Abstract
Orchids constitute the largest and most diverse group of flowering plants and are classified in the family Orchidaceae. Exhibiting significance as the most exotic and ubiquitous flowering plant, the cultivation of orchids on a commercial level is gaining momentum worldwide. In addition to its ornamental and aesthetic value, the orchid industry has successfully generated employment for people in developing countries. Recent advances in biotechnological interventions in orchids have substantially contributed to the development of exotic varieties with novel traits, not to forget the inputs of traditional plant breeding methods and tissue culture approaches. In addition, the scientific developments in orchid biology have remarkably bridged the knowledge gaps in areas of orchid classification, phytochemistry, and cultivation strategies. This has facilitated the commercialization of novel varieties, opening new avenues in the orchid industry, and their global marketing as cut flowers and artificially propagated plants. Orchids constitute the first floriculture crops that revolutionized the orchid industry; however, they also hold several challenges in the natural propagation and conservation of several species that are on the verge of extinction. International organizations like CITES have come forward to address challenges associated with illegal global trade and indiscriminate use of orchid varieties, aiming for conservation and legal commercial goals. This thematic review is one-of-a-kind in providing comprehensive insights into the emerging momentum of orchid biology and how its globalization projects to considerably impact the orchid industry in the coming times. However, it is imperative to understand the challenges in the cultivation and conservation of orchid varieties and ensure legislative guidelines both on domestic and global levels to ensure a multipronged approach to the conservation and commercialization of orchids.
1. Orchid Biology: Recent Aspects and Emerging Perspectives
The emerging benefits and recognition of orchids for mankind have led to substantial research in the present decade. Considered one of the most fascinating and diversified plant families globally, Orchidaceae comprises more than 8000 genera and 35,000 natural and artificial hybrid species distributed globally [1,2]. Within the family Orchidaceae, 70% of species are epiphytic and constitute two-thirds of global epiphytes [3]. Of the remaining orchid species, 25% are terrestrial and 5% require support for growth [4]. With specialized structures and properties, orchids continue to fascinate researchers as they have since time immemorial. The peculiar features demonstrated by orchids comprise specialized pollination, thin non-endospermic seeds, diversified habitats, mycorrhizal-dependent germination, and adaptive mechanisms, among others [5].
Presently, advances in orchid biology and biotechnologies have substantially contributed to the development of new cultivars and hybrids with multiple ornamental values, contributing to the rapidly growing market and demand for exotic varieties. From 2007 to 2012, the cost of fresh orchid cuttings and buds traded was estimated to be 483 million US dollars [6]. According to statistics, orchids are imported and exported by many countries, amounting to 504 million US dollars in 2012 [6]. In addition, orchids are also harvested and traded for medicinal value and food components. On a commercial level, Phalaenopsis is the most important orchid genus, with a market share of 79% among all global orchids. The European market is defined as highly competitive, with multiple cultivation prospects and a commercial value of 182 million potted plants of more than EUR 620 million [7]. The success in the cultivation and marketing of Phalaenopsis has been achieved via micropropagation and breeding approaches in many countries, including the Netherlands, Taiwan, Thailand, etc. [8]. In addition, Oncidium, Vanda, Dendrobium, and Cymbidium are other popular orchid genera grown and marketed worldwide as cut flowers [8]. Vanilla planifolia (vanilla orchid) is an interesting orchid species that produces edible fruit and vanillin, a sought-after commodity across the globe.
The present era has witnessed unprecedented advancements in promoting orchid cultivation, attributed to the dynamic progress in biotechnological interventions. Figure 1 illustrates advanced biotechnologies in orchids facilitating multi-faceted trait improvement. Table 1 provides a comprehensive account of research studies on orchids for generating hybrids with high-value traits. Their multi-faceted importance in floriculture, the food sector, and medicine has substantially contributed to orchid commercialization, with a multi-million-dollar global market [5,6]. The growing demand for exotic varieties and the conservation of threatened species necessitates new scientific technologies such as novel flower varieties, biotic/abiotic stress tolerance, and efficient propagation [5,8]. New frontiers in plant science have focused on advanced studies in orchids including genetic manipulations, proteome studies, and functional genomics, among others [1,2,5] for desired outcomes. Moreover, high-throughput technologies have facilitated studies on orchid phytochemicals, achieved by high-performance liquid chromatography (HPLC), nuclear magnetic resonance (NMR), mass spectrometry (MS), and others. Recent advances in the development of HPLC-DAD and MS have facilitated the precise detection of bioactive components in biological samples and novel compounds with pharmacological attributes.
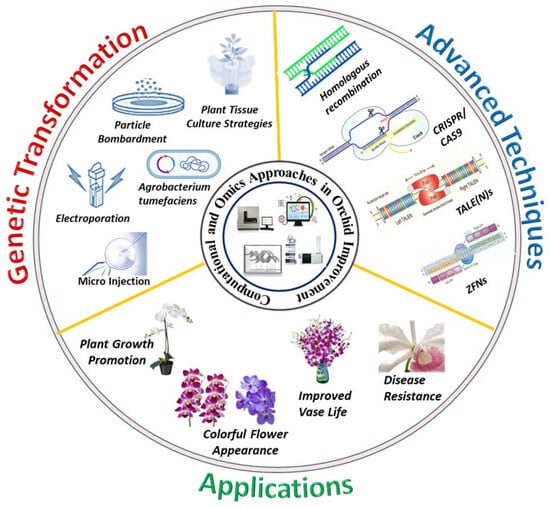
Figure 1.
Advanced biotechnologies in orchids facilitating multi-faceted trait improvement.

Table 1.
A comprehensive account of research studies on orchids for generating hybrids with high-value traits.
However, overexploitation and the complex life cycles of orchids account for key challenges: unsustainable/illegal collection, climatic fluctuations, and threatened habitats. According to IUCN Global Red List statistics for 948 orchid species, 56.5% were classified as threatened in 2017 (The International Union for Conservation of Nature, 2017). Furthermore, attributing to their complicated lifestyles, orchids present a major challenge for restoration/conservation and highlight an immediate requirement for integrated conservation measures on broader levels (Table 1).
In the Cypripedioideae (the slipper orchids) subfamily, 90% of species were reported threatened due to over-harvesting and a decline in habitats by the Global Red List [33]. Vogt-Schilb et al. [34] studied orchid species and distribution on Mediterranean islands and reported a turnover in the composition of the species due to alterations in land usage and variation in species distribution due to changes in worldwide levels [35]. The unsustainable harvesting of orchid species represents a crucial factor. The indiscriminate use and collection of notable orchid genera, namely Renanthera, Paphiopedilum, Cattleya, and Phragmipedium, have resulted in their classification as being severely threatened. To curb the rising illegal trade of commercial varieties, more than 70% of orchid species were categorized in the appendices of the Convention on International Trade in Endangered Species (CITES) [36], a fruitful global initiative toward restoration. However, considering that many other orchid species are smuggled/traded across international borders for food, medicines, and horticulture, attempts are being made to understand/estimate the unauthorized export of orchids (under CITES) [37,38]. The steps toward orchid conservation and the development of new varieties should address the conservation of habitats, proper monitoring, and the implementation of guidelines for protection, and ensure legal marketing/global trade of multi-attribute varieties. The thematic review discusses the growing significance of orchids on a global platform with an emphasis on orchid biotechnologies to develop exotic varieties via conventional and modern strategies. In addition, the contributions of international and domestic organizations in monitoring illegal orchid trade and ensuring guidelines for conservation are extensively discussed. The preservation of orchid biodiversity via scientific approaches, contributions of computational resources in orchid biology, guidelines for marketing/trade at the domestic/global levels, and translational success stories define the underlying themes of the article.
2. Research Methodology: Literature Retrieval, Compilation, and Analysis
The conceptualization, implementation, and review writing spanned a time frame of 3–4 months. For the comprehensive study, an exhaustive literature survey and analysis were performed. Several literature databases, including PubMed (https://pubmed.ncbi.nlm.nih.gov (accessed on 28 January 2024) and Google Scholar (https://scholar.google.com (accessed on 28 January 2024), were used to retrieve research and review papers discussing various aspects of basic orchid biology and emerging biotechnologies for orchid cultivation, conservation, and genetic engineering studies. Efforts were also made to understand and discuss the impact of globalization and legislative guidelines at global/domestic levels for curbing illegal orchid trade and ensuring a legal framework for orchid cultivation and trade.
3. Conventional Versus Modern Approaches for Orchid Cultivation and Conservation
Orchid cultivation is gaining momentum attributed to the development of new orchid varieties with unique features of colors and appearance. To bridge this demand and supply gap, both traditional and molecular breeding approaches are employed with consistent efforts. The traditional breeding approaches of orchids, although time-consuming, have remained the mainstream approach for orchid breeding until now. The traditional breeding of orchid varieties includes their propagation through seeds, division of large clumps, offshoots or keikis, cutting, and air layering [39,40]. However, the increased demands of unique traits like flower/foliage color, morphology, and enhanced shelf life cannot be attained by traditional cultivation approaches including crossbreeding-mediated hybridization and mutation [1]. In recent years, these limitations have been actively addressed with the help of modern breeding approaches via transgenic molecular breeding approaches.
3.1. Classical Breeding Strategies
3.1.1. Crossbreeding
Orchid genera Phalaenopsis and Oncidium are widely grown orchids for commercial production. Orchids are both self-pollinated and cross-pollinated species. However, the self-pollinating species sometimes produce a smaller number of seeds than cross-pollinated flowers [1,41]. Crossbreeding or hybridization using both natural and artificial approaches splendidly integrates the excellent traits of two parents in their hybrid offspring. One of the oldest natural orchid hybrids, Phalaenopsis intermedia, which is a result of a cross between P. aphrodite and P. rosea, was described in 1853. Meanwhile, the first commercial artificial hybrid orchid Calanthe dominyi was developed as a cross between C. masuca and C. furcate [42,43]. The production of these commercial natural and hybrid orchids was achieved using crossbreeding approaches. However, several factors including hybrid combination fertility, targeted traits quality assessment, and superior hybrid offspring selection play an important role and must be considered [44]. The generation F1 derived from the cross between parents with targeted contrasting traits usually has large phenotypic differences from their parents. For example, the F1 generation derived from the parent having a large flower and short flowering time compared to the parent having a small flower and long flowering time, viz. Ionmesa Popcorn ‘Haruri’, produces flowers with distinct notable differences from its parents. However, hybrids sometimes bear problems associated with germination; for example, the occurrence of intraspecific < intrageneric < intergeneric degree of genetic relationship in hybrid Cymbidium seeds results in difficult seed germination and culturing [45]. Similar problems associated with the hybridization process, parent incompatibility, and post-fertilization embryo abortion failing the distinct hybridization have been reported [46]. Commercial orchid cultivation suffers from several associated breeding barriers such as the large and complex polyploid genome, slow growth, and long life cycle; consequently, it takes a long period to generate new cultivars using cultivation through the traditional breeding system. The low transformation efficiency makes the development of new varieties with desired traits a challenging task [47,48]. For example, the high commercial value orchid Phalaenopsis takes more than 2 years to switch from the vegetative to the reproductive phase [49]. Therefore, the applicability of these conditions remains to be studied in major commercial orchid species. Seed germination is one of the key aspects of the traditional breeding system, as it is directly associated with the efficient success of crossbreeding; therefore, in-depth studies are required for a deeper understanding of the seed germination mechanisms and plant developmental characteristics of an effective breeding system. Hence, a suitable cultivation approach is required for hybrid seeds developed from crossbreeding and stable growth of the hybrid population.
3.1.2. Selection Breeding
Selection breeding differentiates from crossbreeding where hybrid selection is based on natural variations in traits [50]. Selection breeding specifically concerns three important genetic parameters: genetic correlations between traits, trait heritability, and interactions between genotypes and the environment [1]. Like classical orchid breeding strategies of crossbreeding and selection breeding, biotechnological interventions have resulted in the origin of mutational breeding and molecular marker-assisted breeding approaches for orchids.
3.1.3. Mutation Breeding
The mutational breeding approach utilizes physical and chemical mutagens to improve individual traits and will shorten the breeding cycle in orchids. Mutational breeding has produced several orchids with improved traits, viz. aroma, higher medicinal content, enhanced shelf life, and stress resistance [43]. Polyploidization using colchicine and other mutagen treatments is one of the key approaches used for mutation in orchids such as Cymbidium, Dendrobium, Oncidium, and Phalaenopsis [51,52,53,54,55,56]. The higher levels of genomic heterozygosity can allow the enhanced mutation rate in a short time duration; however, the random and unpredictable nature of mutagenesis can take place throughout the genome which can lead to other physio-morphological problems in orchid mutants. Therefore, a lot of studies are still required to understand the basis of mutation breeding in orchids for the identification of suitable genotypes, explants, mutagen types, and their optimized dose concentration to produce mutant orchids with desired traits.
3.1.4. Molecular Marker-Assisted Breeding (MMAB)
The molecular marker-assisted breeding approach utilizes the accuracy of molecular biology tools and techniques for fast, accurate, and environmental-influence-free orchid breeding and natural and artificial hybrid selection [57]. The MMAB uses the most prevalent, versatile, and high-potential markers such as restriction fragment length polymerase (RFLP), amplified fragment length polymerase (AFLP), insertional simple sequence repeats (ISSR), and single nucleotide polymorphism (SNP) markers [1]. Most of these markers are widely used in modern orchid breeding and have achieved good results. Li et al. [58] developed a set of wide-range genic SSR markers in Cymbidium ensifolium to evaluate the genetic relationship and trait mapping in the orchid population. These SSR markers facilitate the identification of genetic relationships and have been successively used for the identification of root growth mechanisms and secondary metabolites-related gene identification, along with flower shape and color-related genes in Phalaenopsis [59,60]. Similarly, SNP markers were also utilized to construct integrated genetic maps of the Dendrobium genome along with the identification of several important QTL sites [61]. Although MMAB has played an important role as a modern orchid breeding approach, it mainly targets phylogenetics for determining genetic relationships between orchid species. Therefore, co-integrated approaches of traditional, and modern orchid breeding strategies and biotechnologies are required for improved orchid breeding.
3.2. In Vitro Orchid Propagation in Plant Tissue Culture
In vitro micropropagation provides a convenient and feasible approach for orchids, especially for orchid seeds that are difficult to germinate and grow in a natural environment [40]. Also, it provides a platform for biotechnologies for orchid improvements and genetic engineering. In vitro micropropagation represents a promising tool for the conservation of several threatened and endangered orchids and has been successfully applied in several species such as Paphiopedilum armeniacum, Bulbophyllum nipondhii, Paphiopedilum insigne, and Anoectochilus elatus [62,63,64,65].
In the last decades, plant tissue cultures have been instrumental in the rapid propagation and ex situ conservation of orchids, using different methods and explants including flower stalks, shoot tip nodes, stem bids, root tips, and rhizome segments [66]. The composition of culture media plays a major role in in vitro seed germination and micropropagation using different explants [67,68]. For example, Murashige and Skoog (MS) medium enhances germination in the orchid Geodorum densiflorum, whereas Knudson C medium [69] is more suitable for Paphiopedilum seeds compared to other orchid-specific culture media [70]. It was also observed that a low concentration of mineral salts in MS medium (viz. ½ MS or ¼ MS) promotes seed germination in some terrestrial orchids [71,72]. The constituents of culture media, especially plant growth regulators, auxins, and cytokinins, have a significant impact on the growth, germination, and development of orchid seeds and explants in vitro [73,74]. Along with the plant growth promoters, the addition of organic nutrient sources like coconut water and potato extract in culture media was also found to promote orchid in vitro seed germination [63,75].
The different orchid species show great variations in physiological and morphological features, requiring different conditions for growth in plant tissue culture. Different species respond differently to growth factors (depending on the genotype), and some are recalcitrant in in vitro conditions [76]. In terrestrial orchids, such as Paphiopedilum and Cypripedium, meristem explant survival and PLB induction are difficult. In addition, a method developed for a particular species may not work for another species. Therefore, for targeted genetic transformation, a plant regeneration protocol must be developed for a particular plant genotype [76], and the identification of variations in genotype must occur before plant transformation is necessary. The subsequent discovery of asymbiotic seed germination (for in vitro plant propagation) has been widely employed to develop orchid plants in vitro and direct somatic embryogenesis [77], totipotent callus induction, shoot-bud formation from different explants, PLB formation from cell suspension culture, and others, which have immensely contributed to the micropropagation and creation of transgenic hybrids [5].
3.3. Cryopreservation Techniques
Low temperature and dry storage-based preservation are key approaches to orchid germplasm conservation [78,79]. However, this low temperature and dry storage-based method is successful for 1 to 6 months of germplasm preservation but fails to provide high viability of germplasm under preservation for longer durations [80,81]. Cryopreservation is the best germplasm preservation approach as all the metabolic and physiological processes cease at the temperature −196 °C [82]. The pretreatment of orchid seeds and pollens germplasm through vitrification, desiccation, and encapsulation–dehydration methods of removing the cell water content before cryopreserving in liquid nitrogen are employed [83,84].
3.3.1. Vitrification
Sakai [85] introduced the technique of vitrification typically used for the longer preservation of immature and mature orchid seeds with higher than average water content. The vitrification method uses a high osmolarity vitrification solution containing glycerol, dimethyl sulfoxide, and ethyl glycol as cryoprotectants. The seeds for preservation are kept in this high osmolarity vitrification solution that reduces the intracellular water content of seeds and vitrified by penetration of these cryoprotectants through osmoregulation, thus reducing the freezing temperature and preventing cells from ice nucleation injuries [84,86]. Vitrification has helped in the conservation through cryopreservation of immature and high-water-content seeds of several orchid genera, viz. Bletilla, Cymbidium, Dendrobium, Encyclia, Phaius, and Vanda [82].
3.3.2. Desiccation
Desiccation-based cryopreservation is found to be more suitable for mature orchid seeds. The process of desiccation includes the slow drying of seeds under a controlled desiccation rate under constant relative humidity or drying with silica gel or with CaCl2.6H2O salt solution to reduce the water content of the seeds before preserving them in liquid nitrogen [82]. Several orchids, viz. Bletilla formosana, Caladenia flava, Dactylorhiza fuchsii, Diuris fragrantissima, Eulophia gonychlia, Gymnadenia conopsea, Orchis coriophora, Paphiopedilum rothschildianum, Pterostylis sanguinea, Thelymitra macrophylla, and Dendrobium candidum, were successively cryopreserved using the desiccation technique [82,87].
3.3.3. Encapsulation–Dehydration
Encapsulation–dehydration, a technique developed for artificial seed production, is the third method for cryopreservation [88]. The encapsulation–dehydration approach uses the in vitro cultured orchid plant tissues, seeds, and embryos, partially desiccated with silica beads or airflow of the laminar bench to reduce the cellular water content. These partially dried tissues are trapped and encapsulated in sodium alginate beads before keeping them in liquid nitrogen for cryopreservation [87]. The encapsulation–dehydration techniques are generally applied to a few orchids, viz. Cyrtopodium hatschbachii and Oncidium bifolium [89,90].
3.4. Genetic Engineering and Generation of Hybrids with ‘High-Value’ Traits
Genetic engineering in orchids confers ‘high-value’ traits to the hybrids with desired characteristics and has been attempted in the genera viz. Phalaenopsis, Cattleya, Cymbidium, Dendrobium, Oncidium, Paphiopedilum, and Vanda, with documented translational success. PLBs derived from shoot tip cultures are used as target material for transformation in Phalaenopsis sp., while protocorm has also been frequently used [91]. A relatively large number of transgenic plants can be generated by protocorm transformation due to the presence of thousands of seeds in one fruit of Phalaenopsis sp. However, to achieve a successful outcome, it is indispensable to improve the transformation efficiency for efficient orchid breeding. Furthermore, the genetic engineering procedures in orchids are applied through either particle bombardment or Agrobacterium-mediated transformation to achieve the delivery of the desired gene. The initial genetic transformation studies were limited to the biolistic-mediated transformation [92,93]. The very first successful Agrobacterium-mediated genetic transformation was achieved in the orchid genera Phalaenopsis expressing the GUS gene construct [94]. The Agrobacterium-mediated genetic transformation was found to be more efficient than the biolistic-mediated genetic transformation in terms of incorporation of low copy number genes at transcriptionally active chromosomal regions [95].
The last decade has documented genetically engineered orchids through Agrobacterium-mediated genetic transformation systems in Phalaenopsis, Dendrobium, Cymbidium, Oncidium, and Vanda [18,96,97,98,99]. The genetic engineering approaches in orchids are not only restricted to the overexpression of heterologous genes for the desired traits but also gene silencing to knock out genes in Dendrobium ‘Sonia’ and Oncidium hybrids [100]. Now, with the availability of the complete genomes of Dendrobium officinale and P. equestris, the genetic engineering approaches facilitate CRISPR/Cas9-mediated genome editing in different orchid species [101]. Liu et al. [100] delivered a phytoene synthase-RNAi construct into PLBs of Oncidium hybrid. The downregulation of PSY and geranyl synthase genes was observed in the transgenic orchid, and endogenous levels of abscisic acid and gibberellic acid were decreased in dwarf plants [100]. In another study, Agrobacterium-mediated insertion of KNAT1 (a key gene for bud apical meristem differentiation) and AtRKD4 (a key gene for embryonic differentiation) into the orchid genome induced organogenesis and bud development, thereby improving the yield of native and transgenic orchids.
4. Biotechnological Interventions in Orchids: Existing and Upcoming Trends
Biotechnological approaches play a key role in the development of ornamentals with improved floral and ‘high-value’ attributes and are often employed to alter flower color, fragrance, appearance, disease resistance, and shelf life, among others [79,102].
4.1. Promoting Growth Vigor and Flowering
Desired traits can be selected in orchids with the help of genetic engineering along with controlling the flowering time, fragrance, flower color, and vase life [103]. Table 2 discusses flowering mechanisms in diverse orchids as exemplified by key representative genes and their multiple functions. Figure 2 illustrates the biological roles of MADS-box genes in controlling flowering in the Arabidopsis and Orchid plants.

Table 2.
Flowering mechanisms in diverse orchids as exemplified by key representative genes and their multiple functions.
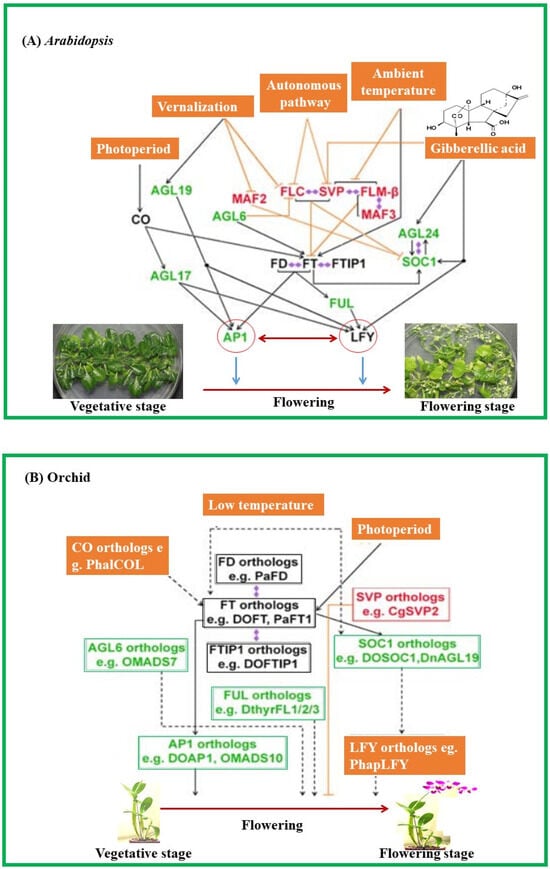
Figure 2.
Biological roles of MADS-box genes in controlling flowering in the Arabidopsis and Orchid plants. (A) In Arabidopsis, the MADS-box genes including SOC1, FLC, SVP, and AGL24 integrate signals for flowering from environmental and endogenous cues. (B) In orchids, orthologous genes of SOC1, AGL6, SVP, and AP1 have been isolated and functionally characterized either in heterologous systems (e.g., Arabidopsis) or orchids and shown to be involved in promoting flowering. MADS-box transcription factors that function as flowering activators and suppressors are shown in green and red, respectively, whereas the remaining flowering regulators are shown in black boxes. Promoting and repressive flowering is indicated by black arrows and orange T bars, respectively. The dashed lines with arrows indicate possible positive regulation based on the studies using a heterologous system. Double-ended diamond arrows indicate protein–protein interactions. AGL6; AGL17; AGL19; AGL24; AP1; CO; FLC; FLM; FT; FTIP1; FUL; LFY, MAF2; SOC1; SVP. Abbreviations: AGL—AGAMOUS-LIKE; AP—APETALA; CO—CONSTANS; FLC—FLOWERING LOCUS; FLM—FLOWERING LOCUS; FTIP—FT-INTERACTING PROTEIN; FUL—FRUITFULL; LFY—LEAFY; MAF—MADS AFFECTING FLOWERING; SOC—SUPPRESSOR OF OVEREXPRESSION OF CONSTANS; SVP— SHORT VEGETATIVE PHASE.
The very first genetic engineering attempts in Oncidium and Odontoglossum orchids were carried out to enhance growth and vase life through the mutant ethylene receptor gene [120]. Oncidium and Odontoglossum are commercially less viable orchids than Phalaenopsis due to their reduced vase life. Raffeiner et al. [120] engineered an ethylene receptor mutant gene etr1-1 from Arabidopsis under the control of a flower-specific promoter to reduce the sensitivity of transgenic orchids toward the exogenous ethylene, thus providing a prolonged vase life. In similar attempts, a virus-induced gene silencing approach was applied in P. equestris by suppressing the P. equestris UDP glucose–flavonoid 3-O-glucosyltransferase (PeUFT3), which significantly faded the flower color by decreasing the anthocyanin content and altered the flower pigmentation due to reduced anthocyanin biosynthesis [121]. Similarly, genetic engineering in orchids was successfully used to generate the adaptive response against cold stress in P. amabilis by introducing the cold-inducible lipid transfer protein (LTP) gene from rice. To alter growth and morphology, genes like the class 1 Knox DOH1 gene and Dendrobium Sonia cytokinin oxidase DSCKX1 gene were introduced and overexpressed in Dendrobium under in vitro conditions, leading to abnormal multiple shoot developments and reduced cytokinin content [122,123,124]. These plants demonstrated slow shoot growth with an improved rooting system. These studies proved the utility of the DSCKX1 gene as a growth rate-manipulating gene in the development of orchids.
4.2. Diagnosis of Pathogens
Most orchid species are faced with challenges of extinction due to global environmental changes and overexploitation; however, microbial pathogens equally threaten orchid cultivation. The most common pathogen–fungal species are namely leaf spots from Nigrospora oryzae, leaf spots from Cladosporium cladosporioides, wilt from Fusarium oxysporum, blight with root rot from Phytophthora capsica, anthracnose from Colletotrichum gloeosporioides, the black spot from Alternaria alternata, leaf spots from Phyllosticta capitalensis, and leaf spots from Phoma multirostrata. Also, sometimes, endophytes can work as conditional pathogens for orchids [79,125,126]. Biotechnological advancements have provided the solution through modern disease diagnosis techniques, allowing detection within the laboratory and in the field.
Droplet polymerase chain reaction (dPCR) is an innovative PCR-based disease diagnosis technique that utilizes the Taq DNA polymerase to unwind targeted DNA sequences from a complex test through a pre-validated primer/probe test [127]. Along with the PCR-based nucleic acid amplification detection technique, an advanced spectroscopy method is also applied for disease diagnosis in orchids. Surface-Enhance Raman Spectroscopy (SERS) is a Raman scrambling-based non-destructive and developing laser-based spectroscopy method that utilizes resistance tests and atomic tests for pathogen detection in plants [128]. DNA hybridization and colorimetric biosensor-based approaches are being extensively researched to establish a rapid, real-time disease diagnosis tool [129]. Similarly, an advanced chip-based integrated microfluidic system has been developed for automated rapid virus detection, through nucleic acid amplification. This approach was utilized to identify the most prevalent orchid virus, Cymbidium mosaic virus (CMV), with the purification of pathogen-specific RNA from the diseased sample. The isolated RNA is amplified to cDNA, and its optical detection is achieved using reverse transcription loop-mediated isothermal amplification (RT-LAMP) for the detection of the pathogen in the sample [130].
4.3. CRISPR/Cas and Advanced Genome Editing in Orchids
Recent advances in the development of genetic manipulation techniques have completely revolutionized orchid biotechnologies and opened new avenues in ‘hybrid’ generation with desired characteristics. Gene editing has been performed in ornamental genera, Dendrobium and Phalaenopsis, with low efficiency; however, CRISPR/Cas9 genome editing research in orchids aims to facilitate the development of key orchid phenotypes. Tong and coworkers [131] attempted a high-efficiency CRISPR/Cas-based editing of Phalaenopsis, wherein two CRISPR/Cas methods were employed to introduce three MAD sites (MADS44, MADS36, and MADS8) together in pYLMADS8_36_44 [132] and vector and single in separate vectors (P1300_MADS8, P1300_MADS36, and P1300_MADS44 [133] for the production of multiple mutants in P. equestris MADS-box genes [131]. The genetic manipulation of orchids has witnessed some success with the establishment of genetic transformation systems and the creation of transgenic mutants with the desired traits; however, the poor transformation efficiency of orchids defines a major challenge, and limited knowledge about the gene function and breeding further adds to the limitations. Further insights and research on achieving better transformation efficiency are needed for the success of transgenic technologies in orchids. Semiarti and coworkers [134] developed a CRISPR/Cas9 KO system for molecular breeding in P. amabilis. The Phytoene desaturase 3 (PDS3) gene was successfully integrated into the transformants and showed pale leaf color, and the study showed prospects for functional gene editing in orchids. As a step further, Xia et al. [135] successfully developed a multiplex genome editing system in Phalaenopsis orchids and protoplast-based screening, highlighting advanced precision breeding in orchids. The CRISPR/Cas9 tool was employed for editing endogenous genes in the D. officinale genome [136]. The study showed efficient genome editing in D. officinale paving the way to create novel varieties.
5. Ethnomedicinal and Edible Importance of Orchids
Represented as the diversified plants among the angiosperms, orchids are cultivated for their attractive flowers and exotic varieties. Initially cultivated as ornamentals for their multi-colored and attractive flowers, the medicinal applications of orchids are gaining momentum in the present time. Studies have reported the initial cultivation of orchids by the Chinese and documented orchids for their medicinal properties and use [137]. Researchers have traced orchid history and their medicinal uses to 120 million years ago, probably cultivated for their health-promoting effects [66]. Early documented evidence was found in the literature of Japan and China, approximately 3000 to 4000 years ago; they are regarded as pioneers in describing the medicinal attributes of different orchid species [138,139]. Shennung, a Chinese legend, described Dendrobium and Bletilla striata species in the book Materia Medica in the 28 century BC [138]. The Indian traditional system also reported the pharmacological uses of orchid species, namely Dendrobium macraei, Orchis latifolia, and Eulophia campestris, among others, in Ayurveda [140]. The key genera of medicinal orchids are as follows: Ephemerantha, Eria, Galeola, Cymbidium, Cypipedium, Nevilia, Thunia, Bletilla, and Anoctochilus, with new orchid varieties being discovered [102,141,142].
The medicinal properties of orchids are gaining popularity and different species are increasingly employed for medicinal attributes, attributed to the presence of diverse phytochemicals present in different species. Alkaloids have been reported in several medicinal orchids and demonstrate antimicrobial functions. Multiple studies in recent times have focused on the isolation of phytochemicals with medicinal attributes from different species and comprise Cypripedin, Orchinol, Nidemin, Hircinol, Loroglossin, and Jibantine. A comprehensive overview of key orchid species, plant parts, phytochemicals, and their ethnobotanical significance has been discussed. Table 3 provides key examples of orchids with medicinal properties and their multi-faceted ethnobotanical attributes.

Table 3.
Key examples of orchids with medicinal properties and their multi-faceted ethnobotanical attributes.
The beneficial effects of phytochemicals from orchids on human health have been well known since ancient times and include anti-inflammatory, neuroprotective, antimicrobial, anticancer, hypoglycemic, anti-rheumatic, and wound-healing properties among other significant effects [141]. Traditional Chinese medicine suggested the routine use of B. striata, D. nobile, and G. elata for medication, with different Dendrobium species widely used in China to cure various diseases [171]. Moreover, the tubers of B. striata were widely used in traditional systems for the treatment of pneumono-phthisis and pneumonorrhagia, in addition to treating gastritis and duodenal ulcers, bleeding, hemoptysis, and tuberculosis. In addition, Ayurvedic literature reported the active use of orchids in herbal formulations, viz. Habenaria intermedia, Malaxis muscifrea, Habenaria edgeworthi, and Malaxis acuminata, which were active ingredients in Chavyanprasa [169]. Similarly, pseudobulbs and tubers of key species, namely Zeuxine strateumatica, Habenaria, Orchis mascula, Eulophia, Orchis latifolia, and Cymbidium aloifolium, were used as restorative therapy in several diseases, with a discussion of key orchid species found in Table 3. Studies have reported the isolation of more than 100 bioactive alkaloids from orchids [141,166,172] and the pharmaceutical constituents comprise Dendrocandin-A, Dendrocandin B, Phenanthrenes, Bulbophythrins A, Bulbophythrins B, Nobilin-E, Nobilin-D, Rotundatin, Ochracinone, Ochracinanthrone, Ochrolic acid, Ochrolone, and many more that have recently been discovered [173,174,175,176,177,178,179].
The flavonol and flavonoid components (of medicinal significance) isolated from orchid species include Apigenin 7-O-glucoside, 8-C-p-hydroxybenzylquercetin, Scutellarein 6-methyl ether, Apigenin-6-O-β-d-glucopyranosil-3-O-α-l-rhamnopyranoside, Quercetin, Chrysin, Homo-isoflavone, etc. [180,181]. The emerging medicinal importance of orchids has necessitated the need for clinical validations [181,182]. A clinical trial by Khouri et al. [183] suggested the efficacy of plant extract (Orchis anatolica) in improving fertility in male mice. In another study, constituents from the orchid Scaphyglottis livida were evaluated as relaxants of heart contractions in mice. The study further showed that stilbenoids limited aortic contractions and led to vasodilation and relaxation and are supposed to have major potential in cardiac treatment [184]. A terrestrial orchid, Eulophia epidendraea, is traditionally used for wound healing, tumors, and antidiarrhoeal activity, attributed to the presence of terpenoids, saponins, alkaloids, etc. [185,186]. Dendrobium and its bioactive constituents display effective hepatoprotective and immunomodulatory functions [187]. Gastrodia elata and its active ingredients have been widely used to treat rheumatism, brain disorders, inflammatory diseases, and headaches, due to the presence of Gastrodin, Gastrodioside, Vanillin, β-sitosterol, Vanillyl alcohol, and ρ-hydroxybenzyl alcohol [188,189].
In China and other Asian countries, multiple orchids have been employed to develop functional food products since 1990. The bioactive constituents and micronutrients present in orchid tubers make them a nutritional supplement in Cameroon and African countries [190]. Fonmboh and coworkers [191] provided literature insights into the medicinal and edible properties of wild root tuber orchids in Cameroon. While the medicinal properties of multiple orchid species are well known, the use of orchids as food constituents and flavoring agents makes them a key economic resource in food industries. In recent times, studies exploring the orchid flora in South Africa have investigated the edible properties and constituents facilitating the orchid trade. Orchid tubers are used as a food source and harvested from the wild species. The tubers are processed as a sausage and locally named chikande and chinaka, relished by the local Mawai population [192]. In addition, tubers are added as ingredients in soups and included in dishes in international hotels [193]. In Cameroon, orchids are mainly epiphytic, and the edible orchids belong to the genera Disa, Satyrium, and Habenaria. Moreover, new food products have been developed from Dendrobium and Gastrodia spp. Food items and healthy drinks as functional food have been processed from the flower and stem of D. officinale and another Dendrobium sp. [194]. A key example is the use of G. elata tubers in soup and Cymbidium flowers in healthy drinks and herbal tea [195,196]. In east and central Africa, tubers of the terrestrial orchids are used to prepare ‘Chikanda’, a traditional cake recipe. ‘Salep’ is another food ingredient in ice cream and drinks, mainly consumed in Turkey and adjoining countries [37]. While edible orchids represent an alternative food source in countries in the Global South, Zambian, Tanzanian, and Malawi people consume orchid tubers for food and trade [197]. In a key study, the nutritional content of wild orchid species from Tanzania was studied and included fiber (2.7%), protein (5.36 g), minerals ash (2.2%), and fat (1.57%), among others, highlighting the use of edible orchids as a functional food. Additionally, the concerns about malnutrition in children/infants can be treated by products developed from orchids [37]. The powdered form of tubers of Orchis mascula is used as a food component in ‘Salep’ beverage or the Turkish food ‘Dondurma’. In Turkey, ice creams from orchid constituents, known as ‘Dondurma’, are relished by the locals. Other popular food items prepared from different edible orchids comprise flavor rum from the dried leaves of Jumellea fragrans in Reunion Island, underground tubers of Gastrodia sesamoides as food in Australia, and Chikanda from Satyrium cursoni [40]. V. planifolia is found in tropical lowlands and inhabits Central America, Brazil, and Mexico [198]. Regarded as endangered by IUCN [199], the plant is the main source of vanilla, a food flavoring agent. While vanillin comprises 80% of total bioactive metabolites in the pods of V. planifolia, vanillic acids such as 4-hydroxybenzoic acid and vanillic alcohols such as Guaiacol, 4-hydroxybenzaldehyde, etc. are the other metabolites [200]. A chromosome-scale V. planifolia genome was reported by Hasing and coworkers [201] that revealed gene variants that affect the pathway of vanillin biosynthesis and bean quality and facilitate orchid breeding for improved traits.
6. Computational and Omics Approaches in Orchid Biology
The discovery of new orchid species and their emerging prospects has necessitated the need to address the knowledge gaps and improve the understanding of key areas in orchid biology. The last few decades have witnessed substantial contributions of computational and omics approaches in providing comprehensive insights into orchid genomes and the molecular and physiological mechanisms, respectively.
Omics refers to a group of disciplines in the areas of genomics, transcriptomics, proteomics, and metabolomics, among others, which contribute to a better understanding of the roles and pathways of different molecules in living organisms [202]. Genomics provides an overview of the complete set of genetic instructions provided by DNA. Orchid genomes are typically larger than those of most model plants and there is remarkable variability in genome size across the family, with the amount of nuclear DNA varying by up to 168-fold [203]. It is difficult to analyze Phalaenopsis orchid via genomics due to its large genome and long life cycle. Lin et al. [204] employed the flow cytometry method for the estimation of DNA content in Doritis pulcherrima Lindl. and Phalaenopsis ‘Blume’ species. The results showed variation in genome size within 18 Phalaenopsis species. Further, new commercial hybrids were produced via chromosome doubling [21] and can be used for the comparative assessment of DNA content, providing insights into the evolution of Phalaenopsis orchids and assisting the orchid breeders in the selection of parent varieties for the hybridization process. Further, to understand the relationship and molecular characterization of different species, sequence-based microsatellite markers were used [205]. Ko and coworkers [206] employed polymorphic microsatellite markers for delimiting species within the Phalaenopsis genus.
Transcriptome studies were performed in C. ensifolium pooled flower buds and mature flowers [207]. The Cymbidium sinense mature plant transcriptome was studied for the identification of genes associated with floral development [208]. MicroRNAs (miRNAs) are short RNA molecules that regulate gene expression in eukaryotes, and they influence physiological mechanisms such as development, cell proliferation, cell death, and differentiation [209,210]. The different techniques used for the study of the entire “miRNome” allow for exploring these novel mechanisms of gene expression regulation [210]. In Orchis italica inflorescence, miRNome revealed the presence of conserved and novel miRNAs. The insilico search for the possible miRNA targets showed a conserved miRNA cleavage site within the four OitaDEF-like transcripts, which experimentally validated for OitaDEF2 [209]. This result reveals that miRNAs play an important role in the diversification of the organs of the perianth in orchids through the inhibitory regulation of the clade-2 DEF-like gene. Different mechanisms might act to regulate the expression level of the other DEF-like genes, suggesting the existence of lineage-specific regulatory mechanisms contributing to the functional specialization of the DEF-like clades in orchids. Advances in next-generation sequencing (NGS) technologies and new algorithms have improved the computational analysis of genome-scale RNA-seq transcriptomes [211,212,213]. The characterization of the plant transcriptome provides useful information into genomic features and functions, including protein-coding/noncoding gene transcripts and alternative splicing for species that lack reference genomes [214,215]. In recent years, genomic and transcriptomics studies have been performed for orchids, namely Dendrobium ssp., Erycina pusilla, Phalaenopsis ssp., and Orchis italica, among others, that provided key insights into the functional role of genes in polyploidy, MADS-box genes diversification, flower development, etc., enriching our knowledge [7].
To accommodate the increasing amount of orchid transcriptome data and house more comprehensive information, the Orchidstra 2.0 database was created with a new database system to store the annotations of 510,947 protein-coding genes and 161,826 noncoding transcripts, including 18 orchid species belonging to 12 genera in 5 subfamilies of Orchidaceae. The Orchidstra 2.0 database showed that RNA-seq-based gene expression data showed that the KNOX genes were highly expressed in the developing flower stalks at its early stage and in germinating seeds in P. phrodite and mesocarp tissues of the developing vanilla six- and eight-week-old pods in V. planifolia [213].
The fungal association affects both the symbiotic and asymbiotic germination of orchids. Liu and coworkers [216] studied the large-scale transcriptome dataset of Anoectochilus roxburghii (Wall.) Lindl. seeds for both symbiotic and asymbiotic germinated seeds. The study identified forty-nine genes involved in regulation, of which six genes were differentially expressed in symbiotic germination vs. asymbiotic germination, suggesting the induction or suppression of these six genes by fungi. Valadares et al. [217] characterized 88 proteins related to energy metabolism, cell rescue and defense, molecular signaling, and secondary metabolism in Oncidium sphacelatum Lindl. at different trophic stages of symbiotic germination. In addition, the proteomic analysis showed the upregulation of proteins that are involved in purine recycling, ribosome biogenesis, energy metabolism, and secretion in O. sphacelatum.
The proteomic studies on orchids focused on flower development and tissue culture studies for mass production. By elucidating developmental processes in orchids, omics approaches can assist with breeding, genetic improvement, conservation, and commercial production in orchids. Comparative proteomics analyses of pollination response in the endangered orchid species Dendrobium chrysanthum were carried out [218]. For a better understanding of the mechanism of pollination in D. chrysanthum, the differentially expressed proteins (DEP) between the self-pollination and cross-pollination pistil of D. chrysanthum were investigated via two-dimensional electrophoresis (2-DE) coupled with tandem mass spectrometry [218]. A total of 54 DEP spots were identified in the two-dimensional electrophoresis (2-DE) maps between the self-pollination and cross-pollination. Gene ontology analysis revealed an array of proteins belonging to the functional categories: metabolic process (8.94%), response to stimulus (5.69%), biosynthetic process (4.07%), protein folding (3.25%), and transport (3.25%). The identification of these DEPs at the early response stage of pollination provides new insights into the mechanism of pollination response and assists in the conservation of the orchid species [218]. By proteomics techniques (LC–MS/MS, LTQ (HPLC)), flower labellum tissues sampled from Ophrys exaltata subsp. Archipelagi, O. garganica, and O. sphegodes were used for the identification of candidate genes for pollinator attraction and reproductive isolation (e.g., genes for hydrocarbon and anthocyanin biosynthesis and regulation and the development of floral morphology) [219]. Proteomic studies (employing 2 DE MALDI ToF/ToF) were performed in the C. ensifolium flower (structures including labellum and inner lateral petals proteins) [220]. In another study, the DNA-binding properties and protein–protein interactions of the floral homeotic MADS-box protein complexes in P. equestris were analyzed by a Yeast two-hybrid system [221].
Reports on the molecular mechanisms of mycorrhizal association and seed germination in orchids are limited. So, the question will be, is there any difference in seed development and germination between orchids and other flowering plants at the molecular level or not? Chen et al. [222] reported that some genes such as PaMADS39 and PaMADS51 belong to the Ma-subclass of type I MADS-box genes and are detected at the cellularization stage of developing endosperm during seed development. These genes are closely related to Arabidopsis (AGL23 and AGL62.1) and they have similar expression patterns in reproductive tissues when fertilization occurs and embryo development initiates. MIKC-type genes were identified from streptophyte lineages, revealing new insights into their evolution and development relationships. The study reported that MIKC-type genes might play a role in seed germination in D. officinale. Some MIKC genes from D. officinale showed different expression during seed germination, including SVP and SQUA subfamily genes, as well as the MIKC gene, and these genes have the same role in other flowering plants [223]. So, it was concluded that the expression pattern in seed germination of orchids was like other plants, like Arabidopsis.
7. Strategies/Guidelines for Orchid Conservation and Utilization
The commercialization of orchid species has witnessed a tremendous upsurge in recent times. The classification of the orchid trade has been performed accordingly: specialist growers-driven market—people who have a collection of orchid species and purchase diverse hybrids and species; and the mass-market trade—comprising casual buyers of potted varieties and easily cultivated varieties. The demand for rare orchid varieties in the floriculture market has partially led to the illegal trade. However, casual buyers sometimes purchase unknowingly with prices like plants that are artificially propagated [224]. Initially, the orchid varieties were sold and purchased in the local markets, but with the advent of e-commerce platforms, the orchid species availability has increased to both online and offline sellers in the networks.
International/National Guidelines for the Preservation of Orchid Biodiversity
On a global level, the trade of all orchid species is monitored by CITES, and legal marketing (to a considerable level) particularly for artificially propagated plants occurs according to the established guidelines. However, the rising incidences of illegal trade have severely hampered the orchid industry. Southeast Asia, an orchid-rich habitat, contributes to a significant share of wild species collection, [225] and countries in Southeast Asia as well as the United States, Europe, and Japan are the emerging specialist markets. However, in the present time, there has been a tremendous upsurge in the illegal trade of commercial orchid varieties; for example, Paphiopedilum spp. highlights the risk of extinction and are categorized as “critically endangered” by CITES owing to extensive reports of over-collection. CITES has three distinct Appendices for different species. Appendix I contains species threatened with extinction conditions, whose international trade is possible only under exceptional conditions. Presently, Appendix I lists two genera of orchids, Paphiopedilum and Phragmipedium, representing 240 species, more than 180 varieties, and 30 natural hybrids, along with Aerangis ellipsis, Dendrobium cruentum, Laelia jongheana, Laelia lobata, Peristeria elata, and Renanthera imschootiana. The rest of the orchids are listed in Appendix II, which includes the species not necessarily threatened with extinction conditions, and Appendix III, which contains species that are protected in one country at least. This extensive protection of orchids makes them the largest plant family protected under CITES [226]. Along with the conservation and protection under CITES, there are several orchid societies around the globe aiding cultivation and conservation efforts under the two major approaches, i.e., in situ conservation and ex situ conservation.
On a worldwide level, several orchid species with ornamental attributes are traded in the legal trade of reasonable potted plants for non-specialist buyers, usually Dendrobium and Phalaenopsis genera, with the key exported countries being the Netherlands, Taiwan, and Thailand. Among the most popular products, cut flowers and orchid plants comprise the maximum share of the global platform, with less expertise and expenses involved in maintenance. The specialized trade includes specialists, and the consumers include specialists’ growers with expertise in orchid cultivation who have a broad collection of exotic germplasms. According to statistics by CITES on legal orchid trade, orchid plants witnessed a large global export between 1996 and 2015 [225]. Considering no in-depth studies exist on the global levels of illegal orchid trade, only commercial harvesting of wild varieties from approximately 10 countries has been documented [225]. In Europe, the legal international market is declining, owing to the increasing costs of propagated orchid species and restrictions by CITES. Given the conservation and protection of several orchid varieties, guidelines have been issued on national/international levels for the preservation of orchid biodiversity. While individual countries have defined parameters and legislation for the cultivation, conservation, and marketing of wild orchids, the legal guidelines are more precise and defined at global levels. Some orchid species may be illegal for local/global trade in a few countries, while in others these may be allowed. On the global platform, 70% of the orchid species are classified in CITES, and a few species, namely Peristeria elata, Dendrobium cruentum, Renanthera imschootiana, Laelia jongheana, and Paphiopedilum sp. are classified in Appendix I, which identifies their trading as prohibited (CITES, Appendices I, II and III. https://cites.org/eng/app/E-Apr27.pdf accessed on 20 October 2023). In an international move, the decision was made to list orchids at the family level, considering the identification of 300–500 new orchid species annually [227]. With guidelines and initiatives being made at domestic and international levels for orchid conservation, the legal framework is still defined by complexity, as it is difficult to clearly define the legal or illegal nature of trade and therefore difficult to implement proper guidelines. In addition, the emergence of e-commerce platforms for online trading has further contributed to the rise in the unauthorized trading of orchids, with access to people across the globe and no clearly defined parameters for orchid trade. In a specific instance on a social media platform (2014), the trading of 46% of species for 150 orchid groups was performed in five languages [228]. In this direction, IUCN Species Survival Commission Orchid Specialist Group’s Global Trade Programme has made efforts to streamline online orchid trading and report these incidents to concerned committees (Orchid Specialist Group Global Trade program, https://globalorchidtrade.wixsite.com/home/about-us (accessed on 28 January 2024)). Floraguard is an established project (established in 2017), aimed at implementing better policies to monitor the online trading of all plant species, including orchids (Floraguard, http://floraguard.org (accessed on 28 January 2024)). According to Article 038 of the Convention on Biological Diversity (1994), the rights of natural resources are under the jurisdiction of respective countries, allowing the maximum utilization of traditional knowledge and resources. Among others, the Nagoya Protocol (2010) includes international consent to deriving shared benefits from the utilization of genetic resources in a transparent manner, while the Cartagena Protocol (2003) on an international platform ensures the safe use and transport of living modified organisms (LMOs).
8. Translational Success, Restoration Initiatives, and Future Research
Research advances in the cultivation and commercialization of orchids have witnessed unprecedented success, witnessing an expanding global market. The Phalaenopsis orchid is cultivated and marketed worldwide, accounting for 500 million USD in the United States, Japan, and the Netherlands, among other countries. The statistics suggest that Phalaenopsis was the most exported orchid in 2018 (76.4%), followed by Oncidium (6.9%) and Cymbidium (3.2%) [8]. From the perspective of the Indian sub-continent, it has about 1350 species classified in 186 genera and represents approximately 5.98% of the orchid flora existing across the world. CITES (administered by the United Nations Environment Programme, located in Geneva, Switzerland) plays a pivotal role in the conservation measures for plant species, including orchids. Moreover, three approaches are primarily adopted for the conservation of genetic resources (in orchids) and are defined as follows: in situ conservation in sanctuaries, legislative guidelines, and ex situ conservation in botanical gardens. In addition to the above conservation measures, there are international laws and guidelines to preserve biodiversity and stop the bio-piracy of natural resources.
While significant success has been achieved on different levels from basic biology studies on orchids to implementing biotechnologies for sustainable living, the future directions in orchid research aim to address the knowledge gaps and bottlenecks in cultivation studies. The advent of genetic engineering and high-throughput sequencing defines a big boost in orchid research, enabling solutions to intriguing puzzles in orchid biology. While to date, 16 orchid genomes have been elucidated, a lot remains to be uncovered. Orchid genome sequencing has opened new avenues in multi-omics-guided studies for gene expression, mapping, and comparative genomics. The progress achieved in distinct areas has enabled information on the origin and diversification of orchids and the molecular mechanisms in growth stages, reproduction, and complex life cycles.
9. Concluding Remarks
In the present decade, the orchid industry has flourished with leaps and bounds on domestic and international platforms. The aesthetic and ornamental attributes of novel orchid species have gained the attention of plant biologists as well as agriculturists. To date, orchids have been cultivated for their cut flowers and artificially propagated varieties, with the multi-faceted applications of orchids in the food sector and medicine gaining global recognition in recent times. Plant tissue culture, breeding approaches, and biotechnologies have made substantial contributions in introducing and improving the plant traits of novel attributes and remarkably improved commercialization on a global platform.
Advances in whole genome sequencing have facilitated the understanding of orchid genomes, providing key insights into the cultivation of new orchid cultivars for commercialization. Furthermore, tools in genomics and proteomics employed for orchid genome assembly provided valuable insights about orchid genomes. The genomic insights showed gene function in heterozygosity, MADS-box gene diversification, and CAM photosynthesis evolution concerning physiological responses, flower development, and the effect of climatic fluctuations on the productivity of orchids [7]. Studies on the function and composition of SSRs and microRNAs in orchids’ growth and development facilitate studies in functional genomics. Recent innovations in biotechnologies have opened new avenues in the development of ornamental plants with desired multi-faceted and novel attributes. Biotechnological interventions such as genetic engineering and in vitro tissue culture along with classical breeding approaches have been regularly employed to alter flower color, fragrance, appearance, disease resistance, and shelf life, as discussed with key examples. The progress in the development of scientific techniques such as HPLC-DAD and MS has facilitated the precise detection of bioactive components from multiple orchids and their emerging prospects in drug discovery and research. Omics biology-guided elucidation of important traits linked to gene families and their regulatory components has key potential to revolutionize orchid improvement. While advances in plant genome editing have enabled precise gene modifications and genome engineering, next-generation tools like the CRISPR-Cas9 system define the key potential for ‘gene stacking’, where multiple gene modification steps can be attempted in a single procedure [229]. Although limited genetic manipulation studies are attempted in orchids, these advanced tools offer great opportunities to design orchids with ‘high-value’ traits in the near future.
The cultivation and export of new orchid varieties are gaining momentum, with an expanding global market generating significant economic returns. However, with the unprecedented rise in exports and global orchid trade, rare and exotic varieties are overharvested and illegally traded by suppliers across the boundaries. Restoration measures have been undertaken to safeguard the interests of people and orchid specialists through guidelines to monitor and prevent the indiscriminate use and trading of orchid species. The guidelines by CITES and the Convention on Biological Diversity and Wildlife Protection Act 1972, by the Government of India, have been successful in reducing bio-piracy and conservation of natural resources to a greater extent, introducing legislative measures and guidelines for monitoring the conservation and trade of wild orchid species. For the orchid industry to flourish, it is imperative to explore orchid biology and biotechnologies and to ensure legal international trade by implementing guidelines towards a multipronged approach for the conservation and commercialization of orchids.
Author Contributions
P.T. and K.-I.P. conceptualized the theme of the manuscript. P.T., A.S. and S.K.B. collected the data and wrote the manuscript. P.T. and K.-I.P. revised and improved the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors extend their gratitude to their organizations for constant encouragement and support.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Li, C.; Dong, N.; Zhao, Y.; Wu, S.; Liu, Z.; Zhai, J. A review for the breeding of orchids: Current achievements and prospects. Hortic. Plant J. 2021, 7, 380–392. [Google Scholar] [CrossRef]
- Vendrame, W.A.; Khoddamzadeh, A.A. Orchid biotechnology. In Horticulture Reviews; Janick, J., Ed.; World Scientific: London, UK, 2017; Volume 44, pp. 173–228. [Google Scholar]
- Hsu, C.C.; Chung, Y.L.; Chen, T.C.; Lee, Y.L.; Kuo, Y.T.; Tsai, W.C.; Hsiao, Y.Y.; Chen, Y.W.; Wu, W.L.; Chen, H.H. An overview of the Phalaenopsis orchid genome through BAC end sequence analysis. BMC Plant Biol. 2011, 11, 3. [Google Scholar] [CrossRef]
- Atwood, J.T. The size of the Orchidaceae and the systematic distribution of epiphytic orchids. Selbyana 1986, 9, 171–186. [Google Scholar]
- Hossain, M.M.; Kant, R.; Van, P.T.; Winarto, B.; Zeng, S.; Teixeira da Silva, J.A. The application of biotechnology to orchids. Crit. Rev. Plant Sci. 2013, 32, 69–139. [Google Scholar] [CrossRef]
- De, L.C.; Pathak, P.; Rao, A.N.; Rajeevan, P.K. Medicinal and Aromatic Orchids: In Commercial Orchids; De Gruyter Open: Warsaw, Poland, 2015. [Google Scholar] [CrossRef]
- Chen, F.-C.; Chin, S.-W. The Orchid Genome. In Compendium of Plant Genomes; Springer International Publishing: Berlin/Heidelberg, Germany, 2021. [Google Scholar] [CrossRef]
- Yuan, S.-C.; Lekawatana, S.; Amore, T.D.; Chen, F.-C.; Chin, S.-W.; Vega, D.M.; Wang, Y.-T. The global orchid markets. In The Orchid Genome, Compendium of Plant Genomes; Chen, F.-C., Chin, S.-W., Eds.; Springer Nature: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Chung, I.M.; Yang, C.H. Overexpression of Oncidium MADS-box (OMADS1) gene promotes early flowering in transgenic orchids (Oncidium gower Ramsey). Acta Physiol. Plant. 2012, 34, 1295–1302. [Google Scholar] [CrossRef]
- Liu, Y.C.; Yeh, C.W.; Chung, J.D.; Tsai, C.Y.; Chiou, C.Y.; Yeh, K.W. Petal-specific RNAi-mediated silencing of the phytoene synthase gene reduces xanthophyll levels to generate new Oncidium orchid varieties with white-colour blooms. Plant Biotechnol. J. 2019, 17, 2035–2037. [Google Scholar] [CrossRef] [PubMed]
- Krittiya, N.; Kisana, B.; Huehne, P. A direct gene transferring system for Oncidium orchids, a difficult crop for genetic transformation. Agric. Nat. Nat. Resour. 2018, 52, 424–442. [Google Scholar]
- Su, V.; Hsu, B.D. Cloning and expression of a putative cytochrome P450 gene that influences the colour of Phalaenopsis flowers. Biotechnol. Lett. 2003, 25, 1933–1939. [Google Scholar] [CrossRef] [PubMed]
- Semiarti, E.; Indrianto, A.; Purwantoro, A.; Suseno, N.; Isminingsih, S.; Yoshioka, Y.; Iwakawa, H.; Machida, Y.; Machida, C. Agrobacterium-mediated transformation of the wild orchid species Phalaenopsis amabilis. Plant Biotechnol. 2007, 24, 265–272. [Google Scholar] [CrossRef]
- Semiarti, E.; Indrianto, A.; Suyono, E.A.; Nurwulan, R.L.; Restiani, R.; Machida, Y.; Machida, C. Genetic transformation of the Indonesian black orchid (Coelogyne pandurata Lindley) through Agrobacterium tumefaciens for micropropagation. In Proceedings of the NIOC, Nagoya, Japan, 18–29 October 2010; pp. 16–20. [Google Scholar]
- Huang, W.T.; Wu, B.W.; Fang, Z.M. Temporal and spatial expression and functional analysis of FT homologous genes in Cymbidium sinense. J. Anhui Agric. Univ. 2017, 1, 141–147. (In Chinese) [Google Scholar]
- Shrestha, B.R.; Chin, D.P.; Tokuhara, K.; Mii, M. Efficient production of transgenic plants of Vanda through sonication-assisted Agrobacterium-mediated transformation of protocorm-like bodies. Plant Biotechnol. 2007, 24, 429–434. [Google Scholar] [CrossRef]
- Cao, Y.; Niimi, Y.; Hu, S.L. Meropenem as an alternative antibiotic agent for suppression of Agrobacterium in genetic transformation of orchid. Agric. Sci. China 2006, 5, 839–846. [Google Scholar] [CrossRef]
- Hsing, H.-X.; Lin, Y.-J.; Tong, C.-G.; Li, M.-J.; Chen, Y.-J.; Ko, S.-S. Efficient and heritable transformation of Phalaenopsis orchids. Bot. Stud. 2016, 57, 30. [Google Scholar] [CrossRef]
- Kantamaht, K.; Alisa, N.; Kamnoon, K.; Amornrat, P. Efficient biolistic transformation and regeneration capacity of an EgTCTP transgene in protocorm-like bodies of Phalaenopsis orchid. Notu-lae Botanicae Horti Agrobotanici Cluj-Napoca 2012, 40, 58–64. [Google Scholar]
- Chen, L.; Kawai, H.; Oku, T.; Takahashi, C.; Niimi, Y. Introduction of Odontoglossum ringspot virus coat protein gene into Cymbidium niveo-marginatum mediated by Agrobacterium tumefaciens to produce transgenic plants. J. Jpn. Soc. Hort. Sci. 2006, 75, 249–255. [Google Scholar] [CrossRef][Green Version]
- Chen, Y.C.; Yang, Y.C.; Chen, Y.H.; Chao, Y.P.; Chang, L.Z.; Chen, Y.W. Modification of Phalaenopsis metabolism by genetic engineering. In International Orchid Symposium; ISHS: Leuven, Belgium, 2010; Volume 878, pp. 473–480. [Google Scholar]
- Lee, S.H.; Li, C.W.; Liau, C.H.; Chang, P.Y.; Liao, L.J.; Lin, C.S.; Chan, M.T. Establishment of an Agrobacterium-mediated genetic transformation procedure for the experimental model orchid Erycina pusilla. Plant Cell Tiss. Org. Cult. 2015, 120, 211–220. [Google Scholar] [CrossRef]
- Ratanasut, K.; Monmai, C.; Piluk, P. Transient hairpin RNAi-induced silencing in floral tissues of Dendrobium sonia ‘Earsakul’ by agroinfiltration for rapid assay of flower color modification. Plant Cell Tiss. Organ. Cult. 2015, 120, 643. [Google Scholar] [CrossRef]
- Zhang, L.; Chin, D.P.; Mii, M. Agrobacterium-mediated transformation of protocorm-like bodies in Cattleya. Plant Cell Tiss. Organ. Cult. 2010, 103, 41–47. [Google Scholar] [CrossRef]
- Qin, X.Y.; Liu, Y.; Mao, S.J.; Li, T.B.; Wu, H.K.; Chu, C.C.; Wang, Y.P. Genetic transformation of lipid transfer protein-encoding gene in Phalaenopsis amabilis to enhance cold resistance. Euphytica 2011, 177, 33–43. [Google Scholar] [CrossRef]
- Chang, L.; Chang, H.H.; Chiu, A.; Chang, J.C.; Hsu, D.W.; Tzean, Y.; Cheng, A.P.; Lu, H.C.; Yeh, H.H. Plant A20/AN1 proteins coordinate different immune responses including RNAi pathway for antiviral immunity. PLoS Pathog. 2019, 14, e1007288. [Google Scholar]
- Hsieh, R.M.; Huang, P.L. Studies on genetic transformation of Phalaenopsis via pollen tube pathway. J. Chin. Soc. Hort. Sci. 1995, 41, 309–324. [Google Scholar]
- Nan, G.L.; Kuehnle, A.R. Genetic transformation in Dendrobium (orchid). In Plant Protoplasts and Genetic Engineering VI. Biotechnology in Agriculture and Forestry; Bajaj, Y.P.S., Ed.; Springer: Berlin/Heidelberg, Germany, 1995; Volume 34, pp. 145–155. [Google Scholar]
- You, S.J.; Liau, C.H.; Huang, H.E.; Feng, T.Y.; Venkatesh, P.; Hsiao, H.H.; Lu, J.C.; Chan, M.T. Sweet pepper ferredoxin-like protein (pflp) gene as a novel selection marker for orchid transformation. Planta 2003, 217, 60–65. [Google Scholar] [CrossRef]
- Chia, T.F.; Lim, A.Y.H.; Luan, Y.; Ng, I. Transgenic Dendrobium (Orchid). In Biotechnology in Agriculture and Forestry 48, Transgenic Crops III; Bajaj, Y.P.S., Ed.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 95–106. [Google Scholar]
- Anzai, H.; Tanaka, M. Transgenic Phalaenopsis (a moth orchid). In Biotechnology in Agriculture and Forestry 48, Trangenic Crops II; Bajaj, Y.P.S., Ed.; Springer: Berlun/Heidelberg, Germany, 2001; pp. 249–264. [Google Scholar]
- Griesbach, R.J. An improved method for transforming plants through Electrophoresis. Plant Sci. 1994, 102, 81–89. [Google Scholar] [CrossRef]
- Fay, M.; Rankou, H. Slipper orchids on the IUCN Red List. In The 2015 Annual Report to the Environment Agency-Abu Dhabi. Framework Support for Implementing the Strategic Plan of the IUCN Species Survival Commission; 2016; pp. 106–111. [Google Scholar]
- Vogt-Schilb, H.; Pradel, R.; Geniez, P.; Hugot, L.; Delage, A.; Richard, F.; Schatz, B. Responses of orchids to habitat change in Corsica over 27 years. Ann. Bot. 2016, 118, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Fay, M.F. British and Irish orchids in a changing world. Curtis’s Bot. Mag. 2015, 32, 3–23. [Google Scholar] [CrossRef]
- Cribb, P.J.; Kell, S.P.; Dixon, K.W.; Barrett, R.L. Orchid conservation: A global perspective. In Orchid Conservation: A Global Perspective; Dixon, K.W., Kell, S.P., Barrett, R.L., Cribb, P.J., Eds.; Natural History Publications (Borneo): Kota Kinabalu, Malaysia, 2003. [Google Scholar]
- Ghorbani, A.; Gravendeel, B.; Zarre, S.; de Boer, H. Illegal wild collection and international trade of CITES-listed terrestrial orchid tubers in Iran. Traffic Bull. 2014, 26, 52–58. [Google Scholar]
- Hinsley, A.; Nuno, A.; Ridout, M.; St John, F.A.V.; Roberts, D.L. Estimating the extent of CITES noncompliance among traders and end-consumers, lessons from the global orchid trade. Cons. Lett. 2017, 10, 602–609. [Google Scholar] [CrossRef]
- Sharma, V. Agrotechniques in orchids. Adv. Tissue Eng. Regen. Med. 2017, 2, 105–108. [Google Scholar]
- Bose, S.K.; Tiwari, P. Orchid biology: A biotechnological perspective on the prospects and challenges in Orchid cultivation. Vitr. Cult. Commer. Flower. Plants Orchid. 2020, 2021, 162–210. [Google Scholar]
- Pansarin, E.; Pansarin, L.; Poleti, M.M.; Gobbo-Neto, L. Self-compatibility and specialization in a fly-pollinated Acianthera (Orchidaceae: Pleurothallidiinae). Aust. J. Bot. 2016, 64, 359–367. [Google Scholar] [CrossRef]
- Veitch, H.J. The hybridization of orchids. The report on the orchid conference held at South Kensignton. J. R. Hortic. Soc. 1886, 1, 22–49. [Google Scholar]
- de Chandra, L.; Pathak, P.; Rao, A.N.; Rajeevan, P.K. Breeding approaches for improved genotypes. In Commercial Orchids; De, L.C., Ed.; De Gruyter Open Poland: Warsaw, Polland, 2019; Volume 300. [Google Scholar]
- Su, J.S.; Jiang, J.F.; Zhang, F.; Liu, Y.; Ding, L.; Chen, S.M.; Chen, F.D. Current achievements and future prospects in the genetic breeding of Chrysanthemum: A review. Hort. Res. 2019, 6, 109. [Google Scholar] [CrossRef]
- Zhang, Z.S.; He, Q.Y.; Fu, X.L.; Ou, X.J.; Lin, W.Q.; Jiang, J.Y. Studies on the wide cross of Chinese orchids and the germination of their hybrid seeds. J. South China Agric. Univ. 2001, 2, 62–65. [Google Scholar]
- Luo, Y.H.; Huang, M.L.; Wu, J.S. Progress in Oncidium breeding study. Acta Agric. Jiangxi 2012, 24, 15–20. [Google Scholar]
- Lu, H.C.; Chen, H.H.; Tsai, W.C.; Chen, W.H.; Su, H.J.; Chang, C.N.; Yeh, H.H. Strategies for functional validation of genes involved in reproductive stages of orchids. Plant Physiol. 2007, 143, 558–569. [Google Scholar] [CrossRef]
- Pan, I.C.; Liao, D.C.; Wu, F.H.; Daniell, H.; Singh, N.D.; Chang, C.; Shih, M.C.; Chan, M.T.; Lin, C.S. Complete chloroplast genome sequence of an orchid model plant candidate: Erycina pusilla apply in tropical Oncidium breeding. PLoS ONE 2012, 7, e34738. [Google Scholar] [CrossRef]
- Wang, H.M.; Tong, C.G.; Jang, S. Current progress in orchid flowering/flower development research. Plant Signal. Behav. 2017, 12, e1322245. [Google Scholar] [CrossRef]
- Osadchuk, V.D.; Saranchuk, I.I.; Lesyk, O.B.; Olifirovych, V.O. Selective breeding in plant growing in Bukovina. Taurian Sci. Herald. 2020, 115, 16. [Google Scholar]
- Li, X.L.; An, D. Induction and identification of autotetraploids in Dendrobium. Hortic. J. 2009, 8, 1239–1242. [Google Scholar]
- Cui, G.R.; Zhang, Z.X.; Zhang, C.Y.; Hu, N.B.; Sui, Y.H.; Li, J.Q. Polyploid induction and identification of Oncidium. Acta Pratacult. Sinica 2010, 19, 184–190. [Google Scholar]
- Cui, G.R.; Zhang, Z.X.; Hu, N.B.; Zhang, C.Y.; Hou, X.L. Tetraploid of Phalaenopsis induction via colchicine treatment from protocorm-like bodies in liquid culture. J. Zhejiang Univ.—Agric. Life Sci. 2010, 1, 49–55. [Google Scholar]
- Zhang, J.X.; Wu, K.L.; Tian, L.N.; Zeng, S.J.; Duan, J. Cloning and characterization of a novel CONSTANS-like gene from Phalaenopsis hybrida. Acta Physiol. Plant. 2011, 33, 409–417. [Google Scholar] [CrossRef]
- Cheng, Q.Q. Studies on Leaf Culture and Induction of Octoploid of Phalaenopsis Cultivars. Master’s Dissertation, Shantou University, Shantou, China, 2011. [Google Scholar]
- Wang, S.Y.; Lee, P.F.; Lee, Y.I.; Hsiao, Y.Y.; Chen, Y.Y.; Pan, Z.J.; Liu, Z.J.; Tsai, W.C. Duplicated C-class MADS-box genes reveal distinct roles in gynostemium development in Cymbidium ensifolium (Orchidaceae). Plant Cell Physiol. 2011, 52, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.L. Molecular marker-assisted breeding: A plant breeder’s review. In Advances in Plant Breeding Strategies: Breed, Biotechnology Molecular Tools; Al-Khayri Shri, J.M., Jain, S.M., Johnson, D.V., Eds.; Springer: New York, NY, USA, 2015; pp. 431–472. [Google Scholar]
- Li, X.; Jin, F.; Jin, L.; Jackson, A.; Huang, C.; Li, K.; Shu, X. Development of Cymbidium ensifolium genic SSR markers and their utility in genetic diversity and population structure analysis in Cymbidiums. BMC Genet. 2014, 15, 124. [Google Scholar] [CrossRef]
- Wu, W.L.; Chung, Y.L.; Kuo, Y.T. Development of SSR markers in Phalaenopsis orchids, their characterization, cross-transferability, and application for identification. In Orchid Biotechnology III; World Scientific: London, UK, 2017; pp. 91–107. [Google Scholar]
- Sudarsono, S.; Haristianita, M.D.; Handini, A.S.; Sukma, D. Molecular marker development based on diversity of genes associated with pigment biosynthetic pathways to support breeding for novel colors in Phalaenopsis. Acta Hortic. 2017, 1167, 305–312. [Google Scholar] [CrossRef]
- Lu, J.J.; Liu, Y.Y.; Xu, J.; Mei, Z.W.; Shi, Y.J.; Liu, P.L.; He, J.B.; Wang, X.T.; Meng, Y.J.; Feng, S.G.; et al. High-density genetic map construction and stem total polysaccharide content related QTL exploration for Chinese endemic Dendrobium (Orchidaceae). Front. Plant Sci. 2018, 9, 398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Wu, K.L.; Zhang, J.X.; Deng, R.F.; Duan, J.; Silva, J.A.T.; Huang, W.C.; Zeng, S.J. Embryo development in association with asymbiotic seed germination in vitro of Paphiopedilum armeniacum. Sci. Rep. 2015, 12, 16356. [Google Scholar] [CrossRef] [PubMed]
- Pakum, W.; Watthana, S.; Srimuang, K.O.; Kongbangkerd, A. Influence of the medium component on in vitro propagation of Thai’s endangered orchid: Bulbophyllum nipondhii Seidenf. Plant Tissue Cult. Biotechnol. 2016, 25, 37–46. [Google Scholar] [CrossRef][Green Version]
- Diengdoh, R.V.; Kumaria, S.; Tandon, P.; Das, M.C. Asymbiotic germination and seed storage of Paphiopedilum insigne, an endangered lady’s slipper orchid. S. Afr. J. Bot. 2017, 112, 215–224. [Google Scholar] [CrossRef]
- Sherif, N.A.; Benjamin, J.H.F.; Kumar, T.S.; Rao, M.V. Somatic embryogenesis, acclimatization, and genetic homogeneity assessment of regenerated plantlets of Anoectochilus elatus Lindl., an endangered terrestrial jewel orchid. Plant Cell Tissue Organ Cult. 2018, 132, 303–316. [Google Scholar] [CrossRef]
- Pant, B. Medicinal orchids and their uses: Tissue culture a potential alternative for conservation. Afr. J. Plant Sci. 2013, 7, 448–467. [Google Scholar] [CrossRef]
- Shekarriz, P.; Kafi, M.; Deilamy, S.D.; Mirmasoumi, M. Coconut water and peptone improve seed germination and protocorm-like body formation of hybrid Phalaneopsis. Agric. Sci. Dev. 2014, 3, 317–322. [Google Scholar]
- Shukla, S.P.; Sharma, A. In vitro seed germination, proliferation, and ISSR marker-based clonal fidelity analysis of Shorea tumbuggaia Roxb.: An endangered and high trade medicinal tree of Eastern Ghats. Vitr. Cell. Dev. Biol. 2017, 53, 200–208. [Google Scholar] [CrossRef]
- Knudson, L. A New Nutrient Solution for Germination of Orchid Seed; American Orchid Society Bulletin: Cambridge, MA, USA, 1946; Volume 15, pp. 14–217. [Google Scholar]
- Long, B.; Niemiera, A.X.; Cheng, Z.; Long, C. In vitro propagation of four threatened Paphiopedilum species (Orchidaceae). Plant Cell Tissue Organ. Cult. 2010, 101, 151–162. [Google Scholar] [CrossRef]
- Parthibhan, S.; Benjamin, J.H.; Muthukumar, M.; Sherif, N.A.; Kumar, T.S.; Rao, M.V. Influence of nutritional media and photoperiods on in vitro asymbiotic seed germination and seedling development of Dendrobium aqueum Lindley. Afr. J. Plant Sci. 2012, 6, 383–393. [Google Scholar] [CrossRef]
- Zeng, S.; Huang, W.; Wu, K.; Zhang, J.; Silva, J.A.T.; Duan, J. In vitro propagation of Paphiopedilum orchids. Crit. Rev. Biotechnol. 2016, 36, 521–534. [Google Scholar] [PubMed]
- Gow, W.P.; Chen, J.T.; Chang, W.C. Influence of growth regulators on direct embryo formation from leaf explants of Phalaenopsis orchids. Acta Physiol. Plant 2008, 30, 507. [Google Scholar] [CrossRef]
- Lindley, C.P.; Arniputri, R.B.; Soliah, L.A.; Cahyono, O. Effects of organic additives and naphthalene acetic acid (NAA) application on the in vitro growth of black orchid hybrid Coelogyne pandurata lindley. Bulg. J. Agric. Sci. 2017, 23, 951–957. [Google Scholar]
- Zhang, Y.; Lee, Y.I.; Deng, L.; Zhao, S. Asymbiotic germination of immature seeds and the seedling development of Cypripedium macranthos Sw., an endangered lady’s slipper orchid. Sci. Hortic. 2013, 164, 130–136. [Google Scholar] [CrossRef]
- Mii, M.; Chin, D.P. Genetic transformation of orchid species: An overview of approaches and methodologies. In Orchid Propagation: From Laboratories to Greenhouses-Methods and Protocols; ISHS: Leuven, Belgium, 2018; pp. 347–365. [Google Scholar]
- Teixeira da Silva, J.A.; Tanaka, M. Multiple regeneration pathways via thin cell layers in hybrid Cymbidium (Orchidaceae). J. Plant Growth Regul. 2006, 25, 203–210. [Google Scholar] [CrossRef]
- Sivasubramaniam, S.; Loh, C.S.; Lee, Y.K.; Hew, C.S. Low-temperature storage of Dendrobium multico white callus tissue. Malay. Orchid. Rev. 1987, 21, 22–26. [Google Scholar]
- Tiwari, P.; Jen Tsung, C. Advances in Orchid Biology, Biotechnology, and Omics; Springer Publication: Berlin/Heidelberg, Germany, 2023; ISBN 13-978-9819910786. [Google Scholar]
- Chang, C.; Sung, P.G.; Chang, C.H.; Chen, Y.C.; Lin, Y.H. Seed development and storage of Bletilla formosana (Hayata) Schltr. Seed Nurs. 2006, 8, 29–38. [Google Scholar]
- Hirano, T.; Godo, T.; Miyoshi, K.; Ishikawa, K.; Ishikawa, M.; Mii, M. Cryopreservation and low-temperature storage of seeds of Phaius tankervilleae. Plant Biotechnol. Rep. 2009, 3, 103–109. [Google Scholar] [CrossRef]
- Wu, R.-Y.; Chang, S.-Y.; Hsieh, T.-F.; Chuang, K.-C.; Ting, I.; Lai, Y.-H.; Chang, Y.-S. Cryopreservation of Orchid Genetic Resources by Desiccation: A Case Study of Bletilla formosana; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Khoddamzadeh, A.A.; Sinniah, U.R.; Lynch, P.; Kadir, M.A.; Kadzimin, S.B.; Mahmood, M. Cryopreservation of protocorm-like bodies (PLBs) of Phalaenopsis bellina (Rchb.f.) Christenson by encapsulation-dehydration. Plant Cell Tissue Organ. Cult. 2011, 107, 4. [Google Scholar] [CrossRef]
- Popova, E.; Kim, H.H.; Saxena, P.K.; Engelmann, F. Frozen beauty: The cryo-biotechnology of orchid diversity. Biotechnol. Adv. 2016, 34, 380–403. [Google Scholar] [CrossRef] [PubMed]
- Sakai, A. Development of cryopreservation techniques. In Cryopreservation of Tropical Plant Germplasm; Engellmann, F., Takagi, H., Eds.; IPGRI: Rome, Italy, 2000; pp. 1–7. [Google Scholar]
- Volk, G.M.; Walters, C. Plant vitrification solution lowers water content and alters freezing behavior in shoot tips during cryoprotection. Cryobiology 2006, 52, 48–61. [Google Scholar] [CrossRef]
- Hirano, T.; Ishikawa, K.; Mii, M. Advances on orchid cryopreservation. In Floriculture Ornamental and Plant Biotechnology: Advances and Topical Issues, 1st ed.; Global Science Book; Teiceira da Silva, J.A., Ed.; Ikenobe: London, UK, 2006; Volume 2, pp. 410–414. [Google Scholar]
- Fabre, J.; Dereuddre, J. Encapsulation-dehydration: A new approach to cryopreservation of Solanum shoot tips. Cryo-Lett. 1990, 11, 413–426. [Google Scholar]
- Flachsland, E.; Terada, G.; Scocchi, A.; Rey, H.; Mroginski, L.; Engelmann, F. Cryopreservation of seeds and in vitro-cultured protocorms of Oncidium bifolium sims. (Orchidaceae) by encapsulation-dehydration. Cryo-Lett. 2006, 27, 235–242. [Google Scholar]
- Surenciski, M.R.; Flachsland, E.A.; Terada, G.; Mroginski, L.A.; Rey, H.Y. Cryopreservation of Cyrtopodium hatschbachii Pabst (Orchidaceae) immature seeds by encapsulation dehydration. Biocell 2012, 36, 31–36. [Google Scholar] [CrossRef]
- Mishiba, K.; Chin, D.P.; Mii, M. Agrobacterium-mediated transformation of Phalaenopsis by targeting protocorms at an early stage after germination. Plant Cell Rep. 2005, 24, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Knapp, J.E.; Kausch, A.P.; Chandlee, J.M. Transformation of three genera of orchid using the bar gene as a selectable marker. Plant Cell Rep. 2000, 19, 893–898. [Google Scholar] [CrossRef]
- Tee, C.S.; Maziah, M.; Tan, C.S.; Abdullah, M.P. Evaluation of different promoters Belarmino, driving the GFP reporter gene and selected target tissues for particle bombardment of Dendrobium Sonia 17. Plant Cell Rep. 2003, 21, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Belarmino, M.M.; Mii, M. Agrobacterium-mediated genetic transformation of a Phalaenopsis orchid. Plant Cell Rep. 2000, 19, 435–442. [Google Scholar] [CrossRef]
- Hiei, Y.; Komari, T.; Ishida, Y.; Saito, H. Development of Agrobacterium-mediated transformation method for monocotyledonous plants. Breed. Res. 2000, 2, 205–213. [Google Scholar] [CrossRef]
- Chin, D.P.; Mishiba, K.; Mii, M. Agrobacterium-mediated transformation of protocorm-like bodies in Cymbidium. Plant Cell Rep. 2007, 26, 735–743. [Google Scholar] [CrossRef]
- Ovcharenko, O.O.; Rudas, V.A. Modern approaches to genetic engineering in the Orchidaceae family. Cytol. Genet. 2023, 57, 142–156. [Google Scholar] [CrossRef]
- Phlaetita, W.; Chin, D.P.; Otang, N.V.; Nakamura, I.; Mii, M. High-efficiency Agrobacterium-mediated transformation of Dendrobium orchid using protocorms as a target material. Plant Biotechnol. 2015, 32, 323–327. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.A.; Dobranszki, J.; Cardoso, J.C.; Chandler, S.F.; Zeng, S. Methods for genetic transformation in Dendrobium. Plant Cell Rep. 2016, 35, 483–504. [Google Scholar] [CrossRef]
- Liu, J.; Chiou, C.; Shen, C.; Chen, P.J.; Liu, Y.C.; Jian, C.D.; Shen, X.L.; Shen, F.Q.; Yeh, K.W. RNA Interference-Based Gene Silencing of Phytoene Synthase Impairs Growth, Carotenoids, and Plastid Phenotype in Oncidium Hybrid Orchid; Springer Plus: Berlin/Heidelberg, Germany, 2014; Volume 478. [Google Scholar]
- Iiyama, C.M.; Vilcherrez-Atoche, J.A.; Germanà, M.A.; Vendrame, W.A.; Cardoso, J.C. Breeding of ornamental orchids with focus on Phalaenopsis: Current approaches, tools, and challenges for this century. Heredity 2024. [Google Scholar] [CrossRef]
- Tiwari, P.; Bose, S.K.; Gautam, A.; Chen, J.-T. Emerging insights into the cultivation strategies, ethnomedicinal uses, and socioeconomic attributes of Orchids. J. Hortic. Sci. Biotechnol. 2023, 20, 420–438. [Google Scholar]
- Hsiao, Y.Y.; Pan, Z.J.; Hsu, C.C.; Yang, Y.P.; Hsu, Y.C.; Chuang, Y.C.; Shih, H.-H.; Chen, W.-h.; Tsai, W.-C.; Chen, H.-H. Research on orchid biology and biotechnology. Plant Cell Physiol. 2011, 52, 1467–1486. [Google Scholar] [CrossRef]
- Yu, H.; Goh, C.J. Identification and characterization of three orchid MADS-box genes of the AP1/AGL9 subfamily during floral transition. Plant Physiol. 2000, 123, 1325–1336. [Google Scholar] [CrossRef]
- Ding, L.; Wang, Y.; Yu, H. Overexpression of DOSOC1, an ortholog of Arabidopsis SOC1, promotes flowering in the orchid Dendrobium Chao Parya Smile. Plant Cell Physiol. 2013, 54, 595–608. [Google Scholar] [CrossRef]
- Liang, S.; Ye, Q.S.; Li, R.H.; Leng, J.Y.; Li, M.R.; Wang, X.J.; Li, H.Q. Transcriptional regulations on the low-temperature-induced floral transition in an Orchidaceae species, Dendrobium nobile: An expressed sequence tags analysis. Int. J. Genom. 2012, 2012, 757801. [Google Scholar]
- Liu, X.R.; Pan, T.; Liang, W.Q.; Gao, L.; Wang, X.J.; Li, H.Q.; Liang, S. Overexpression of an orchid (Dendrobium nobile) SOC1/TM3-Like Ortholog, DnAGL19, in Arabidopsis regulates HOS1-FT expression. Front. Plant Sci. 2016, 7, 99. [Google Scholar] [CrossRef]
- Wang, Y.W.; Liu, L.; Song, S.Y.; Li, Y.; Shen, L.S.; Yu, H. DOFT and DOFTIP1 affect reproductive development in the orchid Dendrobium Chao Praya Smile. J. Exp. Bot. 2017, 68, 5759–5772. [Google Scholar] [CrossRef]
- Sawettalake, N.; Bunnag, S.; Wang, Y.; Shen, L.; Yu, H. DOAP1 promotes flowering in the orchid Dendrobium Chao Praya Smile. Front. Plant Sci. 2017, 8, 400. [Google Scholar] [CrossRef]
- Chen, W.; Qin, Q.; Zheng, Y.; Wang, C.; Wang, S.; Zhou, M.; Zhang, C. Overexpression of Doritaenopsis hybrid EARLY FLOWERING 4-like4 Gene, DhEFL4, postpones flowering in transgenic Arabidopsis. Plant Mol. Biol. Rep. 2016, 34, 103–117. [Google Scholar] [CrossRef]
- Hsu, H.F.; Huang, C.H.; Chou, L.T.; Yang, C.H. Ectopic expression of an orchid (Oncidium Gower Ramsey) AGL6-like gene promotes flowering by activating flowering time genes in Arabidopsis thaliana. Plant Cell Physiol. 2003, 44, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, Y.; Zhang, M.-M.; He, X.; Ahmad, S.; Lan, S.; Liu, Z.-J. Research advances on the gene regulation of floral development and color in orchids. Gene 2023, 888, 147751. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.C.; Lee, P.F.; Chen, H.I.; Hsiao, Y.Y.; Wei, W.J.; Pan, Z.J.; Chuang, M.H.; Kuoh, C.S.; Chen, W.H.; Chen, H.H. PeMADS6, a GLOBOSA/PISTILLATA-like gene in Phalaenopsis equestris involved in petaloid formation, and correlated with flower longevity and ovary development. Plant Cell Physiol. 2005, 46, 1125–1139. [Google Scholar] [CrossRef]
- Pan, Z.J.; Chen, Y.Y.; Du, J.S.; Chen, Y.Y.; Chung, M.C.; Tsai, W.C.; Wang, C.N.; Chen, H.H. Flower development of Phalaenopsis orchid involves functionally divergent SEPALLATA-like genes. New Phytol. 2014, 202, 1024–1042. [Google Scholar] [CrossRef]
- Jang, S.; Choi, S.C.; Li, H.Y.; An, G.; Schmelzer, E. Functional characterization of Phalaenopsis aphrodite flowering genes PaFT1 and PaFD. PLoS ONE 2015, 10, e0134987. [Google Scholar] [CrossRef]
- Wang, S.-L.; Viswanath, K.K.; Tong, C.-G.; An, H.R.; Jang, S.; Chen, F.-C. Floral induction and flower development of Orchids. Front. Plant Sci. 2019, 10, 1258. [Google Scholar] [CrossRef]
- Chen, D.; Guo, B.; Hexige, S.; Zhang, T.; Shen, D.; Ming, F. SQUA-like genes in the orchid Phalaenopsis are expressed in both vegetative and reproductive tissues. Planta 2007, 226, 369–380. [Google Scholar] [CrossRef]
- Su, C.-l.; Chen, W.-C.; Lee, A.-Y.; Chen, C.-Y.; Chang, Y.-C.A.; Chao, Y.-T. A modified ABCDE model of flowering in orchids based on gene expression profiling studies of the moth orchid Phalaenopsis aphrodite. PLoS ONE 2013, 8, e80462. [Google Scholar] [CrossRef]
- Teo, Z.W.N.; Zhou, W.; Shen, L. Dissecting the function of MADS-box transcription factors in Orchid reproductive development. Front. Plant Sci. 2019, 10, 1474. [Google Scholar] [CrossRef]
- Raffeiner, B.; Serek, M.; Winkelmann, T. Agrobacterium tumefaciens mediated transformation of Oncidium and Odontoglossum orchid species with the ethylene receptor mutant gene etr1-1. Plant Cell Tissue Organ Cult. 2009, 98, 125–134. [Google Scholar] [CrossRef]
- Chen, W.H.; Hsu, C.Y.; Cheng, H.Y.; Chang, H.; Chen, H.H.; Ger, M.J. Down-regulation of putative UDP-glucose: Flavonoid 3-O-glucosyltransferase gene alters flower coloring in Phalaenopsis. Plant Cell Rep. 2011, 30, 1007–1017. [Google Scholar] [CrossRef]
- Yu, H.; Yang, S.H.; Goh, C.J. Agrobacterium-mediated transformation of a Dendrobium orchid with the 1 knox gene DOH1. Plant Cell Rep. 2001, 20, 301–305. [Google Scholar] [CrossRef]
- Yang, S.H.; Yu, H.; Goh, C.J. Functional characterization of a cytokinin oxidase gene DSCKX1 in Dendrobium orchid. Plant Mol. Biol. 2003, 51, 237–248. [Google Scholar] [CrossRef]
- Yu, H.; Xu, Y. Orchids. In Biotechnology in Agriculture and Forestry, Transgenic Crops IV; Pua, E.C., Davey, M.R., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 273–286. [Google Scholar]
- Srivastava, S.; Kadooka, C.; Uchida, J.Y. Fusarium species as pathogen on orchids. Microbiol. Res. 2018, 207, 188–195. [Google Scholar] [CrossRef]
- Adil, M.; Tiwari, P.; Chen, J.-T.; Kanwal, S. Major bioactive metabolites and antimicrobial potential of Orchidaceae Fungal endophytes. In Advances in Orchid Biology, Biotechnology, and Omics; Tiwari, P., Chen, J.-T., Eds.; Springer Publishers: Berlin/Heidelberg, Germany, 2023; ISBN 978-9819910786. [Google Scholar]
- Taylor, S.C.; Laperriere, G.; Germain, H. Droplet digital PCR versus q-PCR for gene expression analysis with low abundant targets: From variable nonsense to publication quality data. Sci. Rep. 2017, 7, 2409. [Google Scholar] [CrossRef] [PubMed]
- Chocarro-Ruiz, B.; Fernández-Gavela, A.; Herranz, S.; Lechuga, L.M. Nanophotonic label-free biosensors for environmental monitoring. Curr. Opin. Biotechnol. 2017, 45, 175–183. [Google Scholar] [CrossRef]
- Jain, A.; Sarsaiya, S.; Chen, J.; Wu, Q.; Lu, Y.; Shi, J. Changes in global Orchidaceae disease geographical research trends: Recent incidences, distributions, treatment, and challenges. Bioengineered 2021, 12, 13–29. [Google Scholar] [CrossRef]
- Chang, W.H.; Yang, S.Y.; Lin, C.L.; Wang, C.-H.; Li, P.-C.; Chen, T.-Y.; Jan, F.-J.; Lee, G.-B. Detection of viruses directly from the fresh leaves of a Phalaenopsis orchid using a microfluidic system. Nanomedicine 2013, 9, 1274–1282. [Google Scholar] [CrossRef]
- Tong, C.G.; Wu, F.H.; Yuan, Y.H.; Chen, Y.R.; Lin, C.S. High-efficiency CRISPR/Cas-based editing of Phalaenopsis orchid MADS-genes. Plant Biotechnol. J. 2019, 18, 889–891. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, Q.; Zhu, Q.; Liu, W.; Chen, Y.; Qiu, R.; Wang, B.; Yang, Z.; Li, H.; Lin, Y.; et al. A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Mol. Plant 2015, 8, 1274–1284. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.S.; Hsu, C.T.; Yang, L.H.; Lee, L.Y.; Fu, J.Y.; Cheng, Q.W.; Wu, F.H.; Hisao, H.C.W.; Zhang, Y.S.; Zhang, R.; et al. Application of protoplast technology to CRISPR/Cas9 mutagenesis: From single-cell mutation detection to mutant plant regeneration. Plant Biotechnol. J. 2018, 16, 1295–1310. [Google Scholar] [CrossRef] [PubMed]
- Semiarti, E.; Nopitasari, S.; Setiawati, Y.; Lawrie, M.D.; Purwantoro, A.; Widada, J.; Ninomiya, K.; Asano, Y.; Matsumoto, S.; Yoshioka, Y. Application of CRISPR/Cas9 genome editing system for molecular breeding of orchids. Indones. J. Biotechnol. 2020, 25, 61–68. [Google Scholar] [CrossRef]
- Xia, K.; Zhang, D.; Xu, X.; Liu, G.; Yang, Y.; Chen, Z.; Wang, X.; Zhang, G.-Q.; Sun, H.-X.; Gu, Y. Protoplast technology enables the identification of efficient multiplex genome editing tools in Phalaenopsis. Plant Sci. 2022, 322, 111368. [Google Scholar] [CrossRef]
- Kui, L.; Chen, H.; Zhang, W.; He, S.; Xiong, Z.; Zhang, Y.; Yan, L.; Zhong, C.; He, F.; Chen, J.; et al. Building a genetic manipulation toolbox for orchid biology: Identification of constitutive promoters and application of CRISPR/Cas9 in the orchid, Dendrobium officinale. Front. Plant Sci. 2017, 7, 2036. [Google Scholar] [CrossRef]
- Jalal, J.S.; Kumar, P.; Pangtey, Y. Ethnomedicinal orchids of Uttarakhand, Western Himalaya. Ethnobot. Leafl. 2008, 12, 1227–1230. [Google Scholar]
- Reinikka, M.A. A History of the Orchid; Portland Timber Press: Oregon, Poland, 1995. [Google Scholar]
- Bulpitt, C. The uses and misuses of orchids in medicine. QJM Int. J. Med. 2005, 98, 625–631. [Google Scholar] [CrossRef]
- Li, P.-Y.; Li, L.; Wang, Y.-Z. Traditional uses, chemical compositions and pharmacological activities of Dendrobium: A review. J. Ethnopharmacol. 2023, 310, 116382. [Google Scholar] [CrossRef]
- Gutierrez, R.M.P. Orchids: A review of uses in traditional medicine, its phytochemistry, and pharmacology. J. Med. Plant Res. 2010, 4, 592–638. [Google Scholar]
- Pant, B.; Shrestha, S.; Pradhan, S. In vitro seed germination and seedling development of Phaius tancarvilleae (L’Her.) Blume. Sci. World 2011, 9, 50–52. [Google Scholar] [CrossRef]
- Arditti, J.; Emst, R. Micropropagation of Orchid; Wiley: New York, NY, USA, 1993. [Google Scholar]
- Teoh, E.S. Genus: Calanthe to Cyrtosia. In Medicinal orchids of Asia; Springer: Cham, Switzerland, 2015; pp. 171–250. [Google Scholar]
- Singh, S.; Singh, A.K.; Kumar, S.; Kumar, M.; Pandey, P.K.; Singh, M.C.K. Medicinal properties and uses of orchids: A concise review. Elixir Appl. Bot. 2012, 52, 11627–11634. [Google Scholar]
- Neelam, S.; Chandel, K.P.S. In Vitro conservation of Orchis latifolia: A threatened, medicinal terrestrial orchid. Ind. J. Plant Genet. Resour. 1996, 9, 109–113. [Google Scholar]
- Radice, M.; Scalvenzi, L.; Gutierrez, D. Ethnopharmacology, bioactivity and phytochemistry of Maxillaria densa Lindl. Scientific review and biotrading in the neotropics. Rev. Colomb. For. 2020, 23, 20–33. [Google Scholar]
- Pradhan, S.; Tiruwa, B.; Subedee, B.R.; Pant, B. In vitro germination and propagation of a threatened medicinal orchid, Cymbidium aloifolium (L.) Sw. through artificial seed. Asian Pacific J. Trop. Biomed. 2014, 4, 971–976. [Google Scholar] [CrossRef]
- Natta, S.; Mondol, M.S.A.; Pal, K.; Mandal, S.; Sahana, N.; Pal, R.; Pandit, G.K.; Alam, B.K.; Das, S.S.; Biswas, S.S.; et al. Chemical composition, antioxidant activity, and bioactive constituents of six native endangered medicinal orchid species from north-eastern Himalayan region of India. South Afri. Bot. 2022, 150, 248–259. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, W.; Du, Y.; Su, P.; Qiu, Y.; Ning, J.; Liu, M. Phytochemistry and pharmacological activities of Arundina graminifolia (D. Don) Hochr. and other common Orchidaceae medicinal plants. J. Ethnopharmacol. 2021, 276, 114143. [Google Scholar] [CrossRef]
- Chung, H.-H.; Shi, S.-K.; Huang, B.; Chen, J.-T. Enhanced agronomic traits and medicinal constituents of Autotetraploids in Anoectochilus formosanus Hayata, a top-grade medicinal orchid. Molecules 2017, 22, 1907. [Google Scholar] [CrossRef]
- Rosa, P.W.; Machado, M.S.; de Campos-Buzzi, F.; Niero, R.; Monache, F.D.; Filho, V.C. Seasonal and biological variations of Epidendrum mosenii: Quantification of 24-methylenecycloartanol using gas chromatography. Nat. Prod. Res. 2007, 21, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.-K.; Chern, Y.; Fang, J.-M.; Lin, C.-I.; Chen, W.-P.; Lin, Y.-L. Neuroprotective principles from Gastrodia elata. J. Nat. Prod. 2007, 70, 571–574. [Google Scholar] [CrossRef] [PubMed]
- Nongdam, P. Ethno-medicinal uses of some orchids of Nagaland, North-east India. Res. J. Med. Plants 2014, 8, 126–139. [Google Scholar] [CrossRef]
- Lam, Y.; Ng, T.B.; Yao, R.M.; Shi, J.; Xu, K.; Sze, S.C.W.; Zhang, K.Y. Evaluation of chemical constituents and important mechanism of pharmacological biology in dendrobium plants. Evid.-Based Complement. Alternat. Med. 2015, 2015, 841752. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.C.; Mahajan, R.T. Ethnobotanical potential of Eulophia species for their possible biological activity. Int. J. Pharm. Sci. Rev. Res. 2013, 21, 297–307. [Google Scholar]
- Bose, B.; Choudhury, H.; Tandon, P.; Kumaria, S. Studies on secondary metabolite profiling, anti-inflammatory potential, in vitro photoprotective and skin-aging related enzyme inhibitory activities of Malaxis acuminata, a threatened orchid of nutraceutical importance. J. Photochem. Photobiol. B Biol. 2017, 173, 686–695. [Google Scholar] [CrossRef]
- Uddin, M.J.; Rahman, M.M.; Abdullah-Al-Mamun, M.; Sadik, G. Vanda roxburghii: An experimental evaluation of antinociceptive properties of a traditional epiphytic medicinal orchid in animal models. BMC Complement. Altern. Med. 2015, 15, 305. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, L. Medicinal and pharmaceutical properties of Vanilla planifolia. Int. J. Med. Rev. 2020, 7, 25–29. [Google Scholar]
- Mishra, A.P.; Saklani, S.; Salehi, B.; Parcha, V.; Sharifi-Rad, M.; Milella, L.; Iriti, M.; Sharifi-Rad, J.; Srivastava, M. Satyrium nepalense, a high-altitude medicinal orchid of Indian Himalayan region: Chemical profile and biological activities of tuber extracts. Cell. Mol. Biol. 2018, 64, 35–53. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, X.; Fang, J.; Zhao, Z.; Huang, L.; Guo, H.; Zheng, X. Bletilla striata: Medicinal uses, phytochemistry and pharmacological activities. J. Ethnopharmacol. 2017, 195, 20–38. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Tanaka, R.; Sakurai, H.; Iguchi, K.; Yamada, Y.; Hsu, C.S.; Sakuma, C.; Kikuchi, H.; Shibayama, H.; Kawai, T. Structure of Cymbidine A, a monomeric peptidoglycan-related compound with hypotensive and diuretic activities, isolated from a higher plant, Cymbidium goeringii (Orchidaceae). Chem. Pharm. Bull. 2007, 55, 780–783. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, G.T.; Shi, J.G.; Zhang, J.J. Effects of Coeloglossum viride var. bracteatum extract on memory deficits and pathological changes in senescent mice. Basic Clin. Pharmacol. Toxicol. 2006, 98, 55–60. [Google Scholar] [PubMed]
- Matsuda, H.; Morikawa, T.; Xie, H.; Yoshikawa, M. Antiallergic phenanthrenes and stilbenes from the tubers of Gymnadenia conopsea. Planta Medica 2004, 70, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Arora, M.; Mahajan, A.; Sembi, J.S. A Review on phytochemical and pharmacological potential of family Orchidaceae. Int. Res. J. Pharm. 2017, 8, 9–24. [Google Scholar] [CrossRef]
- Hossain, M.M. Therapeutic orchids: Traditional uses and recent advances-an overview. Fitoterapia 2011, 82, 102–140. [Google Scholar] [CrossRef]
- Akter, S.; Huda, M.K.; Rahman, M.; Hoque, M.M.; Eva, T.A. Phytochemical profiling and bioactivities of Pholidota pallida Lindl. Int. J. Adv. Res. Bot. 2019, 5, 8–13. [Google Scholar]
- Tsering, J.; Tam, N.; Tag, H.; Gogoi, B.J.; Apang, O. Medicinal orchids of Arunachal Pradesh: A review. Bull. Arunachal For. Res. 2017, 32, 1–16. [Google Scholar]
- Singh, A.; Duggal, S. Medicinal Orchids: An overview. Ethnobot. Leafl. 2009, 13, 351–363. [Google Scholar]
- Bhattarai, P.; Adhikari, Y.; Kunwar, R.M.; Bussmann, R.W. Pleione humilis (Sm.) D. Don. Orchidaceae. In Ethnobotany of the Himalayas, Ethnobotany of Mountain Regions, Kunwar, R., Sher, H., Bussmann, R.W., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Chen, C.C.; Wu, L.G.; Ko, F.N.; Teng, C.M. Antiplatelet aggregation principles of Dendrobium loddigesii. J. Nat. Prod. 1994, 57, 1271–1274. [Google Scholar] [CrossRef] [PubMed]
- Chinsamy, M.; Finnie, J.F.; van Staden, J. The ethnobotany of South African medicinal orchids. S. Afr. J. Bot. 2011, 77, 2–9. [Google Scholar] [CrossRef]
- Majumder, P.L.; Pal, S. Rotundatin, a new 9,10-dihydrophenanthrene derivative from Dendrobium rotunatum. Phytochemistry 1992, 31, 3225–3228. [Google Scholar] [CrossRef]
- Honda, C.; Yamaki, M. Phenanthrenes from Dendrobium plicatile. Phytochemistry 2000, 53, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, J.K.; Wang, J.; Li, W.N.; Kurihara, H.; Kitanaka, S.; Yao, X.S. Bioactive bibenzyl derivatives and fluorenones from Dendrobium nobile. J. Nat. Prod. 2007, 70, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, C.L.; Guo, S.X.; Yang, J.S.; Xiao, P.G. Two new compounds from Dendrobium candidum. Chem. Pharm. Bull. 2008, 56, 1477–1479. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yu, H.; Qing, C.; Zhang, Y.; Liu, Y.; Chen, Y. Two new biphenanthrenes with cytotoxic activity from Bulbophyllum odoratissimum. Fitoterapia 2009, 80, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Lalitharani, S.; Mohan, V.R.; Maruthupandian, A. Pharmacognostic investigations on Bulbophyllum albidum (Wight) Hook. f. Int. J. PharmTech Res. 2011, 3, 556–562. [Google Scholar]
- Majumder, P.L.; Guha, B.N.S.; Ray, P.N.S. Four new stilbenoids from the orchids Coelogyne ochracea and Coelogyne cristata. J. Indian Chem. Soc. 2011, 88, 1293–1304. [Google Scholar]
- Williams, C.A. The leaf flavonoids of the Orchidaceae. Phytochemistry 1979, 18, 803–813. [Google Scholar] [CrossRef]
- Gutierrez, R.M.P.; Sosa, I.A.; Vadillo, C.H.; Victoria, T.C. Effect of flavonoids from Prosthechea michuacana on carbon tetrachloride-induced acute hepatotoxicity in mice. Pharma. Biol. 2011, 49, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.T.; Chen, B.C.; Yu, C.C.; Weng, C.M.; Hsu, M.J.; Chen, C.C.; Chen, M.C.; Teng, C.M.; Pan, S.L.; Bien, M.Y.; et al. Apoptosis signal-regulating kinase 1 mediates denbinobin-induced apoptosis in human lung adenocarcinoma cells. J. Biomed. Sci. 2009, 16, 43. [Google Scholar] [CrossRef]
- Khouri, N.A.; Nawasreh, M.; Al-hussain, S.M.; Alkofahi, A.S. Effects of orchids (Orchis anatolica) on reproductive function and fertility in adult male mice. Reprod. Med. Biol. 2006, 5, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Saleem, U. Investigating the medicinal properties of orchids. Columbia Sci. Rev. 2007, 4, 22–23. [Google Scholar]
- Maridass, M. Anti diarrhoeal activity of rare orchid Eulophia epidendraea (Retz.) Fisher. Nat. Pharm. Technol. 2011, 1, 5–10. [Google Scholar]
- Maridass, M.; Raju, G.; Ghanthikumar, S. Tissue-regenerative responses on tuber extracts of Eulophia epidendraea (Retz.) Fischer in Wistar rat. Pharmacology 2008, 3, 631–636. [Google Scholar]
- Ng, T.B.; Liu, J.; Wong, J.H.; Ye, X.; Wing Sze, S.C.; Tong, Y.; Zhang, K.Y. Review of research on Dendrobium, a prized folk medicine. Appl. Microbiol. Biotechnol. 2012, 93, 1795–1803. [Google Scholar] [CrossRef]
- Liu, C.L.; Liu, M.C.; Zhu, R.L. Determination of gastrodin, ρ-hydroxybenzyl alcohol, vanillyl alcohol, ρ-hydroxylbenzaldehyde, and vanillin in tall gastrodia tuber by high-performance liquid chromatography. Chromatographia 2002, 55, 317–320. [Google Scholar] [CrossRef]
- Lee, J.Y.; Jang, Y.W.; Kang, H.S.; Moon, H.; Sim, S.S.; Kim, C.J. Anti-inflammatory action of phenolic compounds from Gastrodia elata root. Arch. Pharmacal Res. 2006, 29, 849–858. [Google Scholar] [CrossRef]
- Szlachetko, D.L. Genera et species Orchidalium. Pol. Bot. J. 2001, 46, 11–26. [Google Scholar]
- Fonmboh, D.J.; Fokunang, T.E.; Ndasi, N.P.; Ngangmou, N.T.; Herve, B.; Tita, B.L.; Nubia, K.C.; Awah, T.M.; Aba, E.R.; Fokunang, C.N. An overview of the ethnobotanic, ethnopharmacological and medicinal importance of edible wild root tuber orchids in Cameroon. Asian J. Biotechnol. Bioresour. Technol. 2021, 7, 11–24. [Google Scholar] [CrossRef]
- Dikanda, P.C. Contribution à l’Étude des Plantes Médicinales de New-Malimba dans l’Arrondissement d’Edéa au Cameroun. Master’s Thesis, Université de Yaoundé I, Yaoundé, Cameroon, 2000; 81p. [Google Scholar]
- Ekole, O.D. Etude de Quelques Plantes Camerounaises Pouvant Être Utilisées Comme Médicinales; Mémoire de DIPES II; Université de Yaoundé I: Yaoundé, Cameroon, 1994; 61p. [Google Scholar]
- Simo, M.; Droissart, V.; Sonke, B.; Stevart, T. The orchid flora of the Mbam Minkom Hills (Yaounde, Cameroon). Belg. J. Bot. 2009, 142, 111–123. [Google Scholar]
- Kasulo, L.; Mwabumba, B.; Munthali, C. A review of edible orchids in Malawi. J. Hortic. For. 2009, 1, 133–139. [Google Scholar]
- Suh, C.E.; Sparks, R.S.J.; Fitton, J.G.; Ayonghe, S.N.; Annen, C.; Nana, R.; Luckman, A. The 1999 and 2000 eruptions of Mt. Cameroon; eruption, behaviour, and petrochemistry of lava. Bull. Volcanicity 2003, 65, 267–281. [Google Scholar] [CrossRef]
- Lalika, M.C.S.; Mende, D.H.; Urio, P.; Gimbi, D.M.; Mwanyika, S.J.; Donati, G. Domestication potential and nutrient composition of wild orchids from two southern regions in Tanzania. Time J. Biol. Sci. Technol. 2013, 1, 1–11. [Google Scholar]
- POWO. Vanilla planifolia Andrews. Plants of the World Online. Royal Botanic Gardens, Kew. 2023. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:262578-2 (accessed on 15 January 2024).
- Vega, M.; Hernández, M.; Herrera-Cabrera, B.E.; Wegier, A. Vanilla planifolia. In IUCN Red List of Threatened Species; E.T103090930A172970359; International Union for Conservation of Nature and Natural Resources: Gland, Switzerland, 2020. [Google Scholar] [CrossRef]
- de Oliveira, R.T.; da Silva Oliveira, J.P.; Macedo, A.F. Vanilla beyond Vanilla planifolia and Vanilla × tahitensis: Taxonomy and historical notes, reproductive biology, and metabolites. Plants 2022, 11, 3311. [Google Scholar] [CrossRef] [PubMed]
- Hasing, T.; Tang, H.; Brym, M.; Khazi, F.; Huang, T.; Chambers, A.H. A phased Vanilla planifolia genome enables genetic improvement of flavor and production. Nat. Food 2020, 1, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Balilashaki, K.; Zakizadeh, H.; Olfati, J.-A.; Vahedi, M.; Kumar, A.; Indracanti, M. Recent advances in Phalaenopsis orchid improvement using Omics approaches. Plant Tissue Cult. Biotechnol. 2019, 29, 133–214. [Google Scholar] [CrossRef]
- Leitch, I.J.; Kahandawala, I.; Suda, J.; Hanson, L.; Ingrouille, M.J.; Chase, M.W.; Fay, M.F. Genome size diversity in orchids: Consequences and evolution. Ann. Bot. 2009, 104, 469–481. [Google Scholar] [CrossRef]
- Lin, S.; Lee, H.C.; Chen, W.H.; Chen, C.C.; Kao, Y.Y.; Fu, Y.M.; Chen, Y.H.; Lin, T.Y. Nuclear DNA contents of Phalaenopsis sp. and Doritis pulcherrima. J. Am. Soc. Hortic. Sci. 2001, 126, 195–199. [Google Scholar] [CrossRef]
- Fattmah; Sukma, D. Development of sequence-based microsatellite marker for Phalaenopsis orchid. HAYATI J. Biosci. 2011, 18, 71–76. [Google Scholar] [CrossRef]
- Ko, Y.Z.; Shih, H.C.; Tsai, C.C.; Ho, H.H.; Liao, P.C.; Chiang, Y.C. Screening transferable microsatellite markers across genus Phalaenopsis (Orchidaceae). Bot. Stud. 2017, 58, 48. [Google Scholar] [CrossRef]
- Ai, Y.; Zheng, Q.D.; Wang, M.J.; Xiong, L.W.; Li, P.; Guo, L.T.; Wang, M.Y.; Peng, D.H.; Lan, S.R.; Liu, Z.J. Molecular mechanism of different flower color formation of Cymbidium ensifolium. Plant Mol. Biol. 2023, 113, 193–204. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, K.; Zeng, S.; Teixeira da Silva, J.A.; Zhao, X.; Tian, C.E.; Xia, H.; Duan, J. Transcriptome analysis of Cymbidium sinense and its application to the identification of genes associated with floral development. BMC Genom. 2013, 14, 279. [Google Scholar] [CrossRef]
- Aceto, S.; Sica, M.; De Paolo, S.; D’Argenio, V.; Cantiello, P.; Salvatore, F.; Gaudio, L. The analysis of the inforescence miRNome of the orchid Orchis italica reveals a DEF-Like MADS-box gene as a new miRNA target. PLoS ONE 2014, 9, e97839. [Google Scholar] [CrossRef]
- Pontrelli, P.; Accetturo, M.; Gesualdo, L. miRNome analysis using real-time PCR. Methods Mol. Biol. 2014, 1186, 201–232. [Google Scholar]
- Kawahara, Y.; Oono, Y.; Wakimoto, H.; Ogata, J.; Kanamori, H.; Sasaki, H.; Mori, S.; Matsumoto, T.; Itoh, T. TENOR: Database for comprehensive mRNA-Seq experiments in rice. Plant Cell Physiol. 2016, 57, e7. [Google Scholar] [CrossRef]
- Szczesniak, M.W.; Rosikiewicz, W.; Makalowska, I. CANTATAdb: A collection of plant long non-coding RNAs. Plant Cell Physiol. 2016, 57, e8. [Google Scholar] [CrossRef]
- Chao, Y.-T.; Yen, S.-H.; Yeh, J.-H.; Chen, W.-C.; Shih, M.-C. Orchidstra 2.0—A transcriptomics resource for the orchid family. Plant Cell Physiol. 2017, 58, e9. [Google Scholar] [CrossRef]
- Aya, K.; Kobayashi, M.; Tanaka, J.; Ohyanagi, H.; Suzuki, T.; Yano, K.; Matsuoka, M. De novo transcriptome assembly of a fern, Lygodium japonicum, and a web resource database, Ljtrans DB. Plant Cell Physiol. 2015, 56, e5. [Google Scholar] [CrossRef]
- Ohyanagi, H.; Ebata, T.; Huang, X.; Gong, H.; Fujita, M.; Mochizuki, T.; Toyoda, A.; Fujiyama, A.; Kaminuma, E.; Nakamura, Y.; et al. Oryza Genome: Genome diversity database of wild Oryza species. Plant Cell Physiol. 2016, 57, e1. [Google Scholar] [CrossRef]
- Liu, S.-S.; Chen, J.; Li, S.-C.; Zeng, X.; Meng, Z.-X.; Guo, S.-X. Comparative transcriptome analysis of genes involved in GA-GID1-DELLA regulatory module in symbiotic and asymbiotic seed germination of Anoectochilus roxburghii (Wall.) Lindl. (Orchidaceae). Int. J. Mol. Sci. 2015, 16, 30190–30203. [Google Scholar] [CrossRef]
- Valadares, R.B.S.; Perotto, S.; Santos, E.C.; Lambais, M.R. Proteome changes in Oncidium sphacelatum (Orchidaceae) at different trophic stages of symbiotic germination. Mycorrhiza 2014, 24, 349–360. [Google Scholar] [CrossRef]
- Wang, W.; Yu, H.; Li, T.; Li, L.; Zhang, G.; Liu, Z.; Huang, T.; Zhang, Y. Comparative proteomics analyses of pollination response in endangered orchid species Dendrobium chrysanthum. Int. J. Mol. Sci. 2017, 18, 2496. [Google Scholar] [CrossRef]
- Sedeek, K.E.; Qi, W.; Schauer, M.A.; Gupta, A.K.; Poveda, L.; Xu, S.; Liu, Z.J.; Grossniklaus, U.; Schiestl, F.P.; Schlüter, P.M. Transcriptome and proteome data reveal candidate genes for pollinator attraction in sexually deceptive orchids. PLoS ONE 2013, 8, e64621. [Google Scholar] [CrossRef]
- Li, X.; Xu, W.; Chowdhury, M.R.; Jin, F. Comparative proteomic analysis of labellum and inner lateral petals in Cymbidium ensifolium flowers. Int. J. Mol. Sci. 2014, 15, 19877–19897. [Google Scholar] [CrossRef]
- Tsai, W.C.; Pan, Z.J.; Hsiao, Y.Y.; Jeng, M.F.; Wu, T.F.; Chen, W.H.; Chen, H.H. Interactions of B-class complex proteins involved in tepal development in Phalaenopsis orchid. Plant Cell Physiol. 2008, 49, 814–824. [Google Scholar] [CrossRef]
- Chen, J.-C.; Wei, M.-J.; Fang, S.-C. Expression analysis of fertilization/early embryogenesis-associated genes in Phalaenopsis orchids. Plant Signal. Behav. 2016, 11, e1237331. [Google Scholar] [CrossRef]
- He, C.M.; Si, C.; Teixeira da Silva, J.A.; Li, M.Z.; Duan, J. Genome-wide identification and classification of MIKC-type MADS-box genes in Streptophyte lineages and expression analyses to reveal their role in seed germination of orchids. BMC Evol. Biol. 2019, 19, 223. [Google Scholar] [CrossRef]
- Williams, S.J.; Gale, S.W.; Hinsley, A.; Gao, J.; St. John, F.A.V. Using consumer preferences to characterize the trade of wild-collected ornamental orchids in China. Conserv. Lett. 2018, 11, e12569. [Google Scholar] [CrossRef]
- Hinsley, A. The Role of Online Platforms in the Illegal Orchid Trade from Southeast Asia; The Global Initiative against Transnational Organized Crime: Geneva, Switzerland, 2018; Available online: www.GlobalInitiative.net (accessed on 25 December 2023).
- Irawati, A. Conservation of orchids, the gems of the tropics. In Conservation of Tropical Plant Species; Normah, M., Chin, H., Reed, B., Eds.; Springer: New York, NY, USA, 2013; pp. 171–187. [Google Scholar]
- Schuiteman, A.; Discovering New Orchids. Royal Botanic Gardens Kew. Available online: https://www.kew.org/read-and-watch/discovering-new-orchids (accessed on 25 December 2023).
- Hinsley, A.; Lee, T.E.; Harrison, J.R.; Roberts, D.L. Estimating the extent and structure of trade in horticultural orchids via social media. Conserv. Biol. 2016, 30, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Soda, N.; Verma, L.; Giri, J. CRISPR-Cas9 based plant genome editing: Significance, opportunities, and recent advances. Plant Physiol. Biochem. 2018, 131, 2–11. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).