Comparative Analysis of Cellulosic Ethanol Production from Lignocellulosic Substrate Moringa oleifera Using Kluyveromyces marxianus and Zymomonas mobilis
Abstract
:1. Introduction
2. Materials and Methodology
2.1. Woody Stem Moringa oleifera
2.2. Pretreatment Methodologies
2.2.1. Sequential Treatment of Biomass
2.2.2. Ultrasonication
2.2.3. Steam Coupled with Acid Hydrolysis Followed by Ultrasonication: Combination Treatment Method
2.3. Micro-organism and Culture Conditions
2.4. Enzyme and Surfactant
2.5. Simultaneous Saccharification and Fermentation
2.6. Analytical Methods
3. Results
3.1. Pretreatment of Woody Stem Moringa oleifera
3.2. Biochemical Composition of Woody Stem Moringa oleifera
3.3. SSF Using Symmons mobilis
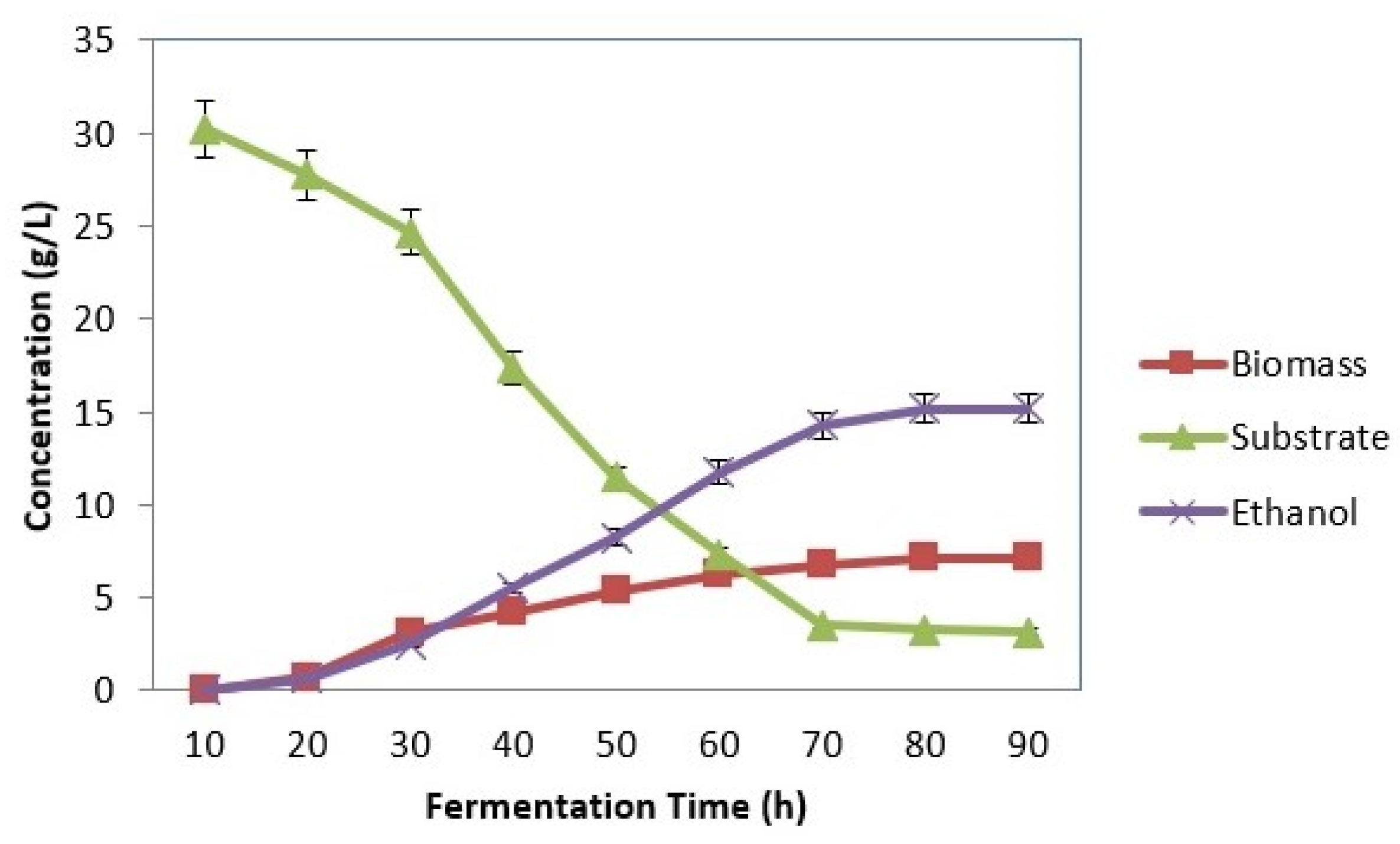
3.3.1. Effect of Substrate Concentration
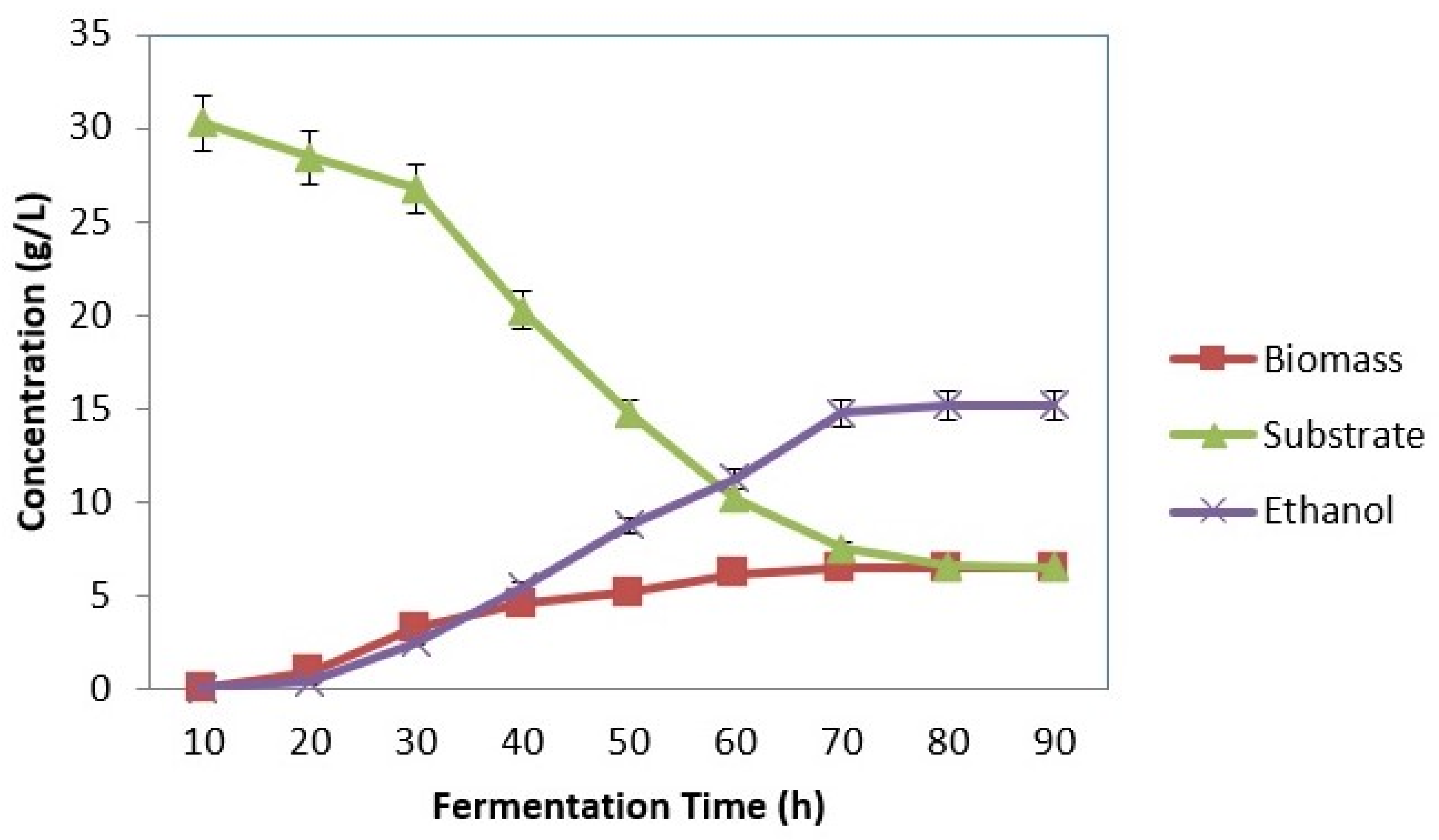
3.3.2. Effect of Inoculum Concentration
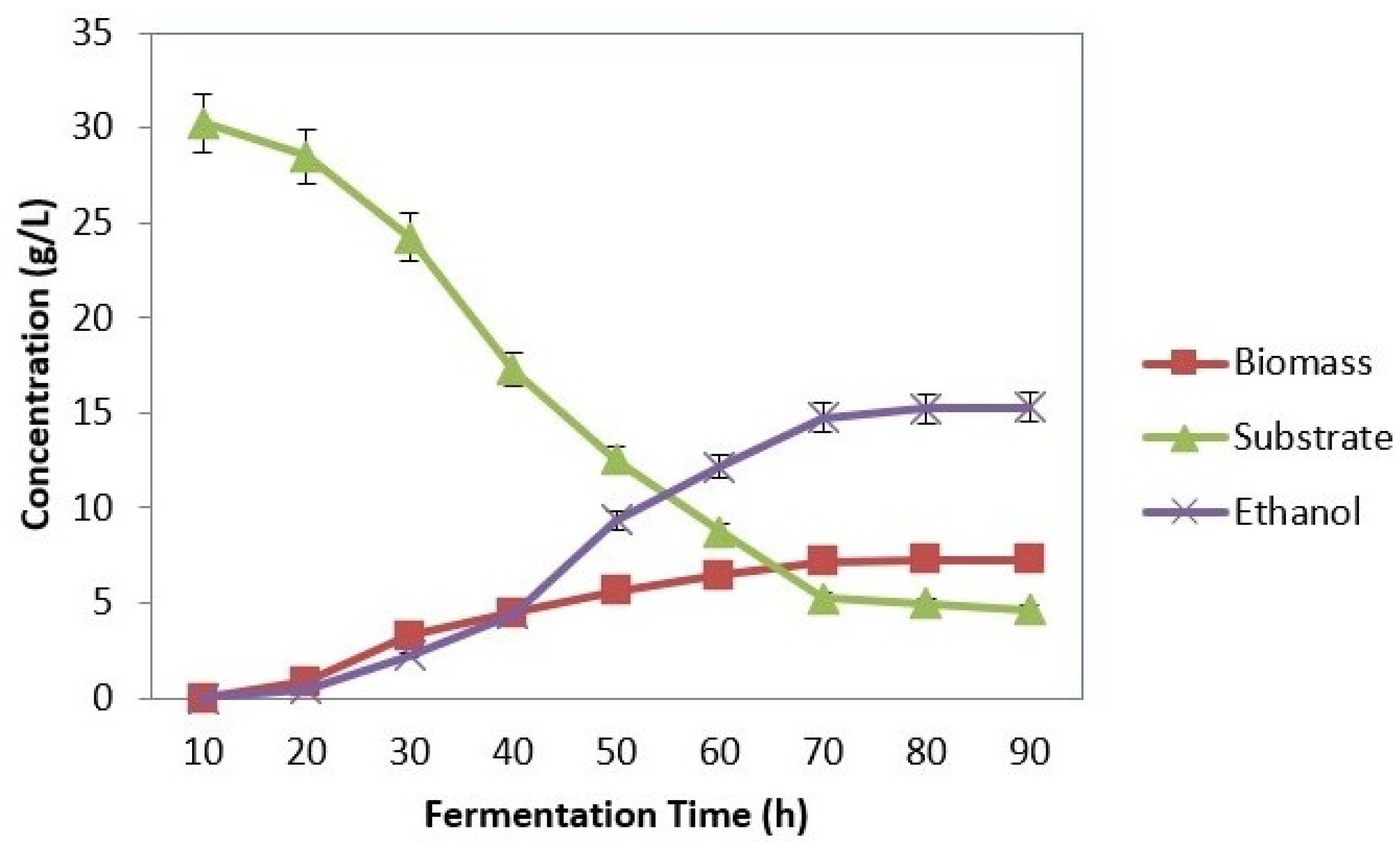
3.3.3. Effect of pH
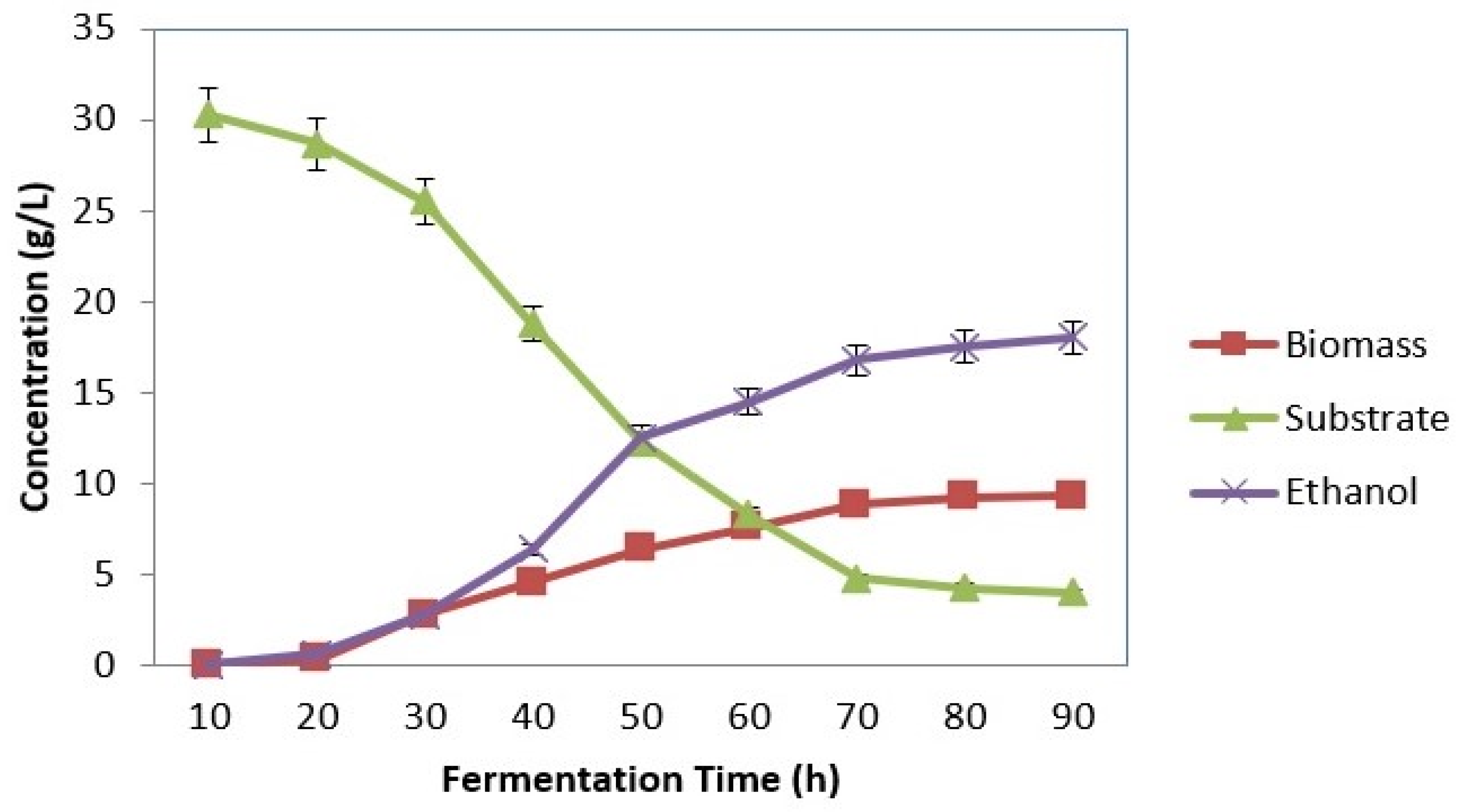
3.3.4. Effect of Temperature
3.4. SSF Using Kluyveromyces marxianus (MTCC 1389)
3.4.1. Effect of Substrate Concentration
3.4.2. Effect of Inoculum Concentration
3.4.3. Effect of pH
3.4.4. Effect of Temperature
3.5. GC-MS & GC-FID Studies of Cellulosic Ethanol Production
3.5.1. Woody Stem of Moringa oleifera and Zymomonas mobilis
3.5.2. Woody Stem Moringa oleifera and Kluyveromyces marxianus
4. Discussion
4.1. SSF Using Zymomonas Mobilis (MTCC 2427)
4.1.1. Effect of Substrate Concentration
4.1.2. Effect of Inoculum Concentration
4.1.3. Effect of pH
4.1.4. Effect of Temperature
4.2. SSF Using Kluyveromyces marxianus (MTCC 1389)
4.2.1. Effect of Substrate Concentration
4.2.2. Effect of Inoculum Concentration
4.2.3. Effect of pH
4.2.4. Effect of Temperature
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clark, D.P.; Pazdernik, N.J. Chapter 12: Environmental Biotechnology. In Biotechnology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 393–418. [Google Scholar]
- Kumar, A.K.; Sharma, S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: A review. Bioresour. Bioprocess. 2017, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Raj, T.; Chandrasekhar, K.; Kumar, A.N.; Banu, J.R.; Yoon, J.J.; Bhatia, S.K.; Yang, Y.H.; Varjani, S.; Kim, S.H. Recent advances in commercial biorefineries for lignocellulosic ethanol production: Current status, challenges and future perspectives. Bioresour. Technol. 2022, 344 Pt B, 126292. [Google Scholar] [CrossRef]
- Láinez, M.; García-Béjar, J.A.; Flores-Cosío, G.; Herrera-López, E.J.; Amaya-Delgado, L. Chapter 4—Advances in fermentative systems for the production of ethanol from lignocellulosic biomass. In Innovations in Fermentation and Phytopharmaceutical Technologies; Elsevier: Amsterdam, The Netherlands, 2022; pp. 47–74. [Google Scholar]
- Sarkar, N.; Ghosh, S.K.; Bannerjee, S.; Aikat, K. Bioethanol production from agricultural wastes: An overview. Renew. Energy 2012, 37, 19–27. [Google Scholar] [CrossRef]
- Shirkavand, E.; Baroutian, S.; Gapes, D.J.; Young, B.R. Combination of fungal and physicochemical processes for lignocellulosic biomass pre-treatment—A review. Renew. Sust. Energ. Rev. 2016, 54, 217–234. [Google Scholar] [CrossRef]
- Gundupalli, M.P.; Cheng, Y.S.; Chuetor, S.; Bhattacharyya, D.; Sriariyanun, M. Effect of dewaxing on saccharification and ethanol production from different lignocellulosic biomass. Bioresour. Technol. 2021, 339, 125596. [Google Scholar] [CrossRef]
- Zhou, X.; Guan, C.; Xu, Y.; Yang, S.; Huang, C.; Sha, J.; Dai, H. Mechanistic insights into morphological and chemical changes during benzenesulfonic acid pretreatment and simultaneous saccharification and fermentation process for ethanol production. Bioresour. Technol. 2022, 360, 127586. [Google Scholar] [CrossRef]
- Bai, F.-W.; Yang, S.; Ho, N.W. 3.05—Fuel Ethanol Production from Lignocellulosic Biomass, Comprehensive Biotechnology, 3rd ed.; Pergamon, Turkey, 2019; pp. 49–65. Available online: https://www.researchgate.net/publication/351468338_Fuel_Ethanol_Production_From_Lignocellulosic_Biomass (accessed on 20 July 2023).
- Hans, M.; Kumar, S.; Chandel, A.K.; Polikarpov, I. A review on bioprocessing of paddy straw to ethanol using simultaneous saccharification and fermentation. Process. Biochem. 2019, 85, 125–134. [Google Scholar] [CrossRef]
- Singh, R.; Shukla, A.; Tiwari, S.; Srivastava, M. A review on delignification of lignocellulosic biomass for enhancement of ethanol production potential. Renew. Sust. Energ. Rev. 2014, 32, 713–728. [Google Scholar] [CrossRef]
- Tutt, M.; Kikas, T.; Olt, J. Comparison of different pretreatment methods on degradation of rye straw. In Proceedings of the 11th International Scientific Conference Engineering for Rural Development, Jelgava, Latvia, 24–25 May 2012; pp. 412–416. [Google Scholar]
- Zhang, R.; Liu, F.; Liu, H. Fast Acidogenic Fermentation of Corn Stover Through a Two-Step Method: Nitric Acid Hydrolysis Combined with the Fermentation of Hydrolysate. BioResources 2013, 8, 4193–4207. [Google Scholar] [CrossRef]
- Kim, I.; Lee, B.; Park, J.Y.; Choi, S.A.; Han, J.I. Effect of nitric acid on pre-treatment and fermentation for enhancing ethanol production of rice straw. Carbohydr. Polym. 2014, 99, 563–567. [Google Scholar] [CrossRef]
- Woiciechowski, A.L.; Neto, C.J.D.; de Souza Vandenberghe, L.P.; de Carvalho Neto, D.P.; Sydney, A.C.N.; Letti, L.A.J.; Karp, S.G.; Torres, L.A.Z.; Soccol, C.R. Lignocellulosic biomass: Acid and alkaline pretreatments and their effects on biomass recalcitrance—Conventional processing and recent advances. Bioresour. Technol. 2020, 304, 122848. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, R.; Sharma, V.; Pracheta, J. A Review on Horse Radish Tree (Moringa oleifera): A Multipurpose Tree with High Economic and Commercial Importance. Asian J. Biotechnol. 2011, 3, 317–328. [Google Scholar] [CrossRef]
- Ali, E.N.; Jamaludin, M.Z. Possibility of Producing Ethanol from Moringa oleifera Pod Husk. J. Adv. Res. 2015, 5, 1–9. [Google Scholar]
- Rehman, M.S.U.; Kim, I.; Chisti, Y.; Han, J.I. Use of ultrasound in the production of bioethanol from lignocellulosic biomass. Energy Educ. Sci. Technol. Part A Energy Sci. Res. 2013, 30, 1391–1410. [Google Scholar]
- Vohra, M.; Manwar, J.; Manmode, R.; Padgilwar, S.; Patil, S. Bioethanol production: Feedstock and current technologies. J. Environ. Chem. Eng. 2014, 2, 573–584. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Enzymatic-based hydrolysis processes for ethanol from lignocellulosic materials: A review. BioResources 2007, 2, 707–738. [Google Scholar] [CrossRef]
- Li, K.; Qin, J.-C.; Liu, C.-G.; Bai, F.-W. Optimization of pretreatment, enzymatic hydrolysis and fermentation for more efficient ethanol production by Jerusalem artichoke stalk. Bioresour. Technol. 2016, 221, 188–194. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Y.; Xue, Y.; Yang, S.; Cheng, Y.; Zhu, W. Ethanol production from lignocellulosic biomass by co-fermentation with Pecoramyces sp. F1 and Zymomonas mobilis ATCC 31821 in an integrated process. Biomass Bioenergy 2022, 161, 106454. [Google Scholar] [CrossRef]
- Szambelan, K.; Szwengiel, A.; Nowak, J.; Jeleń, H.; Frankowski, J. Low-waste technology for the production of bioethanol from sorghum grain: Comparison of Zymomonas mobilis and Saccharomyces cerevisiae in fermentation with stillage reusing. J. Clean. Prod. 2022, 352, 131607. [Google Scholar] [CrossRef]
- Maitan-Alfenas, G.P.; Visser, E.M.; Guimarães, V.M. Enzymatic hydrolysis of lignocellulosic biomass: Converting food waste in valuable products. Curr. Opin. Food Sci. 2015, 1, 44–49. [Google Scholar] [CrossRef]
- Buruiana, C.T.; Garrote, G.; Vizireanu, C. Bioethanol production from residual lignocellulosic materials: A review—Part 2. Ann. Univ. Dunarea Jos Galati Fascicle VI Food Technol. 2013, 37, 25–38. [Google Scholar]
- Baptista, M.; Domingues, L. Kluyveromyces marxianus as a microbial cell factory for lignocellulosic biomass valorisation. Biotechnol. Adv. 2022, 60, 108027. [Google Scholar] [CrossRef] [PubMed]
- Cazetta, M.L.; Celligoi, M.A.P.C.; Buzato, J.B.; Scarmino, I.S. Fermentation of molasses by Zymomonas mobilis: Effects of temperature and sugar concentration on ethanol production. Bioresour. Technol. 2007, 98, 2824–2828. [Google Scholar] [CrossRef]
- Santiago-Gómez, M.; Hernández-Mendoza, A.G.; Martínez-Hernández, S. Ethanol production from Agave salmiana leaves by semi and Simultaneous saccharification and fermentation at high temperature using Kluyveromyces marxianus. Biocatal. Agric. Biotechnol. 2023, 50, 102703. [Google Scholar] [CrossRef]
- Hemansi; Saini, J.K. Enhanced cellulosic ethanol production via fed-batch simultaneous saccharifi-cation and fermentation of sequential dilute acid-alkali pretreated sugarcane bagasse. Bioresour. Technol. 2023, 372, 128671. [Google Scholar] [CrossRef]
- Nikolić, S.; Mojović, L.; Rakin, M.; Pejin, D.; Pejin, J. Ultrasound-assisted production of bioethanol by Simultaneous saccharification and fermentation of corn meal. Food Chem. 2010, 122, 216–222. [Google Scholar] [CrossRef]
- Mohanasrinivasan, V.; Anand, E.; SubathraDevi, C.; JemimahNaine, S.; Karthikeyan, S. Bioethanol production from Anacardium occidentale fruit juice and preparation of blended fuel using in-house developed bioreactor. Appl. Environ. Microbiol. 2015, 3, 44–48. [Google Scholar]
- Ghose, T.K. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Miller, G. L Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Sadasivam, S.; Manickam, A. Biochemical Method; New Age International (P) Ltd. Publishers: New Delhi, India, 1996. [Google Scholar]
- Koshy, B.E.; Pandey, F.; Bhatnagar, T. Quantitative estimation of Bio-ethanol production produced from lingo-cellulosic & house-hold wastes. Int. J. Life Sci. Res. 2014, 2, 130–145. [Google Scholar]
- Sivarathnakumar, S.; Baskar, G.; Kumar, R.P.; Bharathiraja, B. Bioethanol production by the utilisation of Moringa oleifera stem with sono-assisted acid/alkali hydrolysis approach. Int. J. Environ. Sustain. Dev. 2016, 15, 392–403. [Google Scholar] [CrossRef]
- Sivasakthivelan, P.; Saranraj, P.; Sivasakthi, S. Production of Ethanol by Zymomonas mobilis and Saccharomyces cerevisiae Using Sunflower Head Wastes—A Comparative Study. Int. J. Microbiol. Res. 2014, 5, 208–216. [Google Scholar]
- Murugan, C.S.; Rajendran, S. Bioethanol Production from Agave Leaves Using Saccharomyces cerevisiae (MTCC 173) and Zymomonas mobilis (MTCC 2427). Int. J. Microbiol. Res. 2013, 4, 23–26. [Google Scholar]
- Srivastava, A.K.; Agrawal, P.; Rahiman, A. Pretreatment and production of bioethanol from different lignocellulosic biomass. Int. J. Adv. Res. 2014, 2, 888–896. [Google Scholar]
- Morales-Martínez, T.K.; Rios-González, L.J.; Aroca-Arcaya, G.; Rodríguez-de la Garza, J.A. Ethanol production by Zymomonas mobilis NRRL B-806 from enzymatic hydrolysates of Eucalyptus globulus. Rev. Mex. Ing. Quim. 2014, 13, 779–785. [Google Scholar]
- Rai, S.K.; Rajput, L.P.S.; Yogendra, S.; Keerti, T. Bioethanol production from waste potatoes using bacterium Zymomonas mobilis MTCC 2427. Appl. Biol. Res. 2013, 15, 154–158. [Google Scholar]
- Das, S.P.; Deka, D.; Ghosh, A.; Das, D.; Jawed, M.; Goyal, A. Scale up and efficient bioethanol production involving recombinant cellulase (Glycoside hydrolase family 5) from Clostridium thermocellum. Sustain. Chem. Process. 2013, 1, 19. [Google Scholar] [CrossRef]
- Narra, M.; James, J.P.; Balasubramanian, V. Simultaneous saccharification and fermentation of delignified lignocellulosic biomass at high solid loadings by a newly isolated thermotolerant Kluyveromyces sp. for ethanol production. Bioresour. Technol. 2015, 179, 331–338. [Google Scholar] [CrossRef]
- Nachaiwieng, W.; Lumyong, S.; Yoshioka, K.; Watanabe, T.; Khanongnuch, C. Bioethanol production from rice husk under elevated temperature simultaneous saccharification and fermentation using Kluyveromyces marxianus CK8. Biocatal. Agric. Biotechnol. 2015, 4, 543–549. [Google Scholar] [CrossRef]
- Turhan, I.; Demirci, A.; Karhan, M. Ethanol production from carob extract by using Saccharomyces cerevisiae. Bioresour. Technol. 2008, 101, 5290–5296. [Google Scholar] [CrossRef]
- Izmirlioglu, G.; Demirci, A. Ethanol production from waste potato mash by using Saccharomyces cerevisiae. Appl. Sci. 2012, 2, 738–753. [Google Scholar] [CrossRef]
- Ferreira, P.G.; da Silveira, F.A.; dos Santos, R.C.V.; Genier, H.L.A.; Diniz, R.H.S.; Ribeiro, J.I.; da Silveira, W.B. Optimizing ethanol production by thermotolerant Kluyveromyces marxianus CCT 7735 in a mixture of sugar-cane bagasse and ricotta whey. Food Sci. Biotechnol. 2015, 24, 1421–1427. [Google Scholar] [CrossRef]
- Wu, W.-H.; Hung, W.-C.; Lo, K.-Y.; Chen, Y.-H.; Wan, H.-P.; Cheng, K.-C. Bioethanol production from taro waste using thermo-tolerant yeast Kluyveromyces marxianus K21. Bioresour. Technol. 2016, 201, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Kim, N.J.; Jiang, M.; Kang, J.W.; Chang, H.N. Simultaneous saccharification and fermentation of lignocellulosic residues pretreated with phosphoric acid–acetone for bioethanol production. Bioresour. Technol. 2009, 100, 3245–3251. [Google Scholar] [CrossRef]
- Yan, J.; Wei, Z.; Wang, Q.; He, M.; Li, S.; Irbis, C. Bioethanol production from sodium hydroxide/hydrogen peroxide-pretreated water hyacinth via simultaneous saccharification and fermentation with a newly isolated thermotolerant Kluyveromyces marxianu strain. Bioresour. Technol. 2015, 193, 103–109. [Google Scholar] [CrossRef]
- Tomás-Pejó, E.; García-Aparicio, M.; Negro, M.J.; Oliva, J.M.; Ballesteros, M. Effect of different cellulase dosages on cell viability and ethanol production by Kluyveromyces marxianus in SSF processes. Bioresour. Technol. 2009, 100, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Sene, L.; Tavares, B.; Felipe, M.d.G.d.A.; dos Santos, J.C.; Pereira, F.M.; Tominc, G.C.; da Cunha, M.A.A. Ethanol production by Kluyveromyces marxianus ATCC 36907: Fermentation features and mathematical modeling. Biocatal. Agric. Biotechnol. 2023, 51, 102789. [Google Scholar] [CrossRef]
- de Barros, E.M.; Carvalho, V.M.; Rodrigues, T.H.S.; Rocha, M.V.P.; Gonçalves, L.R.B. Comparison of stratgies for the simultaneous saccharification and fermentation of cashew apple bagasse using a thermotolerant Kluyveromyces marxianus to enhance cellulosic ethanol production. Chem. Eng. J. 2017, 307, 939–947. [Google Scholar] [CrossRef]
- Ashour, M.; Al-Souti, A.S.; Hassan, S.M.; Ammar, G.A.G.; Goda, A.M.A.-S.; El-Shenody, R.; Abomohra, A.E.-F.; El-Haroun, E.; Elshobary, M.E. Commercial seaweed liquid extract as strawberry biostimulants and bioethanol production. Life 2022, 13, 85. [Google Scholar] [CrossRef]



| Sample | Weight (gm) | Cellulose | Hemicellulose | Lignin | Glucose | Fructose | Xylose | Arabinose | Ash |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 50 | 38 ± 0.1 | 19 ± 0.06 | 25 ± 0.09 | 0.8 ± 0.12 | 2 ± 0.92 | 9 ± 0.26 | 1 ± 0.9 | 0.29 ± 0.08 |
| 2 | 46.5 | 41 ± 0.6 | 23 ± 0.1 | 21 ± 0.18 | 1 ± 0.13 | 2 ± 0.10 | 10 ± 0.26 | 1 ± 0.00 | 0.21 ± 0.83 |
| 3 | 38.5 | 40 ± 0.5 | 22 ± 0.08 | 20 ± 0.75 | 1 ± 0.75 | 2 ± 0.03 | 11 ± 0.33 | 1 ± 0.25 | 0.22 ± 0.69 |
| 4 | 37 | 41 ± 0.9 | 22 ± 0.09 | 19 ± 0.36 | 2 ± 0.28 | 2 ± 0.07 | 12 ± 0.90 | 1 ± 0.11 | 0.22 ± 0.26 |
| 5 | 44 | 41 ± 0.6 | 18 ± 0.07 | 19 ± 0.85 | 2 ± 0.62 | 2 ± 0.18 | 11 ± 0.80 | 1 ± 0.03 | 0.22 ± 0.74 |
| 6 | 43 | 43 ± 0.8 | 21 ± 0.4 | 19 ± 0.50 | 3 ± 0.02 | 2 ± 0.33 | 11 ± 0.41 | 1 ± 0.02 | 0.03 ± 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivarathnakumar, S.; Al-Ghanim, K.A.; Nicoletti, M.; Govindarajan, M.; Gurunathan, B. Comparative Analysis of Cellulosic Ethanol Production from Lignocellulosic Substrate Moringa oleifera Using Kluyveromyces marxianus and Zymomonas mobilis. Fermentation 2023, 9, 840. https://doi.org/10.3390/fermentation9090840
Sivarathnakumar S, Al-Ghanim KA, Nicoletti M, Govindarajan M, Gurunathan B. Comparative Analysis of Cellulosic Ethanol Production from Lignocellulosic Substrate Moringa oleifera Using Kluyveromyces marxianus and Zymomonas mobilis. Fermentation. 2023; 9(9):840. https://doi.org/10.3390/fermentation9090840
Chicago/Turabian StyleSivarathnakumar, Shanmugam, Khalid A. Al-Ghanim, Marcello Nicoletti, Marimuthu Govindarajan, and Baskar Gurunathan. 2023. "Comparative Analysis of Cellulosic Ethanol Production from Lignocellulosic Substrate Moringa oleifera Using Kluyveromyces marxianus and Zymomonas mobilis" Fermentation 9, no. 9: 840. https://doi.org/10.3390/fermentation9090840






