Enhanced DPPH Radical Scavenging Activity and Enriched γ-Aminobutyric Acid in Mulberry Juice Fermented by the Probiotic Lactobacillus brevis S3
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Medium and Solution Configuration
2.3. Preparation of Mulberry Juice
2.4. Screening and Identification of LAB
2.5. Influence of Nutritional Components on Fermentation of L. brevis S3 in Mulberry Juice
2.6. Safety Evaluation of L. brevis S3
2.6.1. Hemolysis
2.6.2. DNase Production
2.6.3. Biogenic Amine Production
2.6.4. Antibiotic Susceptibility
2.6.5. Autoaggregation Ability
2.6.6. Gastric Acid Tolerance Verification
2.6.7. Simulated Gastric Fluid and Intestinal Fluid Tolerance
2.6.8. Cholate, Hypertonic Stress, and Phenol Tolerance
2.7. Analysis of Mulberry Fermentation
2.7.1. Cell Density and Cell Viability
2.7.2. Measurement of pH
2.7.3. Determination of Polysaccharides
2.7.4. Determination of Flavone
2.7.5. Determination of Polyphenol
2.7.6. Determination of GABA
2.8. Detection of Antioxidant Properties
2.8.1. Reducing Power
2.8.2. DPPH Radical Scavenging Ability
2.8.3. Superoxide Anion Radical Scavenging Ability
2.9. Statistical Analysis
3. Results and Discussion
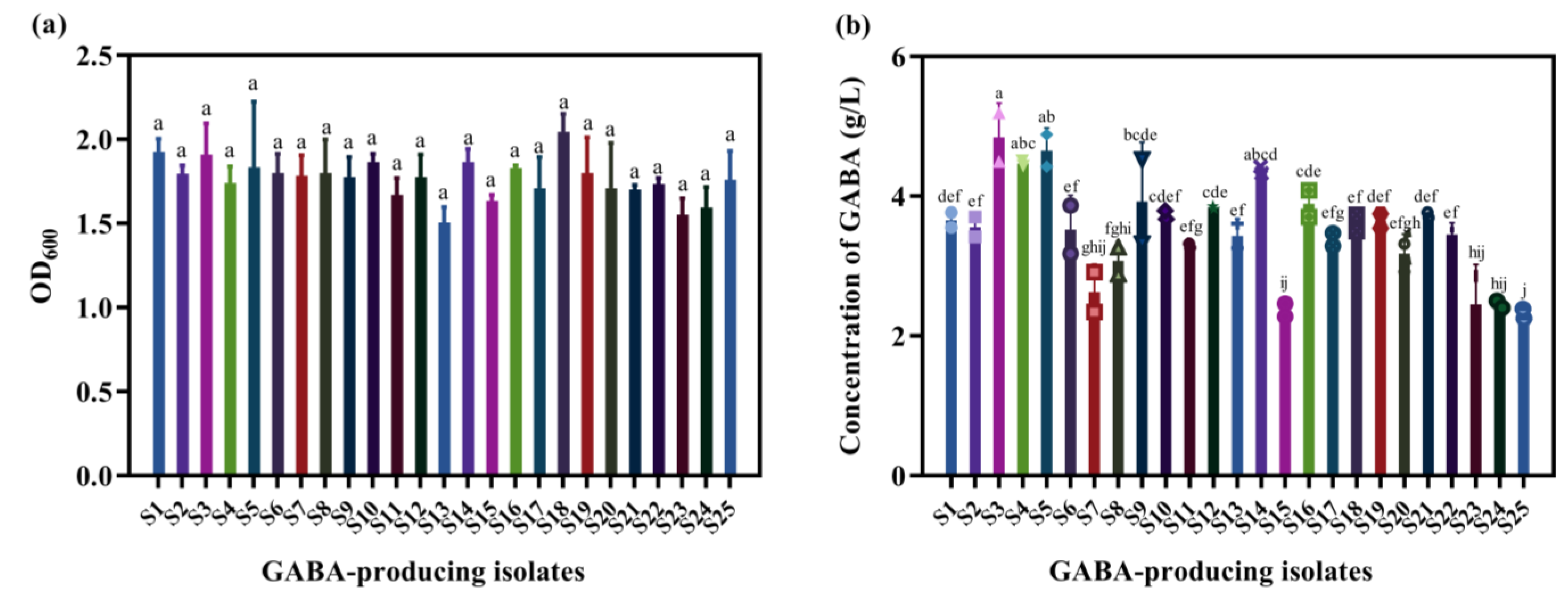
3.1. Screening GABA-Producing LAB Suitable for Mulberry Juice
3.2. Identification and Probiotic Characteristics of GABA-Producing Strain S3
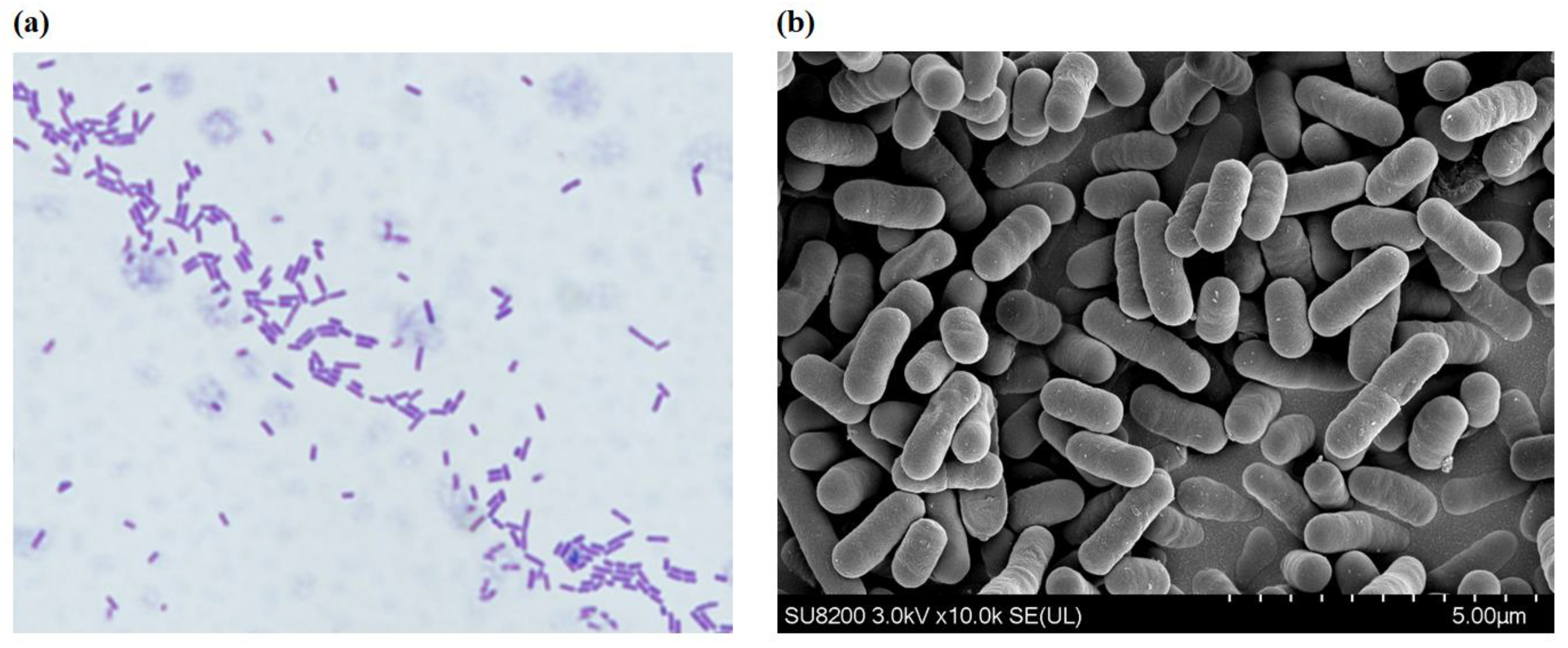
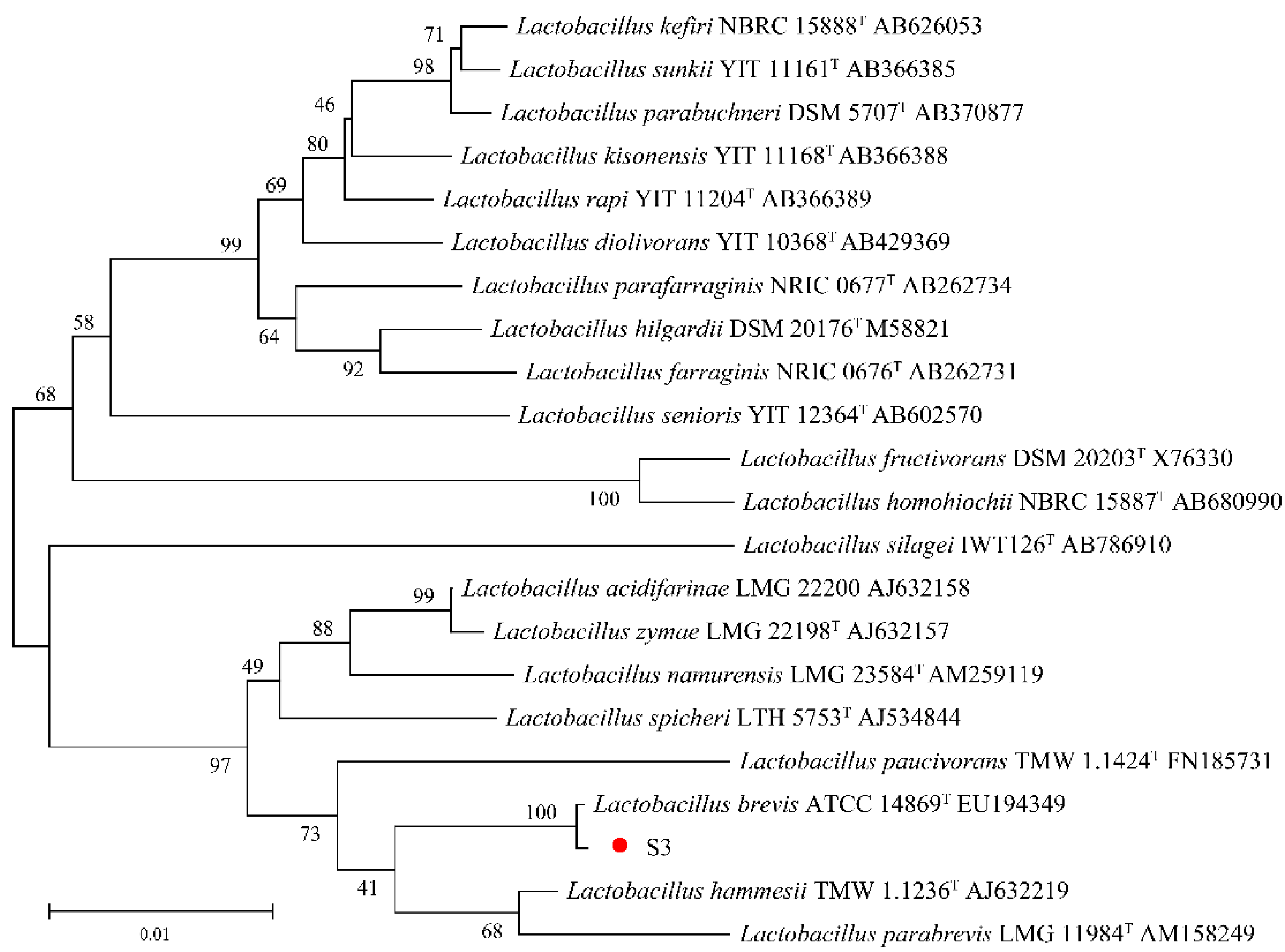
3.2.1. Morphological and Molecular Identification
3.2.2. Safety Evaluation and Potential Probiotic Characteristics of L. brevis S3
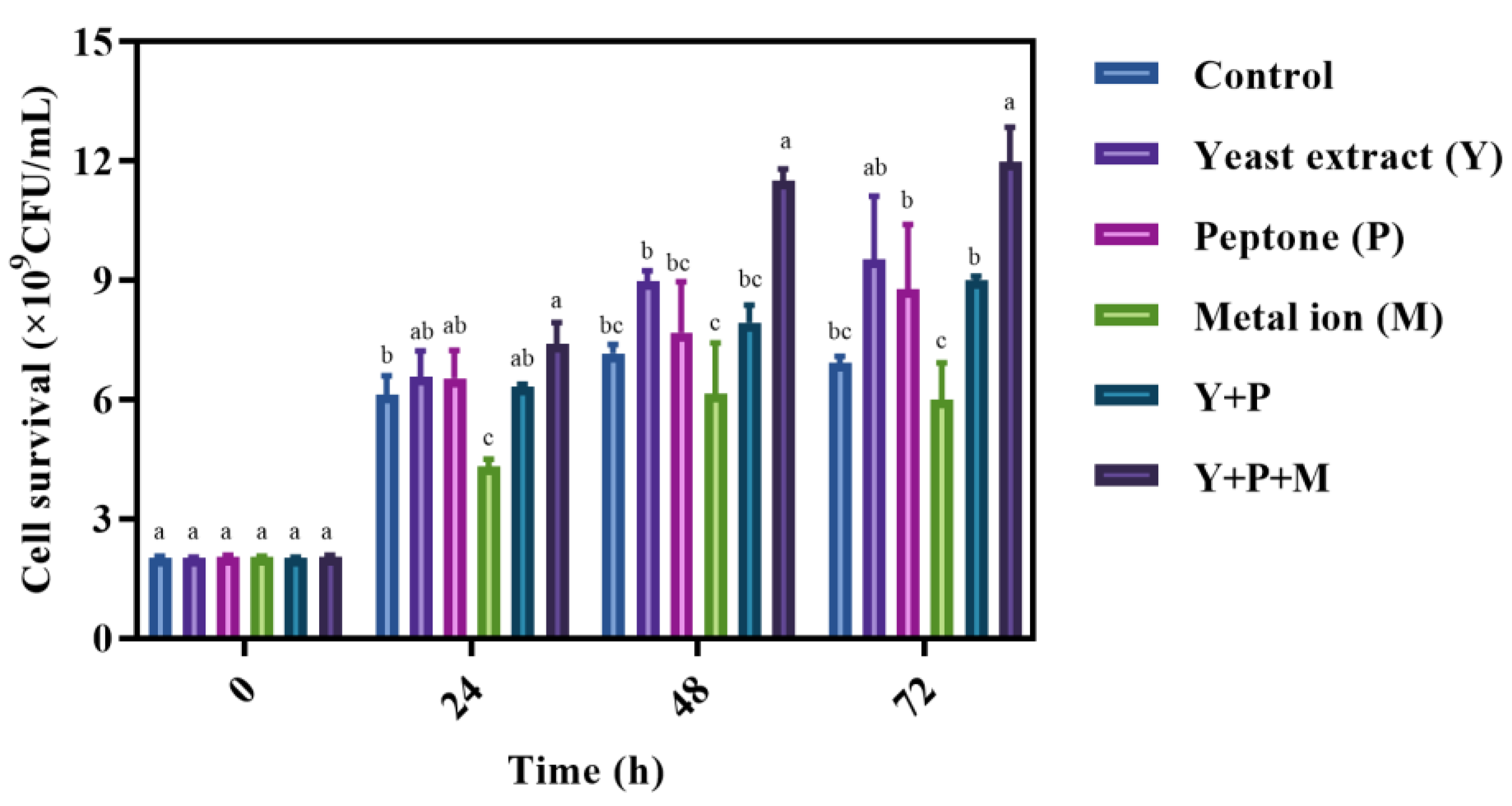
3.3. Influence of Nutritional Components on the Survival of L. brevis S3 in Mulberry Juice
3.4. The Effect of Nutritional Components on the pH of Mulberry Juice Fermented by L. brevis S3
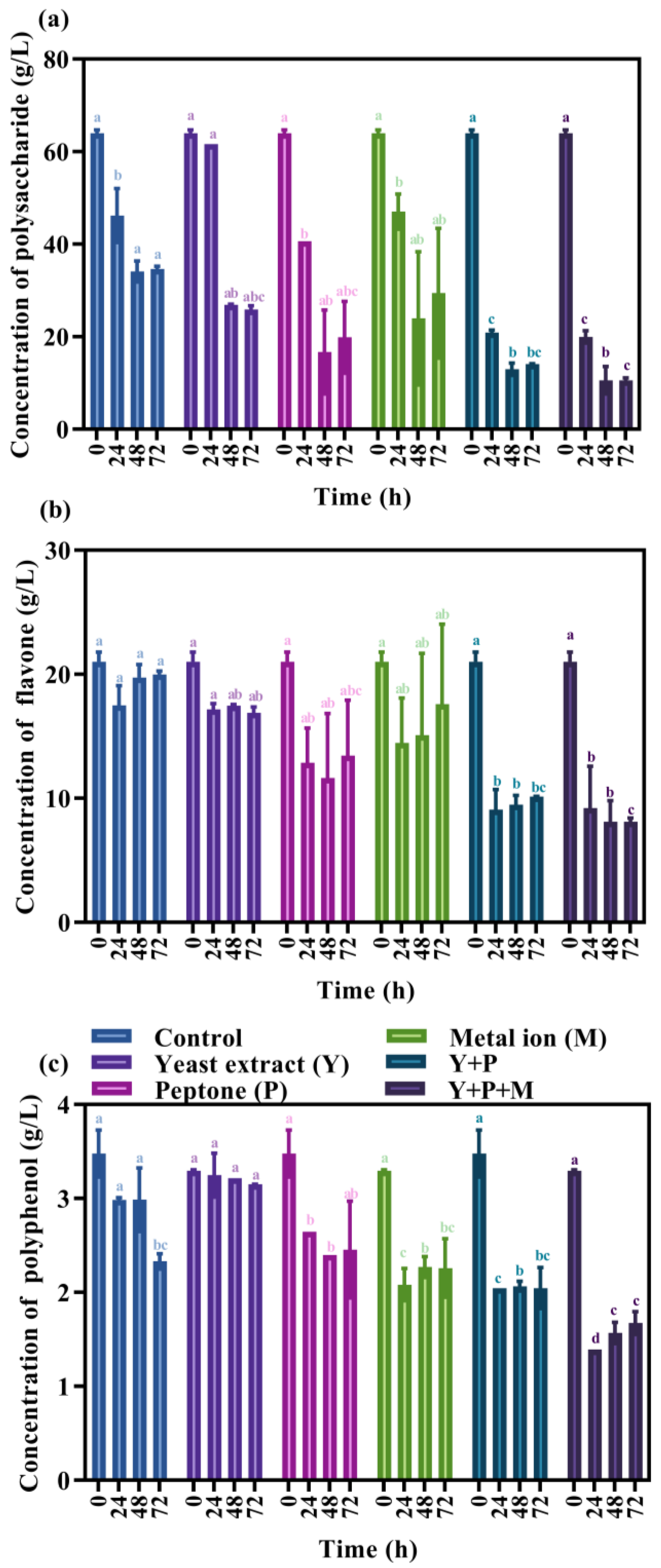
3.5. Effect of Nutritional Components on Bioactive Substances in Fermented Mulberry Juice
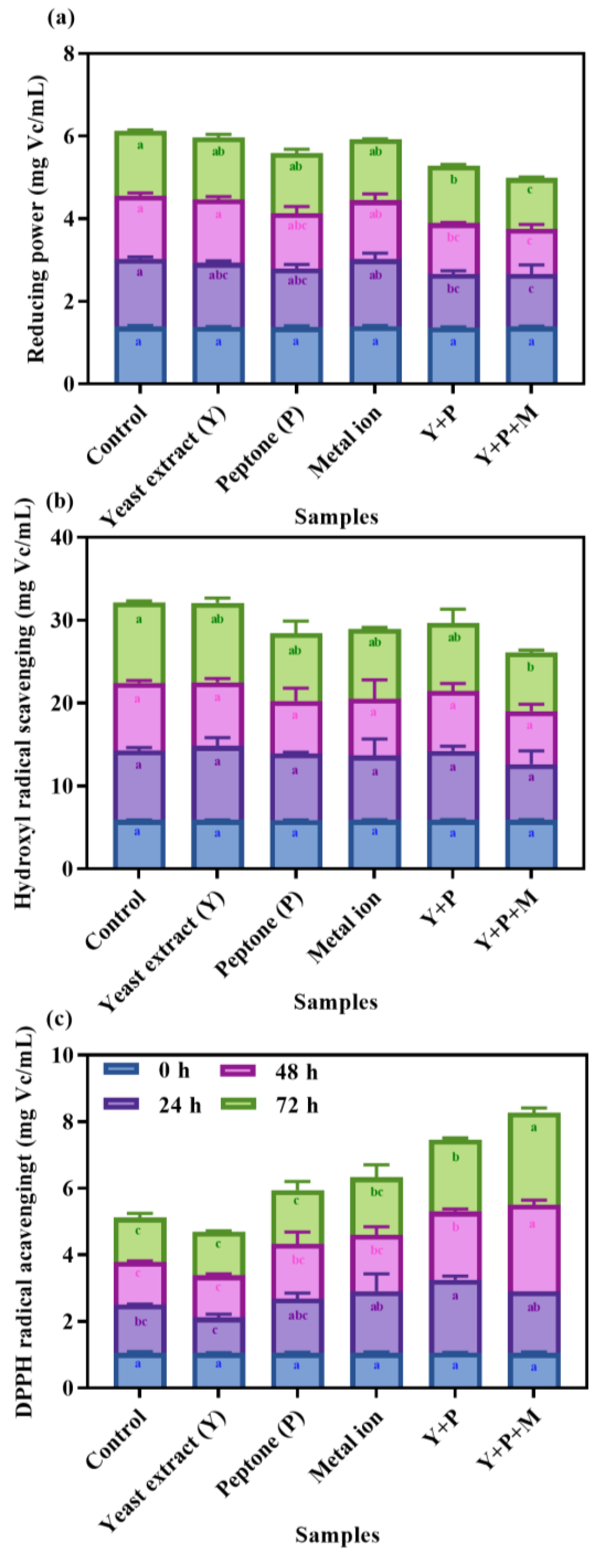
3.6. Influence of Nitrational Components on the Antioxidant Properties of Fermented Mulberry Juice
3.7. Influence of Nutritional Components on GABA Production by L. brevis S3 in Mulberry Juice
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erden, Y. Sour black mulberry (Morus nigra L.) causes cell death by decreasing mutant p53 expression in HT-29 human colon cancer cells. Food Biosci. 2021, 42, 101113. [Google Scholar] [CrossRef]
- Aramwit, P.; Bang, N.; Srichana, T. The properties and stability of anthocyanins in mulberry fruits. Food Res. Int. 2010, 43, 1093–1097. [Google Scholar] [CrossRef]
- Li, X.; Hua, Y.; Yang, C.; Liu, S.; Tan, L.; Guo, J.; Li, Y. Polysaccharides extracted from mulberry fruits (Morus nigra L.): Antioxidant effect of ameliorating H2O2-induced liver injury in HepG2 cells. BMC Complement Med. Ther. 2023, 23, 112. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Huang, Y.; Xu, J.; He, X.; Wang, Y. Anti-neuroinflammatory and antioxidant phenols from mulberry fruit (Morus alba L.). J. Funct. Foods 2020, 68, 103914. [Google Scholar] [CrossRef]
- Mozos, I.; Flangea, C.; Vlad, D.C.; Gug, C.; Mozos, C.; Stoian, D.; Luca, C.T.; Horbańczuk, J.O.; Horbańczuk, O.K.; Atanasov, A.G. Effects of anthocyanins on vascular health. Biomolecules 2021, 11, 811. [Google Scholar] [CrossRef]
- Seo, K.H.; Lee, D.Y.; Jeong, R.H.; Lee, D.S.; Kim, Y.E.; Hong, E.K.; Kim, Y.C.; Baek, N. Neuroprotective effect of prenylated arylbenzofuran and flavonoids from morus alba fruits on glutamate-induced oxidative injury in HT22 hippocampal cells. J. Med. Food 2015, 18, 403–408. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Y.; Qiu, Y.; Song, J. Invesrigation of in vitro and in vivo antioxidant activities of flavonoid extrac from Abelmoschus Manihot (L.) medic flower. Curr. Top. Nutraceut. R. 2017, 15, 179–188. [Google Scholar]
- Teng, H.; Zheng, Y.; Cao, H.; Huang, Q.; Xiao, J.; Chen, L. Enhancement of bioavailability and bioactivity of diet-derived flavonoids by application of nanotechnology: A review. Crit. Rev. Food Sci. Nutr. 2023, 63, 378–393. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhao, L. The mulberry (Morus alba L.) fruit-a review of characteristic components and health benefits. J. Agric. Food Chem. 2017, 65, 10383–10394. [Google Scholar] [CrossRef]
- Wang, R.S.; Dong, P.H.; Shuai, X.X.; Chen, M.S. Evaluation of different black mulberry fruits (Morus nigra L.) based on phenolic compounds and antioxidant activity. Foods 2022, 11, 1252. [Google Scholar] [CrossRef]
- Wang, K.; Qi, J.; Jin, Y.; Li, F.; Wang, J.; Xu, H. Influence of fruit maturity and lactic fermentation on physicochemical properties, phenolics, volatiles, and sensory of mulberry juice. Food Biosci. 2022, 48, 101782. [Google Scholar] [CrossRef]
- Lan, H.; Wang, H.; Chen, C.; Hu, W.; Ai, C.; Chen, L.; Teng, H. Flavonoids and gastrointestinal health: Single molecule for multiple roles. Crit. Rev. Food Sci. Nutr. 2023, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.; Hu, T.G.; Linhardt, R.J.; Liao, S.T.; Wu, H.; Zou, Y.X. Mulberry: A review of bioactive compounds and advanced processing technology. Trends Food Sci. Technol. 2019, 83, 138–158. [Google Scholar] [CrossRef]
- Jeong, H.I.; Jang, S.; Kim, K.H. Morus alba L. for blood sugar management: A systematic review and meta-analysis. Evid. Based Complement Alternat. Med. 2022, 2022, 9282154. [Google Scholar] [CrossRef]
- Lim, H.H.; Lee, S.O.; Kim, S.Y.; Yang, S.J.; Lim, Y. Anti-inflammatory and antiobesity effects of mulberry leaf and fruit extract on high fat diet-induced obesity. Exp. Biol. Med. 2013, 238, 1160–1169. [Google Scholar] [CrossRef]
- Paunović, S.M.; Mašković, P.; Milinković, M. Antioxidant and biological activities of black mulberry (Morus nigra L.) fruit depending on altitude. Erwerbs-Obstbau. 2022, 64, 663–671. [Google Scholar] [CrossRef]
- Sun, Z.H.; Li, L.; Ma, W. Research and prospect of mulberry (Morus alba L.). Agric. Biotech. 2019, 8, 136–139. [Google Scholar] [CrossRef]
- Wang, K.; Kang, S.; Li, F.; Wang, X.; Xiao, Y.; Wang, J.; Xu, H. Relationship between fruit density and physicochemical properties and bioactive composition of mulberry at harvest. J. Food Compost. Anal. 2022, 106, 104322. [Google Scholar] [CrossRef]
- Gao, T.; Chen, J.; Xu, F.; Wang, Y.; Zhao, P.; Ding, Y.; Han, Y.; Yang, J.; Tao, Y. Mixed mulberry fruit and mulberry leaf fermented alcoholic beverages: Assessment of chemical composition, antioxidant capacity in vitro and sensory evaluation. Foods 2022, 11, 3125. [Google Scholar] [CrossRef]
- Wu, C.; Huang, J.; Zhou, R. Genomics of lactic acid bacteria: Current status and potential applications. Crit. Rev. Microbiol. 2017, 43, 393–404. [Google Scholar] [CrossRef]
- Abedin, M.M.; Chourasia, R.; Phukon, L.C.; Sarkar, P.; Ray, R.C.; Singh, S.P.; Rai, A.K. Lactic acid bacteria in the functional food industry: Biotechnological properties and potential applications. Crit. Rev. Food Sci. Nutr. 2023, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, M.; Ghasemi, Y.; Sharifan, A.; Bakhoda, H. Producing and analyzing gamma-aminobutyric acid containing probiotic black grape juice using Lactobacillus plantarum IBRC(10817) and Lactobacillus brevis IBRC(10818). Meas. Food 2022, 8, 100056. [Google Scholar] [CrossRef]
- Cai, L.; Wang, W.; Tong, J.; Fang, L.; He, X.; Xue, Q.; Li, Y. Changes of bioactive substances in lactic acid bacteria and yeasts fermented kiwifruit extract during the fermentation. LWT-Food Sci. Technol. 2022, 164, 113629. [Google Scholar] [CrossRef]
- Hou, D.; Tang, J.; Feng, Q.; Niu, Z.; Shen, Q.; Wang, L.; Zhou, S. Gamma-aminobutyric acid (GABA): A comprehensive review of dietary sources, enrichment technologies, processing effects, health benefits, and its applications. Crit. Rev. Food Sci. Nutr. 2023, 2204373, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Ngo, D.H.; Vo, T.S. An updated review on pharmaceutical properties of gamma-aminobutyric acid. Molecules 2019, 24, 2678. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Wang, J.; Hao, Y.; Zhao, F.; Fu, R.; Yu, Y.; Wang, J.; Niu, R.; Bian, S.; Sun, Z. Exercise ameliorates fluoride-induced anxiety- and depression-like behavior in mice: Role of GABA. Biol. Trace. Elem. Res. 2022, 200, 678–688. [Google Scholar] [CrossRef]
- Chen, H.H.; Cheng, P.W.; Ho, W.Y.; Lu, P.J.; Lai, C.C.; Tseng, Y.M.; Fang, H.C.; Sun, G.C.; Hsiao, M.; Liu, C.P.; et al. Renal denervation improves the baroreflex and GABA system in chronic kidney disease-induced hypertension. Sci. Rep. 2016, 6, 38447. [Google Scholar] [CrossRef]
- Jia, M.; Zhu, Y.; Wang, L.; Sun, T.; Pan, H.; Li, H. pH auto-sustain-based fermentation supports efficient gamma-aminobutyric acid production by Lactobacillus brevis CD0817. Fermentation 2022, 8, 208. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, D.H.; Kang, H.J.; Shin, M.; Yang, S.Y.; Yang, J.; Jung, Y.H. Enhanced production of γ-aminobutyric acid (GABA) using Lactobacillus plantarum EJ2014 with simple medium composition. LWT Food Sci. Technol. 2021, 137, 110443. [Google Scholar] [CrossRef]
- Amatachaya, A.; Siramolpiwat, S.; Kraisorn, M.; Yasiri, A. Gamma-aminobutyric acid (GABA) producing probiotic Lactiplantibacillus pentosus isolated from fermented spider plant (Pak Sian Dong) in Thailand. J. Pure Appl. Microbiol. 2023, 17, 354–361. [Google Scholar] [CrossRef]
- Zhao, A.Q.; Hu, X.Q.; Pan, L.; Wang, X.Y. Isolation and characterization of a gamma-aminobutyric acid producing strain Lactobacillus buchneri WPZ001 that could efficiently utilize xylose and corncob hydrolysate. Appl. Microbiol. Biot. 2015, 99, 3191–3200. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Wu, J.; Hu, D.; Li, J.; Zhu, W.; Yuan, L.; Chen, X.; Yao, J. Gamma-aminobutyric acid-producing Levilactobacillus brevis strains as probiotics in litchi juice fermentation. Foods 2023, 12, 302. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, J.; Li, C.; Zheng, M.; Zhang, Q.; Xiang, W.; Tang, J. The use of gamma-aminobutyric acid-producing Saccharomyces cerevisiae SC125 for functional fermented beverage production from apple juice. Foods 2022, 11, 1202. [Google Scholar] [CrossRef]
- Kanklai, J.; Somwong, T.C.; Rungsirivanich, P.; Thongwai, N. Screening of GABA-producing lactic acid bacteria from Thai fermented foods and probiotic potential of Levilactobacillus brevis F064A for GABA-fermented mulberry juice production. Microorganisms 2020, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Li, H.X.; Cao, Y.S. Lactic acid bacterial cell factories for gamma-aminobutyric acid. Amino Acids 2010, 39, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yue, Y.; Wang, X.; Dai, W.; Piao, C.; Yu, H. Optimization of fermentation for gamma-aminobutyric acid (GABA) production by yeast Kluyveromyces marxianus C21 in okara (soybean residue). Bioprocess Biosyst. Eng. 2022, 45, 1111–1123. [Google Scholar] [CrossRef]
- Kadir, S.A.; Wan-Mohtar, W.A.Q.R.; Mohammad, R.; Lim, S.A.H.; Mohammed, A.S.; Saari, N. Evaluation of commercial soy sauce koji strains of Aspergillus oryzae for gamma-aminobutyric acid (GABA) production. J. Ind. Microbiol. Biotechnol. 2016, 43, 1387–1395. [Google Scholar] [CrossRef]
- Wang, Q.; Xin, Y.; Zhang, F.; Feng, Z.; Fu, J.; Luo, L.; Yin, Z. Enhanced γ-aminobutyric acid-forming activity of recombinant glutamate decarboxylase (gadA) from Escherichia coli. World J. Microbiol. Biotechnol. 2010, 27, 693–700. [Google Scholar] [CrossRef]
- Wu, Q.L.; Shah, N.P. High gamma-aminobutyric acid production from lactic acid bacteria: Emphasis on Lactobacillus brevis as a functional dairy starter. Crit Rev Food Sci. Nutr. 2016, 57, 3661–3672. [Google Scholar] [CrossRef]
- Kim, N.Y.; Kim, S.K.; Ra, C.H. Evaluation of gamma-aminobutyric acid (GABA) production by Lactobacillus plantarum using two-step fermentation. Bioprocess Biosyst. Eng. 2021, 44, 2099–2108. [Google Scholar] [CrossRef]
- Devi, P.B.; Rajapuram, D.R.; Jayamanohar, J.; Verma, M.; Kavitake, D.; Meenachi, A.B.A.; Rani, P.U.; Ravi, R.; Shetty, P.H. Gamma-aminobutyric acid (GABA) production by potential probiotic strains of indigenous fermented foods origin and RSM based production optimization. LWT Food Sci. Technol. 2023, 176, 114511. [Google Scholar] [CrossRef]
- Jones, S.E.; Versalovic, J. Probiotic Lactobacillus reuteri biofilms produce antimicrobial and anti-inflammatory factors. BMC Microbiol. 2009, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Shekh, S.L.; Dave, J.M.; Vyas, B.R.M. Characterization of Lactobacillus plantarum strains for functionality, safety and γ-amino butyric acid production. LWT Food Sci. Technol. 2016, 74, 234–241. [Google Scholar] [CrossRef]
- Pithva, S.; Shekh, S.; Dave, J.; Vyas, B.R. Probiotic attributes of autochthonous Lactobacillus rhamnosus strains of human origin. Appl. Biochem. Biotechnol. 2014, 173, 259–277. [Google Scholar] [CrossRef] [PubMed]
- Svetoslav, D.; Todorov, L.M.T.D. Evaluation of lactic acid bacteria from kefir, molasses and olive brine as possible probiotics based on physiological properties. Ann. Microbiol. 2008, 58, 661–670. [Google Scholar] [CrossRef]
- Krausova, G.; Hyrslova, I.; Hynstova, I. In vitro evaluation of adhesion capacity, hydrophobicity, and auto-aggregation of newly isolated potential probiotic strains. Fermentation 2019, 5, 100. [Google Scholar] [CrossRef]
- Zielinska, D.; Rzepkowska, A.; Radawska, A.; Zielinski, K. In vitro screening of selected probiotic properties of Lactobacillus strains isolated from traditional fermented cabbage and cucumber. Curr. Microbiol. 2015, 70, 183–194. [Google Scholar] [CrossRef]
- Wang, K.; Ren, W.; Jia, X.; Xing, Y.; Wang, Y.; Wang, J.; Xu, H. Physicochemical properties and phytochemical components of white mulberry (Morus alba L.) fruits with different density at harvest. J. Food. Compost. Anal. 2023, 117, 105113. [Google Scholar] [CrossRef]
- Lyu, C.J.; Zhao, W.R.; Peng, C.L.; Hu, S.; Fang, H.; Hua, Y.J.; Yao, S.J.; Huang, J.; Mei, L.H. Exploring the contributions of two glutamate decarboxylase isozymes in Lactobacillus brevis to acid resistance and γ-aminobutyric acid production. Microb. Cell Fact. 2018, 17, 180. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Lan, H.; Wang, K.; Zhao, L.; Hu, Z. Enhanced production of γ-aminobutyric acid in litchi juice fermented by Lactobacillus plantarum HU-C2W. Food Biosci. 2021, 42, 101155. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Q.; Tan, X.; Zhang, S.M.; Zeng, L.; Tang, J.; Xiang, W.L. Characterization of γ-aminobutyric acid (GABA)-producing Saccharomyces cerevisiae and coculture with Lactobacillus plantarum for mulberry beverage brewing. J. Biosci. Bioeng. 2020, 129, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, K.; Alegria, A.; Bron, P.A.; de Angelis, M.; Gobbetti, M.; Kleerebezem, M.; Lemos, J.A.; Linares, D.M.; Ross, P.; Stanton, C.; et al. Stress physiology of lactic acid bacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 837–890. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | Production |
|---|---|
| Hemolysis | - |
| DNase production | - |
| Biogenic amine production | - |
| Antibiotics | Concentration (μg) | Inhibitory Clear Zone (mm) | Sensitive |
|---|---|---|---|
| Erythromycin | 40 | 34 ± 0.8 a | H |
| Chloromycetin | 30 | 0 d | R |
| Cefalexin | 30 | 20 ± 2.1 c | I |
| Vancomycin | 30 | 0 d | R |
| Penicillin | 50 | 24 ± 1.1 b | H |
| Tetracycline | 30 | 20 ± 2.0 c | I |
| Rifampicin | 4 | 26 ± 0.3 b | H |
| Kanamycin | 30 | 0 d | R |
| Time (h) | 2 | 4 | 6 | 8 | 10 | 12 |
|---|---|---|---|---|---|---|
| Autoaggregation (%) | 12.03 ± 0.29 f | 25.92 ± 0.49 e | 37.93 ± 0.15 d | 52.96 ± 0.25 c | 65.75 ± 0.29 b | 75.89 ± 0.48 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, L.; Li, T.; Feng, J.; Yin, J.; Zou, X.; Wang, J.; Wang, B. Enhanced DPPH Radical Scavenging Activity and Enriched γ-Aminobutyric Acid in Mulberry Juice Fermented by the Probiotic Lactobacillus brevis S3. Fermentation 2023, 9, 829. https://doi.org/10.3390/fermentation9090829
Gong L, Li T, Feng J, Yin J, Zou X, Wang J, Wang B. Enhanced DPPH Radical Scavenging Activity and Enriched γ-Aminobutyric Acid in Mulberry Juice Fermented by the Probiotic Lactobacillus brevis S3. Fermentation. 2023; 9(9):829. https://doi.org/10.3390/fermentation9090829
Chicago/Turabian StyleGong, Luchan, Tingting Li, Jian Feng, Jiamin Yin, Xiaozhou Zou, Jun Wang, and Bowen Wang. 2023. "Enhanced DPPH Radical Scavenging Activity and Enriched γ-Aminobutyric Acid in Mulberry Juice Fermented by the Probiotic Lactobacillus brevis S3" Fermentation 9, no. 9: 829. https://doi.org/10.3390/fermentation9090829
APA StyleGong, L., Li, T., Feng, J., Yin, J., Zou, X., Wang, J., & Wang, B. (2023). Enhanced DPPH Radical Scavenging Activity and Enriched γ-Aminobutyric Acid in Mulberry Juice Fermented by the Probiotic Lactobacillus brevis S3. Fermentation, 9(9), 829. https://doi.org/10.3390/fermentation9090829






