Abstract
The suitability of Lactiplantibacillus plantarum (L. plantarum) as a functional starter culture in Nham fermentation was investigated, with a focus on evaluating both its probiotic attributes and fermentation capability. L. plantarum BCC 4352 (LpbBCC4352) exhibited colony-associated antimicrobial activity against Kocuria rhizophila, L. plantarum, Latilactobacillus sakei ssp. sakei, and Pediococcus pentosaceus, as well as the zoonotic Streptococcus suis. LpbBCC4352 exhibited impressive acid (pH 2.5) and bile resistance, coupled with notable survival rates in a simulated human digestive model. In addition, the strain is able to utilize fructo-oligosaccharides in simulated human colon conditions. It also displayed robust adhesion to human colon cell monolayers (Caco-2) and gastric mucin. Furthermore, it showed a promising cholesterol reduction ability in the fermentation medium. The safety of LpbBCC4352 for human consumption was confirmed through a hemolytic activity assay and antibiotic susceptibility testing. Moreover, using LpbBCC4352 as a starter culture not only enhanced the firmness of Nham but also ensured consumer satisfaction. The overall findings emphasize the potential use of LpbBCC4352 as a safe and effective functional starter culture, particularly in the production of Nham.
1. Introduction
Probiotics, as defined by The International Scientific Association for Probiotics and Prebiotics (ISAPP), are “live microorganisms that, when administered in adequate amount, confer a health benefit on the host” [1]. The criteria for assessing probiotic microorganisms intended for use in foods emphasize not only their capability to withstand the harsh conditions of the digestive tract, including resistance to gastric juices and bile, but also the importance of their ability to thrive in the gut [2]. This underscores the necessity of incorporating them into a suitable food vehicle. This incorporation is intended to enhance resilience during passage through the acidic stomach environment, increase resistance to elevated bile salts concentrations, and facilitate the eventual colonization in the intestinal tract [3]. In addition to other criteria, such as reducing cholesterol levels [4] and utilizing prebiotic carbohydrates [5], safety factors such as potential pathogenicity to humans or animals, including hemolytic activities [6], and antibiotic resistance have also been highlighted.
Several bacterial genera have been suggested as probiotics; nevertheless, the most employed probiotic bacteria are lactic acid bacteria (LAB) [7,8,9]. Among LAB strains, Lactobacillus plantarum, which has been reclassified as Lactiplantibacillus plantarum [10], is widely recognized as a probiotic with a well-established safety record. This recognition is endorsed by various studies [11,12,13] and validated by the European Food Safety Authority (EFSA) in 2012 [14]. Nevertheless, sporadic reports of opportunistic infections linked to these bacteria have emerged [15].
L. plantarum plays a significant role in fermenting a wide range of products, including dairy products [16,17,18], fermented meat products [19,20,21,22], and various plant-based products [23,24,25]. Numerous investigations have been conducted to develop functional foods or preparations that incorporate probiotics, such as fermented sausages [26,27,28,29,30,31,32,33,34]. This product is particularly attractive because it remains unheated and maintains high populations of LAB. Moreover, its matrix appears to function as a protective shield, enhancing the survival of probiotic strains through the gastrointestinal tract [35]. In the current study, we considered Nham or Thai fermented pork sausage as an ideal carrier for probiotic cultures due to its consistent fermentation by a dominant microorganism known as lactobacilli [19,36].
Nham, a semi-dry fermented raw pork product, is widely cherished by the Thai population due to its distinct qualities, such as its firm texture, sour flavor, and vibrant red color. These unique characteristics primarily result from the specific ingredients and microorganisms employed during the fermentation process, as indicated by Valyasevi and Rolle [19] and Visessanguan et al. [20]. Nham is typically crafted from a blend of minced raw pork, cooked pork rind, cooked rice, fresh garlic, whole fresh bird’s eye chili, and curing salts. This mixture is tightly packed into plastic casings or banana leaves and left to ferment at an ambient temperature of 30–35 °C for approximately 5 days until the pH level drops below 4.6 [37].
In Thailand, there is a proposed initiative for the incorporation of probiotics into Nham. Phupaboon and colleagues [38] conducted a study in which they illustrated an in vitro probiotic assessment, evaluating the resistance to pH and bile salts of the strains both before and after encapsulation. Furthermore, they employed potent probiotics to ferment a laboratory-scaled Nham by encapsulating probiotic starter cultures.
The present study emphasizes the potential transformation of traditional Thai fermented pork into an innovative sausage product, aiming to enhance its market appeal and nutritional content. Our objective is to develop probiotic enriched Nham. The investigation explores the probiotic characteristics of selected L. plantarum strains, evaluating both their functionality and safety. Additionally, we highlight their suitability as a starter culture for Nham fermentation in a validated industrial-scale production of this kind of fermented pork sausage.
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
L. plantarum BCC 4352, previously isolated from Nham samples, was obtained from the BIOTEC culture collection (BCC), National Center for Genetic Engineering and Biotechnology (BIOTEC) (Khlong Luang, Thailand). The probiotic L. plantarum NCIMB 8826 (LpbNCIMB8826), employed as the positive control in the experiment, was sourced from The National Collection of Industrial Food and Marine Bacteria (NCIMB) (Scotland, UK). All LAB strains were stored at −80 °C in deMan, Rogosa, and Sharpe (MRS) medium with 15% glycerol (Merck, Germany) and propagated in MRS broth at 30 °C for 18 h before being used for testing of their antimicrobial production.
Both Gram-positive and Gram-negative bacteria, as listed in Table 1, were used as bacterial indicators to determine the antimicrobial activity of L. plantarum. Staphylococcus aureus, Listeria innocua, Escherichia coli, and Salmonella Typhimurium were cultured in tryptic soy broth (TSB, Merck, Germany), supplemented with 0.6% yeast extract, and incubated at 37 °C for 18 h with shaking at 200 rpm. Streptococcus suis serotype 2 was cultured in Todd–Hewitt broth with 2% yeast extract at 30 °C for 18 h. The remaining LAB indicator strains were propagated in MRS medium at 30 °C for 18 h.

Table 1.
Antagonistic activity of L. plantarum BCC4352 (LpbBCC4352) against wide ranges of bacterial indicators.
2.2. Determination of Antimicrobial Activity
The antimicrobial activity of L. plantarum strains was assessed using the spot-on-lawn technique as described by Zendo et al. [39]. In assessing the antimicrobial activity of L. plantarum, a 10 µL aliquot of an overnight culture of L. plantarum (8 log CFU/mL) was inoculated onto an indicator plate pre-seeded with 50 µL of the bacterial indicator (7 log CFU/mL). The medium used for each indicator was the same as described in Section 2.1 and supplemented with 1.5% agar. Following a 30-min drying period, the plates were incubated at 30 °C for 24 h. The presence of a clear zone surrounding the culture spots indicated the antagonistic activity of L. plantarum against the bacterial indicator strains.
The antimicrobial activity of the cell-free supernatant (CFS) was evaluated by collecting the CFS samples from the tested strains through centrifugation (8500× g for 10 min at 4 °C), followed by adjusting the pH to 6.5. The neutralized CFS was subsequently sterilized by filtering through a 0.2 µm sterile cellulose acetate filter (ADVANTEC®, Tokyo, Japan) and was then subjected to two-fold serial dilution with sterilized distilled water. A 10 μL of each dilution were applied to agar plates that had been previously overlaid with 5 mL of soft agar (1% agar) containing approximately 7 log CFU/mL of the indicator strain. The medium used for each indicator was the same as described in Section 2.1 and supplemented with 1.5% agar. Plates were then incubated at 30 °C for 24 h. The antimicrobial activity of the sample was expressed in arbitrary units (AU) per milliliter (mL), with one AU defined as the reciprocal of the highest dilution at which the growth inhibition remained detectable.
2.3. Determination of Acid and Bile Tolerance
The tolerance of LpbBCC4352 cells to stress conditions, including low pH and bile salts, was assessed based on the method outlined by Vijayakumar et al. [40]. To evaluate acid tolerance, a 1 mL aliquot of an overnight culture of LpbBCC4352 (9 log CFU/mL) was inoculated into 9 mL of acidified MRS broth (pH 2.5). After 2 h of incubation at 37 °C, viable LpbBCC4352 cells grown under acidic conditions were enumerated and expressed as logarithms of colony-forming units per milliliter (log CFU/mL). The survival rates were calculated as follows:
where C1 is the viable count after acid treatment (log CFU/mL), and C0 denotes the initial viable count (log CFU/mL).
Survival rate (%) = (C1/C0) × 100%
For the bile tolerance test, 1 mL of LpbBCC4352 cells with a concentration of 9 log CFU/mL, was introduced into 9 mL of MRS broth supplemented with varying amounts of oxgall from Sigma-Aldrich (0, 0.1, 0.3, 0.5, 0.7, 1.0, and 2.0% w/v), and the mixture was then incubated at 37 °C for 4 h. The cell viability was measured by standard plating methods and expressed in log CFU/mL. Bile tolerance, expressed as a survival rate of the tested strain after bile salt treatments, were determined using the previously mentioned formula for acid tolerance, where C1 represents the viable count after oxgall treatment, and C0 indicates the initial viable count.
2.4. Resistance to the Simulated Digestion Conditions
The survivability of LpbBCC4352 cells in the human digestive system was assessed using the simulated digestive model established by Minekus et al. [41] with slight modifications. The simulation comprised sequential oral, gastric, and intestinal phases. An overnight culture of LpbBCC4352 was harvested via centrifugation (10,000× g for 10 min at 4 °C) and subsequently washed twice and resuspended with saline solution. The culture suspension, comprising 1 mL aliquots with a bacterial concentration of approximately 9 log CFU/mL, was introduced into 9 mL of simulated salivary fluid (SSF, pH 7) containing amylase enzyme (75 U/mL, pH 7). The mixture was then incubated at 37 °C for 2 min. During the simulated gastric phase, samples from the oral phase were combined with 10 mL simulated gastric fluid (SGF, pH 3) supplemented with pepsin (2000 U/mL, pH 3) and subsequently incubated dynamically at 37 °C for 2 h using a Trayster vertical rotating mixer (IKA, Guangzhou, China) operating at 5 rpm/min. During the simulated intestinal phase, samples from the gastric phase were mixed with 20 mL of simulated intestinal fluid (SIF, pH 7) enriched with bile acid (10 mM) and pancreatin (including trypsin enzyme at 100 U/mL, pH 7). Afterward, the sample was subjected to a 4-h dynamic incubation (5 rpm/min) in an anaerobic chamber (DG500, Whitley Ltd., Surrey, UK) at 37 °C, maintaining anaerobic conditions with 5% carbon dioxide, 5% helium, and 90% nitrogen. Samples were collected at different time intervals, and the viability of LpbBCC4352 cells was evaluated through standard plating on MRS agar plates.
2.5. Detection of the Bile Salt Hydrolase Activity
The qualitative direct plate assay for evaluating the bile salt hydrolase activity of LpbBCC4352 was performed following the procedure described by Allain et al. [42] with slight modifications. In brief, LpbBCC4352 was streaked onto MRS plates containing 0.5% (w/v) taurine conjugates of deoxycholic acid (TDCA) or glycocholic acid conjugates of deoxycholic acid (GDCA), supplemented with 0.37 g of CaCl2 per liter. The plates were subsequently incubated anaerobically at 37 °C for 72 h in an anaerobic chamber, with a gas mixture comprising 5% carbon dioxide, 5% helium, and 90% nitrogen. Control plates of MRS agar medium lacking TDCA or GDCA supplementation were prepared. A positive reaction was determined by the presence of precipitated bile acid or the formation of an opaque halo around the colonies.
2.6. Adhesion Assay
2.6.1. Adhesion to the Human Colon Adenocarcinoma Cell Line (Caco-2)
The adhesion capability of LpbBCC4352 to the human colon adenocarcinoma cell line (Caco-2) was evaluated according to the method of Klingberg et al. [43]. The adhesion assay was performed in a 24-well tissue culture plate (Costar® 3524, Wiesbaden, Germany) with 10-day-old monolayers of Caco-2 cells (4 log cells/well). After rinsing the cells twice with phosphate buffered saline (PBS, pH 7.2), they were then immersed in 2 mL of non-supplemented Modified Eagle’s Medium (MEM) at 37 °C for 1 h. After the incubation, the MEM was replaced with a 200 μL suspension of LpbBCC4352 cells in MEM at a concentration of 7 log CFU/mL. The cell adhesion experiment was performed at 37 °C for 1 h under 5% carbon dioxide and 95% humidity. Following incubation, the monolayer cells treated with bacteria were washed 10 times with sterile PBS solution to eliminate any unattached bacterial cells. Subsequently, 0.05% (v/v) Triton X-100 was introduced into the wells for 10 min to detach the Caco-2 cells along with the adhered bacteria. The quantification of viable adhering bacteria was accomplished by plating serial 10-fold dilutions of the solution containing the detached Caco-2 cells and intact bacterial cells onto MRS-CaCO3 agar, followed by a 48-h incubation at 37 °C. The capability of bacteria to adhere to the Caco-2 cell layer was determined using the following equation:
where, X0 represents the initial number of bacteria added to each well, and Xt represents the number of bacteria after detachment with Triton X-100.
Adhesion (%) = (Xt/X0) × 100
2.6.2. Mucin Adhesion Assay
Mucin adhesion experiments were adapted from Van den Abbeele et al. [44]. The mucin adhesion assay was performed in 96-well polystyrene microtiter plates (Nunc MaxiSorp, Thermo Fisher Scientific, Waltham, MA, USA), using porcine gastric mucin (Sigma-Aldrich®, St. Louis, MO, USA) as a matrix. The wells were coated with 200 µL of mucin solution (1 mg/mL) in PBS, pH 7.0 and incubated at 4 °C for 24 h. Following incubation, the wells were washed twice with 200 µL of PBS to eliminate unbound mucin, and then 200 µL of LpbBCC4352 cell suspension (9 log CFU/mL) was introduced into the wells. Plates were further incubated at 37 °C for 30 min. After the incubation, wells were washed in triplicate with 200 µL of sterile PBS to remove unbound bacteria. Another 300 µL of 0.5% (v/v) Triton X-100 were added and incubated at 37 °C for 30 min to detach the bacterial cells from wells. The number of adhesive bacterial cells was assessed through the standard plating method on MRS agar and expressed as log CFU/well. The capability of bacteria to adhere to the mucin layer was determined using the following equation:
where, X0 represents the initial number of bacteria added to each well, and Xt represents the number of bacteria after detachment with Triton X-100.
Adhesion (%) = (Xt/X0) × 100
2.6.3. Detection of Binding-Related Genes
DNA was extracted from an overnight culture of LpbBCC4352 and LpbNCIMB8826 using the Wizard genomic DNA purification kit (Promega, Charbonnieres, France). PCR was conducted to detect the presence of genes associated with the binding mechanisms to epithelial cells, employing specific primers designed by Turpin et al. [45], using the primer sequences provided in Table S1. The PCR conditions comprised one cycle at 95 °C for 5 min, followed by 40 cycles at 95 °C for 30 s, 54 °C for 15 s, and 72 °C for 30 s, concluding with a final step at 72 °C for 5 min. Verification of the presence of specific PCR products was performed on a 2.0% (w/v) agarose gel.
2.7. Growth of LpbBCC4352 in Prebiotic Medium
The prebiotic oligosaccharide, FOS (fructo-oligosaccharides, F8052), used in this study, was purchased from Sigma-Aldrich®. The growth capacity of LpbBCC4352 in the presence of tested carbohydrates was assessed using the modified standard ileal efflux medium (mSIEM). This medium, designed to simulate the nutritional characteristics of the colon by Gibson et al. [46], was employed in the test following the protocol outlined by Gamonpilas et al. [47] with minor modification. A 0.5 mL culture of LpbBCC4352 cells, containing a cell concentration of 9 log CFU/mL, was cultured in MRS broth for 24 h, followed by centrifugation at 8500× g for 10 min at 4 °C to harvest the cells. The resulting cell pellets were washed twice with 50 mM phosphate-buffered saline (pH 7.2) and then resuspended in 4.5 mL of O2-free basal mSIEM medium without the addition of any carbohydrates. A 0.1 mL aliquot of the culture suspension was introduced into 0.9 mL of the mSIEM medium, which was prepared without glucose and included either 2% (w/w) FOS or control carbohydrates (glucose). Samples were then incubated at 37 °C for 48 h in an anaerobic chamber with a gas mixture consisting of 5% carbon dioxide, 5% helium, and 90% nitrogen. The ability of LpbBCC4352 to utilize the tested carbohydrates was assessed through the analysis of viable cell growth (log CFU/mL), pH changes, and acid production.
2.8. Analysis of Short Chain Fatty Acids (SCFAs)
Analysis of short-chain fatty acids produced by LpbBCC4352 was conducted by modifying the method of Schooley et al. [48]. A 500 µL of the culture supernatant of LpbBCC4352 from Section 2.7 was mixed with trans-cinnamic acid at a concentration of 0.5 mM (internal standard). Subsequently, 100 µL of hydrochloric acid (HCl) and 2 mL of diethyl ether were added to the vial. The mixture was then mixed using a multichannel vortex apparatus at a speed of 2500 rpm for 5 min. Afterward, the sample was centrifuged at 200× g for 5 min, and the upper layer was withdrawn to a volume of 500 µL. Derivatization was performed using N-tert-Butyldimethylsilyl-N-methyltrifluoroacetamide with 1% tert-Butyldimethyl chlorosilane (MTBSTFA + 1%TMCS) at a volume of 50 µL under a temperature of 80 °C for 40 min. Following derivatization, the sample was analyzed for short-chain fatty acids (SCFAs) using a GC-MS instrument in selected ion monitoring (SIM mode), employing a DB1 column and utilizing standard substances to construct a calibration curve for determining the concentration of the target compounds in the sample.
2.9. Cholesterol Assimilation Assay
The experiment was performed based on the method of Liong & Shah [49]. A water-soluble cholesterol (cholesterol-polyethylene glycol 600, Sigma-Aldrich) was added to the sterile MRS broth supplemented with 0.3% oxgall to make a final concentration of 20 µg/mL. An overnight culture of LpbBCC4352 cells (9 log CFU/mL) was then introduced into the broth, and the mixture underwent anaerobic incubation at 37 °C for 72 h using an anaerobic chamber (DG500, Whitley Ltd., UK). Following the incubation period, LpbBCC4352 cell viability was measured by plating methods. The o-phthalaldehyde method, as described by Rudel & Morris [50], was employed to quantify the residual cholesterol levels in the collected cell-free supernatant. In brief, the cell-free supernatant was mixed with 33% (w/v) potassium hydroxide (KOH) and absolute ethanol. The resulting mixture was heated to 60 °C for 15 min, followed by cooling to ambient temperature. After cooling, hexane was added, leading to phase separation. The hexane phase was then collected, transferred to a glass tube, and evaporated under a nitrogen gas flow. Once dried, the remaining residue was treated with the o-phthalaldehyde reagent and subsequently mixed with 98% sulfuric acid. After a 10-min incubation at 30 °C, the absorbance was read at 550 nm using a spectrophotometer. The residual cholesterol concentration in the CFS was calculated according to Rudel & Morris [50]. The capability of the bacterium to assimilate cholesterol was then calculated using the following equation:
where, (Cholesterol)0h represents the residual cholesterol concentration in CFS at 0-h of incubation, and (Cholesterol)72 h represents the residual cholesterol concentration in CFS at 72-h of incubation.
Cholesterol assimilation (%) = [(Cholesterol (µg/mL)0 h − Cholesterol (µg/mL)72 h)/Cholesterol (µg/mL)0 h] × 100
2.10. Safety Evaluation of LpBCC4352
2.10.1. Hemolytic Activity Assay
The hemolytic activity of LpbBCC4352 was assessed using a plate assay following the procedure outlined by Islam et al. [24] with slight modifications. A one-loopful of the overnight culture of LpbBCC4352 was streaked onto 0.5% (v/v) sheep blood agar plate and then incubated at 37 °C for 48 h. The plate was subsequently inspected for the development of a distinct translucent zone surrounding the culture-streaked line, serving as an indicator of a hemolytic reaction. Staphylococcus aureus ATCC 6538 was employed as a beta-hemolytic (positive) control [51], while L. plantarum NCIMB 8826 served as the hemolytic negative control (gamma-hemolytic) [52].
2.10.2. Antibiotic Susceptibility Test
The antibiotic susceptibility profile of LpbBCC4352 against a range of antibiotics, including ampicillin, chloramphenicol, clindamycin, erythromycin, gentamicin, kanamycin, and tetracycline, was determined through the micro-dilution method [53]. Colonies of LpbBCC4352 grown anaerobically on MRS agar at 37 °C for 16–24 h were carefully selected and suspended in 5 mL of sterile 0.85% saline solution to make a cell suspension with an optical density (OD625nm) of 0.16–0.2. Subsequently, the bacterial suspension underwent a 500-fold dilution in the susceptibility test medium, composed of 90% Iso-Sensitest (IST) medium and 10% MRS medium, before being distributed into each well of a 96-well plate that contained varying concentrations of antibiotics. The minimum inhibitory concentration (MIC) values were then visually assessed after 48 h of anaerobic incubation at 37 °C.
2.11. Evaluating the Performance of LpbBCC4352 as a Starter Culture in Nham Production
2.11.1. Starter Culture Preparation
LpbBCC4352 cells cultured overnight in MRS broth were harvested by centrifugation at 8500× g for 10 min at 4 °C. Subsequently, the harvested cells were washed twice with a saline solution (0.85% NaCl) and then resuspended in the same diluent to make a final cell concentration of 9 log CFU/mL.
2.11.2. Nham Preparation
Nham was prepared in a private meat processing plant located in Bangkok, Thailand, following the formulation detailed by Visessanguan et al. [20]. In summary, two different batches of Nham were prepared with LpbBCC4352 inoculation: one with an initial count of 4 log CFU/g (Nham-Lp4) and the other with 6 log CFU/g (Nham-Lp6). A control group, which consisted of un-inoculated naturally fermented Nham, was labeled as Nham-NF. The fermentation process for all Nham samples took place at a temperature of 30 °C for 96 h. Samples of Nham were collected and analyzed every 12 h throughout the fermentation period.
2.11.3. Determination of Physicochemical Properties of Nham
At each sampling point, Nham samples were analyzed for titratable acidity (TA), pH, texture, and color. Titratable acidity (TA) was determined following the AOAC method [54] and expressed as grams of lactic acid per 100 g of Nham (on a dry weight basis). The pH was measured using a standard pH meter (Mettler Toledo, Greifensee, Switzerland). The texture profile analysis (TPA) of Nham was conducted using a TA-XT2i texture analyzer (Stable Micro Systems, Godalming, UK) equipped with a cylindrical aluminum probe (50 mm diameter) as detailed by Visessanguan et al. [20]. Color was evaluated in the CIE L*a*b* color space using a Minolta Colour Meter CR300 (Minolta Camera Ltd., Osaka, Japan). To address color variations, three readings were averaged from the surface of each sample as described by Visessanguan et al. [20]. Total color difference between control sample (Nham-NF) and treatments (Nham-Lp4 and Nham-Lp6) was calculated according to Sharma [55].
2.11.4. Microbiological Analysis of Nham
The total LAB count in Nham was determined using the standard plate count technique. Triplicate samples of Nham (25 g each) were mixed with 225 mL of sterilized 1% peptone solution and homogenized for 1 min at 200 rpm using a stomacher (Seward, West Sussex, UK). Ten-fold serial dilutions were prepared, and the dilutions were plated on MRS agar plates supplemented with 0.75% (w/v) CaCO3. The plates were then incubated at 30 °C for 48 h before enumerating the colonies.
2.11.5. Sensory Analysis
The sensory analysis of Nham was conducted following the procedure outlined in our previous study [21]. Briefly, a group of 30 individuals, regular consumers of Nham, were tasked with assessing the sensory characteristics of Nham. Before the evaluation, Nham samples with a pH of not more than 4.6 were left to reach room temperature for a minimum of 30 min and then randomly distributed to the panelists. Each sensory aspect was assessed on a hedonic scale ranging from 1 to 9, where panelists indicated their score based on their perception, with 1 signifying extreme dislike, 5 indicating neither liking nor disliking, and 9 representing extreme liking.
2.12. Statistical Analysis
Statistical data differentiation was conducted through one-way analysis of variance (ANOVA). Significance in mean differences among data groups was determined when p-value ≤ 0.05. Duncan’s multiple range test was employed to assess these mean differences.
3. Results and Discussion
3.1. Antimicrobial Activity
Lactobacilli are recognized for their ability to produce diverse antimicrobial compounds that effectively inhibit the growth of pathogenic bacteria, thereby contributing significantly to their probiotic activity [56]. LpbBCC4352 exhibited antimicrobial activity against K. rhizophila NBRC 12708T, L. plantarum ATCC 14917T, L. sakei JCM 1157T, P. pentosaceus JCM 5885T, and the zoonotic S. suis strains P1/7 through the cell-to-cells spot-on-lawn assay. Nonetheless, its neutralized cell-free supernatant did not show antimicrobial activity against any of the tested bacterial indicators (Table 1).
These results suggest that antimicrobial production by LpbBCC4352 requires co-cultivation with specific bacterial strains. Antimicrobial production of lactobacilli through co-culture fermentation has been widely reported [57,58,59,60,61]. Through the investigation, S. suis is recognized for its high contagiousness, with the potential to cause permanent hearing impairment and fatality in humans [62,63,64,65]. Although documented outbreaks of S. suis are not prevalent in Thailand, concerns regarding consumer safety have arisen due to reported instances of S. suis contamination in raw pork meat, a major ingredient of Nham [65,66,67]. In this study, the observed capability of LpbBCC4352 to inhibit S. suis suggests a potential application of these bacteria to enhance the safety of Nham consumption. Ongoing research is expected to further investigate this prospect.
3.2. Resistance to pH, Bile Salts, and the Simulated Digestive Conditions
Evaluating the ability of LAB strains to withstand physical and chemical stress barriers in the gastrointestinal tract, such as gastric acidity and bile toxicity, is commonly recommended for identifying potential probiotic strains [68]. In this study, LpbBCC4352 displayed remarkable acid tolerance, with a cell viability of 6.3 log CFU/mL after exposure to pH 2.5 for 2 h and achieving a survival rate exceeding 96.9 ± 0.1% (Figure 1a,b) while LpbNCIMB 8826, a positive control, exhibited resilience to pH 2.5 with a cell viability of 6.6 log CFU/mL and a survival rate of 98.9 ± 0.1% (Figure 1a,b). The ability of Lactiplantibacillus to withstand low pH values is a significant aspect of pH homeostasis, a process in which H+-ATPase, glutamate-decarboxylase, and arginine-deiminase play essential roles [69,70,71,72].

Figure 1.
Acid tolerance test of L. plantarum strains. (a) viable cell count after acid exposure for 2 h, (b) % survivability after acid treatment. Data represent the means of triplicates. Significant differences were analyzed by one-way ANOVA with Duncan’s multiple range test. Different lowercase letters indicate significant differences among tested strains at the same exposure time (p ≤ 0.05).
During the human digestive process, bile salts are initially released from the gallbladder, with concentrations ranging from 1.5% to 2.0% during the first hour of digestion and gradually decreasing to around 0.3% (w/v) or lower [73]. Therefore, a concentration range of 0.1% to 2.0% (w/v) of bile salts was employed in the test medium to assess the survival of tested strains. The results indicated that variations in oxgall concentration in the fermentation medium had a significant impact on the growth of tested strains. The viability of both LpbBCC4352 and LpbNCIMB8826 decreased as the concentrations of oxgall in the medium increased, particularly at concentrations higher than 0.3% (Figure 2a). After a 4-h exposure to the tested medium, LpbBCC4352 demonstrated resistance against the tested bile salt. Even when exposed to the highest concentration of oxgall (2%), LpbBCC4352 maintained a noteworthy viability of 75.2 ± 2.8% (Figure 2b) and a cell viability of 7.2 log CFU/mL (Figure 2a). In contrast, LpbNCIMB8826 exhibited a survivability of 79.4 ± 0.7% (Figure 2b) and displayed a cell viability count of 7.9 log CFU/mL (Figure 2a). These findings suggest that LpbBCC4352 exhibits notable in vitro bile tolerance when compared to the probiotic reference strain.
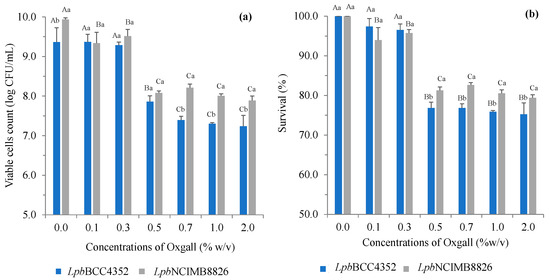
Figure 2.
The effects of bile salts concentrations on growth of L. plantarum strains. The strains were exposed to the tested bile at 37 °C for 4 h. (a) the viable cell counts after bile salt exposure for 4 h and (b) the % survivability after bile salt treatments are shown. Data represent the means of triplicates. Significant differences were analyzed by one-way ANOVA with Duncan’s multiple range test. Uppercase letters represent significant differences among the oxgall concentrations of the same strain (p ≤ 0.05). Lowercase letters represent significant differences between the strains at the same oxgall concentration (p ≤ 0.05).
Contrary to expectations, our findings revealed that LpbBCC4352 lacked in bile salt hydrolase (BSH) activity in various test media, such as MRS-TDCA and MRS-GDCA (Figure S1). This was surprising given the commonly established correlation between bile tolerance in lactobacilli and BSH activity. This implies that the bile tolerance observed in LpbBCC4352 may be linked to metabolic mechanisms beyond BSH activity, possibly related to the roles of proteins that impact cell envelope architecture or uphold intracellular homeostasis [74,75,76,77].
The survivability of LpbBCC4352 cells in a simulated human digestive model is illustrated in Figure 3. When exposed to SSF containing amylase, LpbBCC4352 demonstrated a viability exceeding 90%, with a retained cell count of 8.6 log CFU/mL. However, sequential exposure to SGF for 2 h resulted in a further slight reduction in the survival count by 1.0 log cycles, with the count retaining 7.6 log CFU/mL. After an additional 4 h of exposure to SIF, viability decreased further by an average of 1.2 log cycles, and the final cell count reached 6.4 log CFU/mL. Similar results were obtained when LpbNCIMB8826 was subjected to the tested model; a decline in cell count was observed when the strain was sequentially exposed to SIF. The cell viability is retained at 6.9 log CFU/mL at this stage. The obtained results are aligned with those from experiments on acid and bile tolerance. These results suggest that LpbBCC4352 has the potential to survive passage through the digestive tract.
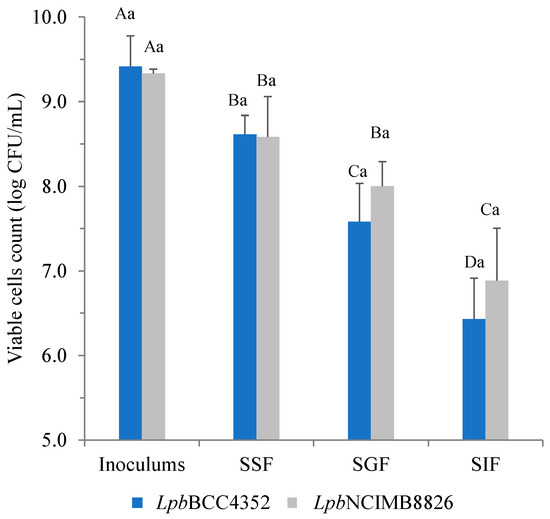
Figure 3.
Survival of L. plantarum strains during transit to the simulated conditions of the human digestive tract. Data represent the means of triplicates. Significant differences were analyzed by one-way ANOVA with Duncan’s multiple range test. Different uppercase letters indicate significant differences among the stages of the same strain (p ≤ 0.05). Different lowercase letters indicate significant differences between the tested strains at the same stage (p ≤ 0.05).
3.3. Adhesion to Caco-2 Cells and Mucin
The ability of strains to adhere to intestinal epithelial cells and mucosal surfaces, crucial in conferring health benefits by competing with pathogens to colonize epithelial cells and modulating the host immune system, is considered as a primary criterion in the selection of probiotic microorganisms [78,79,80]. Figure 4a reveals a significantly higher adhesion level of LpbBCC4352 when compared with LpbNCIMB8826 (p < 0.05). LpbBCC4352 cells demonstrated an adhesion rate of 84.5 ± 0.1%, while LpbNCIMB8826 exhibited a lower rate of 77.7 ± 0.1%. However, LpbBCC4352 exhibited lower mucin adherence (74.8 ± 1.1%) than LpbNCIMB8826 (79.9 ± 1.9%), as shown in Figure 4b.
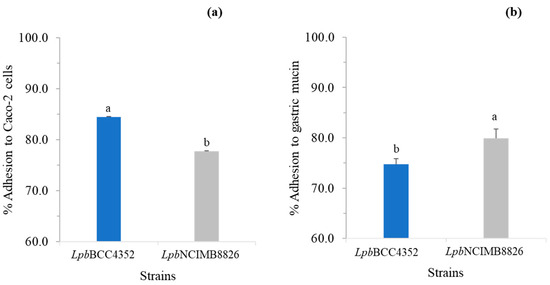
Figure 4.
Bacterial adherence to Caco-2 cells (a) and gastric mucin (b). The capability of bacteria to adhere to Caco-2 cells and mucin was expressed as a percentage of adhesion. Data represent the means of triplicates. Significant differences were analyzed by one-way ANOVA with Duncan’s multiple range test. Different lowercase letters indicate significant differences between the tested strains in a specific adhesion test (p ≤ 0.05).
We investigated the presence or absence of genes related to the binding ability of lactobacilli to the gastrointestinal tract, as proposed by Turpin et al. [45]. The analysis revealed the presence of binding-related genes in LpbBCC4352, including aggregation-promoting factors (apf), collagen-binding protein (cnb), fibronectin-binding protein (fpbA), mucus adhesion promoting protein (mapA), glucan synthase (gtf), mannose-specific adhesion (msa), mucin-binding protein (mub1, mub2), and surface layer protein A (slpA) as shown in Table 2. The agarose gel electrophoresis of PCR products is presented in Figure S2.

Table 2.
The distribution of genes involved in binding to the gastrointestinal tract in the selected L. plantarum.
Regarding adherence to Caco-2 cells, LpbBCC4352 exhibits a significantly higher adhesion rate compared to LpbNCIMB8826. This is possibly due to the presence of the SlpA, which is absent in LpbNCIMB8826. SlpA was reported to be involved in the attachment of bacterium to Caco-2. SlpA from L. acidophilus NCFM was shown to engage in binding with both Caco-2 cells and dendritic cells (DC), influencing the modulation of DC and T-cell functions, as demonstrated in studies by Buck et al. [81] and Ashida et al. [82].
Furthermore, the obtained results indicate a potential involvement of mucin-binding proteins (mub1, mub2) and the collagen-binding S-layer (cbsA) in enhancing the binding capabilities of the tested strains to mucin. In particular, the adhesive strength of LpbNCIMB8826 seems to be enhanced by the presence of mub1, mub2, and cbsA. The significant difference in the binding capacity of LpbNCIMB8826 and LpbBCC4352 emphasizes the impact of the absence of mub1 and cbsA in LpbBCC4352. Nevertheless, further study of the expression of the corresponding genes (SlpA, mub1, mub2, and cbsA) is needed to confirm their involvement in the binding mechanism in LpbBCC4352.
3.4. Growth in the Prebiotic Medium
The results demonstrated that LpbBCC4352 could utilize FOS in the medium tested, as evidenced by observed cell growth, pH reduction, and acid production. LpbBCC4352 displayed robust growth behavior, manifesting a significant rise in cell counts from the initial 5.2 to 7.2 log CFU/mL within 24 h of incubation, followed by a slight increase to 7.5 log CFU/mL at 48 h of incubation (Figure 5a). Besides the observed growth, the decline in pH value from 6.7 to 4.3 during the growth phase (Figure 5b) and the concurrent rise in the production of acids at 48 h (Figure 6) of fermentation consistently support the fermentability of the fructo-oligosaccharides by LpbBCC4352.

Figure 5.
Changes in the growth of L. plantarum BCC4352 (a) and pH (b) during growth in mSIEM medium with and without the addition of tested carbohydrates. The strain was grown at 37 °C for 48 h. Values represent the means ± S.D. of triplicate determinations. Significant differences were analyzed by one-way ANOVA with Duncan’s multiple range test. Different lowercase letters indicate significant differences among the culture media at the same fermentation time (p ≤ 0.05).
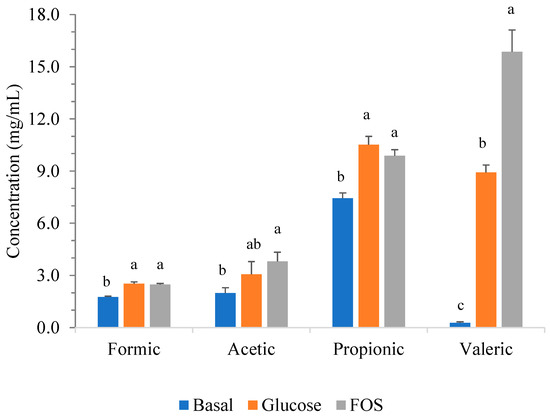
Figure 6.
Short-chain fatty acid (SCFA) production during the growth of LpbBCC4352 in mSIEM medium with and without the addition of tested carbohydrates. The strain was grown at 37 °C for 48 h, and all measurements were performed in triplicate. Significant differences were analyzed by one-way ANOVA with Duncan’s multiple range test. Different lowercase letters indicate significant differences among the tested carbohydrates of the same SCFA (p ≤ 0.05).
Similar results were observed when growing LpbBCC4352 in mSIEM medium containing 2% glucose. There was a rise in LpbBCC4352 cell counts from the initial values of 5.2 to 7.3 log CFU/mL within 24 h of incubation, although a slight decrease of 0.46 log cycles was observed at 48-h of fermentation (Figure 5a). The rapid decline in pH value from 6.6 to 3.7 during the growth phase was noted (Figure 5b). The production of targeted SCFAs was found to be similar to that detected in the mSIEM medium with 2% FOS. However, a lower amount of valeric acid was investigated (Figure 6).
Compared to the basal mSIEM medium, LpbBCC4352 cell viability consistently remained at 5 log CFU/mL throughout the fermentation period (Figure 5a). The quantity of the targeted SCFAs produced was determined to be considerably lower than those observed in the mSIEM medium containing FOS and glucose (Figure 6).
LpbBCC4352 was found to generate notably high concentrations of targeted short-chain fatty acids (SCFAs), with a particular emphasis on valeric acid production. LpbBCC4352 exhibited outstanding performance, achieving a production level of 15.8 ± 1.25 mg/mL within a 48-h growth period. Valeric acid is recognized as a potential therapeutic target for various disease pathologies, such as cancer and colitis [83].
3.5. Cholesterol Assimilation
Cholesterol assimilation refers to the mechanisms involved in the removal of cholesterol, which represents a desirable characteristic of probiotics [84]. The findings indicated that LpbBCC4352 displayed a cholesterol assimilation of 42.27 ± 1.69%, resulting in the removal of 9.09 ± 0.80 μg/mL of cholesterol. In comparison, LpbNCIMB8826 demonstrated a higher cholesterol assimilation of 55.85 ± 7.80%, leading to the removal of 12.20 ± 1.21 μg/mL of cholesterol compared to the control medium without bacterial inoculation (Table 3). Our findings align with earlier studies [49,85,86], which documented the capability of lactobacilli to assimilate cholesterol from the fermentation medium. Lactobacilli are believed to remove cholesterol by integrating it into their cellular membrane, as suggested by various studies [49,85,87,88,89,90]. Further to these, alternative or additional mechanisms for cholesterol removal have been reported, including enzymatic deconjugation of bile acids, co-precipitation of cholesterol with deconjugated bile, cholesterol binding to cell walls [49,91,92], conversion of cholesterol into coprostanol [89], and the production of short-chain fatty acids by probiotics [93].

Table 3.
Cholesterol reduction capability of L. plantarum strains.
3.6. Hemolytic Activity
The hemolytic activity assay demonstrated that LpbBCC4352 displayed a gamma-hemolytic property (non-hemolytic) on sheep blood agar, even after 48 h of incubation (Figure 7). Our findings align with previous studies by Osmanagaoglu et al. [94] and Gao et al. [95], supporting the notion that hemolysis is not a prevalent feature in lactobacilli. This observation underscores that LpbBCC4352 satisfies the essential safety criteria for probiotics, as outlined in the FAO/WHO guidelines [96].
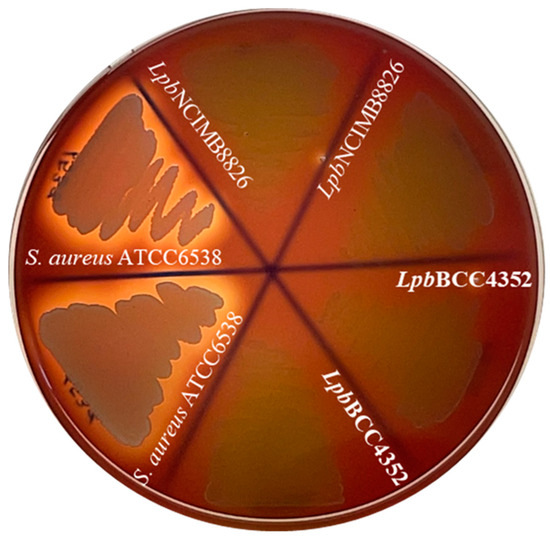
Figure 7.
Hemolytic activity assay on sheep blood agar plates. Plates were incubated at 37 °C for 48 h before inspecting for hemolysis. The translucent zone surrounding the culture-streaked line indicates a positive beta-hemolytic reaction. Staphylococcus aureus ATCC6538 was employed as a beta-hemolytic (positive) control, while L. plantarum NCIMB8826 used as the gamma-hemolytic (negative) control.
3.7. Antibiotics Susceptibility Test
According to the Qualified Presumption of Safety (QPS) approach established by the European Food Safety Authority [97], it is imperative to assess the antibiotic resistance determinant in a probiotic candidate. The results revealed that LpbBCC4352 exhibited sensitivity to all tested antibiotics listed in Table 4. These findings indicate that LpbBCC4352 may be considered a safe strain for use as a probiotic or food starter culture, given its lack of antibiotic resistance to commonly used antibiotics.

Table 4.
Antibiotics susceptibility profile of L. plantarum BCC4352 (LpbBCC4352).
3.8. Effect of LpbBCC4352 on the Fermentation Characteristics of Nham
Physicochemical characterizations were employed to evaluate the fermentation characteristics of Nham, both in its natural fermentation product (Nham-NF) and when inoculated with LpbBCC4352 (Nham-Lp4 and Nham-Lp6). Nham-Lp6 completed fermentation (pH ≤ 4.6) within 36 h, whereas both Nham-Lp4 and Nham-NF completed fermentation within 48 h (Figure 8a). These findings suggest that the pH development in Nham is influenced by the initial number of starter cultures added. Similar results regarding pH development in Nham, fermented with L. curvatus at initial inoculations of 4 and 6 log CFU/g, were reported by Visessanguan et al. [20]. An increase in titratable acidity was noted throughout the fermentation period (Figure 8b). The results indicate that the progression of titratable acidity in Nham-Lp6 surpassed that in both Nham-Lp4 and Nham-NF during the entire fermentation process.
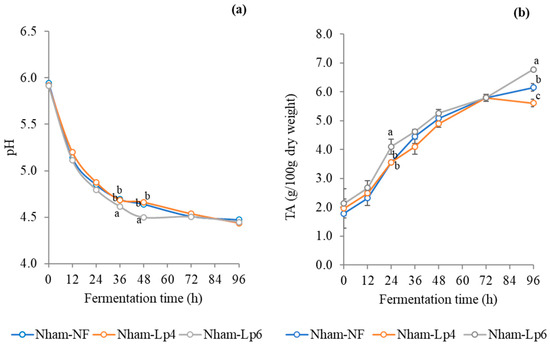
Figure 8.
Changes in pH (a) and titratable acidity (b) of Nham, both with and without inoculation of LpbBCC4352, were monitored during fermentation at 30 °C. The measurements were performed in triplicate. Significant differences were analyzed by one-way ANOVA with Duncan’s multiple range test. Different lowercase letters indicate significant differences (p ≤ 0.05) among sample at the same fermentation time.
Inoculation with 6 log CFU/g of LpbBCC4352 demonstrated the potential to enhance the firmness of Nham, as evidenced by the higher hardness value observed at 72–96 h of fermentation, compared to Nham-Lp4 and Nham-NF (Figure 9).
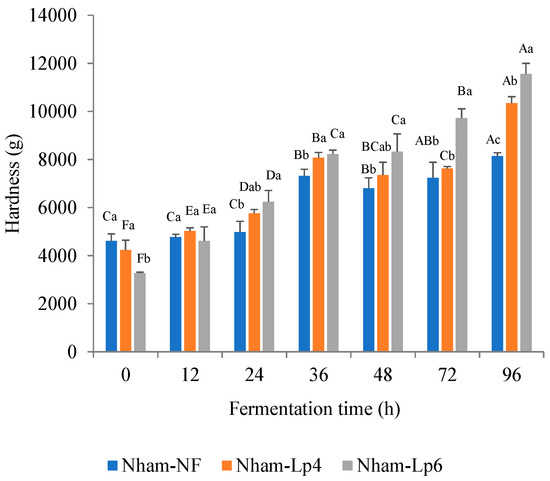
Figure 9.
Changes in hardness of Nham, with and without inoculation of LpbBCC4352, were monitored during fermentation at 30 °C. The measurements were performed in triplicates. Significant differences were analyzed by one-way ANOVA with Duncan’s multiple range test. Different uppercase letters indicate significant differences between fermentation times of the same sample (p ≤ 0.05). Different lowercase letters indicate significant differences between samples at the same fermentation time (p ≤ 0.05).
No significant differences in the color of Nham were detected among different levels of the LpbBCC4352 starter culture (p > 0.05) (Table S2). At the end of the fermentation period, the total color differences (ΔE) values of Nham-Lp4 and Nham-Lp6 compared with Nham-NF were below 2.3, indicating that there were no noticeable differences in color between the samples that could be visualized by the naked eye [55]. Similar results regarding color development in Nham inoculated with Lactobacillus curvatus have been previously documented [20,37].
The numbers of LAB detected in the LpbBCC4352-inoculated Nham increased drastically to a maximum of 10 log CFU/g within 24 h of fermentation and remained stable until 96 h of fermentation. In Nham-NF, there was a rise in the number of LAB to a maximum of 10 log CFU/g observed at 24 h of fermentation, and it tended to decrease after 48 h throughout the fermentation period. During 72–96 h of fermentation, LAB count in Nham-NF was significantly lower than those of Nham-Lp4 and Nham-Lp6 (p < 0.05) (Figure 10).
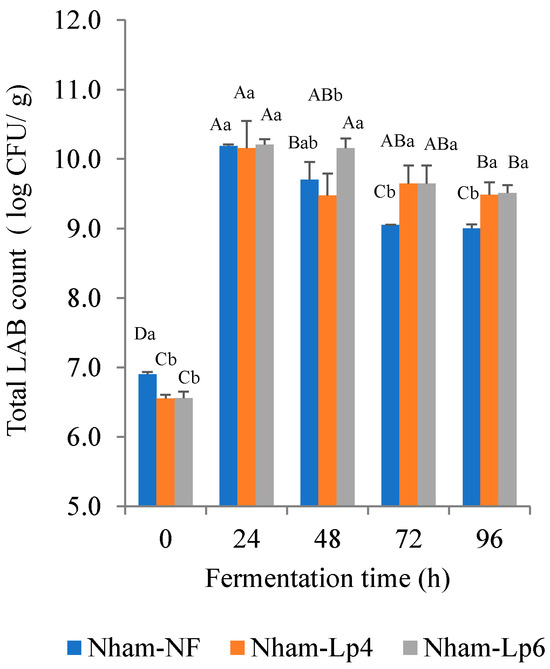
Figure 10.
Changes in LAB count during fermentation of Nham at 37 °C for 96 h. The measurements were performed in triplicate. Significant differences were analyzed by one-way ANOVA with Duncan’s multiple range test. Different uppercase letters indicate significant differences between fermentation times of the same sample (p ≤ 0.05). Different lowercase letters indicate significant differences between samples at the same fermentation time (p ≤ 0.05).
3.9. Acceptability Testing of the Finished Products
The results presented in Figure 11 indicate that there were no significant differences (p > 0.05) in consumer acceptability for the overall characteristics among three groups of Nham. The findings suggest that inoculating LpbBCC4352 at various levels did not negatively impact the organoleptic properties of Nham. Consequently, LpbBCC4352 could be used as a starter culture for Nham fermentation without compromising the distinctive quality of the product.
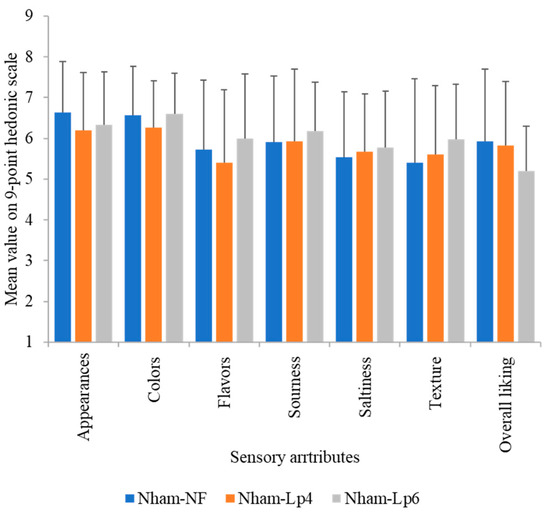
Figure 11.
Sensory analysis of the final fermented Nham (pH < 4.6) with and without inoculation of LpbBCC4352. Data are means of consumer judgements on a nine-point hedonic scale (n = 30).
4. Conclusions
L. plantarum BCC4352 exhibited desirable in vitro probiotic properties. Its ability to survive during transit through the simulated conditions of the human digestive process, along with a capability to adhere to epithelial cells and gastric mucin, indicates the possibility of its ability to proliferate and colonize in the gut. In addition, the ability to produce antimicrobials, utilize prebiotic carbohydrates, and remove cholesterol would be beneficial for its probiotic functions. In a food model, LpbBCC4352 does not significantly affect the unique character of the final fermented Nham when applied as a starter culture, indicating the potential application of this strain as a starter for Nham fermentation. Furthermore, Nham possesses the capability to act as a means of delivering a potentially probiotic strain LpbBCC4352 to consumers.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation10030145/s1, Figure S1: Bile salts hydrolase (Bsh) activity assay of L. plantarum BCC4352; Figure S2: Agarose gel electrophoresis of PCR products from L. plantarum BCC4352 (a), L. plantarum NCIMB8826 (b), and negative control (c), respectively; Table S1. Primers used to detect the potential binding related genes in L. plantarum strains; Table S2. Changes in color of Nham before (t = 0 h) and after (t = 96 h) fermentation.
Author Contributions
Conceptualization, Y.K., A.P. (Atikorn Panya), A.P. (Awanwee Petchkongkaew) and W.W.; Methodology, Y.K., L.P., S.A., K.C., P.S., M.K., T.J., K.K., N.P., A.P. (Atikorn Panya), W.V., A.P. (Awanwee Petchkongkaew) and W.W.; Validation, Y.K., A.P. (Atikorn Panya), W.V., A.P. (Awanwee Petchkongkaew) and W.W.; Formal Analysis, Y.K., A.P. (Atikorn Panya), A.P. (Awanwee Petchkongkaew) and W.W.; Investigation, Y.K., L.P., S.A., K.C., P.S., M.K., T.J., K.K., N.P., A.P. (Atikorn Panya), W.V., A.P. (Awanwee Petchkongkaew) and W.W.; Resources, Y.K., A.P. (Atikorn Panya), W.V., A.P. (Awanwee Petchkongkaew) and W.W.; Writing—Original Draft Preparation, Y.K. and W.W.; Writing—Review & Editing, Y.K., W.V., A.P. (Atikorn Panya), A.P. (Awanwee Petchkongkaew) and W.W.; Supervision, W.V. and A.P. (Awanwee Petchkongkaew); Project Administration, Y.K. and W.W.; Funding Acquisition, W.V., A.P. (Awanwee Petchkongkaew) and W.W. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge the financial support provided by Faculty of Science and Technology, Thammasat University Contract No. SciGR4/2563, National Center for Genetic Engineering and Biotechnology (BIOTEC), and the Thailand Science Research and Innovation Fundamental Fund.
Institutional Review Board Statement
Ethical review and approval were waived for sensory evaluation experiment because the study was performed before the Institutional Review Board was established.
Informed Consent Statement
Inform consent was obtained from all subjects involved in the sensory evaluation study.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Acknowledgments
This research was supported by the National Center for Genetic Engineering and Biotechnology (BIOTEC), National Science and Technology Development Agency (NSTDA), Thailand. We express our sincere gratitude to Suganya Yongkiettrakul of BIOTEC and Potjanee Srimanote from the Graduate Program in Biomedical Sciences, Faculty of Allied Health Sciences, Thammasat University, Pathum Thani, Thailand, for generously supplying the S. suis serotype 2 strain.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- 2001. Available online: http://www.who.int/foodsafety/publications/fs_management/en/probiotics.pdf (accessed on 16 January 2023).
- Pieniz, S.; Andreazza, R.; Anghinoni, T.; Camargo, F.; Brandelli, A. Probiotic potential, antimicrobial and antioxidant activities of Enterococcus durans strain LAB18s. Food Control 2014, 37, 251–256. [Google Scholar] [CrossRef]
- Li, C.; Nie, S.P.; Ding, Q.; Zhu, K.X.; Wang, Z.J.; Xiong, T.; Gong, J.; Xie, M.-Y. Cholesterol-lowering effect of Lactobacillus plantarum NCU116 in a hyperlipidemic rat model. J. Funct. Foods 2014, 8, 340–347. [Google Scholar] [CrossRef]
- Hernández-Hernández, O.; Muthaiyan, A.; Moreno, F.J.; Montilla, A.; Sanz, M.L.; Ricke, S.C. Effect of prebiotic carbohydrates on the growth and tolerance of Lactobacillus. Food Microbiol. 2012, 30, 355–361. [Google Scholar] [CrossRef]
- Ji, Y.; Li, J.; Qin, Z.; Li, A.; Gu, Z.; Liu, X.; Lin, L.; Zhou, Y. Contribution of nuclease to the pathogenesis of Aeromonas hydrophila. Virulence 2015, 6, 515–522. [Google Scholar] [CrossRef]
- Rodgers, S. Novel applications of live bacteria in food services: Probiotics and protective cultures. Trends Food Sci. Technol. 2008, 19, 188–197. [Google Scholar] [CrossRef]
- Buntin, N.; Chanthachum, S.; Hongpattarakere, T. Screening of lactic acid bacteria from gastrointestinal tracts of marine fish for their potential use as probiotics. Songklanakarin J. Sci. Technol. 2008, 30 (Suppl. S1), 141–148. [Google Scholar]
- De Vos, P.; Marijke, M.F.; Milica, S.; Jan, S. Encapsulation for preservation of functionality and targeted delivery of bioactive food components. Int. Dairy J. 2010, 20, 292–302. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.; Harris, H.M.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Ouwehand, A.C.; Isolauri, E. Clinical applications of probiotic bacteria. Int. Dairy J. 1998, 8, 563–572. [Google Scholar] [CrossRef]
- Borriello, S.P.; Hammes, W.P.; Holzapfel, W.; Marteau, P.; Schrezenmeir, J.; Vaara, M.; Valtonen, V. Safety of probiotics that contain lactobacilli or bifidobacteria. Clin. Infect. Dis. 2003, 36, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Bernardeau, M.; Vernoux, J.P.; Henri-Dubernet, S.; Guéguen, M. Safety assessment of dairy microorganisms: The Lactobacillus genus. Int. J. Food Microbiol. 2008, 126, 278–285. [Google Scholar] [CrossRef]
- EFSA. Scientific opinion on the maintenance of the list of QPS biological agents intentionally added to food and feed. EFSA J. 2012, 10, 3020. [Google Scholar] [CrossRef]
- Salminen, S.; Playne, M.; Lee, Y.K. Successful probiotic lactobacilli: Human studies on probiotic efficacy. In Handbook of Functional Dairy Products; Shortt, C., O’Brien, J., Eds.; CRC Press: New York, NY, USA, 2004. [Google Scholar]
- Ngamsomchat, A.; Kaewkod, T.; Konkit, M.; Tragoolpua, Y.; Bovonsombut, S.; Chitov, T. Characterisation of Lactobacillus plantarum of dairy-product origin for probiotic chèvre cheese production. Foods 2022, 11, 934. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Wen, J.; Wu, Z.; Pan, D.; Wang, L. Evaluation of probiotic yoghurt by the mixed culture with Lactobacillus plantarum A3. Food Sci. Hum. Wellness 2022, 11, 323–331. [Google Scholar] [CrossRef]
- El-Sayed, M.I.; Aly, E.; El-Dee, A.M. Improving the physicochemical and antioxidative properties of fermented goat milk using carob molasses and some probiotic Strains. Food Sci. Biotechnol. 2023, 33, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Valyasevi, R.; Rolle, R.S. An overview of small-scale food fermentation technologies in developing countries with special reference to Thailand: Scope for their improvement. Int. J. Food Microbiol. 2002, 75, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Visessanguan, W.; Benjakul, S.; Smitinont, T.; Kittikun, C.; Thepkasikul, P.; Panya, A. Changes in microbiological, biochemical and physical-chemical properties of Nham inoculated with different inoculum levels of Lactobacillu curvatus. LWT-Food Sci. Technol. 2006, 39, 814–826. [Google Scholar] [CrossRef]
- Kingcha, Y.; Tosukhowong, A.; Zendo, T.; Roytrakul, S.; Luxananil, P.; Chareonpornsook, K.; Valyasevi, R.; Sonomoto, K.; Visessanguan, W. Anti-listeria activity of Pediococcus pentosaceus BCC 3772 and application as starter culture for Nham, a traditional fermented pork sausage. Food Control 2012, 25, 190–196. [Google Scholar] [CrossRef]
- Ba, H.V.; Seo, H.W.; Seong, P.N.; Kang, S.M.; Kim, Y.S.; Cho, S.-H.; Park, B.-Y.; Ham, J.-S.; Kim, J.-H. Lactobacillus plantarum (KACC 92189) as a Potential Probiotic Starter Culture for Quality Improvement of Fermented Sausages. Korean J. Food Sci. Anim. Resour. 2018, 38, 189–202. [Google Scholar] [CrossRef]
- Beganović, J.; Pavunc, A.L.; Gjuračić, K.; Špoljarec, M.; Šušković, J.; Kos, B. Improved sauerkraut production with probiotic strain Lactobacillus plantarum L4 and Leuconostoc mesenteroides LMG 7954. J. Food Sci. 2011, 76, M124–M129. [Google Scholar] [CrossRef]
- Islam, S.; Biswas, S.; Jabin, T.; Moniruzzaman, M.; Biswas, J.; Uddin, S.; Ekram, A.E.; Elgorban, A.M.; Ghodake, G.; Syed, A.; et al. Probiotic potential of Lactobacillus plantarum DMR14 for preserving and extending shelf life of fruits and fruit juice. Heliyon 2023, 9, e17382. [Google Scholar] [CrossRef]
- Atsadawut, A.; Apinun, W.; Jantima, T.; Dolnapa, K.; Chiu-Hsia, C. Potential of infrared drying and cell-protective agent efficiency on survival of Lactobacillus plantarum probiotic in fermented soybean meal. Biocatal. Agric. Biotechnol. 2023, 53, 102843. [Google Scholar] [CrossRef]
- Baruzzi, F.; Poltronieri, P.; Quero, G.M.; Morea, M.; Morelli, L. An in vitro protocol for direct isolation of potential probiotic lactobacilli from raw bovine milk and traditional fermented milks. Appl. Microbiol. Biotechnol. 2011, 90, 331–342. [Google Scholar] [CrossRef]
- Zago, M.; Fornasari, M.E.; Carminati, D.; Burns, P.; Suàrez, V.; Vinderola, G.; Reinheimer, J.; Giraffa, G. Characterization and probiotic potential of Lactobacillus plantarum strains isolated from cheeses. Food Microbiol. 2011, 28, 1033–1040. [Google Scholar] [CrossRef]
- Bautista-Gallego, J.; Arroyo-López, F.N.; Rantsiou, K.; Jiménez-Díaz, R.; Garrido-Fernández, A.; Cocolin, L. Screening of lactic acid bacteria isolated from fermented table olives with probiotic potential. Food Res. Int. 2013, 50, 135–142. [Google Scholar] [CrossRef]
- Jofré, A.; Aymerich, T.; Garriga, M. Probiotic fermented sausages: Myth or reality? Procedia Food Sci. 2015, 5, 133–136. [Google Scholar] [CrossRef]
- Trabelsi, I.; Slima, S.B.; Ktari, N.; Triki, M.; Abdehedi, R.; Abaza, W.; Moussa, H.; Abdeslam, A.; Salah, R.B. Incorporation of probiotic strain in raw minced beef meat: Study of textural modification, lipid and protein oxidation and color parameters during refrigerated storage. Meat Sci. 2019, 154, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Blaiotta, G.; Murru, N.; Di Cerbo, A.; Romano, R.; Aponte, M. Production of probiotic bovine salami using Lactobacillus plantarum 299v as adjunct. J. Sci. Food Agric. 2018, 98, 2285–2294. [Google Scholar] [CrossRef] [PubMed]
- Pavli, F.G.; Argyri, A.A.; Chorianopoulos, N.G.; Nychas, G.J.E.; Tassou, C.C. Effect of Lactobacillus plantarum L125 strain with probiotic potential on physicochemical, microbiological, and sensorial characteristics of dry-fermented sausages. LWT 2020, 118, 108810. [Google Scholar] [CrossRef]
- Cavalheiro, C.P.; Ruiz-Capillas, C.; Herrero, A.M.; Jiménez-Colmenero, F.; Pintado, T.; de Menezes, C.R.; Fries, L.L.M. Effect of encapsulated Lactobacillus plantarum as probiotic on dry sausages during chilled storage. Int. J. Food Sci. Technol. 2020, 55, 3613–3621. [Google Scholar] [CrossRef]
- Sirini, N.; Loyeau, P.; Ruiz, M.; Stegmayer, M.; Soto, L.; Werning, M.; Frizzo, L.; Ordoñez, V.; Fernández-López, J.; Rosmini, M. Development of probiotic fermented sausages and viability monitoring of supplemented Lactiplantibacillus plantarum BFL strain. Fermentation 2022, 8, 526. [Google Scholar] [CrossRef]
- Klingberg, T.D.; Budde, B.B. The survival and persistence in the human gastrointestinal tract of five potential probiotic lactobacilli consumed as freeze-dried cultures or as probiotic sausage. Int. J. Food Microbiol. 2006, 109, 157–159. [Google Scholar] [CrossRef]
- Tanasupawat, S.; Komagata, K. Lactic acid bacteria in fermented foods in Thailand. World J. Microbiol. Biotechnol. 1995, 11, 253–256. [Google Scholar] [CrossRef]
- Visessanguan, W.; Benjakul, S.; Riebroy, S.; Thepkasikul, P. Changes in composition and functional properties of proteins and their contributions to Nham characteristics. Meat Sci. 2004, 66, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Phupaboon, S.; Kontongdee, P.; Hashim, F.J.; Kanpipit, N.; Matra, M.; Totakul, P.; Prommachart, R.; Phesatcha, B.; Wanapat, M. Bioaccessibility and Microencapsulation of Lactobacillus sp. to Enhance Nham Protein Hydrolysates in Thai Fermented Sausage. Foods 2022, 11, 3846. [Google Scholar] [CrossRef] [PubMed]
- Zendo, T.; Fukao, M.; Ueda, K.; Higuchi, T.; Nakayama, J.; Sonomoto, K. Identification of the lantibiotic nisin Q, a new natural nisin variant produced by Lactococcus lactis 61-14 isolated from a river in Japan. Biosci. Biotechnol. Biochem. 2003, 67, 1616–1619. [Google Scholar] [CrossRef]
- Vijayakumar, M.; Ilavenil, S.; Kim, D.H.; Arasu, M.V.; Priya, K.; Choi, K.C. In vitro assessment of the probiotic potential of Lactobacillus plantarum KCC-24 isolated from Italian rye-grass (Lolium multiflorum) forage. Anaerobe 2015, 32, 90–97. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardized static in vitro digestion method suitable for food–an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Allain, T.; Chaouch, S.; Thomas, M.; Travers, M.A.; Valle, I.; Langella, P.; Grellier, P.; Polack, B.; Florent, I.; Bermúdez-Humarán, L.G. Bile salt hydrolase activities: A novel target to screen anti-Giardia lactobacilli. Front. Microbiol. 2018, 9, 89. [Google Scholar] [CrossRef]
- Klingberg, T.D.; Axelsson, L.; Naterstad, K.; Elsser, D.; Budde, B.B. Identification of potential probiotic starter cultures for Scandinavian-type fermented sausages. Int. J. Food Microbiol. 2005, 105, 419–431. [Google Scholar] [CrossRef]
- Van den Abbeele, P.; Grootaert, C.; Possemiers, S.; Verstraete, W.; Verbeken, K.; Van de Wiele, T. In vitro model to study the modulation of the mucin-adhered bacterial community. Appl. Microbiol. Biotechnol. 2009, 83, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Turpin, W.; Humblot, C.; Noordine, M.L.; Thomas, M.; Guyot, J.P. Lactobacillaceae and Cell Adhesion: Genomic and Functional Screening. PLoS ONE 2012, 7, e38034. [Google Scholar] [CrossRef]
- Gibson, G.R.; Cummings, J.H.; Macfarlane, G.T. Use of a three-stage continuous culture system to study the effect of mucin on dissimilatory sulfate reduction and methanogenesis by mixed populations of human gut bacteria. Appl. Environ. Microbiol. 1988, 54, 2750–2755. [Google Scholar] [CrossRef]
- Gamonpilas, C.; Buathongjan, C.; Sangwan, W.; Rattanaprasert, M.; Weizman, K.C.; Klomtun, M.; Phonsatta, N.; Methacanon, P. Production of low molecular weight pectins via electron beam irradiation and their potential prebiotic functionality. Food Hydrocoll. 2021, 113, 106551. [Google Scholar] [CrossRef]
- Schooley, D.L.; Kubiak, F.M.; Evans, J.V. Capillary gas chromatographic analysis of volatile and non-volatile organic acids from biological samples as the t-butyldimethylsilyl derivatives. J. Chromatogr. Sci. 1985, 23, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Liong, M.T.; Shah, N.P. Optimization of cholesterol removal by probiotics in the presence of prebiotics by using a response surface method. Appl. Environ. Microbiol. 2005, 71, 1745–1753. [Google Scholar] [CrossRef] [PubMed]
- Rudel, L.L.; Morris, M.D. Determination of cholesterol using O-phthalaldehyde. J. Lipid Res. 1973, 14, 364–366. [Google Scholar] [CrossRef] [PubMed]
- Speck, S.; Wenke, C.; Feßler, A.T.; Kacza, J.; Geber, F.; Scholtzek, A.D.; Hanke, D.; Eichhorn, I.; Schwarz, S.; Rosolowski, M.; et al. Borderline resistance to oxacillin in Staphylococcus aureus after treatment with sub-lethal sodium hypochlorite concentrations. Heliyon 2020, 6, e04070. [Google Scholar] [CrossRef] [PubMed]
- Vesa, T.; Pochart, P.; Marteau, P. Pharmacokinetics of Lactobacillus plantarum NCIMB 8826, Lactobacillus fermentum KLD, and Lactococcus lactis MG 1363 in the human gastrointestinal tract. Aliment. Pharmacol. Ther. 2000, 14, 823–828. [Google Scholar] [CrossRef] [PubMed]
- ISO 10932:2010; Milk and Milk Products-Determination of the Minimal Inhibitory Concentration (MIC) of Antibiotics Applicable to Bifidobacteria and Non-Enterococcal Lactic Acid Bacteria (LAB). International Organization for Standardization: Geneva, Switzerland, 2010.
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis International, 17th ed.; AOAC: Washington, DC, USA, 2000. [Google Scholar]
- Sharma, G. Digital color imaging. IEEE Trans. Image Process. 1997, 6, 901–932. [Google Scholar] [CrossRef] [PubMed]
- Nespolo, C.R.; Brandelli, A. Production of bacteriocin-like substances by lactic acid bacteria isolated from regional ovine cheese. Braz. J. Microbiol. 2010, 41, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Rojo-Bezares, B.; Saenz, Y.; Navarro, L.; Zarazaga, M.; Ruiz-Larrea, F.; Torres, C. Coculture-inducible bacteriocin activity of Lactobacillus plantarum strain J23 isolated from grape must. Food Microbiol. 2007, 24, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Tabasco, R.; García-Cayuela, T.; Peláez, C.; Requena, T. Lactobacillus acidophilus La-5 increases lactacin B production when it senses live target bacteria. Int. J. Food Microbiol. 2009, 132, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; De Angelis, M.; Calasso, M.; Vincentini, O.; Vernocchi, P.; Ndagijimana, M.; De Vincenzi, M.; Dessì, M.R.; Guerzoni, M.E.; Gobbetti, M. Quorum sensing in sourdough Lactobacillus plantarum DC400: Induction of plantaricin A (PlnA) under co-cultivation with other lactic acid bacteria and effect of PlnA on bacterial and Caco-2 cells. Proteomics 2010, 10, 2175–2190. [Google Scholar] [CrossRef]
- Maldonado-Barragán, A.; Caballero-Guerrero, B.; Luis Ruiz-Barba, J. Induction of bacteriocin production by coculture is widespread among plantaricin-producing Lactobacillus plantarum strains with different regulatory operons. Food Microbiol. 2013, 33, 40–47. [Google Scholar] [CrossRef]
- Ding, T.; Li, Y. Study on the promotive effect and mechanism of exogenous nucleotides on Lactobacillus casei antagonism against Salmonella enterica by coculture. LWT 2023, 182, 114821. [Google Scholar] [CrossRef]
- Fongcom, A.; Pruksakorn, S.; Mongkol, R.; Tharavichitkul, P.; Yoonim, N. Streptococcus suis infection in northern Thailand. J. Med. Assoc. Thail. 2001, 84, 1502–1508. [Google Scholar]
- Khadthasrima, N.; Hannwong, T.; Thammawitjaya, P.; Pingsusean, D.; Akkanij, B.; Jaikhar, A.; Paungmali, P.; Yudee, P.; Wongyai, S.; Samerchea, S.; et al. Human Streptococcus suis Outbreak in Phayao Province, Thailand, 2007. Outbreak Surveill. Investig. Rep. (OSIR) e-J. 2008, 1, 4–7. Available online: http://www.osirjournal.net/issue.php?id=3 (accessed on 16 January 2023). [CrossRef]
- Navacharoen, N.; Chantharochavong, V.; Hanprasertpong, C.; Kangsanarak, J.; Lekagul, S. Hearing and vestibular loss in Streptococcus suis infection from swine and traditional raw pork exposure in northern Thailand. J. Laryngol. Otol. 2009, 123, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Noppon, B.; Khaeng-air, S.; Sopa, A.; Phuaram, P.; Wongsan, R.; Laohasinnurak, T. Streptococcus suis serotype 2 in Uncooked Pork Meat Products in Khon Kaen, Northeastern Thailand, and their Antimicrobial Profiles. Int. J. Sci. Eng. Res. 2014, 5, 1130–1133, ISSN 2229-5518. [Google Scholar]
- Burintramart, U.; Chantarasakha, K.; Chokesajjawatee, N. Diversity of lactic acid bacteria in Thai fermented pork (Nham) during fermentation assessed by Denaturing Gradient Gel Electrophoresis. KKU Res. J. 2014, 19, 19–25. [Google Scholar]
- Wongnak, P.; Wiratsudakul, A.; Nuanualsuwan, S. A risk assessment of pathogenic Streptococcus suis in pork supply chains and markets in Thailand. Food Control 2020, 118, 1071432. [Google Scholar] [CrossRef]
- Schillinger, U.; Guigas, C.; Holzapfel, W.H. In vitro adherence and other properties of lactobacilli used in probiotic yoghurt-like products. Int. Dairy J. 2005, 15, 1289–1297. [Google Scholar] [CrossRef]
- Nannen, N.L.; Hutkins, R.W. Proton-translocating adenosine triphosphate activity in lactic acid bacteria. J. Dairy Sci. 1991, 74, 747–751. [Google Scholar] [CrossRef]
- Cotter, P.D.; Hill, C. Surviving the acid test: Responses of gram-positive bacteria to low pH. Microbiol. Mol. Biol. Rev. 2003, 67, 429–453. [Google Scholar] [CrossRef] [PubMed]
- Jaichumjai, P.; Valyasevi, R.; Assavanig, A.; Kurdi, P. Isolation and characterization of acid-sensitive Lactobacillus plantarum with application as starter culture for Nham production. Food Microbiol. 2010, 27, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Tian, X.; Huang, R.; Tao, X.; Shah, N.P.; Wei, H.; Wan, C. A physiological comparative study of acid tolerance of Lactobacillus plantarum ZDY 2013 and L. plantarum ATCC 8014 at membrane and cytoplasm levels. Ann. Microbiol. 2017, 67, 669–677. [Google Scholar] [CrossRef]
- Noriega, L.; Gueimonde, M.; Sánchez, B.; Margolles, A.; delosReyes-Gavilán, C.G. Effect of the adaptation to high bile salts concentrations on glycosidic activity, survival at low pH and cross-resistance to bile salts in Bifidobacteirum. Int. J. Food Microbiol. 2004, 94, 79–86. [Google Scholar] [CrossRef]
- Kheroua, O.; Belleville, J. Behaviour of digestive enzymes in the pancreatic juice and pancreas of rats fed on a low-protein diet than on a balanced diet. Reprod. Nutr. Dev. 1981, 21, 901–917. [Google Scholar] [CrossRef]
- De Smet, I.; Van Hoorde, L.; Vande Woestyne, M.; Christiaens, H.; Verstraete, W. Significance of bile salt hydrolytic activities of Lactobacilli. J. Appl. Bacteriol. 1995, 79, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Moser, S.A.; Savage, D.C. Bile salt hydrolase activity and resistance to toxicity of conjugated bile salts are unrelated properties in Lactobacilli. Appl. Environ. Microbiol. 2001, 67, 3476–3480. [Google Scholar] [CrossRef] [PubMed]
- Begley, M.; Gahan, C.G.M.; Hill, C. The interaction between bacteria and bile. FEMS Microbiol. Rev. 2005, 29, 625–651. [Google Scholar] [CrossRef] [PubMed]
- Dunne, C.; O’Mahony, L.; Murphy, L.; Thornton, G.; Morrissey, D.; O’halloran, S.; Feeney, M.; Flynn, S.; Fitzgerald, G.; Daly, C.; et al. In vitro selection criteria for probiotic bacteria of human origin: Correlation with in vivo findings. Am. J. Clin. Nutr. 2001, 73, 386s–392s. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Surono, I.; Meriluoto, J.; Salminen, S. Indigenous dadih lactic acid bacteria: Cell-surface properties and interactions with pathogens. J. Food Sci. 2007, 72, M89–M93. [Google Scholar] [CrossRef] [PubMed]
- Bergillos-Meca, T.; Costabile, A.; Walton, G.; Moreno-Montoro, M.; Ruiz-Bravo, A.; Ruiz-López, M.D. In vitro evaluation of the fermentation properties and potential probiotic activity of Lactobacillus plantarum C4 in batch culture systems. LWT-Food Sci. Technol. 2015, 60, 420–426. [Google Scholar] [CrossRef]
- Buck, B.L.; Altermann, E.; Svingerud, T.; Klaenhammer, T.R. Functional analysis of putative adhesion factors in Lactobacillus acidophilus NCFM, Appl. Environ. Microbiol. 2005, 71, 8344–8351. [Google Scholar] [CrossRef]
- Ashida, N.; Yanagihara, S.; Shinoda, T.; Yamamoto, N. Characterization of adhesive molecule with affinity to Caco-2 cells in Lactobacillus acidophilus by proteome analysis. J. Biosci. Bioeng. 2011, 112, 333–337. [Google Scholar] [CrossRef]
- Yuille, S.; Reichardt, N.; Panda, S.; Dunbar, H.; Mulder, I.E. Human gut bacteria as potent class I histone deacetylase inhibitors in vitro through production of butyric acid and valeric acid. PLoS ONE 2018, 13, e0201073. [Google Scholar] [CrossRef]
- Ishimwe, N.; Daliri, E.B.; Lee, B.H.; Fang, F.; Du, G. The perspective on cholesterol-lowering mechanisms of probiotics. Mol. Nutr. Food Res. 2015, 59, 94–105. [Google Scholar] [CrossRef]
- Lye, H.; Khoo, B.Y.; Karim, A.A.; Rusul, G.; Liong, M.T. Growth properties and cholesterol removal ability of electroporated Lactobacillus acidophilus BT 1088. J. Microbiol. Biotechnol. 2012, 22, 981–989. [Google Scholar] [CrossRef]
- Kingkaew, E.; Konno, H.; Hosaka, Y.; Phongsopitanun, W.; Tanasupawat, S. Characterization of lactic acid bacteria from fermented fish (pla-paeng-daeng) and their cholesterol-lowering and immunomodulatory effects. Microbes Environ. 2023, 38, ME22044. [Google Scholar] [CrossRef]
- Lin, M.Y.; Chen, T.W. Reduction of Cholesterol by Lactobacillus acidophilus in Culture Broth. J. Food Drug Anal. 2000, 8, 4. [Google Scholar] [CrossRef]
- Lye, H.S.; Rahmat-Ali, G.R.; Liong, M.T. Mechanisms of cholesterol removal by lactobacilli under conditions that mimic the human gastrointestinal tract. Int. Dairy J. 2010, 20, 169–175. [Google Scholar] [CrossRef]
- Lye, H.S.; Rusul, G.; Liong, M.T. Removal of cholesterol by lactobacilli via incorporation and conversion to coprostanol. J. Dairy Sci. 2010, 93, 1383–1392. [Google Scholar] [CrossRef]
- Kriaa, A.; Bourgin, M.; Potiron, A.; Mkaouar, H.; Jablaoui, A.; Gérard, P.; Maguin, E.; Rhimi, M. Microbial impact on cholesterol and bile acid metabolism: Current status and future prospects. J. Lipid Res. 2019, 60, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Liong, M.T.; Shah, N.P. Effects of a Lactobacillus casei synbiotic on serum lipoprotein, intestinal microflora, and organic acids in rats. J. Dairy Sci. 2006, 89, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.M.; Bongers, R.S.; de Vos, W.M.; Kleerebezem, M. Functional analysis of four bile salt hydrolase and penicillin acylase family members in Lactobacillus plantarum WCFS1. Appl. Environ. Microbiol. 2008, 74, 4719–4726. [Google Scholar] [CrossRef] [PubMed]
- De Preter, V.; Vanhoutte, T.; Huys, G.; Swings, J.; De Vuyst, L.; Rutgeerts, P.; Verbeke, K. Effects of Lactobacillus casei Shirota, Bifidobacterium breve, and oligofructose-enriched inulin on colonic nitrogen-protein metabolism in healthy humans. Am. J. Physiol.-Gastrointest. Liver Physiol. 2007, 292, G358–G368. [Google Scholar] [CrossRef] [PubMed]
- Osmanagaoglu, O.; Kiran, F.; Ataoglu, H. Evaluation of in vitro probiotic potential of Pediococcus pentosaceus OZF isolated from human breast milk. Probiotics Antimicrob. Proteins 2010, 2, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, D.; Liu, S.; Liu, Y. Probiotic potential of L. sake C2 isolated from traditional Chinese fermented cabbage. Eur. Food Res. Technol. 2012, 234, 45–51. [Google Scholar] [CrossRef]
- FAO/WHO. Joint FAO/WHO Working Group Report on Draft Guidelines for the Evaluation of Probiotics in Food; FAO/WHO: London, ON, Canada, 2002. Available online: https://www.mhlw.go.jp/file/05-Shingikai-11121000-Iyakushokuhinkyoku-Soumuka/0000197343.pdf (accessed on 16 January 2023).
- EFSA. Update of the criteria used in the assessment of bacterial resistance to antibiotics of human or veterinary importance. EFSA J. 2008, 6, 732. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).