Blue and Red Light Downconversion Film Application Enhances Plant Photosynthetic Performance and Fruit Productivity of Rubus fruticosus L. var. Loch Ness
Abstract
1. Introduction
2. Materials and Methods
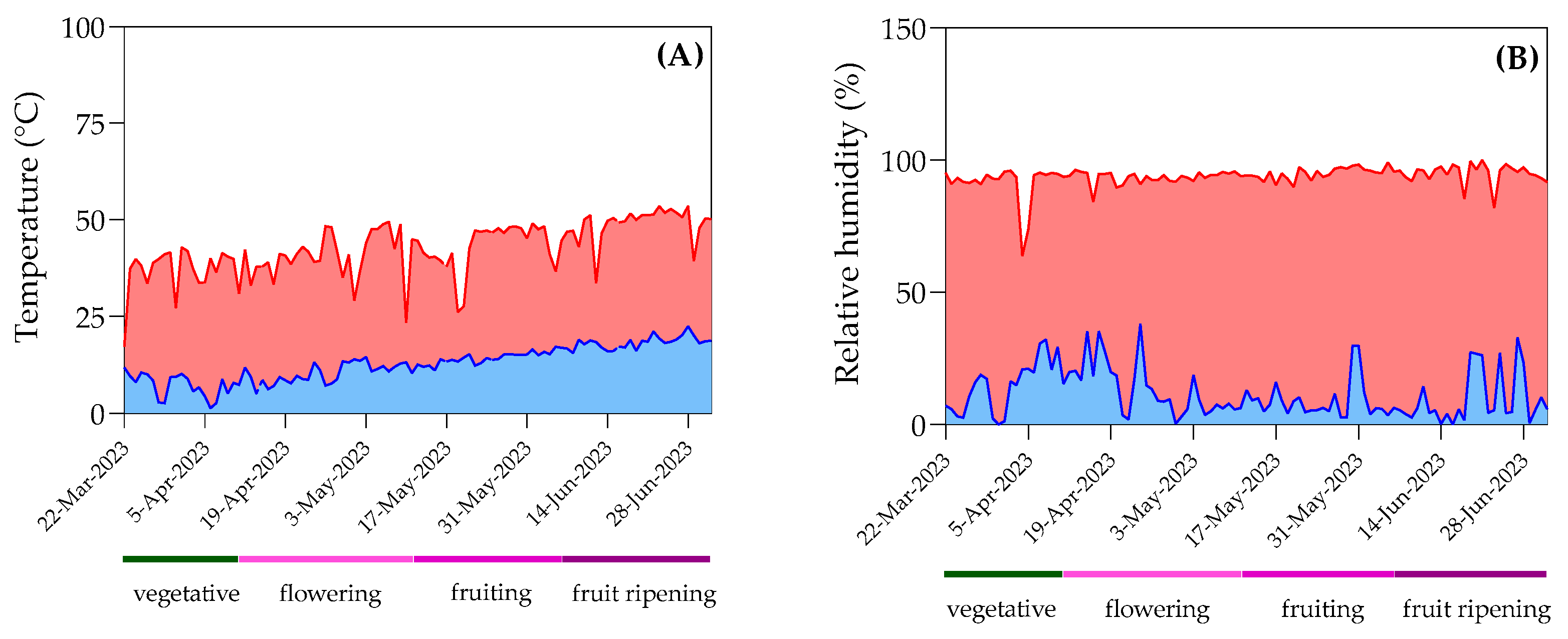
2.1. Plant Material and Fruit Yield Measurement
2.2. Plant Morphological Parameters
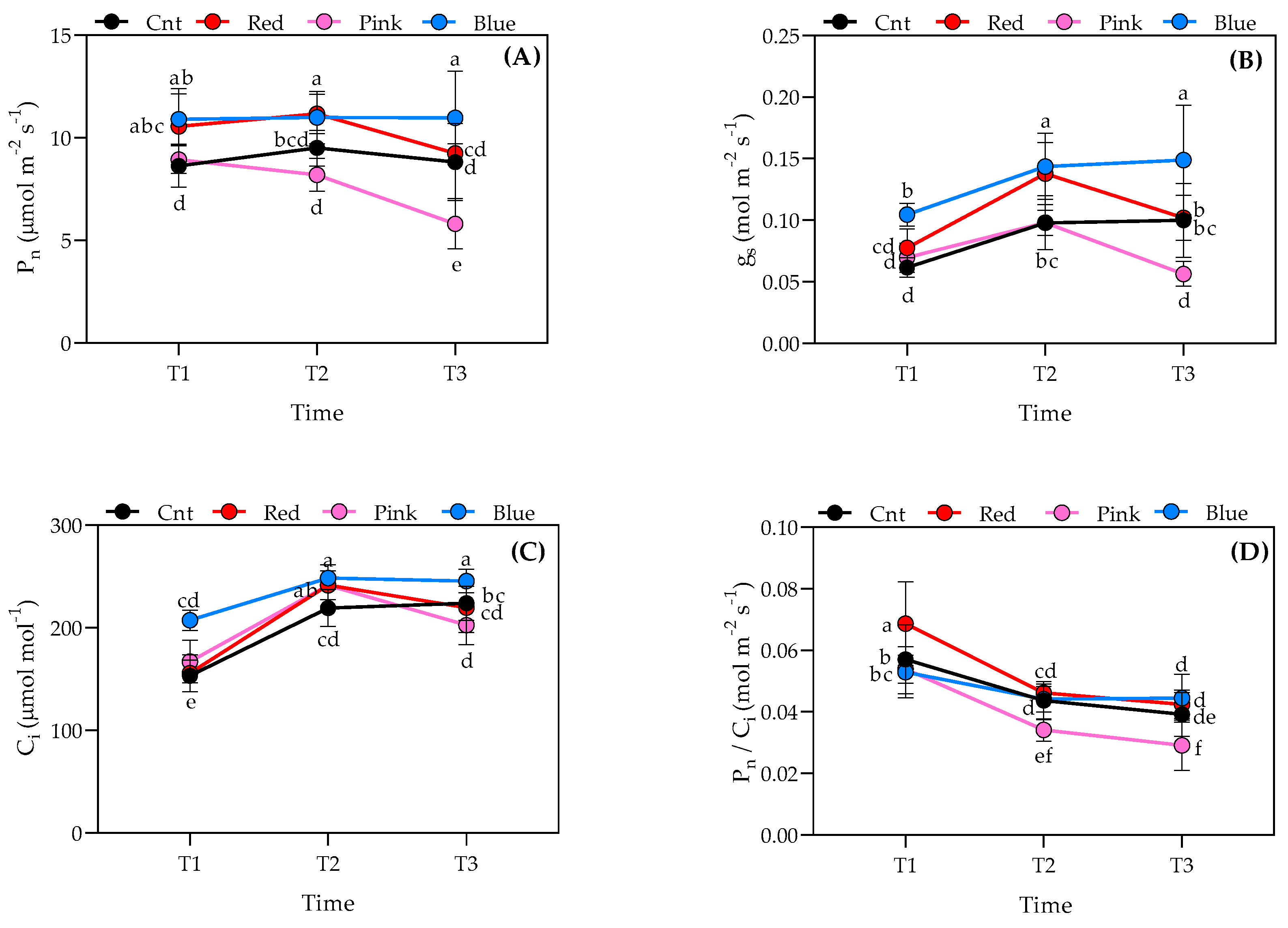
2.3. Gas Exchange Analysis
2.4. Leaf Pigment Content
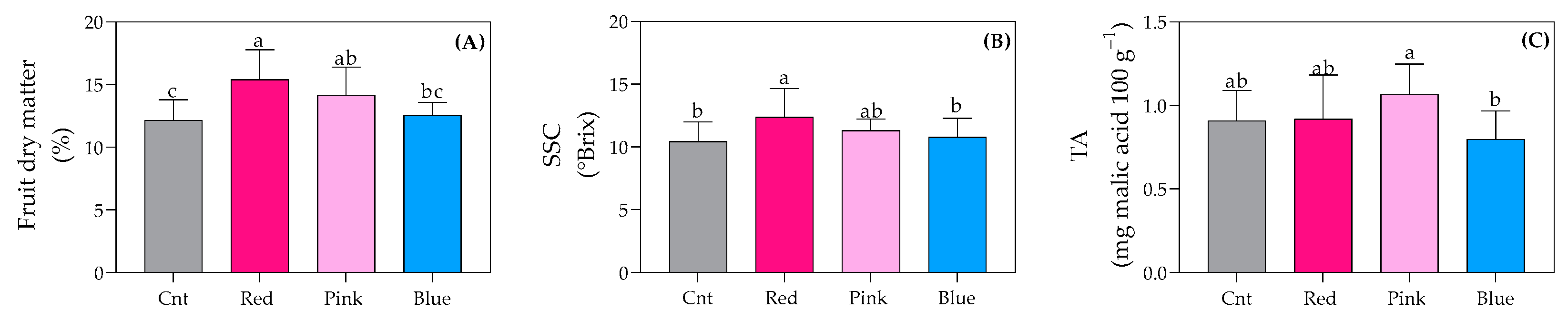
2.5. Fruit Dry Matter, Soluble Solid Content (SSC), and Titratable Acidity (TA)
2.6. Fruit Extraction for Flavonoid (TFC) Content and Antioxidant Activity Assays
2.7. TFC Analysis
2.8. Antioxidant Activity and Total Anthocyanin Content (TAC) Assays
2.9. UHPLC-HR-ESI-Orbitrap/MS Chemical Profile
2.10. Statistical Analysis
3. Results
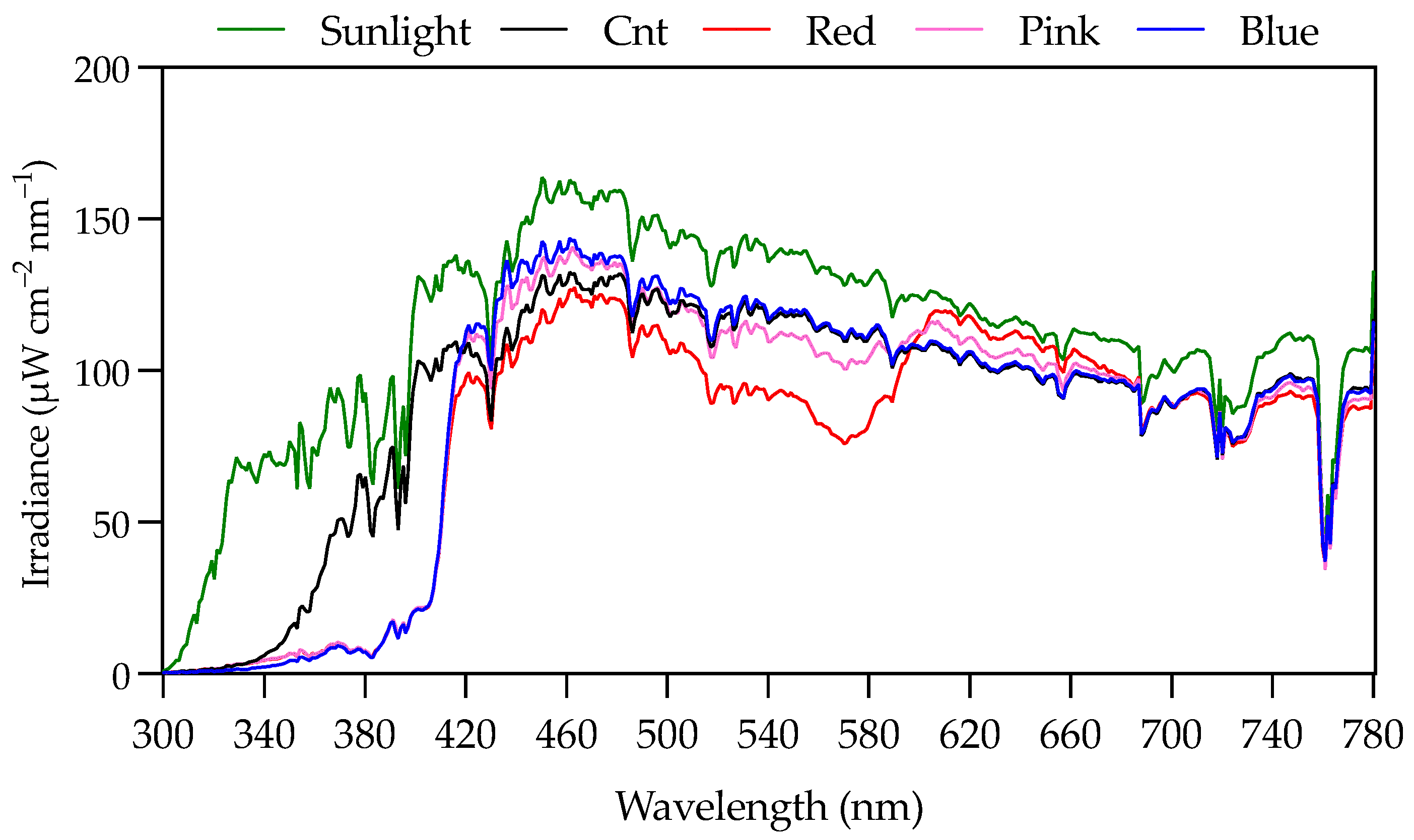
3.1. Light Downconversion Technology Characteristics
3.2. Results of Plant Morphological Parameters
3.3. Gas Exchange Analysis Results
3.4. Leaf Pigment Content Results
3.5. Fruit Yield and Morphological Parameters
3.6. Fruit Organoleptic Quality
3.7. Fruit Nutraceutical Quality
3.8. Chemical Fingerprint of Fruit Using UHPLC-HR-ESI-Orbitrap/MS Analysis
4. Discussion
4.1. Red and Blue Films Preserved Plant Morphology, Boosting Photosynthesis and Flower Fertility
4.2. Red and Blue Films Did Not Alter Blackberry Fruit Organoleptic and Nutraceutical Quality, Enhancing Fruit Productivity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peng, X.; Wang, B.; Wang, X.; Ni, B.; Zuo, Z. Effects of Different Colored Light-Quality Selective Plastic Films on Growth, Photosynthetic Abilities, and Fruit Qualities of Strawberry. Hortic. Sci. Technol. 2020, 38, 462–473. [Google Scholar] [CrossRef]
- Roosta, H.R.; Bikdeloo, M.; Ghorbanpour, M. The Growth, Nutrient Uptake and Fruit Quality in Four Strawberry Cultivars under Different Spectra of LED Supplemental Light. BMC Plant Biol. 2024, 24, 179. [Google Scholar] [CrossRef] [PubMed]
- Jishi, T. Estimation of Time Course in Phytochrome Photostationary State under Artificial Light for Controlling Plant Growth. Front. Plant Sci. 2024, 14, 1305182. [Google Scholar] [CrossRef]
- Yang, C.; Liu, W.; You, Q.; Zhao, X.; Liu, S.; Xue, L.; Sun, J.; Jiang, X. Recent Advances in Light-Conversion Phosphors for Plant Growth and Strategies for the Modulation of Photoluminescence Properties. Nanomaterials 2023, 13, 1715. [Google Scholar] [CrossRef]
- Devlin, P.F.; Christie, J.M.; Terry, M.J. Many Hands Make Light Work. J. Exp. Bot. 2007, 58, 3071–3077. [Google Scholar] [CrossRef]
- Zotti, M.; Mazzoleni, S.; Mercaldo, L.V.; Della Noce, M.; Ferrara, M.; Veneri, P.D.; Diano, M.; Esposito, S.; Cartenì, F. Testing the Effect of Semi-Transparent Spectrally Selective Thin Film Photovoltaics for Agrivoltaic Application: A Multi-Experimental and Multi-Specific Approach. Heliyon 2024, 10, e26323. [Google Scholar] [CrossRef] [PubMed]
- Landi, M.; Zivcak, M.; Sytar, O.; Brestic, M.; Allakhverdiev, S.I. Plasticity of Photosynthetic Processes and the Accumulation of Secondary Metabolites in Plants in Response to Monochromatic Light Environments: A Review. Biochim. Biophys. Acta BBA-Bioenerg. 2020, 1861, 148131. [Google Scholar] [CrossRef]
- Lauria, G.; Lo Piccolo, E.; Ceccanti, C.; Paoli, L.; Giordani, T.; Guidi, L.; Malorgio, F.; Massai, R.; Nali, C.; Pellegrini, E.; et al. Supplemental Red Light More than Other Wavebands Activates Antioxidant Defenses in Greenhouse-Cultivated Fragaria × Ananassa Var. Elsanta Plants. Sci. Hortic. 2023, 321, 112319. [Google Scholar] [CrossRef]
- Smith, H.L.; McAusland, L.; Murchie, E.H. Don’t Ignore the Green Light: Exploring Diverse Roles in Plant Processes. J. Exp. Bot. 2017, 68, 2099–2110. [Google Scholar] [CrossRef]
- Chia, P.-L.; Kubota, C. End-of-Day Far-Red Light Quality and Dose Requirements for Tomato Rootstock Hypocotyl Elongation. HortScience 2010, 45, 1501–1506. [Google Scholar] [CrossRef]
- Jishi, T.; Kimura, K.; Matsuda, R.; Fujiwara, K. Effects of Temporally Shifted Irradiation of Blue and Red LED Light on Cos Lettuce Growth and Morphology. Sci. Hortic. 2016, 198, 227–232. [Google Scholar] [CrossRef]
- Dale, A.; Blom, T.J. Far-Red Light Alters Primostem Morphology of Red Raspberry. HortScience 2004, 39, 973–974. [Google Scholar] [CrossRef]
- He, Y.; Wang, Y.; Guo, Y.; Ma, L. Fine Adjustment of Emission Wavelength, Light-Conversion Quality, Photostability of Blue-Violet Light Conversion Agents Based on FRET Effect. Dyes Pigments 2023, 217, 111429. [Google Scholar] [CrossRef]
- Kambalapally, V.R.; Rajapakse, N.C. Spectral Filters Affect Growth, Flowering, and Postharvest Quality of Easter Lilies. HortTechnology 1999, 9, 134a. [Google Scholar] [CrossRef]
- Gao, Y.; Li, G.; Cai, B.; Zhang, Z.; Li, N.; Liu, Y.; Li, Q. Effects of Rare-Earth Light Conversion Film on the Growth and Fruit Quality of Sweet Pepper in a Solar Greenhouse. Front. Plant Sci. 2022, 13, 989271. [Google Scholar] [CrossRef]
- Park, Y.; Runkle, E.S. Spectral-Conversion Film Potential for Greenhouses: Utility of Green-to-Red Photons Conversion and Far-Red Filtration for Plant Growth. PLoS ONE 2023, 18, e0281996. [Google Scholar] [CrossRef]
- El Horri, H.; Vitiello, M.; Ceccanti, C.; Lo Piccolo, E.; Lauria, G.; De Leo, M.; Braca, A.; Incrocci, L.; Guidi, L.; Massai, R.; et al. Ultraviolet-to-Blue Light Conversion Film Affects Both Leaf Photosynthetic Traits and Fruit Bioactive Compound Accumulation in Fragaria × Ananassa. Agronomy 2024, 14, 1491. [Google Scholar] [CrossRef]
- Miao, L.; Zhang, Y.; Yang, X.; Xiao, J.; Zhang, H.; Zhang, Z.; Wang, Y.; Jiang, G. Colored Light-Quality Selective Plastic Films Affect Anthocyanin Content, Enzyme Activities, and the Expression of Flavonoid Genes in Strawberry (Fragaria×ananassa) Fruit. Food Chem. 2016, 207, 93–100. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, Z.; Wu, Y.; Wu, W.; Lyu, L.; Li, W. Effects of Nitrogen Application Level on the Physiological Characteristics, Yield and Fruit Quality of Blackberry. Sci. Hortic. 2023, 313, 111915. [Google Scholar] [CrossRef]
- Lemarié, S.; Guérin, V.; Sakr, S.; Jouault, A.; Caradeuc, M.; Cordier, S.; Guignard, G.; Gardet, R.; Bertheloot, J.; Demotes-Mainard, S.; et al. Impact of Innovative Optically Active Greenhouse Films on Melon, Watermelon, Raspberry and Potato Crops. Acta Hortic. 2019, 1252, 191–200. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Simakin, A.V.; Bunkin, N.F.; Shafeev, G.A.; Astashev, M.E.; Glinushkin, A.P.; Grinberg, M.A.; Vodeneev, V.A. Development and Application of Photoconversion Fluoropolymer Films for Greenhouses Located at High or Polar Latitudes. J. Photochem. Photobiol. B 2020, 213, 112056. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, Y.; Wada, E.; Fukumoto, Y.; Aruga, H.; Shimoi, Y. The Effect of Spectrum Conversion Covering Film on Cucumber in Soilless Culture. Acta Hortic. 2012, 956, 481–487. [Google Scholar] [CrossRef]
- Blum, M.A.; Parrish, C.H.; Hebert, D.; Houck, D.; Makarov, N.; Ramasamy, K.; McDaniel, H.; Giacomelli, G.A.; Bergren, M.R. Enhancing Light Use Efficiency and Tomato Fruit Yield with Quantum Dot Films to Modify the Light Spectrum. Acta Hortic. 2023, 1377, 227–234. [Google Scholar] [CrossRef]
- Wei, Z.; Yang, H.; Duan, Y.; Wu, W.; Lyu, L.; Li, W. Physiological and Metabolomic Analyses Reveal the Effects of Different NH4+:NO3− Ratios on Blackberry Fruit Quality. Sci. Hortic. 2023, 318, 112124. [Google Scholar] [CrossRef]
- Gradillas, A.; Martínez-Alcázar, M.P.; Gutiérrez, E.; Ramos-Solano, B.; García, A. A Novel Strategy for Rapid Screening of the Complex Triterpene Saponin Mixture Present in the Methanolic Extract of Blackberry Leaves (Rubus Cv. Loch Ness) by UHPLC/QTOF-MS. J. Pharm. Biomed. Anal. 2019, 164, 47–56. [Google Scholar] [CrossRef]
- Shi, C.; Fang, D.; Huang, C.; Zhou, A.; Lu, T.; Wang, J.; Song, Y.; Lyu, L.; Wu, W.; Li, W. Active Electrospun Nanofiber Packaging Maintains the Preservation Quality and Antioxidant Activity of Blackberry. Postharvest Biol. Technol. 2023, 199, 112300. [Google Scholar] [CrossRef]
- Silva, S.D.; Feliciano, R.P.; Boas, L.V.; Bronze, M.R. Application of FTIR-ATR to Moscatel Dessert Wines for Prediction of Total Phenolic and Flavonoid Contents and Antioxidant Capacity. Food Chem. 2014, 150, 489–493. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Vitiello, M.; Pecoraro, M.; De Leo, M.; Camangi, F.; Parisi, V.; Donadio, G.; Braca, A.; Franceschelli, S.; De Tommasi, N. Chemical Profiling, Antioxidant, and Anti-Inflammatory Activities of Hyoseris Radiata L., a Plant Used in the Phytoalimurgic Tradition. Antioxidants 2024, 13, 111. [Google Scholar] [CrossRef] [PubMed]
- Gasperotti, M.; Masuero, D.; Vrhovsek, U.; Guella, G.; Mattivi, F. Profiling and Accurate Quantification of Rubus Ellagitannins and Ellagic Acid Ccnjugates Using Direct UPLC-Q-TOF HDMS and HPLC-DAD Analysis. J. Agric. Food Chem. 2010, 58, 4602–4616. [Google Scholar] [CrossRef] [PubMed]
- Gil-Martínez, L.; Mut-Salud, N.; Ruiz-García, J.A.; Falcón-Piñeiro, A.; Maijó-Ferré, M.; Baños, A.; De La Torre-Ramírez, J.M.; Guillamón, E.; Verardo, V.; Gómez-Caravaca, A.M. Phytochemicals Determination, and Antioxidant, Antimicrobial, Anti-Inflammatory and Anticancer Activities of Blackberry Fruit. Foods 2023, 12, 1505. [Google Scholar] [CrossRef]
- Oszmiański, J.; Nowicka, P.; Teleszko, M.; Wojdyło, A.; Cebulak, T.; Oklejewicz, K. Analysis of Phenolic Compounds and Antioxidant Activity in Wild Blackberry Fruit. Int. J. Mol. Sci. 2015, 16, 14540–14553. [Google Scholar] [CrossRef]
- Tzima, K.; Putsakum, G.; Rai, D.K. Antioxidant Guided Fractionation of Blackberry Polyphenols Show Synergistic Role of Catechins and Ellagitannins. Molecules 2023, 28, 1933. [Google Scholar] [CrossRef]
- Dewir, Y.H.; Al-Ali, A.M.; Rihan, H.Z.; Alshahrani, T.; Alwahibi, M.S.; Almutairi, K.F.; Naidoo, Y.; Fuller, M.P. Effects of Artificial Light Spectra and Sucrose on the Leaf Pigments, Growth, and Rooting of Blackberry (Rubus Fruticosus) Microshoots. Agronomy 2022, 13, 89. [Google Scholar] [CrossRef]
- Folta, K.M.; Carvalho, S.D. Photoreceptors and Control of Horticultural Plant Traits. HortScience 2015, 50, 1274–1280. [Google Scholar] [CrossRef]
- Maimaitituerxun, R.; Abdusalam, A. Exploring the Influence of Petal and Stamen Color on Pollinator and Reproductive Success in Punica Granatum. Biodivers. Sci. 2023, 31, 22633. [Google Scholar] [CrossRef]
- Lauria, G.; Lo Piccolo, E.; Davini, A.; Ruffini Castiglione, M.; Pieracci, Y.; Flamini, G.; Martens, S.; Angeli, A.; Ceccanti, C.; Guidi, L.; et al. Modulation of VOC Fingerprint and Alteration of Physiological Responses after Supplemental LED Light in Green- and Red-Leafed Sweet Basil (Ocimum Basilicum L.). Sci. Hortic. 2023, 315, 111970. [Google Scholar] [CrossRef]
- Khramov, R.; Kosobryukhov, A.; Kreslavski, V.; Balakirev, D.; Khudyakova, A.; Svidchenko, E.; Surin, N.; Ponomarenko, S.; Luponosov, Y. Luminescence of Agrotextiles Based on Red-Light-Emitting Organic Luminophore and Polypropylene Spunbond Enhances the Growth and Photosynthesis of Vegetable Plants. Front. Plant Sci. 2022, 13, 827679. [Google Scholar] [CrossRef]
- Minich, A.S.; Minich, I.B.; Zelen’chukova, N.S.; Karnachuk, R.A.; Golovatskaya, I.F.; Efimova, M.V.; Raida, V.S. The Role of Low Intensity Red Luminescent Radiation in the Control of Arabidopsis Thaliana Morphogenesis and Hormonal Balance. Russ. J. Plant Physiol. 2006, 53, 762–767. [Google Scholar] [CrossRef]
- Freitas, I.S.D.; Roldán, G.Q.; Macedo, A.C.; Mello, S.D.C. The Responses of Photosynthesis, Fruit Yield and Quality of Mini-Cucumber to LED-Interlighting and Grafting. Hortic. Bras. 2021, 39, 86–93. [Google Scholar] [CrossRef]
- Almeida, J.M.D.; Calaboni, C.; Rodrigues, P.H.V. Pigments in Flower Stems of Lisianthus under Different Photoselective Shade Nets. Ornam. Hortic. 2021, 27, 535–543. [Google Scholar] [CrossRef]
- Da Cunha Honorato, A.; Nohara, G.A.; De Assis, R.M.A.; Maciel, J.F.A.; De Carvalho, A.A.; Pinto, J.E.B.P.; Bertolucci, S.K.V. Colored Shade Nets and Different Harvest Times Alter the Growth, Antioxidant Status, and Quantitative Attributes of Glandular Trichomes and Essential Oil of Thymus Vulgaris L. J. Appl. Res. Med. Aromat. Plants 2023, 35, 100474. [Google Scholar] [CrossRef]
- Damour, G.; Simonneau, T.; Cochard, H.; Urban, L. An Overview of Models of Stomatal Conductance at the Leaf Level: Models of Stomatal Conductance. Plant Cell Environ. 2010, 33, 1419–1438. [Google Scholar] [CrossRef]
- Yoshida, H.; Hikosaka, S.; Goto, E.; Takasuna, H.; Kudou, T. Effects of Light Quality and Light Period on Flowering of Everbearing Strawberry in a Closed Plant Production System. Acta Hortic. 2012, 956, 107–112. [Google Scholar] [CrossRef]
- Thwe, A.A.; Kasemsap, P.; Vercambre, G.; Gay, F.; Phattaralerphong, J.; Gautier, H. Impact of Red and Blue Nets on Physiological and Morphological Traits, Fruit Yield and Quality of Tomato (Solanum Lycopersicum Mill.). Sci. Hortic. 2020, 264, 109185. [Google Scholar] [CrossRef]
- Huang, X.; Wu, Y.; Zhang, S.; Yang, H.; Wu, W.; Lyu, L.; Li, W. Variation in Antioxidant Enzyme Activity and Key Gene Expression during Fruit Development of Blackberry and Blackberry–Raspberry Hybrids. Food Biosci. 2023, 54, 102892. [Google Scholar] [CrossRef]
- Karaklajic-Stajic, Z.; Tomic, J.; Pesakovic, M.; Paunovic, S.M.; Stampar, F.; Mikulic-Petkovsek, M.; Grohar, M.C.; Hudina, M.; Jakopic, J. Black Queens of Fruits: Chemical Composition of Blackberry (Rubus Subg. Rubus Watson) and Black Currant (Ribes Nigrum L.) Cultivars Selected in Serbia. Foods 2023, 12, 2775. [Google Scholar] [CrossRef]


| Cnt | Red | Pink | Blue | |
|---|---|---|---|---|
| Plant biomass (kg plant−1) | 0.17 ± 0.02 ab | 0.20 ± 0.02 a | 0.14 ± 0.04 b | 0.21 ± 0.02 a |
| Leaf dry matter (%) | 40.0 ± 1.3 a | 40.9 ± 1.0 a | 35.7 ± 1.4 b | 40.5 ± 0.4 a |
| Stem dry matter (%) | 37.8 ± 1.3 a | 38.0 ± 5.8 a | 34.2 ± 6.0 a | 37.6 ±2.1 a |
| LMA (g m−2) | 36.3 ± 8.8 ab | 30.6 ± 3.1 b | 25.7 ± 8.4 b | 45.1 ± 4.4 a |
| Leaf thickness (mm) | 0.39 ± 0.01 d | 0.51 ± 0.01 a | 0.48 ± 0.03 b | 0.43 ± 0.01 c |
| Stem length (m) | 1.5 ± 0.1 ab | 1.4 ± 0.2 bc | 1.5 ± 0.2 a | 1.3 ± 0.1 c |
| Flower number (n° plant−1) | 289.0 ± 31.8 a | 288.2 ± 12.2 a | 315.3 ± 13.9 a | 206.6 ± 14.0 b |
| Flower fecundity rate (%) | 11.4 ± 2.2 c | 28.2 ± 0.7 a | 26.6 ± 2.7 a | 18.3 ± 3.3 b |
| Cnt | Red | Pink | Blue | |
|---|---|---|---|---|
| Fruit yield (g plant−1) | 80.2 ± 13.4 b | 186.0 ± 31.4 a | 110.8 ± 33.3 b | 171.0 ± 38.7 a |
| Fruit number (n° plant−1) | 36.7 ± 4.9 b | 73.0 ± 12.2 a | 34.7 ± 2.1 b | 48.3 ± 5.9 b |
| Single fruit weight (g) | 2.3 ± 0.1 b | 2.6 ± 0.4 b | 2.4 ± 0.2 b | 3.2 ± 0.1 a |
| Fruit width (mm) | 13.8 ± 0.4 a | 14.8 ± 1.4 a | 13.8 ± 1.0 a | 15.6 ± 0.6 a |
| Fruit height (mm) | 15.5 ± 0.7 b | 16.7 ± 1.9 ab | 15.4 ± 0.5 b | 18.1 ± 0.7 a |
| Berry shape index | 1.11 ± 0.01 b | 1.14 ± 0.01 b | 1.11 ± 0.03 b | 1.24 ± 0.07 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Horri, H.; Vitiello, M.; Braca, A.; De Leo, M.; Guidi, L.; Landi, M.; Lauria, G.; Lo Piccolo, E.; Massai, R.; Remorini, D.; et al. Blue and Red Light Downconversion Film Application Enhances Plant Photosynthetic Performance and Fruit Productivity of Rubus fruticosus L. var. Loch Ness. Horticulturae 2024, 10, 1046. https://doi.org/10.3390/horticulturae10101046
El Horri H, Vitiello M, Braca A, De Leo M, Guidi L, Landi M, Lauria G, Lo Piccolo E, Massai R, Remorini D, et al. Blue and Red Light Downconversion Film Application Enhances Plant Photosynthetic Performance and Fruit Productivity of Rubus fruticosus L. var. Loch Ness. Horticulturae. 2024; 10(10):1046. https://doi.org/10.3390/horticulturae10101046
Chicago/Turabian StyleEl Horri, Hafsa, Maria Vitiello, Alessandra Braca, Marinella De Leo, Lucia Guidi, Marco Landi, Giulia Lauria, Ermes Lo Piccolo, Rossano Massai, Damiano Remorini, and et al. 2024. "Blue and Red Light Downconversion Film Application Enhances Plant Photosynthetic Performance and Fruit Productivity of Rubus fruticosus L. var. Loch Ness" Horticulturae 10, no. 10: 1046. https://doi.org/10.3390/horticulturae10101046
APA StyleEl Horri, H., Vitiello, M., Braca, A., De Leo, M., Guidi, L., Landi, M., Lauria, G., Lo Piccolo, E., Massai, R., Remorini, D., & Ceccanti, C. (2024). Blue and Red Light Downconversion Film Application Enhances Plant Photosynthetic Performance and Fruit Productivity of Rubus fruticosus L. var. Loch Ness. Horticulturae, 10(10), 1046. https://doi.org/10.3390/horticulturae10101046












