–97. [Google Scholar] [CrossRef]
- Yuan, R.; Li, J. Effect of Sprayable 1-MCP, AVG, and NAA on Ethylene Biosynthesis, Preharvest Fruit Drop, Fruit Maturity, and Quality of ‘Delicious’ Apples. HortScience 2008, 43, 1454–1460. [Google Scholar] [CrossRef]
- Boyacı, S. Effect of Aminoethoxyvinylglycine (AVG) Applications on Pre-Harvest Drop and Fruit Quality of ‘Red Delicious, Red Chief’ Apple Cultivar. Erwerbs-Obstbau 2022, 64, 395–400. [Google Scholar] [CrossRef]
- Wang, Z.; Dilley, D.R. Aminoethoxyvinylglycine, Combined with Ethephon, Can Enhance Red Color Development without over-Ripening Apples. HortScience 2001, 36, 328–331. [Google Scholar] [CrossRef]
- Blankenship, S.M.; Unrath, C.R. PAL and Ethylene Content during Maturation of Red and Golden Delicious Apples. Phytochemistry 1988, 27, 1001–1003. [Google Scholar] [CrossRef]
- Whale, S.K.; Singh, Z. Endogenous Ethylene and Color Development in the Skin of ‘Pink Lady’ Apple. J. Am. Soc. Hortic. Sci. 2007, 132, 20–28. [Google Scholar] [CrossRef]
- Farcuh, M.; Hopfer, H. Aroma Volatiles as Predictors of Chilling Injury Development during Peach (Prunus persica (L) Batsch) Cold Storage and Subsequent Shelf-Life. Postharvest Biol. Technol. 2023, 195, 112137. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Farcuh, M.; Cohen, Y.; Crisosto, C.; Sadka, A.; Blumwald, E. Non-Climacteric Ripening and Sorbitol Homeostasis in Plum Fruits. Plant Sci. 2015, 231, 30–39. [Google Scholar] [CrossRef]
- Infante, R.; Farcuh, M.; Meneses, C. Monitoring the Sensorial Quality and Aroma through an Electronic Nose in Peaches during Cold Storage. J. Sci. Food Agric. 2008, 88, 2073–2078. [Google Scholar] [CrossRef]
- Ziosi, V.; Noferini, M.; Fiori, G.; Tadiello, A.; Trainotti, L.; Casadoro, G.; Costa, G. A New Index Based on Vis Spectroscopy to Characterize the Progression of Ripening in Peach Fruit. Postharvest Biol. Technol. 2008, 49, 319–329. [Google Scholar] [CrossRef]
- Blanpied, G.D.; Silsby, J. Predicting Harvest Date Windows for Apples. In A Cornell Cooperative Extension; Cornell University: Ithaca, NY, USA, 1992; pp. 7–9. [Google Scholar]
- Dong, Y.H.; Mitra, D.; Kootstra, A.; Lister, C.; Lancaster, J. Postharvest Stimulation of Skin Color in Royal Gala Apple. J. Am. Soc. Hortic. Sci. 1995, 120, 95–100. [Google Scholar] [CrossRef]
- Charles, M.T.; Arul, J. UV Treatment of Fresh Fruits and Vegetables for Improved Quality: A Status Report. Stewart Postharvest Rev. 2007, 3, 1–8. [Google Scholar] [CrossRef]
- Vimolmangkang, S.; Zheng, D.; Han, Y.; Khan, M.A.; Soria-Guerra, R.E.; Korban, S.S. Transcriptome Analysis of the Exocarp of Apple Fruit Identifies Light-Induced Genes Involved in Red Color Pigmentation. Gene 2014, 534, 78–87. [Google Scholar] [CrossRef]
- Greene, D.W. Preharvest Drop Control and Maturity of ‘Delicious’ Apples as Affected by Aminoethoxyvinylglycine (AVG). J. Tree Fruit Prod. 2002, 3, 1–10. [Google Scholar] [CrossRef]
- Layne, D.R.; Jiang, Z.; Rushing, J.W. Tree Fruit Reflective Film Improves Red Skin Coloration and Advances Maturity in Peach. Horttechnology 2001, 11, 234–242. [Google Scholar] [CrossRef]
- Overbeck, V.; Schmitz-Eiberger, M.A.; Blanke, M.M. Reflective Mulch Enhances Ripening and Health Compounds in Apple Fruit. J. Sci. Food Agric. 2013, 93, 2575–2579. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, S.; Fallahi, E.; Fazio, G. The Influence of Rootstock on Fruit Ethylene, Respiration, Index of Absorbance Difference, Fruit Quality, and Production of ‘Aztec Fuji’Apple under a Full-Crop Condition. HortScience 2022, 57, 1–9. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).