Aguamiel Enhance Proteolytic Activity and Survival of Lactiplantibacillus pentosus ABHEAU-05 during Refrigerated Storage of a Fermented Milk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Obtaining and Analysis of Aguamiel
2.2. Adaptation of Lactiplantibacillus pentosus ABHEAU-05
2.3. Fermentation Kinetics
2.4. Proteolysis by Tri-Nitro Benzyl Sulfonic (TNBS)
2.5. Evaluation of Refrigerated Fermented Milk
2.6. Separation of Hydrolyzed Milk Proteins by Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.7. Statistical Analysis
3. Results and Discussion
3.1. Aguamiel Analysis
3.2. Fermentation Kinetics
3.3. Determination of Free Amino Groups during Fermentation
3.4. Metabolism during Refrigeration
3.4.1. Viability
3.4.2. Production of Free Amino Groups
3.4.3. Identification of Protein Hydrolysis by SDS-PAGE
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Granato, D.; Barba, F.J.; Bursać Kovačević, D.; Lorenzo, J.M.; Cruz, A.G.; Putnik, P. Functional foods: Product development, technological trends, efficacy testing, and safety. Annu. Rev. Food Sci. Technol. 2020, 11, 93–118. [Google Scholar] [CrossRef]
- Ari, S. Studies on functional foods in Japan—State of the art. Biosci. Biotechnol. Biochem. 1996, 60, 9–15. [Google Scholar] [CrossRef]
- Domínguez, D.L.; Fernández, R.V.; Cámara, M. An international regulatory review of food health-related claims in functional food products labeling. J. Funct. Foods 2020, 68, 103896. [Google Scholar] [CrossRef]
- Savaiano, D.A.; Hutkins, R.W. Yogurt, cultured fermented milk, and health: A systematic review. Nutr. Rev. 2021, 79, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Zendeboodi, F.; Khorshidian, N.; Mortazavian, A.M.; G da Cruz, A. Probiotic: Conceptualization from a new approach. Curr. Opin. Food Sci. 2020, 32, 103–123. [Google Scholar] [CrossRef]
- Chen, W.; Hang, F. Lactic Acid Bacteria Starter. Lactic Acid Bacteria: Bioengineering and Industrial Applications; Springer: Berlin/Heidelberg, Germany, 2019; pp. 93–143. [Google Scholar]
- Palanivelu, J.; Thanigaivel, S.; Vickram, S.; Dey, N.; Mihaylova, D.; Desseva, I. Probiotics in Functional Foods: Survival Assessment and Approaches for Improved Viability. Appl. Sci. 2022, 12, 455. [Google Scholar] [CrossRef]
- Aoun, A.; Darwish, F.; Hamod, N. The influence of the gut microbiome on obesity in adults and the role of probiotics, prebiotics, and synbiotics for weight loss. Prev. Nutr. Food Sci. 2020, 25, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Olvera-Rosales, L.-B.; Cruz-Guerrero, A.-E.; Ramírez-Moreno, E.; Quintero-Lira, A.; Contreras-López, E.; Jaimez-Ordaz, J.; Castañeda-Ovando, A.; Añorve-Morga, J.; Calderón-Ramos, Z.-G.; Arias-Rico, J.; et al. Impact of the Gut Microbiota Balance on the Health–Disease Relationship: The Importance of Consuming Probiotics and Prebiotics. Foods 2021, 10, 1261. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, D.H.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Yilmaz, S.; Yilmaz, E.; Dawood, M.A.; Ringø, E.; Ahmadifar, E.; Abdel-Latif, H.M. Probiotics, prebiotics, and synbiotics used to control vibriosis in fish: A review. Aquaculture 2022, 547, 737514. [Google Scholar] [CrossRef]
- Fan, S.; Zhang, Z.; Zhao, Y.; Daglia, M.; Zhang, J.; Zhu, Y.; Bai, J.; Zhu, L.; Xiao, X. Recent advances in targeted manipulation of the gut microbiome by prebiotics: From taxonomic composition to metabolic function. Curr. Opin. Food Sci. 2022, 49, 100959. [Google Scholar] [CrossRef]
- Pang, J.; Zhang, Y.; Tong, X.; Zhong, Y.; Kong, F.; Li, D.; Liu, X.; Qiao, Y. Recent Developments in Molecular Characterization, Bioactivity, and Application of Arabinoxylans from Different Sources. Polymers 2023, 15, 225. [Google Scholar] [CrossRef]
- Ortiz-Basurto, R.I.; Pourcelly, G.; Doco, T.; Williams, P.; Dornier, M.; Belleville, M.P. Analysis of the main components of the aguamiel produced by the maguey-pulquero (Agave mapisaga) throughout the harvest period. J. Agric. Food Chem. 2008, 56, 3682–3687. [Google Scholar] [CrossRef]
- Romero-López, M.R.; Osorio-Díaz, P.; Flores-Morales, A.; Robledo, N.; Mora-Escobedo, R. Chemical composition, antioxidant capacity and prebiotic effect of aguamiel (Agave atrovirens) during in vitro fermentation. Rev. Mex. Ing. Quim. 2015, 14, 281–292. [Google Scholar]
- Villarreal-Morales, S.L.; Muñiz-Márquez, D.B.; Michel-Michel, M.; González-Montemayor, Á.M.; Escobedo-García, S.; Salas-Tovar, J.A.; Flores, C.; Rodríguez-Herrera, R. Aguamiel a fresh beverage from Agave spp. sap with functional properties. In Natural Beverages; Academic Press: Cambridge, MA, USA, 2019; pp. 179–208. [Google Scholar]
- Peralta-García, I.; González-Muñoz, F.; Rodríguez-Alegría, M.E.; Sánchez-Flores, A.; López-Munguía, A. Evolution of fructans in aguamiel (Agave sap) during the plant production lifetime. Front. Nutr. 2020, 7, 566950. [Google Scholar] [CrossRef] [PubMed]
- Jaimez-Ordaz, J.; Martinez-Ramirez, X.; Cruz-Serrano, A.E.; Contreras-López, E.; Ayala-Nino, A.; Castro-Rosas, J.; González-Olivares, L.G. Survival and proteolytic capacity of probiotics in a fermented milk enriched with agave juice and stored in refrigeration. Food Sci. Technol. 2019, 39, 188–194. [Google Scholar] [CrossRef]
- Ramírez-Godínez, J.; Gutiérrez-Rodríguez, J.F.; Contreras-López, E.; Rodríguez-Serrano, G.M.; Castañeda-Ovando, A.; Jaimez-Ordaz, J.; González-Olivares, L.G. Agave juice improves survival and proteolytic activity of Lactobacillus rhamnosus GG during ripening of semi-ripened mexican cheese. Food Sci. Technol. 2021, 42, e30820. [Google Scholar] [CrossRef]
- Escalante, A.; Giles-Gómez, M.; Esquivel-Flores, G.; Matus Acuña, V.; Moreno-Terrazas, R.; López-Munguía, A.; Lappe-Oliveras, P. Pulque fermentation. In Handbook of Plant-Based Fermented Food and Beverage Technology; Routledge: London, UK, 2012; Volume 2, pp. 691–706. [Google Scholar]
- Escalante, A.; López Soto, D.R.; Velázquez Gutiérrez, J.E.; Giles-Gómez, M.; Bolívar, F.; López-Munguía, A. Pulque, a traditional Mexican alcoholic fermented beverage: Historical, microbiological, and technical aspects. Front. Microbiol. 2016, 7, 1026. [Google Scholar] [CrossRef] [PubMed]
- de la Rosa, O.; Flores-Gallegos, A.C.; Muñíz-Márquez, D.; Contreras-Esquivel, J.C.; Teixeira, J.A.; Nobre, C.; Aguilar, C.N. Successive Fermentation of Aguamiel and Molasses by Aspergillus oryzae and Saccharomyces cerevisiae to Obtain High Purity Fructooligosaccharides. Foods 2022, 11, 1786. [Google Scholar] [CrossRef]
- Carrillo-López, A.; Silos-Espino, H.; Flores-Benitez, S.; Espinoza-Sánchez, E.A.; Ornelas-Tavares, J.R.; Flores-Chávez, L.; Tovar-Robles, C.; Méndez-Gallegos, J.; Rossel-Kipping, D. Some evidences on effect of intake aguamiel (Agave sap). Sustain. Agric. Res. 2016, 5, 49–55. [Google Scholar] [CrossRef]
- Cervantes-Elizarrarás, A.; Cruz-Cansino, N.d.S.; Ramírez-Moreno, E.; Vega-Sánchez, V.; Velázquez-Guadarrama, N.; Zafra-Rojas, Q.Y.; Piloni-Martini, J. In vitro probiotic potential of lactic acid bacteria isolated from aguamiel and pulque and antibacterial activity against pathogens. Appl. Sci. 2019, 9, 601. [Google Scholar] [CrossRef]
- Moreno-Vilet, L.; Bostyn, S.; Flores-Montaño, J.L.; Camacho-Ruiz, R.M. Size-exclusion chromatography (HPLC-SEC) technique optimization by simplex method to estimate molecular weight distribution of agave fructans. Food Chem. 2017, 237, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Ramírez, M.C.; Jaimez-Ordaz, J.; Escorza-Iglesias, V.A.; Rodríguez-Serrano, G.M.; Contreras-López, E.; Ramírez-Godínez, J.; Castañeda-Ovando, A.; Morales-Estrada, A.I.; Felix-Reyes, N.; González-Olivares, L.G. Lactobacillus pentosus ABHEAU-05: An in vitro digestion resistant lactic acid bacterium isolated from a traditional fermented Mexican beverage. Rev. Argent. Microbiol. 2020, 52, 305–314. [Google Scholar] [CrossRef] [PubMed]
- González-Olivares, L.G.; Añorve-Morga, J.; Castañeda-Ovando, A.; Contreras-López, E.; Jaimez-Ordaz, J. Peptide separation of commercial fermented milk during refrigerated storage. Food Sci. Technol. 2014, 34, 674–679. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lainez, C.; López-Velázquez, G.; de Vos, P. Health benefits of Inulin and Agavin-type Fructans in Food: Impact on Microbiota, Immune and Gut Barrier Function. In The Book of Fructans; Academic Press: Cambridge, MA, USA, 2023; pp. 211–234. [Google Scholar]
- Zalán, Z.; Hudáček, J.; Štětina, J.; Chumchalová, J.; Halász, A. Production of organic acids by Lactobacillus strains in three different media. Eur. Food Res. Technol. 2010, 230, 395–404. [Google Scholar] [CrossRef]
- Gámez, H.J.; Ramírez, C.; Aguirre, D. Cinética de fermentación de Lactobacillus plantarum en un medio de cultivo enriquecido como potencial probiótico. Rev. Vet. Zootec. 2013, 7, 37–53. [Google Scholar]
- Concha, J.L.H.; Agudelo, C.; Ortega, R. Determinación de parámetros cinéticos de dos inóculos lácticos: Lactobacillus plantarum A6 y bacterias ácido lácticas de yogurt. Biotecnol. Sect. Agropecu. Agroind. 2010, 8, 8–16. [Google Scholar]
- Mueller, M.; Reiner, J.; Fleischhacker, L.; Viernstein, H.; Loeppert, R.; Praznik, W. Growth of selected probiotic strains with fructans from different sources relating to degree of polymerization and structure. J. Funct. Foods 2016, 24, 264–275. [Google Scholar] [CrossRef]
- Böger, M.; Van Leeuwen, S.S.; Lammerts van Bueren, A.; Dijkhuizen, L. Structural identity of galactooligosaccharide molecules selectively utilized by single cultures of probiotic bacterial strains. J. Agric. Food Chem. 2019, 67, 13969–13977. [Google Scholar] [CrossRef]
- Kneifel, W. In vitro growth behaviour of probiotic bacteria in culture media with carbohydrates of prebiotic importance. Microb. Ecol. Health Dis. 2000, 12, 27–34. [Google Scholar]
- Di Lodovico, S.; Gasparri, F.; Di Campli, E.; Di Fermo, P.; D’Ercole, S.; Cellini, L.; Di Giulio, M. Prebiotic combinations effects on the colonization of Staphylococcal skin strains. Microorganisms 2021, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Oluwatosin, S.O.; Tai, S.L.; Fagan-Endres, M.A. Sucrose, maltodextrin and inulin efficacy as cryoprotectant, preservative and prebiotic–towards a freeze dried Lactobacillus plantarum topical probiotic. Biotechnol. Rep. 2022, 33, e00696. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, H.; Hutkins, R.W. Fermentation of fructooligosaccharides by lactic acid bacteria and bifidobacteria. Appl. Environ. Microbiol. 2000, 66, 2682–2684. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Serrano, G.M.; García-Garibay, M.; Cruz-Guerrero, A.E.; Gómez-Ruiz, L.; Ayala-Niño, A.; Castañeda-Ovando, A.; González-Olivares, L.G. Proteolytic System of Streptococcus thermophilus. J. Microbiol. Biotechnol. 2018, 28, 1581–1588. [Google Scholar] [CrossRef]
- Toldrá, F.; Reig, M.; Aristoy, M.C.; Mora, L. Generation of bioactive peptides during food processing. Food Chem. 2018, 267, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Nighswonger, B.D.; Brashears, M.M.; Gilliland, S.E. Viability of Lactobacillus acidophilus and Lactobacillus casei in fermented milk products during refrigerated storage. J. Dairy Sci. 1996, 79, 212–219. [Google Scholar] [CrossRef]
- Znamirowska, A.; Szajnar, K.M.; Pawlos, M. Probiotic Fermented Milk with Collagen. Dairy 2020, 1, 126–134. [Google Scholar] [CrossRef]
- Van De Guchte, M.; Serror, P.; Chervaux, C.; Smokvina, T.; Ehrlich, S.D.; Maguin, E. Stress responses in lactic acid bacteria. In Lactic Acid Bacteria: Genetics, Metabolism and Applications, Proceedings of the Seventh Symposium on Lactic Acid Bacteria: Genetics, Metabolism and Applications, Egmond aan Zee, The Netherlands, 1–5 September 2002; Springer: Dordrecht, The Netherlands, 2002; pp. 187–216. [Google Scholar]
- Nielsen, M.S.; Martinussen, T.; Flambard, B.; Sørensen, K.I.; Otte, J. Peptide profiles and angiotensin-I-converting enzyme inhibitory activity of fermented milk products: Effect of bacterial strain, fermentation pH, and storage time. Int. Dairy J. 2009, 19, 155–165. [Google Scholar] [CrossRef]
- Shi, J.; Luo, Y.; Xiao, Y.; Li, Z.; Xu, Q.; Yao, M. Effects of fermentation by Lactobacillus casei on the antigenicity and allergenicity of four bovine milk proteins. Int. Dairy J. 2014, 35, 75–80. [Google Scholar] [CrossRef]
- Bu, G.; Luo, Y.; Zhang, Y.; Chen, F. Effects of fermentation by lactic acid bacteria on the antigenicity of bovine whey proteins. J. Sci. Food Agric. 2010, 90, 2015–2020. [Google Scholar] [CrossRef] [PubMed]
- Olvera-Rosales, L.B.; Pérez-Escalante, E.; Castañeda-Ovando, A.; Contreras-López, E.; Cruz-Guerrero, A.E.; Regal-López, P.; Cardelle-Cobas, A.; González-Olivares, L.G. ACE-Inhibitory Activity of Whey Proteins Fractions Derived of Fermentation by Lacticaseibacillus rhamnosus GG and Streptococcus thermophilus SY-102. Foods 2023, 12, 2416. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ma, Y.; Zheng, Y.; Zhao, W.; Zhao, X.; Luo, T.; Zhang, J.; Yang, Z. Cold-stress response of probiotic Lactobacillus plantarum K25 by iTRAQ proteomic analysis. J. Microbiol. Biotechnol. 2020, 30, 187. [Google Scholar] [CrossRef] [PubMed]
- González-Olivares, L.G.; Jiménez-Guzmán, J.; Cruz-Guerrero, A.; Rodríguez-Serrano, G.; Gómez-Ruiz, L.; García-Garibay, M. Bioactive peptides released by lactic acid bacteria in commercial fermented milks. Rev. Mex. Ing. Quím. 2011, 10, 179–188. [Google Scholar]
- Sebastián-Nicolas, J.L.; Contreras-López, E.; Ramírez-Godínez, J.; Cruz-Guerrero, A.E.; Rodríguez-Serrano, G.M.; Añorve-Morga, J.; Jaimez-Ordaza, J.; Castañeda-Ovando, A.; Pérez-Escalante, E.; Ayalo-Niño, A.; et al. Milk fermentation by Lacticaseibacillus rhamnosus GG and Streptococcus thermophilus SY-102: Proteolytic profile and ace-inhibitory activity. Fermentation 2021, 7, 215. [Google Scholar] [CrossRef]
- Donkor, O.N.; Henriksson, A.; Singh, T.K.; Vasiljevic, T.; Shah, N.P. ACE-inhibitory activity of probiotic yoghurt. Int. Dairy J. 2007, 17, 1321–1331. [Google Scholar] [CrossRef]
- Moslehishad, M.; Ehsani, M.R.; Salami, M.; Mirdamadi, S.; Ezzatpanah, H.; Naslaji, A.N.; Moosavi-Movahedi, A.A. The comparative assessment of ACE-inhibitory and antioxidant activities of peptide fractions obtained from fermented camel and bovine milk by Lactobacillus rhamnosus PTCC 1637. Int. Dairy J. 2013, 29, 82–87. [Google Scholar] [CrossRef]
- Mbye, M.; Baig, M.A.; AbuQamar, S.F.; El-Tarabily, K.A.; Obaid, R.S.; Osaili, T.M.; Al-Nabulsi, A.A.; Turner, M.S.; Shah, N.P.; Ayyash, M.M. Updates on understanding of probiotic lactic acid bacteria responses to environmental stresses and highlights on proteomic analyses. Compr. Rev. Food Sci. Food. Saf. 2020, 19, 1110–1124. [Google Scholar] [CrossRef]
- Fonseca, F.; Pénicaud, C.; Tymczyszyn, E.E.; Gómez-Zavaglia, A.; Passot, S. Factors influencing the membrane fluidity and the impact on production of lactic acid bacteria starters. Appl. Microbiol. Biotechnol. 2019, 103, 6867–6883. [Google Scholar] [CrossRef]
- Chávez de la Vega, M.I.; Alatorre-Santamaría, S.; Gómez-Ruiz, L.; García-Garibay, M.; Guzmán-Rodríguez, F.; González-Olivares, L.G.; Cruz-Guerrero, A.E.; Rodríguez-Serrano, G.M. Influence of Oat β-Glucan on the Survival and Proteolytic Activity of Lactobacillus rhamnosus GG in Milk Fermentation: Optimization by Response Surface. Fermentation 2021, 7, 210. [Google Scholar] [CrossRef]
- Adamberg, K.; Kask, S.; Laht, T.M.; Paalme, T. The effect of temperature and pH on the growth of lactic acid bacteria: A pH-auxostat study. Int. J. Food Microbiol. 2003, 85, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Ovando, A.; Moreno-Vilet, L.; Jaimez-Ordaz, J.; Ramírez-Godínez, J.; Pérez-Escalante, E.; Cruz-Guerrero, A.E.; Contreras-López, E.; Alatorre-Santamaría, S.A.; Guzmán-Rodríguez, F.J.; González-Olivares, L.G. Aguamiel syrup as a technological diversification product: Composition, bioactivity and present panorama. Future Foods 2023, 8, 100249. [Google Scholar] [CrossRef]
 aguamiel and
aguamiel and  inulin.
inulin.
 aguamiel and
aguamiel and  inulin.
inulin.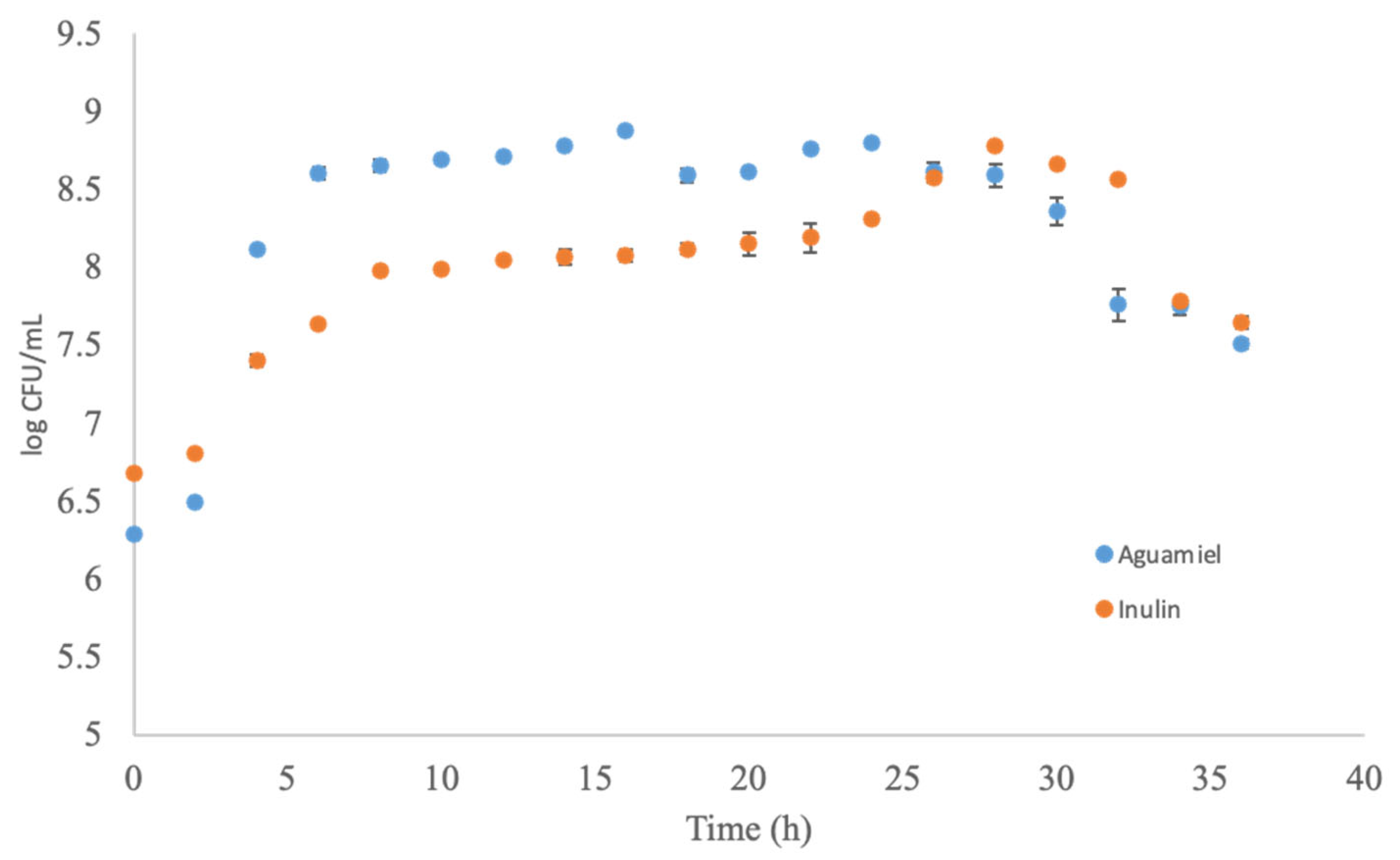

| Carbon Source | µ (h−1) | g (h) |
|---|---|---|
| Aguamiel | a 0.98 ±0.04 | a 0.71 ±0.0 |
| Inulin | b 0.43 ±0.03 | b 1.61 ±0.01 |
| Stage of Fermentation | Amino Group Concentration Aguamiel (mg/L) | Stage of Fermentation | Amino Group Concentration Inulin (mg/L) |
|---|---|---|---|
| Beginning (0 h) | a 156.78 ±3.14 | Beginning (0 h) | a 182.33 ±6.66 |
| Middle (8 h) | b 255.56 ±4.23 | Middle (18 h) | b 263.44 ±0.98 |
| End (18 h) | a 162.05 ±7.01 | End (36 h) | c 113.72 ±5.39 |
| Refrigeration Time (Week) | Aguamiel (log CFU/mL) | Inulin (log CFU/mL) |
|---|---|---|
| End of fermentation | a 8.68 ±0.04 | a 8.65 ±0.01 |
| 1 | a 8.65 ±0.04 | a 8.62 ±0.02 |
| 2 | b 8.58 ±0.02 | b 8.46 ±0.02 |
| 3 | b 8.47 ±0.05 | c 8.30 ±0.07 |
| Refrigeration Time (Week) | Aguamiel (mg/L) | Inulin (mg/L) |
|---|---|---|
| End of fermentation | a 162.05 ±0.01 | a 113.72 ±5.74 |
| 1 | b 203.890 ±0.01 | b 206.81 ±9.20 |
| 2 | b 212.97 ±0.01 | b 208.58 ± 0.60 |
| 3 | b 215.87 ±0.008 | b 209.97 ±1.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contreras-López, E.; Félix-Reyes, N.L.; González-Olivares, L.G.; Jaimez-Ordaz, J.; Castañeda-Ovando, A.; Añorve-Morga, J.; López-Hernández, B.A.; Vélez-Rivera, N.; Ramírez-Godínez, J. Aguamiel Enhance Proteolytic Activity and Survival of Lactiplantibacillus pentosus ABHEAU-05 during Refrigerated Storage of a Fermented Milk. Fermentation 2023, 9, 841. https://doi.org/10.3390/fermentation9090841
Contreras-López E, Félix-Reyes NL, González-Olivares LG, Jaimez-Ordaz J, Castañeda-Ovando A, Añorve-Morga J, López-Hernández BA, Vélez-Rivera N, Ramírez-Godínez J. Aguamiel Enhance Proteolytic Activity and Survival of Lactiplantibacillus pentosus ABHEAU-05 during Refrigerated Storage of a Fermented Milk. Fermentation. 2023; 9(9):841. https://doi.org/10.3390/fermentation9090841
Chicago/Turabian StyleContreras-López, Elizabeth, Nancy Lizeth Félix-Reyes, Luis Guillermo González-Olivares, Judith Jaimez-Ordaz, Araceli Castañeda-Ovando, Javier Añorve-Morga, Blanca Azalia López-Hernández, Nayeli Vélez-Rivera, and Juan Ramírez-Godínez. 2023. "Aguamiel Enhance Proteolytic Activity and Survival of Lactiplantibacillus pentosus ABHEAU-05 during Refrigerated Storage of a Fermented Milk" Fermentation 9, no. 9: 841. https://doi.org/10.3390/fermentation9090841
APA StyleContreras-López, E., Félix-Reyes, N. L., González-Olivares, L. G., Jaimez-Ordaz, J., Castañeda-Ovando, A., Añorve-Morga, J., López-Hernández, B. A., Vélez-Rivera, N., & Ramírez-Godínez, J. (2023). Aguamiel Enhance Proteolytic Activity and Survival of Lactiplantibacillus pentosus ABHEAU-05 during Refrigerated Storage of a Fermented Milk. Fermentation, 9(9), 841. https://doi.org/10.3390/fermentation9090841






