Optimized Fertilizer–Water Management Improves Carrot Quality and Soil Nutrition and Reduces Greenhouse Gas Emissions on the North China Plain
Abstract
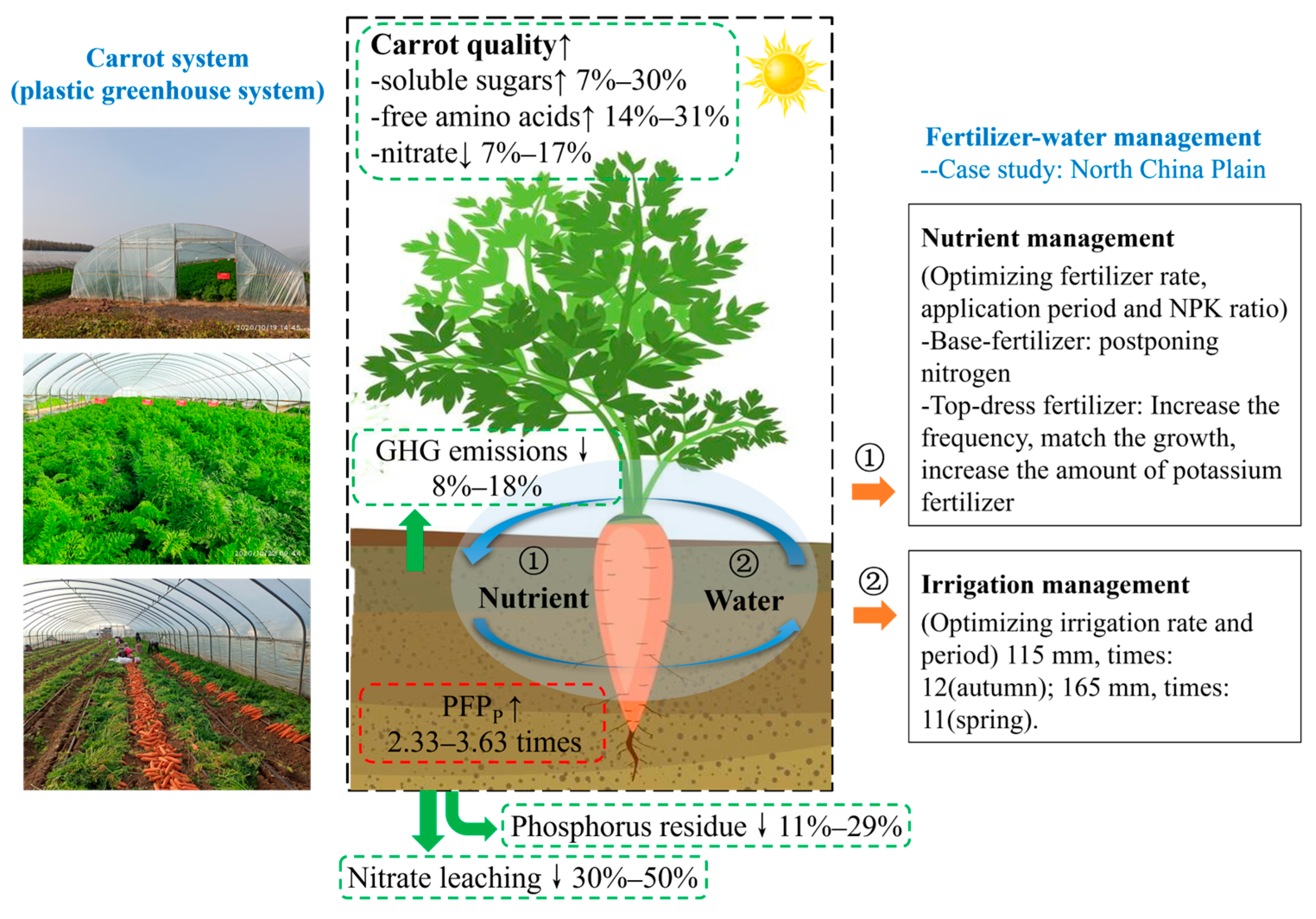
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Soil Parameters
2.2. Experimental Design
2.3. Plant Sampling and Measurements
2.4. Soil Sampling and Measurements
2.5. Nutrient and Water Use Calculations
2.6. GHG Emissions
2.7. Statistical Analyses
3. Results
3.1. Yield and Growth Parameters
3.2. Biochemical Parameters, Dry Matter Accumulation, and Nutrient Accumulation
3.3. Soil N Content, Residual P Levels, and GHG Emissions
3.4. Nutrient and Water Utilization Efficiency
3.5. RDA for Root Quality and Nutrient Contents
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 27 January 2024).
- Li, T.F.; Zhou, P.Y.; Ding, Y.C.; Zhou, S.S.; Liu, Y. Distribution characteristics and source analysis of nitrogen and phosphorus in different rivers in two water period: A case study of Pi river and shiting river in the upper reaches of tuo river in China. Int. J. Environ. Res. Public Health 2022, 19, 12433. [Google Scholar] [CrossRef]
- Sikora, J.; Niemiec, M.; Szeląg-Sikora, A.; Gródek-Szostak, Z.; Kuboń, M.; Komorowska, M. The impact of a controlled-release fertilizer on greenhouse gas emissions and the efficiency of the production of chinese cabbage. Energies 2020, 13, 2063. [Google Scholar] [CrossRef]
- Wang, X.Z.; Dou, Z.X.; Shi, X.J.; Zou, C.Q.; Liu, D.Y.; Wang, Z.Y.; Guan, X.L.; Sun, Y.X.; Wu, G.; Zhang, B.G.; et al. Innovative management programme reduces environmental impacts in chinese vegetable production. Nat. Food 2021, 2, 47–53. [Google Scholar] [CrossRef]
- Colombari, L.F.; Lanna, N.B.; Guimarães, L.R.; Cardoso, A.I.I. Production and quality of carrot in function of split application of nitrogen doses in top dressing. Hortic. Bras. 2018, 36, 306–312. [Google Scholar] [CrossRef]
- Sikora, J.; Niemiec, M.; Tabak, M.; Gródek-Szostak, Z.; Szeląg-Sikora, A.; Kuboń, M.; Komorowska, M. Assessment of the efficiency of nitrogen slow-release fertilizers in integrated production of carrot depending on fertilization strategy. Sustainability 2020, 12, 1982. [Google Scholar] [CrossRef]
- Afrin, A.; Islam, M.A.; Hossain, M.M.; Hafiz, M.M.H. Growth and yield of carrot influenced by organic and inorganic fertilizers with irrigation interva: Fertilizer and irrigation affect yield of carrot. J. Bangladesh. Agril. Univ. 2019, 17, 338–343. [Google Scholar] [CrossRef]
- Bender, I.; Edesi, L.; Hiiesalu, I.; Ingver, A.; Kaart, T.; Kaldmäe, H.; Talve, T.; Tamm, I.; Luik, A. organic carrot (Daucus carota L.) production has an advantage over conventional in quantity as well as in quality. Agronomy 2020, 10, 1420. [Google Scholar] [CrossRef]
- Diallo, F.; Legros, S.; Diarra, K.; Feder, F. Varying effects of organic waste products on yields of market garden crops in a 4-year field experiment under tropical conditions. Agronomy 2022, 12, 32. [Google Scholar] [CrossRef]
- Kiran, M.; Jilani, M.S.; Waseem, K.; Haq, F.; Khan, M.S.; Nadim, M.A.; Rahman, K.; Hussain, K. Growth and yield enhancement of carrot through integration of NPK and organic manures. J. Hortic. Sci. 2022, 17, 341–346. [Google Scholar] [CrossRef]
- Çakmakçı, S.; Çakmakçı, R. Quality and Nutritional Parameters of Food in Agri-Food Production Systems. Foods 2023, 12, 351. [Google Scholar] [CrossRef]
- Li, J.J.; Li, C.Z.; Gao, L.J.; Liu, D.S.; Zou, G.Y.; Xu, J.X.; Li, S.M.; Sun, Q.P. Impact of different organic fertilizers on soil humus, microbiomass and quality of cabbage. Adv. Mater. 2014, 955–959, 628–632. [Google Scholar] [CrossRef]
- Li, F.L.; Kong, Q.B.; Zhang, Q.; Wang, H.P.; Wang, L.M.; Luo, T. Spent mushroom substrates affect soil humus composition, microbial biomass and functional diversity in paddy fields. Appl. Soil Ecol. 2020, 149, 103489. [Google Scholar] [CrossRef]
- Pascual-Seva, N.; San Bautista, A.; López-Galarza, S.; Maroto, J.V.; Pascual, B. Influence of different drip irrigation strategies on irrigation water use efficiency on chufa (Cyperus esculentus L. var. Sativus Boeck.) crop. Agric. Water Manag. 2018, 208, 406–413. [Google Scholar] [CrossRef]
- Yavuz, D.; Seymen, M.; Yavuz, N.; Türkmen, Ö. Effects of irrigation interval and quantity on the yield and quality of confectionary pumpkin grown under field conditions. Agric. Water Manag. 2015, 159, 290–298. [Google Scholar] [CrossRef]
- Lopes, J.; Medeiros, D.L.; Kiperstok, A. Combining cleaner production and life cycle assessment for reducing the environmental impacts of irrigated carrot production in brazilian semi-arid region. J. Clean. Prod. 2018, 170, 924–939. [Google Scholar] [CrossRef]
- Léllis, B.C.; Carvalho, D.F.; Martínez-Romero, A.; Tarjuelo, J.M.; Domínguez, A. Effective management of irrigation water for carrot under constant and optimized regulated deficit irrigation in Brazil. Agric. Water Manag. 2017, 192, 294–305. [Google Scholar] [CrossRef]
- Gutezeit, B. Yield and quality of carrots as affected by soil moisture and N-fertilization. J. Hortic. Sci. Biotechnol. 2001, 76, 732–738. [Google Scholar] [CrossRef]
- Cheng, M.H.; Wang, H.D.; Zhang, F.C.; Wang, X.K.; Liao, Z.Q.; Zhang, S.H.; Yang, Q.L.; Fan, J.L. Effects of irrigation and fertilization regimes on tuber yield, water-nutrient uptake and productivity of potato under drip fertigation in sandy regions of northern China. Agric. Water Manag. 2023, 287, 108459. [Google Scholar] [CrossRef]
- Liu, Q.M.; Ding, Y.Q.; Chi, R.P.; Dong, P.Y. Safe and High-efficient cultivation technology for facility carrot industrialization. Asian Agric. Res. 2018, 10, 65–67. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022. [Google Scholar]
- Wang, G.L.; Huang, W.; Li, M.Y.; Xu, Z.S.; Wang, F.; Xiong, A.S. Expression profiles of genes involved in jasmonic acid biosynthesis and signaling during growth and development of carrot. Acta Biochim. Biophys. Sin. 2016, 48, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Moore, S. Amino acid analysis: Aqueous dimethyl sulfoxide as solvent for the ninhydrin reaction. J. Biol. Chem. 1968, 243, 6281–6283. [Google Scholar] [CrossRef] [PubMed]
- Barnes, R.B.; Richardson, D.; Berry, J.W.; Hood, R.L. Flame photometry a rapid analytical procedure. Ind. Eng. Chem. Anal. Ed. 1945, 17, 605–611. [Google Scholar] [CrossRef]
- Bremner, J.M. Methods of soil analysis. Agronomy 1965, 9, 1195–1198. [Google Scholar]
- Cavell, A.J. The colorimetric determination of phosphorous in plant materials. J. Sci. Food Agric. 1955, 6, 479–481. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid Colorimetric Determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Hernández, Y.; Lobo, M.G.; González, M. Determination of vitamin c in tropical fruits: A comparative evaluation of methods. Food Chem. 2006, 96, 654–664. [Google Scholar] [CrossRef]
- Sreenivasan, A.; Vaidya, R.M. Determination of carotene in plant materials. Anal. Chem. 1948, 20, 720–722. [Google Scholar] [CrossRef]
- Ramagli, L.S.; Rodriguez, L.V. Quantitation of microgram amounts of protein in two-dimensional polyacrylamide gel electrophoresis sample buffer. Electrophoresis 1985, 6, 559–563. [Google Scholar] [CrossRef]
- Ebell, L.F. Variation in total soluble sugars of conifer tissues with method of analysis. Phytochemistry 1969, 8, 227–233. [Google Scholar] [CrossRef]
- Mussa, S.A.B.; Elferjani, H.S.; Haroun, F.A.; Abdelnabi, F.F. Determination of available nitrate, phosphate and sulfate in soil samples. Int. J. PharmTech Res. 2009, 1, 598–604. [Google Scholar]
- Yang, F.; Zhai, W.; Li, Z.; Huang, Y.; Manzoor, M.; Yang, B.; Hou, Y.; Lei, L.; Tang, X. Immobilization of lead and cadmium in agricultural soil by bioelectrochemical reduction of sulfate in underground water. Chem. Eng. J. 2021, 422, 130010. [Google Scholar] [CrossRef]
- Huang, J.; Wang, C.; Qi, L.; Zhang, X.; Tang, G.; Li, L.; Guo, J.; Jia, Y.; Dou, X.; Lu, M. Phosphorus is more effective than nitrogen in restoring plant communities of heavy metals polluted soils. Environ. Pollut. 2020, 266, 115259. [Google Scholar] [CrossRef]
- Mulvaney, R.L.; Khan, S.A.; Stevens, W.B.; Mulvaney, C.S. Improved diffusion methods for determination of inorganic nitrogen in soil extracts and water. Biol. Fertil. Soils 1997, 24, 413–420. [Google Scholar] [CrossRef]
- Chen, X.C.; Chen, F.J.; Chen, Y.L.; Gao, Q.; Yang, X.L.; Yuan, L.X.; Zhang, F.S.; Mi, G.H. Modern maize hybrids in Northeast China exhibit increased yield potential and resource use efficiency despite adverse climate change. Glob. Chang. Biol. 2013, 19, 923–936. [Google Scholar] [CrossRef]
- Lombardo, S.; Pandino, G.; Mauromicale, G. Optimizing Nitrogen Fertilization to Improve Qualitative Performances and Physiological and Yield Responses of Potato (Solanum tuberosum L.). Agronomy 2020, 10, 352. [Google Scholar] [CrossRef]
- Luo, H.H.; Wang, Q.; Zhang, J.K.; Wang, L.S.; Li, Y.B.; Yang, G.Z. Minimum fertilization at the appearance of the first flower benefits cotton nutrient utilization of nitrogen, phosphorus and potassium. Sci. Rep. 2020, 10, 6815. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.Z.; Nishiyama, S.; Kang, Y. Effects of different irrigation regimes on the growth and yield of drip-irrigated potato. Agric. Water Manag. 2003, 63, 153–167. [Google Scholar] [CrossRef]
- Li, H.H.; Liu, H.; Gong, X.W.; Li, S.; Pang, J.; Chen, Z.F.; Sun, J.S. Optimizing irrigation and nitrogen management strategy to trade off yield, crop water productivity, nitrogen use efficiency and fruit quality of greenhouse grown tomato. Agric. Water Manag. 2021, 245, 106570. [Google Scholar] [CrossRef]
- Wang, X.Z.; Liu, B.; Wu, G.; Sun, Y.X.; Guo, X.S.; Jin, G.Q.; Jin, Z.H.; Zou, C.Q.; Chadwick, D.; Chen, X.P. Cutting carbon footprints of vegetable production with integrated soil—Crop system management: A case study of greenhouse pepper production. J. Clean. Prod. 2020, 254, 120158. [Google Scholar] [CrossRef]
- IPCC (Intergovernmental Panel on Climate Change). Climate change 2014: Impacts, Adaptation and Vulnerability: Regional Aspects; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- ISO 14040: 2006; Environmental Management Life Cycle Assessment Principles and Framework. ISO (International Organization for Standardization): London, UK, 2006. Available online: https://www.iso.org/standard/37456.html (accessed on 27 January 2024).
- ISO 14044: 2006; Environmental Management Life Cycle Assessment Requirements and Guidelines. ISO (International Organization for Standardization): London, UK, 2006. Available online: https://www.iso.org/standard/38498.html (accessed on 27 January 2024).
- Zhang, W.F.; Dou, Z.X.; He, P.; Ju, X.T.; Powlson, D.; Chadwick, D.; Norse, D.; Lu, Y.L.; Zhang, Y.; Wu, L.; et al. New technologies reduce greenhouse gas emissions from nitrogenous fertilizer in China. Proc. Natl. Acad. Sci. USA 2013, 110, 8375–8380. [Google Scholar] [CrossRef] [PubMed]
- Yue, S.C. Optimum Nitrogen Management for High-Yielding Wheat and Maize Cropping System; China Agricultural University Press: Beijing, China, 2013; p. 80. [Google Scholar]
- Ji, C.L.; Ding, M.; Wang, B.X.; Wang, C.M.; Zhao, Y.W. Comparative evaluation of chemical and organic fertilizer on the base of life cycle analysis methods. Chin. J. Soil Sci. 2012, 43, 412–417, (In Chinese with English abstract). [Google Scholar]
- Clark, S.; Khoshnevisan, B.; Sefeedpari, P. Energy efficiency and greenhouse gas emissions during transition to organic and reduced-input practices: Student farm case study. Ecol. Eng. 2016, 88, 186–194. [Google Scholar] [CrossRef]
- Cui, Z.L.; Yue, S.C.; Wang, G.L.; Meng, Q.F.; Wu, L.; Yang, Z.P.; Zhang, Q.; Li, S.Q.; Zhang, F.S.; Chen, X.P. In-season root-zone N management for mitigating greenhouse gas emission and reactive N losses in intensive wheat production. Environ. Sci. Technol. 2013, 47, 6015–6022. [Google Scholar] [CrossRef] [PubMed]
- Pishgar-Komleh, S.H.; Omid, M.; Heidari, M.D. On the study of energy use and GHG (greenhouse gas) emissions in greenhouse cucumber production in Yazd province. Energy 2013, 59, 63–71. [Google Scholar] [CrossRef]
- Liang, L.; Ridoutt, B.G.; Lal, R.; Wang, D.; Wu, W.L.; Peng, P.; Hang, S.; Wang, L.Y.; Zhao, G.S. Nitrogen footprint and nitrogen use efficiency of greenhouse tomato production in North China. J. Clean. Prod. 2019, 208, 285–296. [Google Scholar] [CrossRef]
- Chen, W.; Yin, X.; Ma, D. A bottom-up analysis of China’s iron and steel industrial energy consumption and CO2 emissions. Appl. Energ. 2014, 136, 1174–1183. [Google Scholar] [CrossRef]
- Tian, Y.; Zhu, Q.; Geng, Y. An analysis of energy-related greenhouse gas emissions in the Chinese iron and steel industry. Energ. Policy 2013, 56, 352–361. [Google Scholar] [CrossRef]
- Wang, L.F.; Zhang, L.S. Life cycle assessment of environmental impacts for the whole steel production process. China Popul. Resour. Environ. 2012, 22, 239–244, (In Chinese with English abstract). [Google Scholar]
- Perrin, A.; Basset-Mens, C.; Gabrielle, B. Life cycle assessment of vegetable products: A review focusing on cropping systems diversity and the estimation of field emissions. Int. J. Life Cycle Assess 2014, 19, 1247–1263. [Google Scholar] [CrossRef]
- Guo, Y.J. Study on Regulation Rules of a Nitrification Inhibitor, Dicyandiamide (DCD) on Fertilizer Nitrogen Losses from a Greenhouse Vegetable Soil. Ph.D. Thesis, Agricultural University of Hebei, Baoding, China, 2012. [Google Scholar]
- Wang, X.Z.; Zou, C.Q.; Gao, X.P.; Guan, X.L.; Zhang, W.; Zhang, Y.Q.; Shi, X.J.; Chen, X.P. Nitrous oxide emissions in Chinese vegetable systems: A meta-analysis. Environ. Pollut. 2018, 239, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Ti, C.P.; Luo, Y.X.; Yan, X.Y. Characteristics of nitrogen balance in open-air and greenhouse vegetable cropping systems of China. Environ. Sci. Pollut. Res. 2015, 22, 18508–18518. [Google Scholar] [CrossRef]
- Lu, K.P. Optimized Management of Nitrogen Fertilizer and Strategies for Reducing Nitrogen Leaching Loss in Greenhouse Vegetable Field in Taihu Region. Ph.D. Thesis, Nanjing Agricultural University, Nanjing, China, 2012. [Google Scholar]
- Min, J.; Zhang, H.; Shi, W. Optimizing nitrogen input to reduce nitrate leaching loss in greenhouse vegetable production. Agric. Water Manag. 2012, 111, 53–59. [Google Scholar] [CrossRef]
- Yu, F.B.; Luo, X.P.; Song, C.F.; Zhang, M.X.; Shan, S.D. Concentrated biogas slurry enhanced soil fertility and tomato quality. Acta Agric. Scand. Sect. B Soil Plant Sci. 2010, 60, 262–268. [Google Scholar] [CrossRef]
- Zheng, J.; Qi, X.Y.; Shi, C.; Yang, S.H.; Wu, Y. Tomato comprehensive quality evaluation and irrigation mode optimization with biogas slurry based on the combined evaluation model. Agronomy 2022, 12, 1391. [Google Scholar] [CrossRef]
- Carvalho, A.D.; Silva, G.O.; Resende, F.V. Adaptabilidade e estabilidade de populações de cenoura pelo método REML/BLUP. Hortic. Bras. 2017, 35, 69–74. [Google Scholar] [CrossRef][Green Version]
- Liu, B.; Wang, X.; Ma, L.; Chadwick, D.; Chen, X. Combined applications of organic and synthetic nitrogen fertilizers for improving crop yield and reducing reactive nitrogen losses from China’s vegetable systems: A meta-analysis. Environ. Pollut. 2021, 269, 116143. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, A.; Khan, A.A.; Alharby, H.F.; Bamagoos, A.A.; Alabdallah, N.M.; Hakeem, K.R. Optimizing Nitrogen Application in Root Vegetables from Their Growth, Biochemical and Antioxidant Response to Urea Fertilizer. Agriculture 2021, 11, 704. [Google Scholar] [CrossRef]
- Mokgehle, S.N.; Tesfay, S.Z.; Araya, H.T.; Plooy, C.P.D. Antioxidant activity and soluble sugars of African ginger (Siphonochilus aethiopicus) in response to irrigation regimen and nitrogen levels. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2017, 67, 425–434. [Google Scholar] [CrossRef]
- Hole, C.C.; Barnes, A.; Thomas, T.H.; Scott, P.A.; Rankin, W.E.F. Dry matter distribution between the shoot and storage root of carrot (Daucus carota L.): I. Comparison of Varieties. Ann. Bot. 1983, 51, 175–187. [Google Scholar] [CrossRef]
- Filho, A.; Peixoto, F. Accumulation and exportation of nutrient by carrot ‘Forto’. Rev. Caatinga 2013, 26, 64–70. [Google Scholar]
- Selijasen, R.; Kristensen, K.L.; Lauridsen, C.; Wyss, G.S.; Kretzscmar, U.; Brilouez-Aragone, I.; Kahl, J. Quality of carrot as affected by pre- and postharvest factors and processing. J. Sci. Food Agric. 2013, 93, 2611–2626. [Google Scholar] [CrossRef]
- Aquino, R.F.B.A.; Assunção, N.S.; Aquino, L.A.; Aquino, P.M.D.; Oliveira, G.A.D.; Carvalho, A.M.X.D. Nutrient demand by carrot crop is influenced by the cultivar. Rev. Bras. Ciência Solo 2015, 39, 541–552. [Google Scholar] [CrossRef][Green Version]
- Niemiec, M.; Cupiał, M.; Szeląg-Sikora, A. Evaluation of the efficiency of celeriac fertilization with the use of slow-acting fertilizers. Agric. Agric. Sci. Procedia 2015, 7, 177–183. [Google Scholar] [CrossRef]
- Maucieri, C.; Zhang, Y.; McDaniel, M.D.; Borin, M.; Adams, M.A. Short-term effects of biochar and salinity on soil greenhouse gas emissions from a semi-arid australian soil after re-wetting. Geoderma 2017, 307, 267–276. [Google Scholar] [CrossRef]
- Malcolm, B.J.; Cameron, K.C.; Curtin, D.; Di, H.J.; Beare, M.H.; Johnstone, P.R.; Edwards, G.R. Organic matter amendments to soil can reduce nitrate leaching losses from livestock urine under simulated fodder beet grazing. Agric. Ecosyst. Environ. 2019, 272, 10–18. [Google Scholar] [CrossRef]
- Fan, Y.N.; Zhang, Y.X.; Hess, F.; Huang, B.; Chen, Z.K. Nutrient balance and soil changes in plastic greenhouse vegetable production. Nutr. Cycl. Agroecosyst. 2020, 117, 77–92. [Google Scholar] [CrossRef]
- Hussain, T.; Gollany, H.T.; Mulla, D.J.; Ben, Z.; Tahir, M.; Ata-Ul-Karim, S.T.; Liu, K.; Maqbool, S.; Hussain, N.; Duangpan, S. Assessment and Application of EPIC in Simulating Upland Rice Productivity, Soil Water, and Nitrogen Dynamics under Different Nitrogen Applications and Planting Windows. Agronomy 2023, 13, 9. [Google Scholar] [CrossRef]



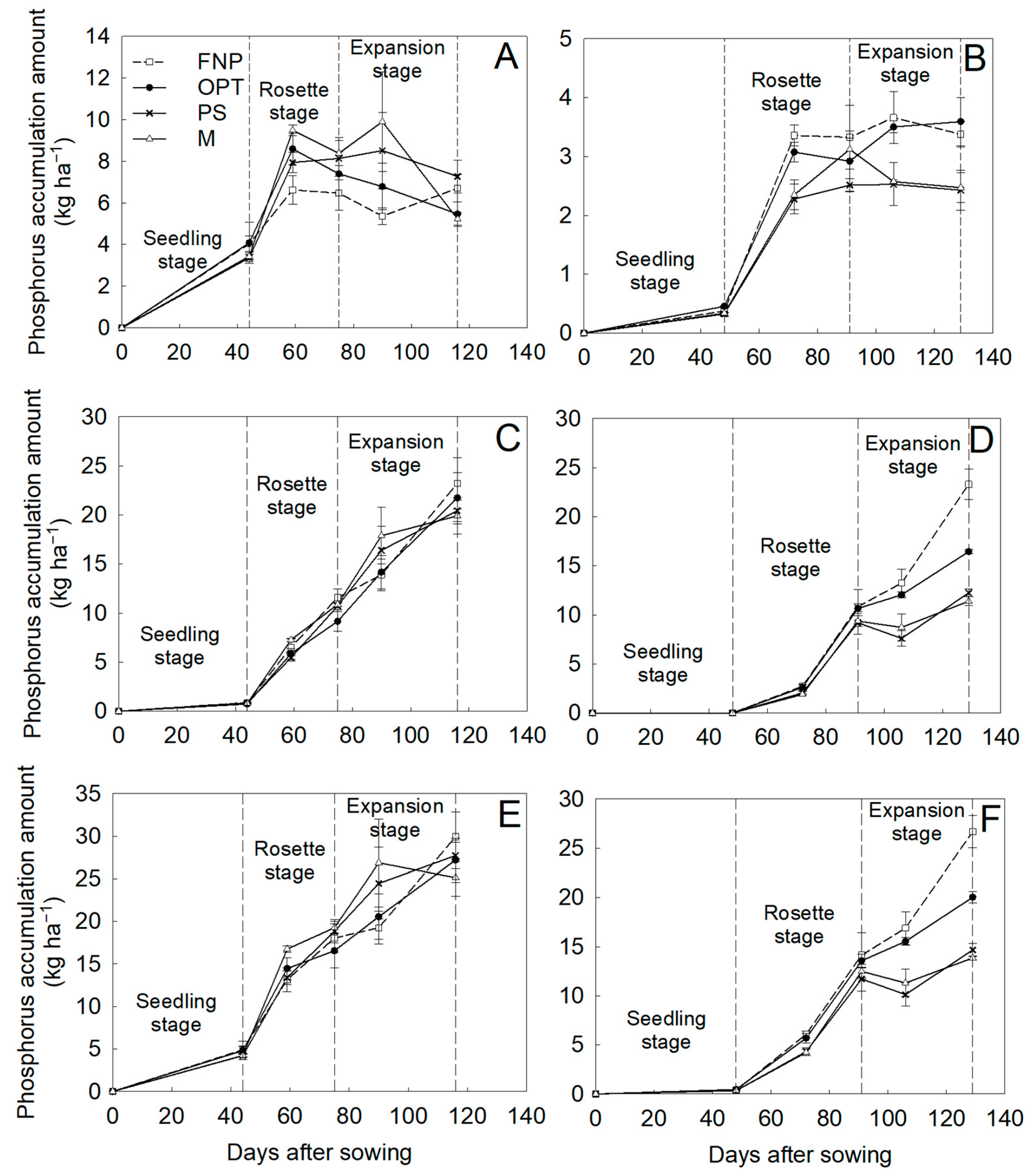
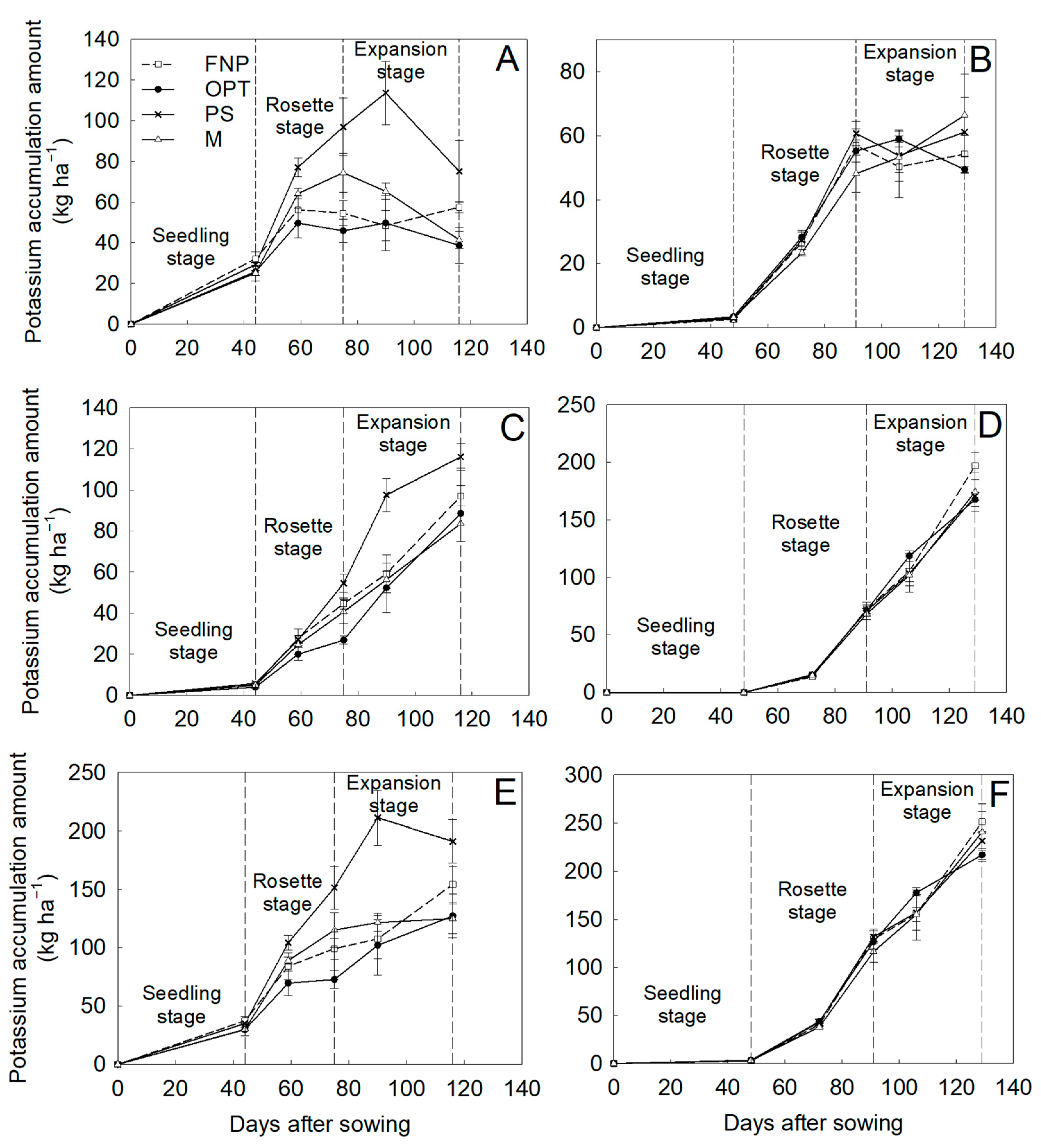

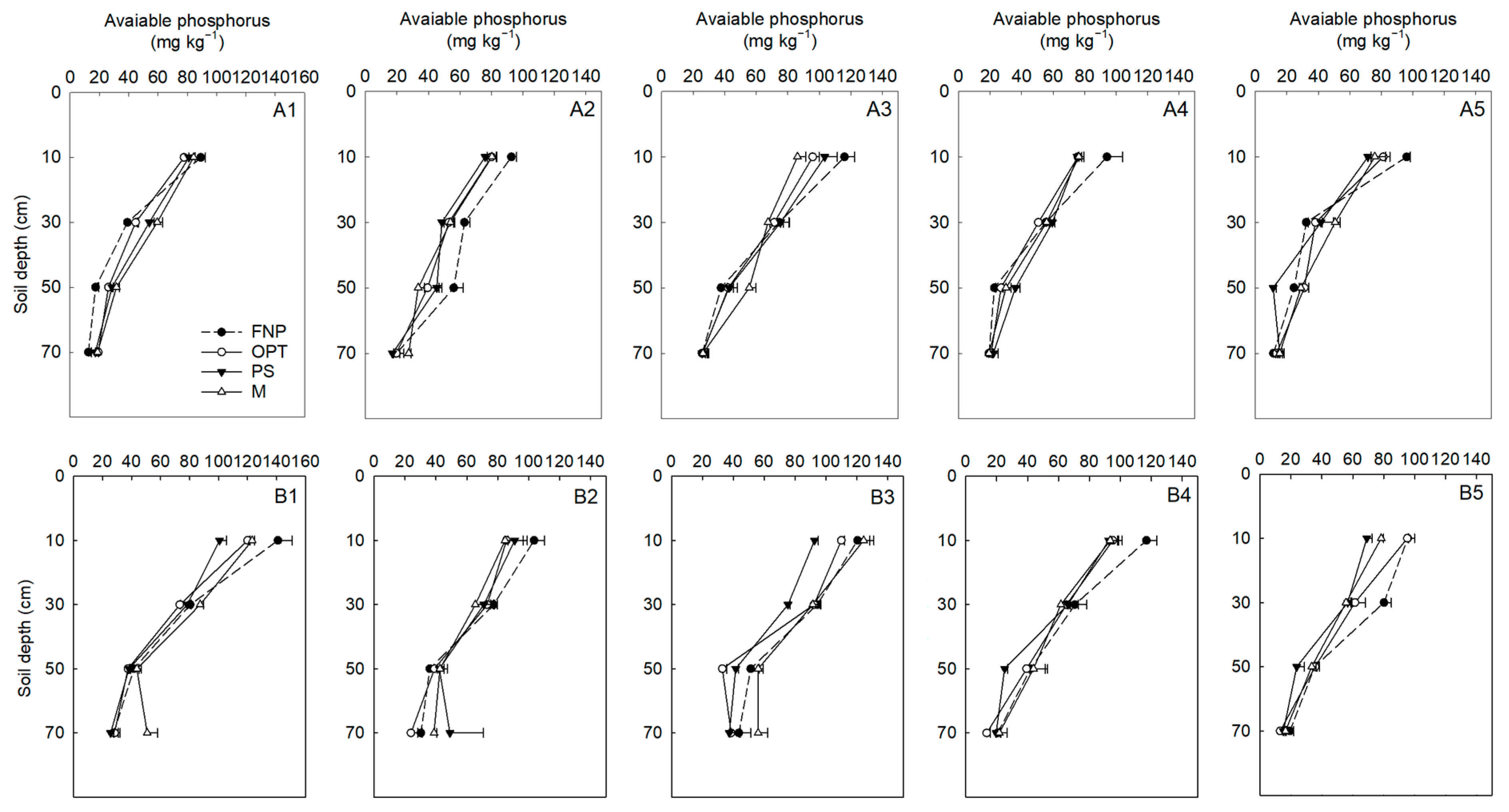

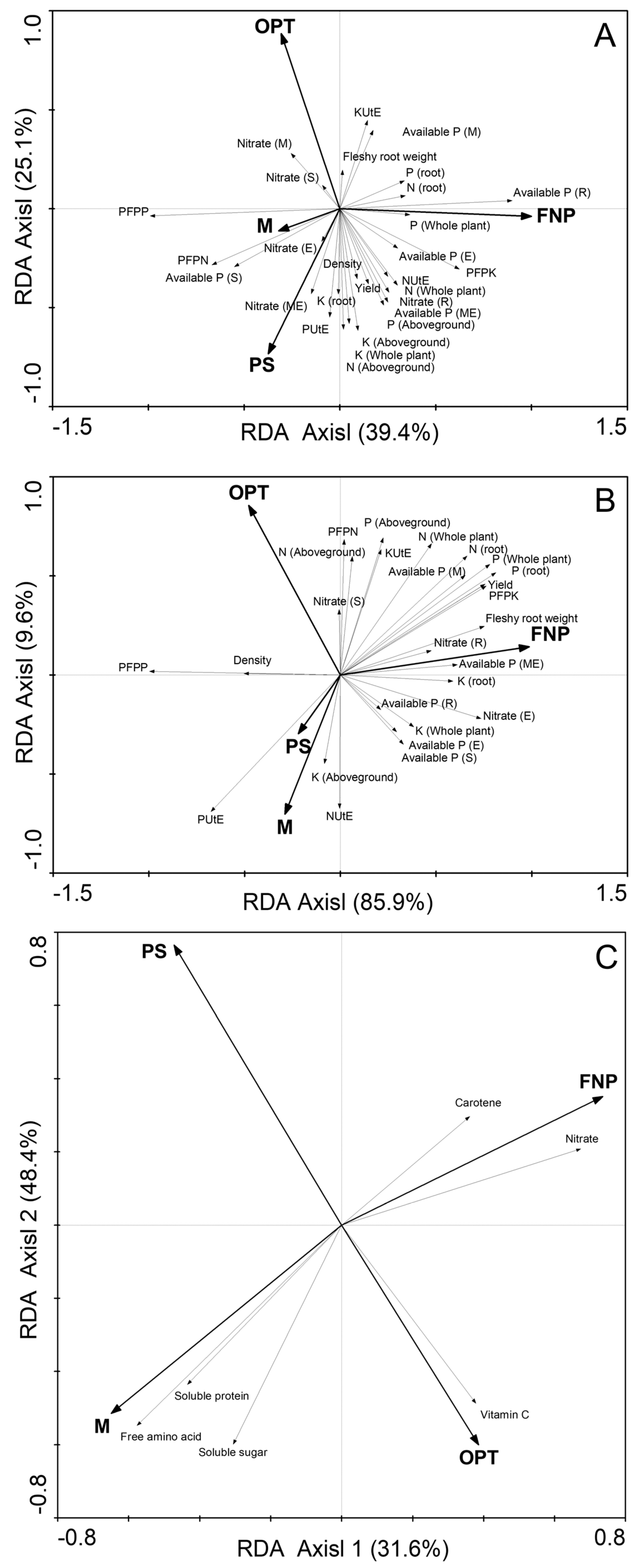
| Years | Treatments | Yield (t ha−1) | Fleshy Root Weight (g Plant−1) | Density (Plant m−2) |
|---|---|---|---|---|
| 2020 Autumn | FNP | 70.1 ± 3.62 a | 198 ± 6.45 ab | 35.6 ± 2.64 a |
| OPT | 66.3 ± 3.21 a | 208 ± 6.79 a | 32.1 ± 2.42 a | |
| PS | 71.6 ± 0.51 a | 204 ± 4.44 a | 35.2 ± 0.97 a | |
| M | 67.1 ± 1.36 a | 181 ± 5.36 b | 37.2 ± 0.86 a | |
| 2021 Spring | FNP | 91.9 ± 4.95 a | 278 ± 32.00 a | 33.8 ± 2.52 a |
| OPT | 78.1 ± 1.15 b | 195 ± 3.25 b | 40.0 ± 0.30 a | |
| PS | 67.3 ± 0.69 c | 199 ± 18.20 b | 34.5 ± 2.82 a | |
| M | 69.5 ± 1.20 bc | 170 ± 3.26 b | 40.9 ± 0.09 a |
| Treatments | Soluble Sugar (%) | Carotene (mg g−1) | Vitamin C (mg kg−1) | Soluble Protein (mg g−1) | Free Amino Acid (mg g−1) | Nitrate (mg kg−1) |
|---|---|---|---|---|---|---|
| FNP | 6.32 ± 0.45 c | 0.50 ± 0.05 a | 157 ± 41 a | 2.41 ± 0.20 a | 12.4 ± 0.43 b | 105 ± 2.80 a |
| OPT | 7.99 ± 0.23 a | 0.47 ± 0.03 a | 161 ± 45 a | 2.42 ± 0.22 a | 14.9 ± 0.85 a | 98 ± 1.06 ab |
| PS | 7.07 ± 0.22 bc | 0.48 ± 0.04 a | 147 ± 40 a | 2.38 ± 0.26 a | 14.1 ± 0.56 ab | 93 ± 3.63 ab |
| M | 7.65 ± 0.14 ab | 0.45 ± 0.03 a | 155 ± 43 a | 2.73 ± 0.16 a | 15.6 ± 1.12 a | 87 ± 8.27 b |
| Years | Treatments | Harvest Index (%) | Physiological Utilization Efficiency (kg kg−1) | Partial Productivity of Fertilizer (kg kg−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | P | K | N | P | K | N | P | K | ||
| 2020 Autumn | FNP | 57 ± 2.1 a | 77 ± 2.2 a | 62 ± 3.0 a | 36 ± 1.5 a | 218 ± 10.2 a | 42 ± 2.7 ab | 217 ± 11.2 b | 312 ± 16.1 b | 246 ± 12.7 a |
| OPT | 61 ± 3.6 a | 80 ± 2.6 a | 70 ± 4.1 a | 33 ± 0.5 b | 198 ± 16.0 a | 43 ± 3.7 ab | 242 ± 11.7 ab | 1339 ± 64.9 a | 210 ± 10.2 b | |
| PS | 53 ± 3.1 a | 74 ± 2.1 a | 62 ± 4.8 a | 35 ± 1.0 ab | 229 ± 11.5 a | 34 ± 3.6 b | 261 ± 1.9 a | 1446 ± 10.3 a | 227 ± 1.6 ab | |
| M | 52 ± 1.8 a | 79 ± 0.6 a | 67 ± 0.3 a | 36 ± 0.3 a | 233 ± 9.1 a | 45 ± 2.9 a | 244 ± 5.0 ab | 1355 ± 27.5 a | 213 ± 4.3 b | |
| 2021 Spring | FNP | 64 ± 1.3 a | 87 ± 1.0 a | 78 ± 2.4 a | 56 ± 2.1 ab | 316 ± 11.2 c | 34 ± 2.2 a | 285 ± 15.4 ab | 408 ± 22.0 c | 322 ± 17.4 a |
| OPT | 59 ± 1.4 a | 82 ± 1.6 a | 77 ± 0.7 a | 53 ± 2.7 b | 375 ± 5.9 b | 35 ± 1.8 a | 303 ± 4.5 a | 1577 ± 23.2 a | 274 ± 4.0 b | |
| PS | 60 ± 2.2 a | 84 ± 1.7 a | 74 ± 3.8 a | 59 ± 1.3 ab | 453 ± 19.0 a | 29 ± 0.6 a | 262 ± 2.7 b | 1360 ± 13.9 b | 236 ± 2.4 c | |
| M | 58 ± 2.3 a | 82 ± 2.1 a | 73 ± 2.6 a | 60 ± 1.7 a | 488 ± 10.4 a | 29 ± 2.8 a | 269 ± 4.6 b | 1404 ± 24.2 b | 244 ± 4.2 bc | |
| Years | Treatments | Yield Water Use Efficiency (g mm−1) | Biomass Water Use Efficiency (g mm−1) |
|---|---|---|---|
| 2020 Autumn | FNP | 53 ± 2.74 b | 7.7 ± 0.40 a |
| OPT | 57 ± 2.78 ab | 7.3 ± 0.67 a | |
| PS | 63 ± 0.45 a | 9.0 ± 0.36 a | |
| M | 59 ± 1.20 a | 7.9 ± 0.22 a | |
| 2021 Spring | FNP | 50 ± 2.72 a | 5.8 ± 0.30 a |
| OPT | 47 ± 0.70 a | 5.9 ± 0.18 a | |
| PS | 40 ± 0.41 b | 5.0 ± 0.24 a | |
| M | 41 ± 0.71 b | 5.1 ± 0.10 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, W.; Zhang, C.; Zhang, P.; Zhao, Y.; Guo, M.; Li, Y.; Chi, R.; Chen, Y. Optimized Fertilizer–Water Management Improves Carrot Quality and Soil Nutrition and Reduces Greenhouse Gas Emissions on the North China Plain. Horticulturae 2024, 10, 151. https://doi.org/10.3390/horticulturae10020151
Tang W, Zhang C, Zhang P, Zhao Y, Guo M, Li Y, Chi R, Chen Y. Optimized Fertilizer–Water Management Improves Carrot Quality and Soil Nutrition and Reduces Greenhouse Gas Emissions on the North China Plain. Horticulturae. 2024; 10(2):151. https://doi.org/10.3390/horticulturae10020151
Chicago/Turabian StyleTang, Wenhui, Cuiyue Zhang, Peiqiang Zhang, Ying Zhao, Mengyao Guo, Yun Li, Ruiping Chi, and Yanling Chen. 2024. "Optimized Fertilizer–Water Management Improves Carrot Quality and Soil Nutrition and Reduces Greenhouse Gas Emissions on the North China Plain" Horticulturae 10, no. 2: 151. https://doi.org/10.3390/horticulturae10020151
APA StyleTang, W., Zhang, C., Zhang, P., Zhao, Y., Guo, M., Li, Y., Chi, R., & Chen, Y. (2024). Optimized Fertilizer–Water Management Improves Carrot Quality and Soil Nutrition and Reduces Greenhouse Gas Emissions on the North China Plain. Horticulturae, 10(2), 151. https://doi.org/10.3390/horticulturae10020151





