Green Alternatives for the Control of Fungal Diseases in Strawberry: In-Field Optimization of the Use of Elicitors, Botanical Extracts and Essential Oils
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Growth Conditions and Tested Products
2.2. Experimental Design
2.3. Disease Assessment and Inoculation Procedure of Podosphaera aphanis
2.4. Yield and Fruit Quality
2.5. Gene Expression Analysis
2.6. Statistical Analysis
3. Results
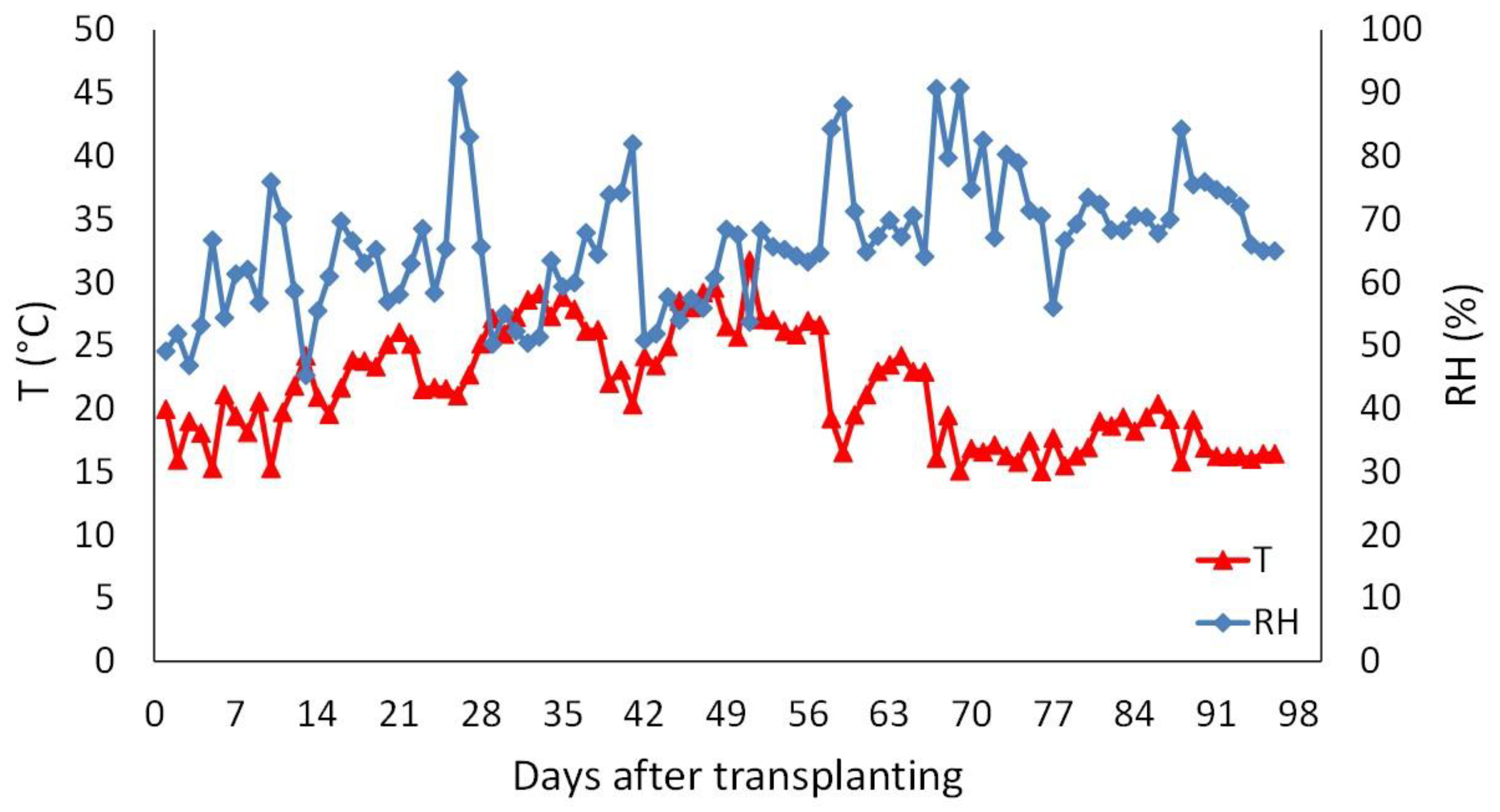
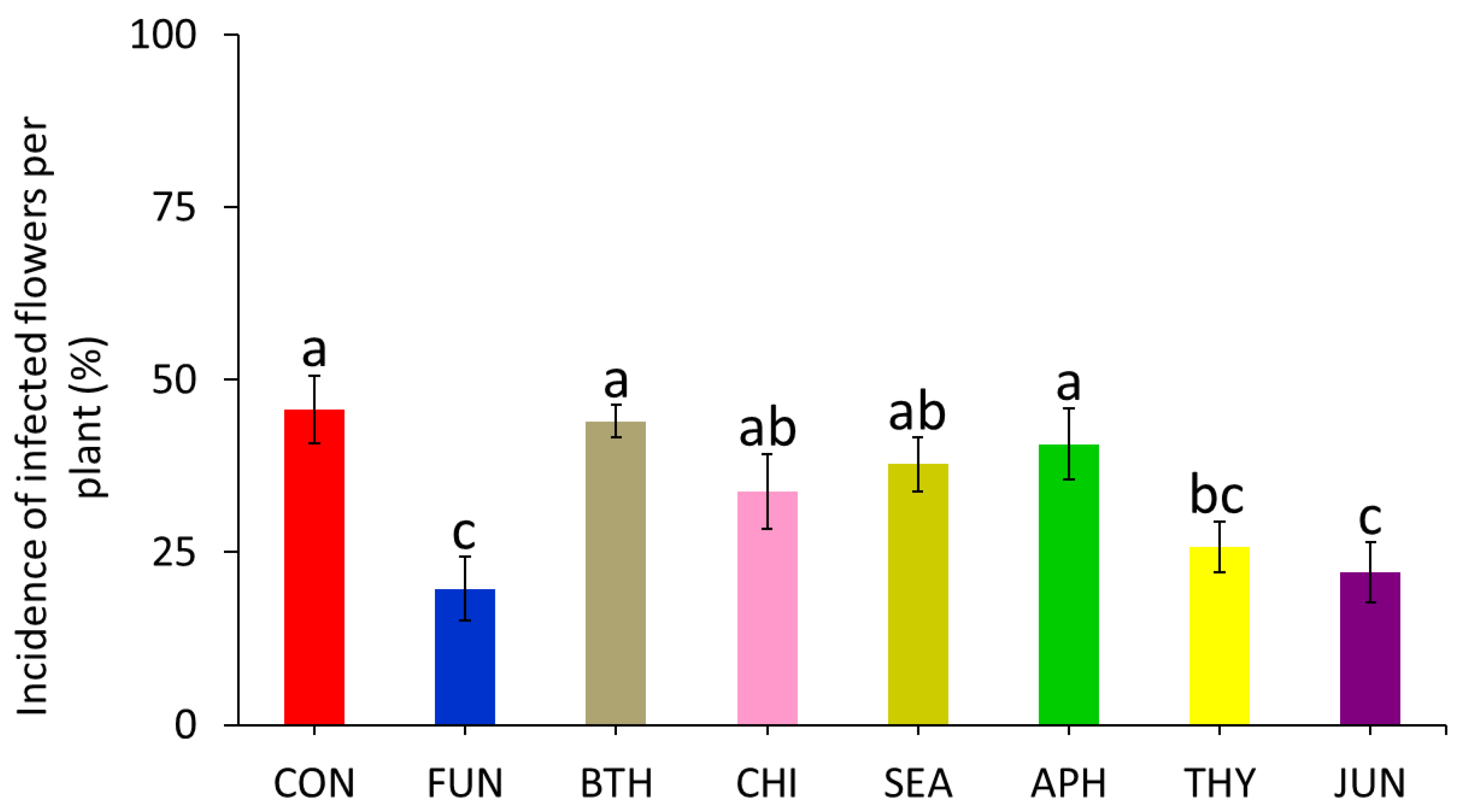
3.1. Efficacy against Natural Infection by Botrytis Cinerea (Experiment 1)
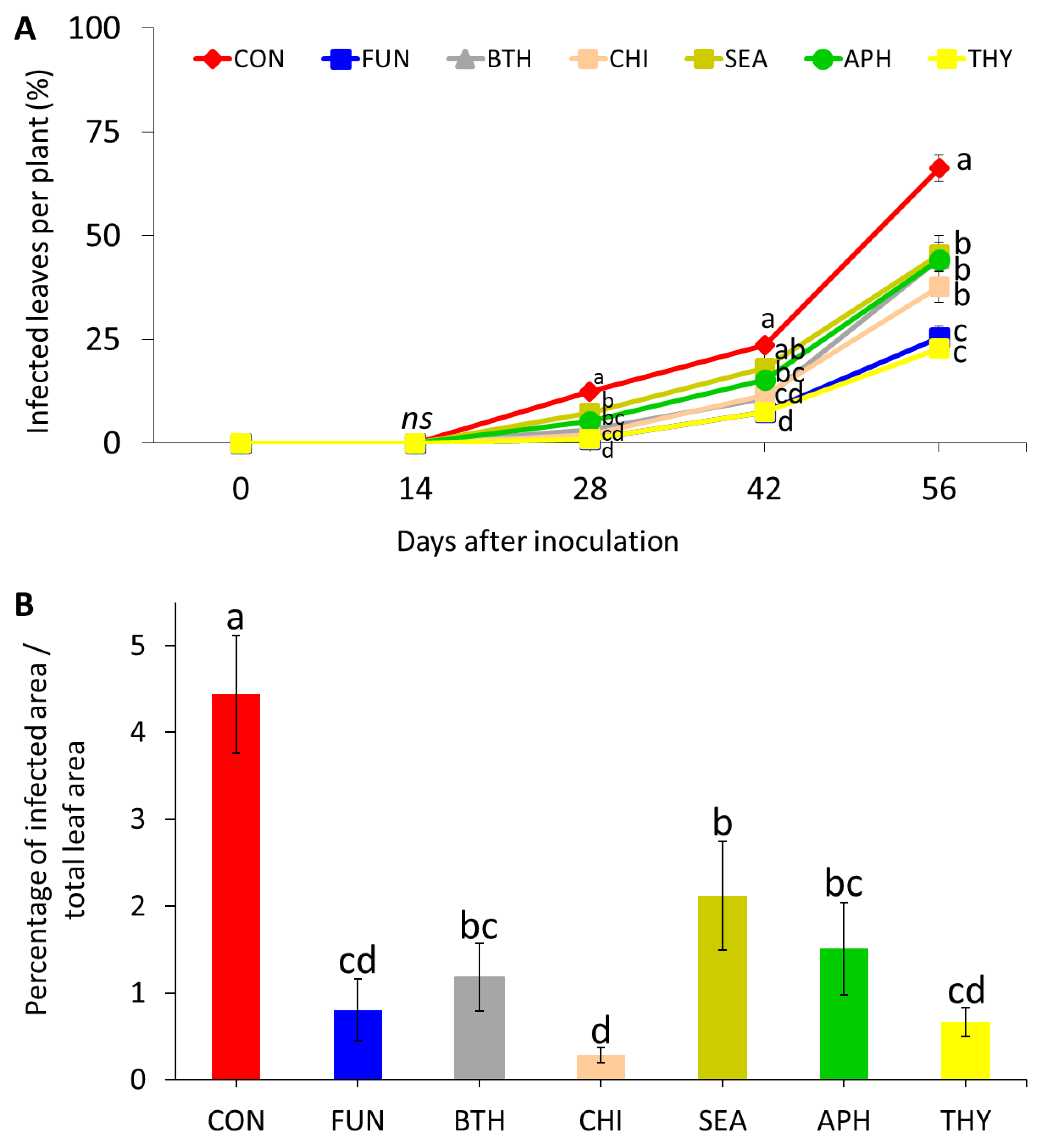
3.2. Preventive Effects against Powdery Mildew Incidence and Severity on Leaves (Experiment 2)
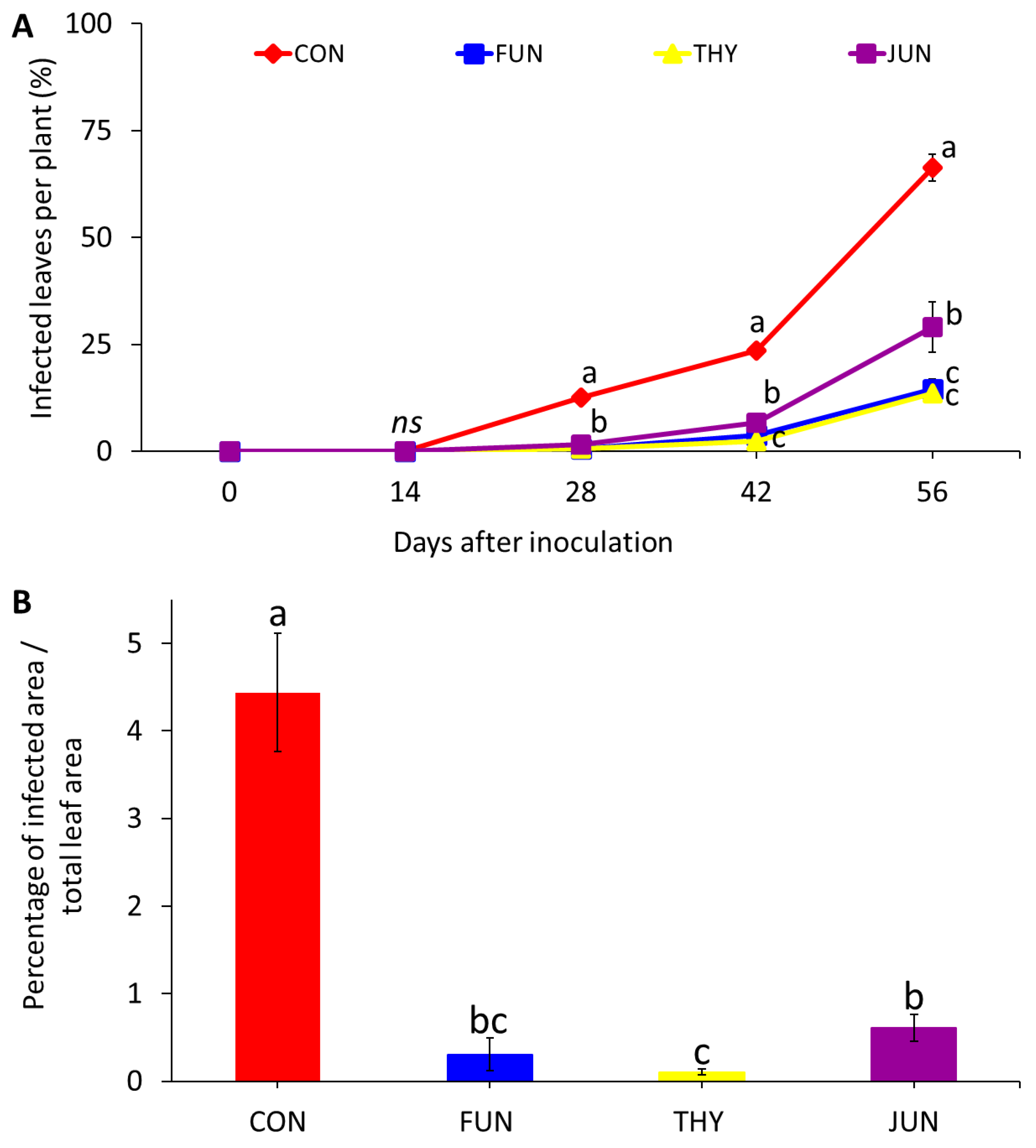
3.3. Curative Effects against Powdery Mildew Incidence and Severity on Leaves (Experiment 3)
3.4. Fruit Production and Quality
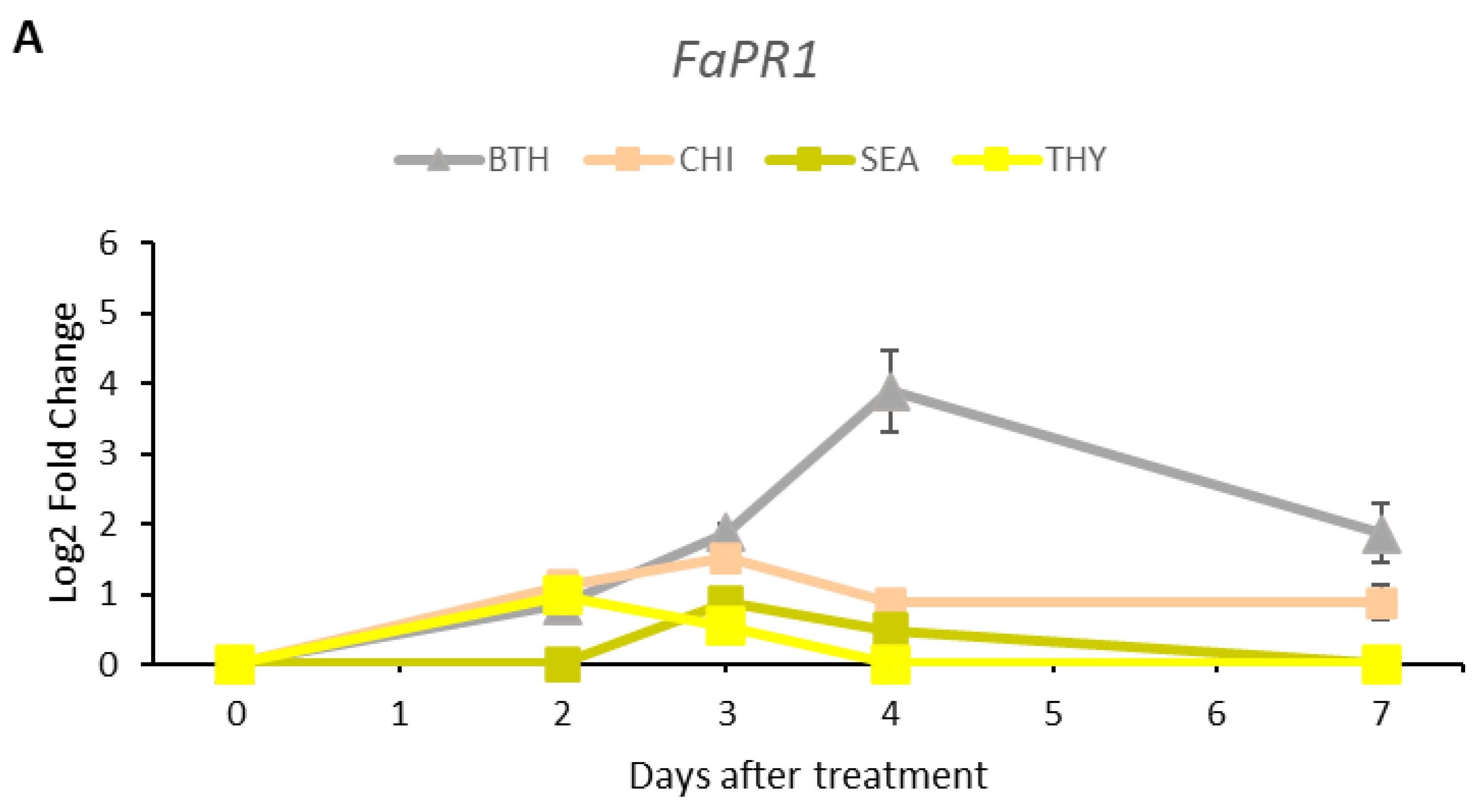
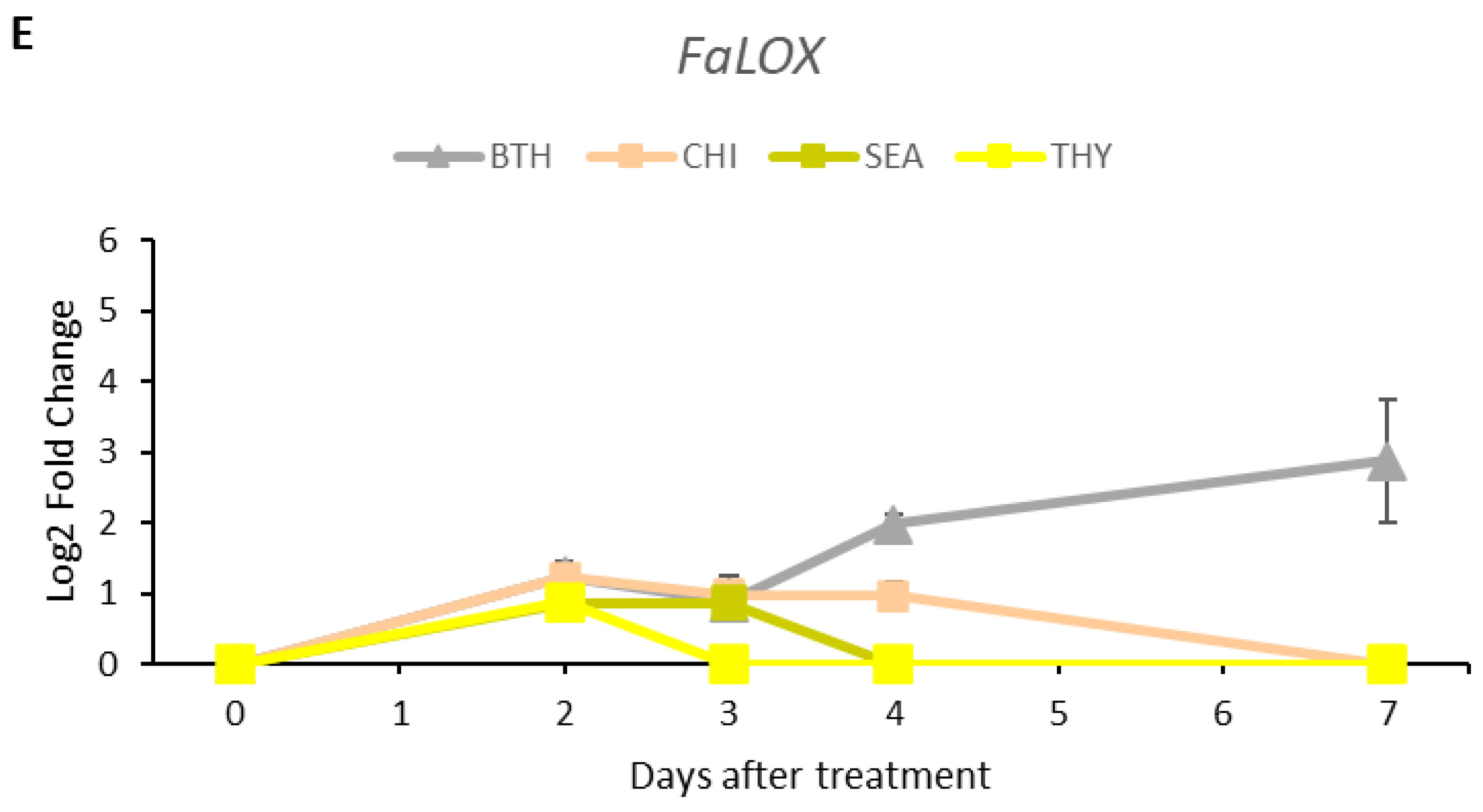
3.5. Gene Expression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 20 October 2022).
- IndexBox, Inc. EU—Strawberries—Market Analysis, Forecast, Size, Trends and Insights; IndexBox, Inc.: Luxembourg, 2021. [Google Scholar]
- van der Wolf, J.M.; Evenhuis, A.; Kastelein, P.; Krijger, M.C.; Funke, V.Z.; van den Berg, W.; Moene, A.F. Risks for Infection of Strawberry Plants with an Aerosolized Inoculum of Xanthomonas fragariae. Eur. J. Plant Pathol. 2018, 152, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Rosello, C.; Bélanger, R.; Ratti, C. Fate of Residual Pesticides in Fruit and Vegetable Waste (FVW) Processing. Foods 2020, 9, 1468. [Google Scholar] [CrossRef]
- Kim, J.-O.; Shin, J.-H.; Gumilang, A.; Chung, K.; Choi, K.Y.; Kim, K.S. Effectiveness of Different Classes of Fungicides on Botrytis cinerea Causing Gray Mold on Fruit and Vegetables. Plant Pathol. J. 2016, 32, 570–574. [Google Scholar] [CrossRef]
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of Pesticides Use in Agriculture: Their Benefits and Hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef]
- Wightwick, A.; Walters, R.; Allinson, G.; Reichman, S.; Menzies, N. Environmental Risks of Fungicides Used in Horticultural Production Systems. In Fungicides; InTech: Rijeka, Croatia, 2010. [Google Scholar]
- Hukkanen, A.T.; Kokko, H.I.; Buchala, A.J.; McDougall, G.J.; Stewart, D.; Kärenlampi, S.O.; Karjalainen, R.O. Benzothiadiazole Induces the Accumulation of Phenolics and Improves Resistance to Powdery Mildew in Strawberries. J. Agric. Food Chem. 2007, 55, 1862–1870. [Google Scholar] [CrossRef] [PubMed]
- Elad, Y.; Malathrakis, N.E.; Dik, A.J. Biological Control of Botrytis-Incited Diseases and Powdery Mildews in Greenhouse Crops. Crop Prot. 1996, 15, 229–240. [Google Scholar] [CrossRef]
- Sylla, J.; Alsanius, B.W.; Krüger, E.; Becker, D.; Wohanka, W. In Vitro Compatibility of Microbial Agents for Simultaneous Application to Control Strawberry Powdery Mildew (Podosphaera aphanis). Crop Prot. 2013, 51, 40–47. [Google Scholar] [CrossRef]
- EUROSTAT. European Statistics. Agri-Environmental Indicator—Consumption of Pesticides; EUROSTAT: Luxembourg, 2021. [Google Scholar]
- EUROSTAT. Statistics on Agricultural Use of Pesticides in the EU; EUROSTAT: Luxembourg, 2019. [Google Scholar]
- EEA. How Pesticides Impact Human Health and Ecosystems in Europe (Briefing No. 06/2023); The European Environment Agency (EEA): Copenhagen, Denmark, 2023. [Google Scholar]
- EFSA. The 2019 European Union Report on Pesticide Residues in Food. EFSA J. 2021, 19, e06491. [Google Scholar] [CrossRef]
- Cernava, T.; Erlacher, A.; Soh, J.; Sensen, C.W.; Grube, M.; Berg, G. Enterobacteriaceae Dominate the Core Microbiome and Contribute to the Resistome of Arugula (Eruca sativa Mill.). Microbiome 2019, 7, 13. [Google Scholar] [CrossRef]
- Sangiorgio, D.; Cellini, A.; Donati, I.; Ferrari, E.; Tanunchai, B.; Fareed Mohamed Wahdan, S.; Sadubsarn, D.; Farneti, B.; Checcucci, A.; Buscot, F.; et al. Taxonomical and Functional Composition of Strawberry Microbiome Is Genotype-Dependent. Adv. Plant Genet. Genom. 2022, 42, 189–204. [Google Scholar] [CrossRef]
- Borges, A.A.; Sandalio, L.M. Induced Resistance for Plant Defense. Front. Plant Sci. 2015, 6, 109. [Google Scholar] [CrossRef][Green Version]
- Romanazzi, G.; Sanzani, S.M.; Bi, Y.; Tian, S.; Gutiérrez Martínez, P.; Alkan, N. Induced Resistance to Control Postharvest Decay of Fruit and Vegetables. Postharvest Biol. Technol. 2016, 122, 82–94. [Google Scholar] [CrossRef]
- Terry, L. Elicitors of Induced Disease Resistance in Postharvest Horticultural Crops: A Brief Review. Postharvest Biol. Technol. 2004, 32, 1–13. [Google Scholar] [CrossRef]
- Reglinski, T.; Vanneste, J.; Wurms, K.; Gould, E.; Spinelli, F.; Rikkerink, E. Using Fundamental Knowledge of Induced Resistance to Develop Control Strategies for Bacterial Canker of Kiwifruit Caused by Pseudomonas syringae pv. actinidiae. Front. Plant Sci. 2013, 4, 24. [Google Scholar] [CrossRef]
- Phuntumart, V.; Marro, P.; Métraux, J.-P.; Sticher, L. A Novel Cucumber Gene Associated with Systemic Acquired Resistance. Plant Sci. 2006, 171, 555–564. [Google Scholar] [CrossRef]
- Kazan, K.; Lyons, R. Intervention of Phytohormone Pathways by Pathogen Effectors. Plant Cell 2014, 26, 2285–2309. [Google Scholar] [CrossRef]
- Mishina, T.E.; Zeier, J. The Arabidopsis Flavin-Dependent Monooxygenase FMO1 Is an Essential Component of Biologically Induced Systemic Acquired Resistance. Plant Physiol. 2006, 141, 1666–1675. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.S.; Borza, T.; Critchley, A.T.; Prithiviraj, B. Carrageenans from Red Seaweeds As Promoters of Growth and Elicitors of Defense Response in Plants. Front. Mar. Sci. 2016, 3, 81. [Google Scholar] [CrossRef]
- Amil-Ruiz, F.; Garrido-Gala, J.; Gadea, J.; Blanco-Portales, R.; Muñoz-Mérida, A.; Trelles, O.; de los Santos, B.; Arroyo, F.T.; Aguado-Puig, A.; Romero, F.; et al. Partial Activation of SA- and JA-Defensive Pathways in Strawberry upon Colletotrichum acutatum Interaction. Front. Plant Sci. 2016, 7, 1036. [Google Scholar] [CrossRef]
- Cui, H.; Gobbato, E.; Kracher, B.; Qiu, J.; Bautor, J.; Parker, J.E. A Core Function of EDS1 with PAD4 Is to Protect the Salicylic Acid Defense Sector in Arabidopsis Immunity. New Phytol. 2017, 213, 1802–1817. [Google Scholar] [CrossRef]
- Carr, J.P.; Lewsey, M.G.; Palukaitis, P. Chapter 3—Signaling in Induced Resistance. In Advances in Virus Research; Carr, J.P., Loebenstein, G., Eds.; Academic Press: Cambridge, MA, USA, 2010; Volume 76, pp. 57–121. ISBN 0065-3527. [Google Scholar]
- Ebrahim, S.; Usha, K.; Singh, B. Pathogenesis Related (PR) Proteins in Plant Defense Mechanism. Sci. Microb. Pathog. 2011, 2, 1043–1054. [Google Scholar]
- Chernin, L.; Glick, B.R. The Use of ACC Deaminase to Increase the Tolerance of Plants to Various Phytopathogens. In Bacteria in Agrobiology: Stress Management; Maheshwari, D.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 279–299. ISBN 978-3-642-23465-1. [Google Scholar]
- Pieterse, C.M.J.; Van Pelt, J.A.; Ton, J.; Parchmann, S.; Mueller, M.J.; Buchala, A.J.; Métraux, J.-P.; Van Loon, L.C. Rhizobacteria-Mediated Induced Systemic Resistance (ISR) in Arabidopsis Requires Sensitivity to Jasmonate and Ethylene but Is Not Accompanied by an Increase in Their Production. Physiol. Mol. Plant Pathol. 2000, 57, 123–134. [Google Scholar] [CrossRef]
- Turner, J.G.; Ellis, C.; Devoto, A. The Jasmonate Signal Pathway. Plant Cell 2002, 14, S153–S164. [Google Scholar] [CrossRef]
- Creelman, R.A.; Mullet, J.E. Biosynthesis and Action of Jasmonates in Plants. Annu. Rev. Plant Biol. 1997, 48, 355–381. [Google Scholar] [CrossRef]
- Vellosillo, T.; Aguilera, V.; Marcos, R.; Bartsch, M.; Vicente, J.; Cascon, T.; Hamberg, M.; Castresana, C. Defense Activated by 9-Lipoxygenase-Derived Oxylipins Requires Specific Mitochondrial Proteins. Plant Physiol. 2013, 161, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Iriti, M.; Rossoni, M.; Borgo, M.; Ferrara, L.; Faoro, F. Induction of Resistance to Gray Mold with Benzothiadiazole Modifies Amino Acid Profile and Increases Proanthocyanidins in Grape: Primary versus Secondary Metabolism. J. Agric. Food Chem. 2005, 53, 9133–9139. [Google Scholar] [CrossRef] [PubMed]
- Korkina, L.G. Phenylpropanoids as Naturally Occurring Antioxidants: From Plant Defense to Human Health. Cell. Mol. Biol. 2007, 53, 15–25. [Google Scholar]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Jakopic, J.; Cunja, V.; Veberic, R.; Munda, A.; Stampar, F. Phenolic Compounds as Defence Response of Pepper Fruits to Colletotrichum coccodes. Physiol. Mol. Plant Pathol. 2013, 84, 138–145. [Google Scholar] [CrossRef]
- Ruiz-García, Y.; Gómez-Plaza, E. Elicitors: A Tool for Improving Fruit Phenolic Content. Agriculture 2013, 3, 33–52. [Google Scholar] [CrossRef]
- Sivankalyani, V.; Feygenberg, O.; Diskin, S.; Wright, B.; Alkan, N. Increased Anthocyanin and Flavonoids in Mango Fruit Peel Are Associated with Cold and Pathogen Resistance. Postharvest Biol. Technol. 2016, 111, 132–139. [Google Scholar] [CrossRef]
- Boubakri, H.; Poutaraud, A.; Wahab, M.A.; Clayeux, C.; Baltenweck-Guyot, R.; Steyer, D.; Marcic, C.; Mliki, A.; Soustre-Gacougnolle, I. Thiamine Modulates Metabolism of the Phenylpropanoid Pathway Leading to Enhanced Resistance to Plasmopara Viticolain Grapevine. BMC Plant Biol. 2013, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Cluzet, S.; Torregrosa, C.; Jacquet, C.; Lafitte, C.; Fournier, J.; Mercier, L.; Salamagne, S.; Briand, X.; Esquerre’-Tugaye’, M.-T.; Dumas, B. Gene Expression Profiling and Protection of Medicago Truncatula against a Fungal Infection in Response to an Elicitor from Green Algae Ulva spp. Plant Cell Environ. 2004, 27, 917–928. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Rabea, E.I. Potential of the Biopolymer Chitosan with Different Molecular Weights to Control Postharvest Gray Mold of Tomato Fruit. Postharvest Biol. Technol. 2009, 51, 110–117. [Google Scholar] [CrossRef]
- Ali, A.; Mohamed, M.T.M.; Siddiqui, Y. Control of Anthracnose by Chitosan through Stimulation of Defence-Related Enzymes in Eksotika II Papaya (Carica papaya L.) Fruit. J. Biol. Life Sci. 2012, 3, 114–126. [Google Scholar] [CrossRef]
- Meng, X.; Tian, S. Effects of Preharvest Application of Antagonistic Yeast Combined with Chitosan on Decay and Quality of Harvested Table Grape Fruit. J. Sci. Food Agric. 2009, 89, 1838–1842. [Google Scholar] [CrossRef]
- El Hadrami, A.; Adam, L.R.; El Hadrami, I.; Daayf, F. Chitosan in Plant Protection. Mar. Drugs 2010, 8, 968–987. [Google Scholar] [CrossRef] [PubMed]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural Uses of Plant Biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed Extracts as Biostimulants of Plant Growth and Development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Sivasankari, S.; Venkatesalu, V.; Anantharaj, M.; Chandrasekaran, M. Effect of Seaweed Extracts on the Growth and Biochemical Constituents of Vigna Sinensis. Bioresour. Technol. 2006, 97, 1745–1751. [Google Scholar] [CrossRef]
- Vera, J.; Castro, J.; Gonzalez, A.; Moenne, A. Seaweed Polysaccharides and Derived Oligosaccharides Stimulate Defense Responses and Protection against Pathogens in Plants. Mar. Drugs 2011, 9, 2514–2525. [Google Scholar] [CrossRef]
- Elmer, P.A.G.; Reglinski, T. Biosuppression of Botrytis cinerea in Grapes. Plant Pathol. 2006, 55, 155–177. [Google Scholar] [CrossRef]
- Klarzynski, O.; Descamps, V.; Plesse, B.; Yvin, J.-C.; Kloareg, B.; Fritig, B. Sulfated Fucan Oligosaccharides Elicit Defense Responses in Tobacco and Local and Systemic Resistance against Tobacco Mosaic Virus. Mol. Plant Microbe Interact. 2003, 16, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Stadnik, M.J.; Freitas, M.B. de Algal Polysaccharides as Source of Plant Resistance Inducers. Trop. Plant Pathol. 2014, 39, 111–118. [Google Scholar] [CrossRef]
- Schaafsma, G. Safety of Protein Hydrolysates, Fractions Thereof and Bioactive Peptides in Human Nutrition. Eur. J. Clin. Nutr. 2009, 63, 1161. [Google Scholar] [CrossRef]
- Ertani, A.; Sambo, P.; Nicoletto, C.; Santagata, S.; Schiavon, M.; Nardi, S. The Use of Organic Biostimulants in Hot Pepper Plants to Help Low Input Sustainable Agriculture. Chem. Biol. Technol. Agric. 2015, 2, 11. [Google Scholar] [CrossRef]
- Matsubayashi, Y.; Sakagami, Y. Peptide Hormones in Plants. Annu. Rev. Plant Biol. 2006, 57, 649–674. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; Giordano, M.; El-Nakhel, C.; Kyriacou, M.C.; De Pascale, S. Foliar Applications of a Legume-Derived Protein Hydrolysate Elicit Dose-Dependent Increases of Growth, Leaf Mineral Composition, Yield and Fruit Quality in Two Greenhouse Tomato Cultivars. Sci. Hortic. 2017, 226, 353–360. [Google Scholar] [CrossRef]
- Boubaker, H.; Karim, H.; El Hamdaoui, A.; Msanda, F.; Leach, D.; Bombarda, I.; Vanloot, P.; Abbad, A.; Boudyach, E.H.; Ait Ben Aoumar, A. Chemical Characterization and Antifungal Activities of Four Thymus Species Essential Oils against Postharvest Fungal Pathogens of Citrus. Ind. Crops Prod. 2016, 86, 95–101. [Google Scholar] [CrossRef]
- Cindi, M.D.; Soundy, P.; Romanazzi, G.; Sivakumar, D. Different Defense Responses and Brown Rot Control in Two Prunus Persica Cultivars to Essential Oil Vapours after Storage. Postharvest Biol. Technol. 2016, 119, 9–17. [Google Scholar] [CrossRef]
- Soppelsa, S.; Van Hemelrijck, W.; Bylemans, D.; Andreotti, C. Essential Oils and Chitosan Applications to Protect Apples against Postharvest Diseases and to Extend Shelf Life. Agronomy 2023, 13, 822. [Google Scholar] [CrossRef]
- Farzaneh, M.; Kiani, H.; Sharifi, R.; Reisi, M.; Hadian, J. Chemical Composition and Antifungal Effects of Three Species of Satureja (S. hortensis, S. spicigera, and S. khuzistanica) Essential Oils on the Main Pathogens of Strawberry Fruit. Postharvest Biol. Technol. 2015, 109, 145–151. [Google Scholar] [CrossRef]
- Ben-Jabeur, M.; Ghabri, E.; Myriam, M.; Hamada, W. Thyme Essential Oil as a Defense Inducer of Tomato against Gray Mold and Fusarium Wilt. Plant Physiol. Biochem. 2015, 94, 35–40. [Google Scholar] [CrossRef]
- de Lira Mota, K.; de Oliveira Pereira, F.; de Oliveira, W.; Lima, I.; de Oliveira Lima, E. Antifungal Activity of Thymus Vulgaris L. Essential Oil and Its Constituent Phytochemicals against Rhizopus oryzae: Interaction with Ergosterol. Molecules 2012, 17, 14418–14433. [Google Scholar] [CrossRef]
- Wu, Y.-X.; Zhang, Y.-D.; Li, N.; Wu, D.-D.; Li, Q.-M.; Chen, Y.-Z.; Zhang, G.-C.; Yang, J. Inhibitory Effect and Mechanism of Action of Juniper Essential Oil on Gray Mold in Cherry Tomatoes. Front. Microbiol. 2022, 13, 1000526. [Google Scholar] [CrossRef]
- Maia, A.J.; Oliveira, J.S.B.; Schwan-Estrada, K.R.F.; Faria, C.M.R.; Batista, A.F.; Costa, W.F.; Batista, B.N. The Control of Isariopsis Leaf Spot and Downy Mildew in Grapevine cv. Isabel with the Essential Oil of Lemon Grass and the Activity of Defensive Enzymes in Response to the Essential Oil. Crop Prot. 2014, 63, 57–67. [Google Scholar] [CrossRef]
- Soppelsa, S.; Kelderer, M.; Casera, C.; Bassi, M.; Robatscher, P.; Matteazzi, A.; Andreotti, C. Foliar Applications of Biostimulants Promote Growth, Yield and Fruit Quality of Strawberry Plants Grown under Nutrient Limitation. Agronomy 2019, 9, 483. [Google Scholar] [CrossRef]
- Bulger, M.A.; Ellis, M.A.; Madden, L.V. Influence of Temperature and Wetness Duration on Infection of Strawberry Flowers by Botrytis cinerea and Disease Incidence of Fruit Originating from Infected. Am. Phytopathol. Soc. 1987, 77, 1225–1230. [Google Scholar] [CrossRef]
- Wang, S.Y.; Tzeng, D.D.-S. Methionine-Riboflavin Mixtures with Surfactants and Metal Ions Reduce Powdery Mildew Infection in Strawberry Plants. J. Am. Soc. Hortic. Sci. 1998, 123, 987–991. [Google Scholar] [CrossRef]
- Meyers, K.J.; Watkins, C.B.; Pritts, M.P.; Liu, R.H. Antioxidant and Antiproliferative Activities of Strawberries. J. Agric. Food Chem. 2003, 51, 6887–6892. [Google Scholar] [CrossRef]
- Wolfe, K.; Wu, X.; Liu, R.H. Antioxidant Activity of Apple Peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef]
- Cellini, A.; Buriani, G.; Rocchi, L.; Rondelli, E.; Savioli, S.; Rodriguez Estrada, M.T.; Cristescu, S.M.; Costa, G.; Spinelli, F. Biological Relevance of Volatile Organic Compounds Emitted during the Pathogenic Interactions between Apple Plants and Erwinia amylovora. Mol. Plant Pathol. 2017, 19, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Hammer, K.A.; Carson, C.F. Antibacterial and Antifungal Activities of Essential Oils. In Lipids and Essential Oils as Antimicrobial Agents; Thormar, H., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2010. [Google Scholar]
- Sivakumar, D.; Bautista-Baños, S. A Review on the Use of Essential Oils for Postharvest Decay Control and Maintenance of Fruit Quality during Storage. Crop Prot. 2014, 64, 27–37. [Google Scholar] [CrossRef]
- Bhatia, R.; Shreaz, S.; Khan, N.; Muralidhar, S.; Basir, S.F.; Manzoor, N.; Khan, L.A. Proton Pumping ATPase Mediated Fungicidal Activity of Two Essential Oil Components. J. Basic Microbiol. 2011, 52, 504–512. [Google Scholar] [CrossRef]
- Freiesleben, S.H.; Jager, A. Correlation between Plant Secondary Metabolites and Their Antifungal Mechanisms—A Review. Med. Aromat. Plants 2014, 3. [Google Scholar] [CrossRef]
- Ghannoum, M.A.; Rice, L.B. Antifungal Agents: Mode of Action, Mechanisms of Resistance, and Correlation of These Mechanisms with Bacterial Resistance. Clin. Microbiol. Rev. 1999, 12, 501–517. [Google Scholar] [CrossRef]
- Li, D.; Calderone, R. Exploiting Mitochondria as Targets for the Development of New Antifungals. Virulence 2017, 8, 159–168. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Feo, V.D. Essential Oils and Antifungal Activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef]
- Tian, J.; Ban, X.; Zeng, H.; He, J.; Chen, Y.; Wang, Y. The Mechanism of Antifungal Action of Essential Oil from Dill (Anethum graveolens L.) on Aspergillus flavus. PLoS ONE 2012, 7, e30147. [Google Scholar] [CrossRef]
- Benhamou, N.; Bélanger, R.R. Benzothiadiazole-Mediated Induced Resistance to Fusarium oxysporum f. sp. radicis-lycopersici in Tomato. Plant Physiol. 1998, 118, 1203–1212. [Google Scholar] [CrossRef]
- Hien Dao, T.T.; Puig, R.C.; Kim, H.K.; Erkelens, C.; Lefeber, A.W.M.; Linthorst, H.J.M.; Choi, Y.H.; Verpoorte, R. Effect of Benzothiadiazole on the Metabolome of Arabidopsis thaliana. Plant Physiol. Biochem. 2009, 47, 146–152. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, W.; Bi, Y.; Luo, Y. Postharvest BTH Treatment Induces Resistance of Peach (Prunus persica L. cv. Jiubao) Fruit to Infection by Penicillium Expansum and Enhances Activity of Fruit Defense Mechanisms. Postharvest Biol. Technol. 2005, 35, 263–269. [Google Scholar] [CrossRef]
- Skłodowska, M.; Gajewska, E.; Kuźniak, E.; Mikiciński, A.; Sobiczewski, P. BTH-Mediated Antioxidant System Responses in Apple Leaf Tissues. Sci. Hortic. 2010, 125, 34–40. [Google Scholar] [CrossRef]
- Terry, L.A.; Joyce, D.C. Suppression of Grey Mould on Strawberry Fruit with the Chemical Plant Activator Acibenzolar. Pest Manag. Sci. Former. Pestic. Sci. 2000, 56, 989–992. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Qiu, X.; Moore, P.H.; Borth, W.; Hu, J.; Ferreira, S.; Albert, H.H. Systemic Acquired Resistance Induced by BTH in Papaya. Physiol. Mol. Plant Pathol. 2003, 63, 237–248. [Google Scholar] [CrossRef]
- Cellini, A.; Fiorentini, L.; Buriani, G.; Yu, J.; Donati, I.; Cornish, D.A.; Novak, B.; Costa, G.; Vanneste, J.L.; Spinelli, F. Elicitors of the Salicylic Acid Pathway Reduce Incidence of Bacterial Canker of Kiwifruit Caused by Pseudomonas syringae pv. actinidae. Ann. Appl. Biol. 2014, 165, 441–453. [Google Scholar] [CrossRef]
- Bokshi, A.I.; Jobling, J.; McConchie, R. A Single Application of Milsana® Followed by Bion® Assists in the Control of Powdery Mildew in Cucumber and Helps Overcome Yield Losses. J. Hortic. Sci. Biotechnol. 2008, 83, 701–706. [Google Scholar] [CrossRef]
- Wei, Y.; Wei, Y.; Xu, F.; Shao, X. The Combined Effects of Tea Tree Oil and Hot Air Treatment on the Quality and Sensory Characteristics and Decay of Strawberry. Postharvest Biol. Technol. 2018, 136, 139–144. [Google Scholar] [CrossRef]
- Soppelsa, S.; Kelderer, M.; Testolin, R.; Zanotelli, D.; Andreotti, C. Effect of Biostimulants on Apple Quality at Harvest and After Storage. Agronomy 2020, 10, 1214. [Google Scholar] [CrossRef]
- Sturchio, E.; Donnarumma, L.; Annesi, T.; Milano, F.; Casorri, L.; Masciarelli, E.; Zanellato, M.; Meconi, C.; Boccia, P. Essential Oils: An Alternative Approach to Management of Powdery Mildew Diseases. Phytopathol. Mediterr. 2014, 53, 385–395. [Google Scholar]
- Mostafa, Y.S.; Hashem, M.; Alshehri, A.M.; Alamri, S.; Eid, E.M.; Ziedan, E.-S.H.E.; Alrumman, S.A. Effective Management of Cucumber Powdery Mildew with Essential Oils. Agriculture 2021, 11, 1177. [Google Scholar] [CrossRef]
- Iriti, M.; Vitalini, S.; Di Tommaso, G.; D’Amico, S.; Borgo, M.; Faoro, F. New Chitosan Formulation Prevents Grapevine Powdery Mildew Infection and Improves Polyphenol Content and Free Radical Scavenging Activity of Grape and Wine. Aust. J. Grape Wine Res. 2011, 17, 263–269. [Google Scholar] [CrossRef]
- Faoro, F.; Maffi, D.; Cantu, D.; Iriti, M. Chemical-Induced Resistance against Powdery Mildew in Barley: The Effects of Chitosan and Benzothiadiazole. BioControl 2008, 53, 387–401. [Google Scholar] [CrossRef]
- Jayaraj, J.; Wan, A.; Rahman, M.; Punja, Z.K. Seaweed Extract Reduces Foliar Fungal Diseases on Carrot. Crop Prot. 2008, 27, 1360–1366. [Google Scholar] [CrossRef]
- Franssen, H.J.; Bisseling, T. Peptide Signaling in Plants. Proc. Natl. Acad. Sci. USA 2001, 98, 12855–12856. [Google Scholar] [CrossRef]
- Lindsey, K.; Casson, S.; Chilley, P. Peptides: New Signalling Molecules in Plants. Trends Plant Sci. 2002, 7, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Sonawala, U.; Dinkeloo, K.; Danna, C.H.; McDowell, J.M.; Pilot, G. Review: Functional Linkages between Amino Acid Transporters and Plant Responses to Pathogens. Plant Sci. 2018, 277, 79–88. [Google Scholar] [CrossRef]
- Cappelletti, M.; Perazzolli, M.; Nesler, A.; Giovannini, O.; Pertot, I. The Effect of Hydrolysis and Protein Source on the Efficacy of Protein Hydrolysates as Plant Resistance Inducers against Powdery Mildew. J. Bioprocess. Biotech. 2017, 7, 1000306.1–1000306.10. [Google Scholar] [CrossRef]
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]

| Treatment | Name | Active Ingredient | Concentration | Commercial Name |
|---|---|---|---|---|
| CON | Untreated | water | - | - |
| FUN | Fungicide | penconazole | 0.25 mL L−1 | Topas® 200 EW, Syngenta Crop protection, Basel, Switzerland |
| BTH | Benzothiadiazole | acibenzolar-S-methyl | 0.4 g L−1 | Bion® 50 WG, Syngenta Crop protection, Basel, Switzerland |
| CHI | Chitosan solution | chitosan | 10 mL L−1 | ChitoPlant Solution®, Agritalia, Italy |
| SEA | Seaweed extract (Ascophyllum nodosum) | mix of components | 4 g L−1 | Experimental product, ILSA S.p.a., Arzignano, Italy |
| APH | Alfalfa protein hydrolysate | mix of components | 4 g L−1 | Experimental product, ILSA S.p.a., Arzignano, Italy |
| THY | Thyme essential oil (Thymus vulgaris) | thymol | 1 mL L−1 | Essential oil, Vitalis Dr. Joseph, Brunico, Italy |
| JUN | Juniper essential oil (Juniperus communis) | α-pinene | 1 mL L−1 | Essential oil, Vitalis Dr. Joseph, Brunico, Italy |
| Gene Name | Gene Description | Forward Primer Sequence [5′-3′] | Reverse Primer Sequence [5′-3′] |
|---|---|---|---|
| FaPR1 | Pathogenesis-related protein 1 | TGCTAATTCACATTATGGCG | GTTAGAGTTGTAATTATAGTAGG |
| FaPR5 | Pathogenesis-related protein 5 | CGATGCCCCTGCTTACAGTTACCCTAAGGATG | CCTCGTAATTGCTTCAAGGGCAGAACACAACC |
| FaPR10 | Pathogenesis-related protein 10 | CGAGGAATACAACTAAACCTTGCCGTCT | TACAATTTGCCACACATACACCGAAGTG |
| FaEDS1 | Enhanced disease susceptibility 1 | AAAAGAGAGACTTCAATGCCAATGTG | CTTCGTTCTTTGCGTGTCTGTAGTAGTT |
| FaLOX | Lipoxygenase | CGACGACGACTGGATACACCGCAGGG | GAGGTTGGCCGCTGTTTCTTGCACCGTA |
| FaGAPDH2 | (housekeeping) | CCCAAGTAAGGATGCCCCCATGTTCG | TTGGCAAGGGGAGCAAGACAGTTGGTAG |
| Treatment | Total Yield (kg plant−1) | Number Fruits Plant−1 (N°) | Mean Fruit Weight (g) | Total Soluble Solids (°Brix) | Titratable Acidity (g citric acid L−1) | Total Phenol Content (mg GAE 100 g−1 DW) | ||
|---|---|---|---|---|---|---|---|---|
| CON | 0.120 ± 0.002 | e | 11.63 ± 0.71 | de | 10.62 ± 0.57 | 13.13 ± 0.27 | 4.29 ± 0.49 | 2139.09 ± 63.54 |
| FUN | 0.161 ± 0.004 | a | 14.00 ± 0.46 | ab | 11.57 ± 0.38 | 11.43 ± 0.52 | 3.75 ± 0.61 | 2602.84 ± 303.15 |
| BTH | 0.121 ± 0.003 | de | 12.00 ± 0.60 | cde | 10.21 ± 0.29 | 12.73 ± 1.06 | 3.48 ± 0.17 | 2250.58 ± 145.74 |
| CHI | 0.135 ± 0.007 | c | 12.38 ± 0.80 | bcde | 11.10 ± 0.48 | 11.23 ± 1.62 | 2.95 ± 0.33 | 2286.84 ± 125.48 |
| SEA | 0.133 ± 0.003 | cd | 13.13 ± 0.90 | abcd | 10.44 ± 0.72 | 10.68 ± 0.72 | 3.11 ± 0.17 | 2204.47 ± 92.48 |
| APH | 0.127 ± 0.005 | cde | 11.25 ± 0.67 | e | 11.54 ± 0.54 | 12.53 ± 0.17 | 3.24 ± 0.14 | 2431.74 ± 57.76 |
| THY | 0.148 ± 0.003 | b | 13.50 ± 0.63 | abc | 11.17 ± 0.55 | 11.60 ± 0.69 | 3.12 ± 0.24 | 2499.77 ± 256.05 |
| JUN | 0.157 ± 0.005 | ab | 14.38 ± 0.32 | a | 10.98 ± 0.26 | 11.38 ± 0.36 | 2.91 ± 0.14 | 2235.11 ± 114.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soppelsa, S.; Cellini, A.; Donati, I.; Buriani, G.; Spinelli, F.; Andreotti, C. Green Alternatives for the Control of Fungal Diseases in Strawberry: In-Field Optimization of the Use of Elicitors, Botanical Extracts and Essential Oils. Horticulturae 2024, 10, 1044. https://doi.org/10.3390/horticulturae10101044
Soppelsa S, Cellini A, Donati I, Buriani G, Spinelli F, Andreotti C. Green Alternatives for the Control of Fungal Diseases in Strawberry: In-Field Optimization of the Use of Elicitors, Botanical Extracts and Essential Oils. Horticulturae. 2024; 10(10):1044. https://doi.org/10.3390/horticulturae10101044
Chicago/Turabian StyleSoppelsa, Sebastian, Antonio Cellini, Irene Donati, Giampaolo Buriani, Francesco Spinelli, and Carlo Andreotti. 2024. "Green Alternatives for the Control of Fungal Diseases in Strawberry: In-Field Optimization of the Use of Elicitors, Botanical Extracts and Essential Oils" Horticulturae 10, no. 10: 1044. https://doi.org/10.3390/horticulturae10101044
APA StyleSoppelsa, S., Cellini, A., Donati, I., Buriani, G., Spinelli, F., & Andreotti, C. (2024). Green Alternatives for the Control of Fungal Diseases in Strawberry: In-Field Optimization of the Use of Elicitors, Botanical Extracts and Essential Oils. Horticulturae, 10(10), 1044. https://doi.org/10.3390/horticulturae10101044









