Preparation and Impact of Fermented Sheep Bone Powder on Sausage Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.2.1. Preparation of Enzymatic Bone Powder Solution
2.2.2. Activation and Domestication of Strains
2.2.3. Preparation of Fermented Sheep Bone Powder
2.2.4. Preparation of Sausage
2.3. Degree of Hydrolysis
2.4. Calcium Content
2.5. Scanning Electron Microscope (SEM) and Particle Size Analysis
2.6. Basic Nutrients
2.7. Amino Acid Assay
2.8. Color Evaluation
2.9. Texture Determination (TLP)
2.10. Sensory Evaluation
2.11. Statistical Analysis
3. Results and Discussion
3.1. Optimization of Fermented Sheep Bone Sludge
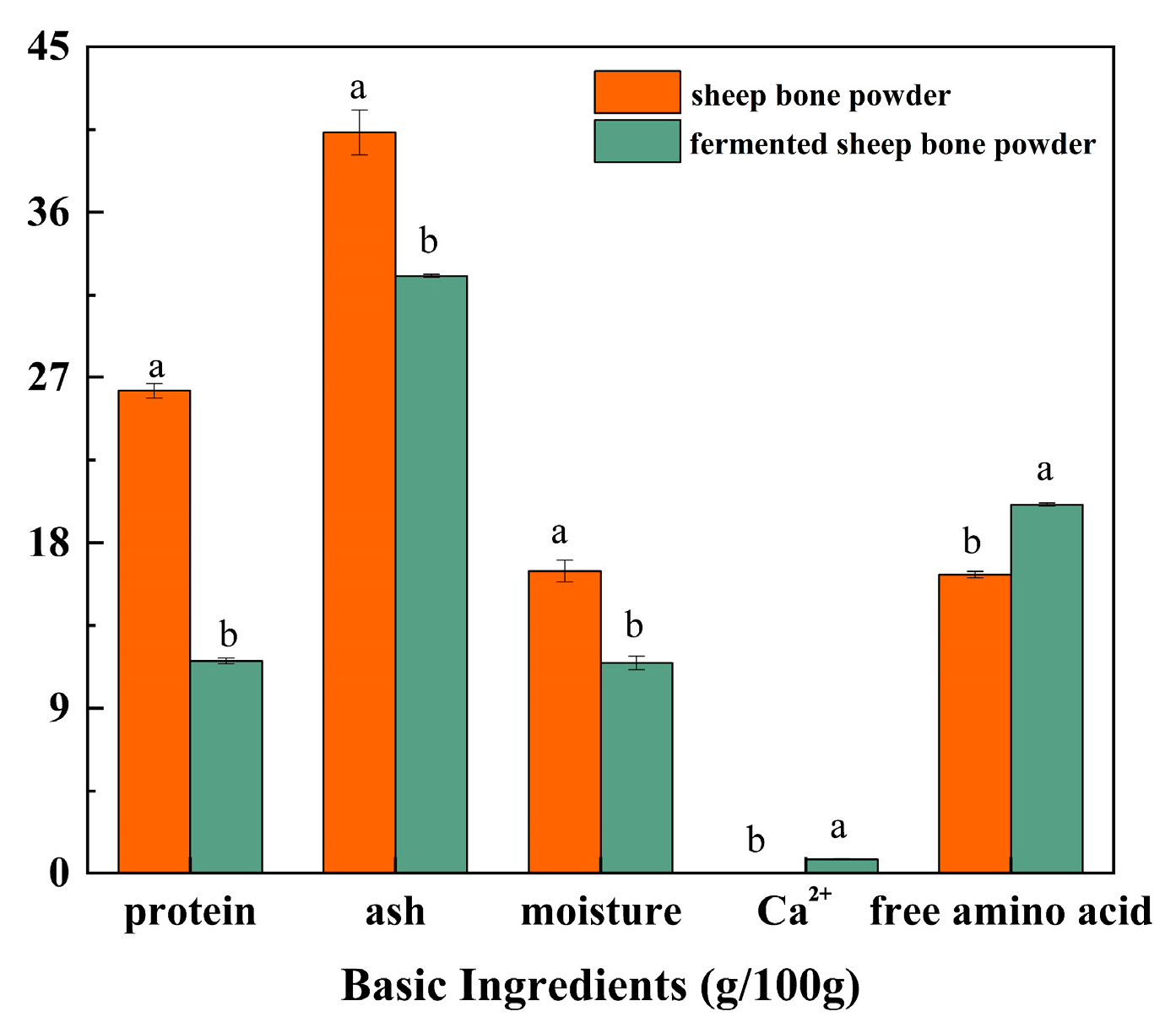
3.2. Basic Ingredients of Fermented Sheep Bone Powder
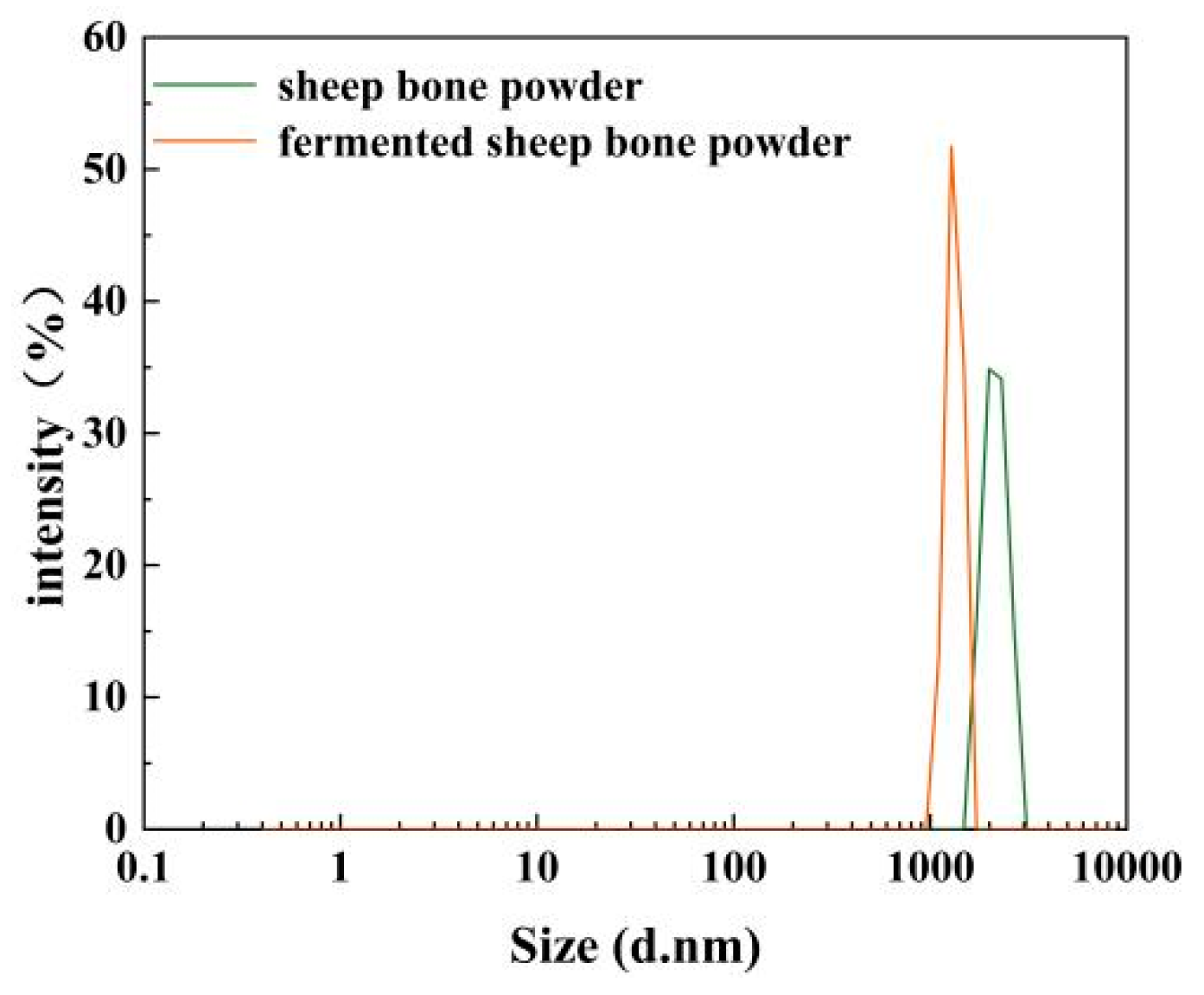
3.3. Microstructural Analysis of Fermented Sheep Bone Powder
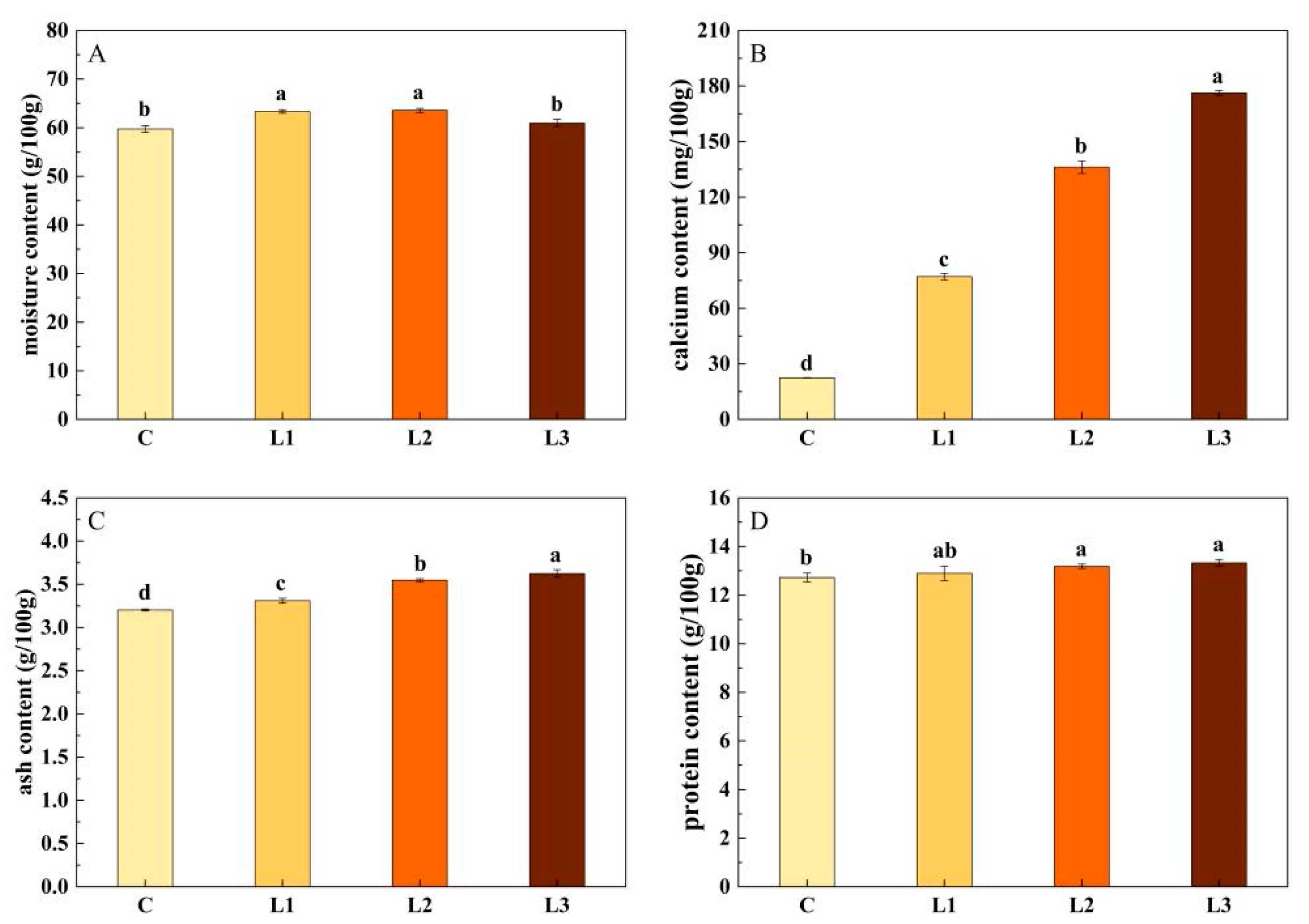
3.4. Effect of Fermented Sheep Bone Powder on the Basic Composition of Sausages
3.5. Effect of Fermented Sheep Bone Powder on the Amino Acid Content of Sausages
3.6. Effect of Fermented Sheep Bone Powder on the Texture of Sausages
3.7. Effect of Fermented Sheep Bone Powder on the Color of Sausages
3.8. Effect of Fermented Sheep Bone Powder on the Sensory Evaluation of Sausages
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Weaver, C.M.; Peacock, M. Calcium. Adv. Nutr. 2019, 10, 546–548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.; Venkitasamy, C.; Guo, S.; Pan, Z.; Ke, H.; Tang, H.; Huang, W.; Zhao, L. Improving the Flavor of Microbone Meal with Flavourzyme by Response Surface Method. J. Food Process Eng. 2019, 42, e13040.1–e13040.11. [Google Scholar] [CrossRef]
- Cordeiro, A.R.; Bezerra, T.K.; Madruga, M.S. Valuation of Goat and Sheep By-Products: Challenges and Opportunities for Their Use. Animals 2022, 12, 3277. [Google Scholar] [CrossRef] [PubMed]
- ur Rahman, U.; Sahar, A.; Khan, M.A. Recovery and Utilization of Effluents from Meat Processing Industries. Food Res. Int. 2014, 100, 4884–4886. [Google Scholar] [CrossRef]
- Yin, T.; Du, H.; Zhang, J. Preparation and Characterization of Ultrafine Fish Bone Powder. J. Aquat. Food Prod. Technol. 2016, 25, 1045–1055. [Google Scholar] [CrossRef]
- Kakimov, A.K.; Kabulov, B.B.; Yessimbekov, Z.S.; Kuderinova, N.A. Use of meat-bone paste as a protein source in meat product production. Teor. Prakt. Pererab. Mâsa 2016, 1, 42–50. [Google Scholar] [CrossRef]
- Liang, L.; Zhou, C.; Yuyu, Z.; Sun, B. Effect of Welsh Onion on Taste Components and Sensory Characteristics of Porcine Bone Soup. Foods 2021, 10, 2968. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, M.; Bhandari, B.; Gao, Z. Novel Technologies in Utilization of Byproducts of Animal Food Processing: A Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 3420–3430. [Google Scholar] [CrossRef]
- Qin, X.; Shen, Q.; Guo, Y.; Li, X.; Liu, J.; Ye, M.; Wang, H.; Jia, W.; Zhang, C. Physicochemical Properties, Digestibility and Anti-Osteoporosis Effect of Yak Bone Powder with Different Particle Sizes. Food Res. Int. Ott. Ont. 2021, 145, 110401. [Google Scholar] [CrossRef]
- Han, K.; Pang, P.; Cao, J.; Huo, N.; Zhang, H.; Chang, H. Fermentation of sheep bone enzymatic hydrolysates by Lactobacillus plantarum. Chin. J. Biotechnol. 2018, 34, 11. [Google Scholar]
- Zhang, J.; Li, X.; Zhao, K.; Li, H.; Liu, J.; Da, S.; Ciren, D.; Tang, H. In Vitro Digestion and Fermentation Combined with Microbiomics and Metabolomics Reveal the Mechanism of Superfine Yak Bone Powder Regulating Lipid Metabolism by Altering Human Gut Microbiota. Food Chem. 2023, 410, 135441. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Qiao, Y.; Zou, Y.; Huang, M.; Kang, Z.; Zhou, G. Effect of Flavourzyme on Proteolysis, Antioxidant Capacity and Sensory Attributes of Chinese Sausage. Meat Sci. 2014, 98, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Wang, D.; Sun, L.; Su, R.; Corazzin, M.; Sun, X.; Dou, L.; Zhang, M.; Zhao, L.; Su, L. Isolation, Purification and Structure Identification of a Calcium-Binding Peptide from Sheep Bone Protein Hydrolysate. Foods 2022, 11, 2655. [Google Scholar] [CrossRef] [PubMed]
- Venuste, M.; Zhang, X.; Shoemaker, C.F.; Karangwa, E.; Abbas, S.; Kamdem, P.E. Influence of Enzymatic Hydrolysis and Enzyme Type on the Nutritional and Antioxidant Properties of Pumpkin Meal Hydrolysates. Food Funct. 2013, 4, 811–820. [Google Scholar] [CrossRef]
- Hu, H.; Li, Y.; Zhang, L.; Tu, H.; Wang, X.; Ren, L.; Dai, S.; Wang, L. Use of Tremella as Fat Substitute for the Enhancement of Physicochemical and Sensory Profiles of Pork Sausage. Foods 2021, 10, 2167. [Google Scholar] [CrossRef]
- Mirzapour-Kouhdasht, A.; Garcia-Vaquero, M.; Eun, J.-B.; Simal-Gandara, J. Influence of Enzymatic Hydrolysis and Molecular Weight Fractionation on the Antioxidant and Lipase/α-Amylase Inhibitory Activities In Vitro of Watermelon Seed Protein Hydrolysates. Molecules. 2022, 27, 7897. [Google Scholar] [CrossRef]
- Rigdon, M.; Stelzleni, A.M.; Mckee, R.W.; Pringle, T.D.; Thippareddi, H. Texture and Quality of Chicken Sausage Formulated with Woody Breast Meat. Poult. Sci. 2020, 100, 100915. [Google Scholar] [CrossRef]
- Meng, H.; Fan, J.; Yang, Y. Development of Bean Curd and Fish Sausage. Sci. Technol. Food Ind. 2006, 27, 3. [Google Scholar]
- Toldrá, F.; Reig, M.; Mora, L. Management of Meat By- and Co-Products for an Improved Meat Processing Sustainability. Meat Sci. 2021, 181, 108608. [Google Scholar] [CrossRef]
- Zhang, L. Research Status and Development Trend of Comprehensive Utilization of Livestock and Poultry Bones. Mod. Foods 2021, 86–90 + 94. [Google Scholar] [CrossRef]
- Han, K.; Cao, J.; Wang, J.; Chen, J.; Yuan, K.; Pang, F.; Gu, S.; Huo, N. Effects of Lactobacillus Helveticus Fermentation on the Ca2+ Release and Antioxidative Properties of Sheep Bone Hydrolysate. Food Sci. Anim. Resour. 2018, 38, 1144–1154. [Google Scholar] [CrossRef] [PubMed]
- Zong, H.; Bin, Z.; Qin, B.; Fang, H.; Sun, J.; Feng, Q.; Lou, X. Study on the Functional Fermentation Process of Pork Bone Paste. China Condiment 2014, 000, 31–35,46. [Google Scholar]
- Wang, X.; Zhang, Z.; Xu, H.; Li, X.; Hao, X. Preparation of Sheep Bone Collagen Peptide–Calcium Chelate Using Enzymolysis-Fermentation Methodology and Its Structural Characterization and Stability Analysis. R. Soc. Chem. 2020, 10, 11624–11633. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Zhang, M.; Mujumdar, A.S.; Liu, Y. Effect of Different Drying Methods Combined with Fermentation and Enzymolysis on Nutritional Composition and Flavor of Chicken Bone Powder. Dry Technol. Int. J. 2021, 39, 1240–1250. [Google Scholar] [CrossRef]
- Atef, M.; Ojagh, S.M.; Latifi, A.M.; Esmaeili, M.; Udenigwe, C.C. Biochemical and Structural Characterization of Sturgeon Fish Skin Collagen (Huso Huso). J. Food Biochem. 2020, 44, e13256. [Google Scholar] [CrossRef]
- Lu, D.; Peng, M.; Yu, M.; Jiang, B.; Chen, J. Effect of Enzymatic Hydrolysis on the Zinc Binding Capacity and in Vitro Gastrointestinal Stability of Peptides Derived From Pumpkin (Cucurbita pepo L.) Seeds. Front. Nutr. 2021, 8, 647782. [Google Scholar] [CrossRef]
- Yu, L.; Chai, M.; Zeng, M.; He, Z.; Chen, J. Effect of Lipid Oxidation on the Formation of N ε -Carboxymethyl-Lysine and N ε-Carboxyethyl-Lysine in Chinese-Style Sausage during Storage. Food Chem. 2018, 269, 466–472. [Google Scholar] [CrossRef]
- Su, C.; Xu, J. The Effects of Sulphur Amino Acids on the Characteristic of Meat Flavors by Maillard Reaction. J. Suzhou Univ. 2008, 4, 114–116 + 120. [Google Scholar]
- Careri, M.; Mangia, A.; Barbieri, G.; Bouoni, L.; Virgili, R.; Parolari, G. Sensory Property Relationships to Chemical Data of Italian-type Dry-cured Ham. J. Food Sci. 2006, 58, 968–972. [Google Scholar] [CrossRef]
- Lee, N.; Park, J.W. Calcium Compounds to Improve Gel Functionality of Pacific Whiting and Alaska Pollock Surimi. J. Food Sci. 2006, 63, 969–974. [Google Scholar] [CrossRef]
- Yin, T.; Park, J.W. Effects of Nano-Scaled Fish Bone on the Gelation Properties of Alaska Pollock Surimi. Food Chem. 2014, 150, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Hemung, B.O.; Yongsawatdigul, J.; Chin, K.B.; Limphirat, W.; Siritapetawee, J. Silver Carp Bone Powder as Natural Calcium for Fish Sausage. J. Aquat. Food Prod. Technol. 2018, 27, 305–315. [Google Scholar] [CrossRef]
- Abreu, V.K.G.; Pereira, A.L.F.; de Freitas, E.R.; Trevisan, M.T.S.; da Costa, J.M.C.; Cruz, C.E.B. Lipid and Color Stability of the Meat and Sausages of Broiler Fed with Calcium Anacardate. J. Sci. Food Agric. 2019, 99, 2124–2131. [Google Scholar] [CrossRef] [PubMed]
- Daengprok, W.; Garnjanagoonchorn, W.; Mine, Y. Fermented Pork Sausage Fortified with Commercial or Hen Eggshell Calcium Lactate. Meat Sci. 2002, 62, 199–204. [Google Scholar] [CrossRef]
- Teixeira, A.; Silva, S.; Guedes, C.; Rodrigues, S. Sheep and Goat Meat Processed Products Quality: A Review. Foods 2020, 9, 960. [Google Scholar] [CrossRef]
- Choi, Y.M.; Jung, K.C.; Jo, H.M.; Nam, K.W.; Choe, J.H.; Rhee, M.S.; Kim, B.C. Combined Effects of Potassium Lactate and Calcium Ascorbate as Sodium Chloride Substitutes on the Physicochemical and Sensory Characteristics of Low-Sodium Frankfurter Sausage. Meat Sci. 2014, 96, 21–25. [Google Scholar] [CrossRef]
- Yang, X.; Sebranek, J.G.; Luo, X.; Zhang, W.; Zhang, M.; Xu, B.; Zhang, Y.; Liang, R. Effects of Calcium Salts on the Physicochemical Quality of Cured Beef Sausages during Manufacturing and Storage: A Potential Calcium Application for Sausages with Alginate Casings. Foods 2021, 10, 2783. [Google Scholar] [CrossRef]





| Item | Total Score | Score Criteria |
|---|---|---|
| Flavor | 5 | strong flavor in meat; smooth in taste; no impurities (4–5); slightly strong flavor in meat; slightly rough taste; a few impurities (2–3); light flavor in meat; rough in taste; lots of impurities (1–2). |
| Texture | 3 | Firm and elastic (3); slightly firm and elastic (2); loose and non-elastic (1). |
| Color | 2 | Bright, rose-red in color (2); slightly dull, dark red in color (1); very grayish color (0). |
| Item | C | L1 | L2 | L3 |
|---|---|---|---|---|
| Asp | 0.95 ± 0.033 b | 1.05 ± 0.011 a | 1.08 ± 0.037 a | 1.05 ± 0.011 a |
| Thr * # | 0.51 ± 0.024 a | 0.52 ± 0.013 a | 0.51 ± 0.011 a | 0.52 ± 0.010 a |
| Ser | 0.48 ± 0.019 a | 0.50 ± 0.08 a | 0.49 ± 0.009 a | 0.50 ± 0.008 a |
| Glu | 1.92 ± 0.074 a | 2.02 ± 0.068 a | 1.91 ± 0.057 a | 1.97 ± 0.048 a |
| Pro # | 0.48 ± 0.015 bc | 0.47 ± 0.016 c | 0.51 ± 0.009 ab | 0.52 ± 0.011 a |
| Gly | 0.56 ± 0.018 b | 0.54 ± 0.014 b | 0.67 ± 0.009 a | 0.69 ± 0.025 a |
| Ala # | 0.67 ± 0.019 b | 0.69 ± 0.008 ab | 0.71 ± 0.008 a | 0.72 ± 0.005 a |
| Val * # | 0.44 ± 0.014 a | 0.42 ± 0.014 a | 0.42 ± 0.010 a | 0.42 ± 0.010 a |
| Met * # | 0.18 ± 0.004 b | 0.24 ± 0.002 a | 0.24 ± 0.004 a | 0.24 ± 0.008 a |
| Ile * # | 0.85 ± 0.022 a | 0.87 ± 0.018 a | 0.84 ± 0.015 a | 0.86 ± 0.023 a |
| Leu * # | 0.39 ± 0.014 a | 0.38 ± 0.018 a | 0.37 ± 0.007 a | 0.38 ± 0.009 a |
| Tyr | 0.37 ± 0.013 a | 0.38 ± 0.014 a | 0.37 ± 0.014 a | 0.36 ± 0.0152 a |
| Phe * # | 0.30 ± 0.014 b | 0.31 ± 0.007 b | 0.31 ± 0.008 b | 0.35 ± 0.001 a |
| His * | 0.41 ± 0.009 a | 0.41 ± 0.011 a | 0.41 ± 0.009 a | 0.41 ± 0.006 a |
| Lys * | 0.94 ± 0.022 a | 0.93 ± 0.009 a | 0.92 ± 0.011 a | 0.92 ± 0.008 a |
| Arg | 0.67 ± 0.023 b | 0.68 ± 0.009 ab | 0.69 ± 0.014 ab | 0.70 ± 0.009 a |
| Total Amino Acid | 10.11 ± 0.338 b | 10.44 ± 0.234 ab | 10.48 ± 0.232 ab | 10.62 ± 0203 a |
| Essential Amino Acid (EAA) | 4.02 ± 0.122 a | 4.10 ± 0.093 a | 4.03 ± 0.074 a | 4.10 ± 0.075 a |
| Hydrophobic Amino Acid | 3.82 ± 0.126 b | 3.91 ± 0.096 ab | 3.93 ± 0.071 ab | 4.01 ± 0.077 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Zhao, S.; Wang, X.; Le, W.; Hu, G.; Liu, T.; Zhao, C.; Jin, Y.; Su, L. Preparation and Impact of Fermented Sheep Bone Powder on Sausage Quality. Fermentation 2023, 9, 842. https://doi.org/10.3390/fermentation9090842
Wang C, Zhao S, Wang X, Le W, Hu G, Liu T, Zhao C, Jin Y, Su L. Preparation and Impact of Fermented Sheep Bone Powder on Sausage Quality. Fermentation. 2023; 9(9):842. https://doi.org/10.3390/fermentation9090842
Chicago/Turabian StyleWang, Chenlei, Siyu Zhao, Xiaolin Wang, Wenjia Le, Guanhua Hu, Ting Liu, Congying Zhao, Ye Jin, and Lin Su. 2023. "Preparation and Impact of Fermented Sheep Bone Powder on Sausage Quality" Fermentation 9, no. 9: 842. https://doi.org/10.3390/fermentation9090842
APA StyleWang, C., Zhao, S., Wang, X., Le, W., Hu, G., Liu, T., Zhao, C., Jin, Y., & Su, L. (2023). Preparation and Impact of Fermented Sheep Bone Powder on Sausage Quality. Fermentation, 9(9), 842. https://doi.org/10.3390/fermentation9090842








