Abstract
This study aimed to investigate the beneficial effects of Momordica charantia (MC) extract and MC fermented with Leuconostoc mesenteroides MKSR (FMC) on high-fat and high-cholesterol diet-induced metabolic complications. Male C57BL/6 mice were divided into six groups: normal diet (ND), high-fat and high-cholesterol diet (HFCD), HFCD with 1% MC extract (HFCD + 1M), HFCD with 4% MC extract (HFCD + 4M), HFCD with 1% fermented MC (HFCD + 1F), and HFCD with 4% fermented MC (HFCD + 4F). After 12 weeks of dietary intervention, the consumption of MC fermented with L. mesenteroides MKSR resulted in significant decreases in white adipose tissue weights (epididymal adipose tissue and retroperitoneal adipose tissue), serum alanine aminotransferase activity, and hepatic triglyceride levels. FMC also lowered total hepatic cholesterol content, improved glucose clearance during the oral glucose tolerance and insulin tolerance tests, and increased fecal cholesterol efflux from the enterohepatic circulation. Furthermore, the FMC notably increased hepatic mRNA expressions, which may indicate a compensatory mechanism against induced cholesterol efflux. Moreover, FMC induced both adipogenic (sterol regulatory element-binding protein 1c) and lipolytic (lipoprotein lipase, peroxisome proliferator-activated receptor alpha, and adiponectin) mRNA expressions. These findings suggest that fermentation with the probiotic L. mesenteroides MKSR enhances the beneficial effects of MC, preventing metabolic complications associated with a high-fat diet.
1. Introduction
Metabolic syndrome (MetS) is a combination of multiple risk factors that significantly contribute to the development of metabolic complications, including excessive fat accumulation [1], type 2 diabetes mellitus [2], atherosclerotic cardiovascular disease [3], and hypertension [4,5]. The onset and development of MetS are closely intertwined with various factors, such as dietary choices [6], energy expenditure throughout life events [7], and genetic backgrounds [8]. Individuals living a contemporary lifestyle are increasingly busy and lead sedentary lives, resulting in less time for meal preparation and physical activity [9]. Consequently, such people tend to rely on convenient and energy-dense foods that are quick to consume but often lack nutritional density [10]. Fatty acids play a significant role in increasing the energy density of foods, given their high energy content (9 kcal/g) compared to that of other macronutrients like carbohydrates and proteins, which provide 4 kcal/g [11]. Ideally, transitioning away from a high-fat diet is the recommended approach to prevent metabolic complications associated with high fat intake, which include insulin resistance and excess white adipose tissue (WAT) accumulation [12]. However, if adopting an alternative diet is not feasible or preventive, individuals at risk for MetS may consider incorporating functional nutrients to help modulate energy metabolism [13].
MC, commonly known as bitter melon, is a tropical and subtropical edible plant widely used in traditional medicine to modulate metabolic complications, including diabetes, obesity, and dyslipidemia [14,15,16]. Charantin has been widely accepted as a major functional compound in MC that improves insulin sensitivity by increasing cellular glucose uptake and promoting hepatic and muscular glycogen synthesis [17]. Additionally, charantin exhibits antioxidant [18] and anti-inflammatory properties [19], which may contribute to other potential health benefits in preventing complications associated with metabolic disorders. MetS, including obesity, insulin resistance, and dyslipidemia, is often associated with low-grade chronic inflammation, which can be influenced by several factors and contribute to the development of chronic inflammation and pathological processes [20]. Therefore, charantin supplementation to mitigate MetS-induced adipocyte and systemic inflammation could potentially prevent prolonged inflammation-mediated oxidative and endoplasmic reticulum stress, which can exacerbate the development of MetS-related complications [21].
However, there are disadvantages to isolating charantin from MC. Given the hydrophobic nature of charantin, organic solvents need to be used during the extraction process [22]. Unfortunately, the use of organic solvents to extract compounds from edible plants has negative implications for product development. One crucial issue related to organic solvent use is safety, particularly concerning the presence of residual solvents in the final product [23]. Even after completing the extraction process, traces of organic solvents may remain in the extracted compound, including charantin. These residual solvents are undesirable, especially when the compounds are intended for consumption and their biological functionality is of importance. Therefore, ensuring that all purification steps are thoroughly performed is important to remove any residual solvents from the product. Additionally, the organic solvents typically used for extraction, such as methanol, ethanol, or acetone, can negatively impact the environment [24]. Improper disposal of organic solvents and spills can contribute to environmental contamination, potentially harming ecosystems and human health [25]. As such, implementing proper waste management practices is crucial to minimizing the environmental impact associated with the use of organic solvents during extraction processes.
An alternative and sustainable method using hot water extraction was employed to overcome the safety, technical, and environmental disadvantages of using organic solvents for MC extracts. Therefore, the MC extract used in this study may possess different physicochemical and biological functional properties compared to the conventional charantin-containing MC extract obtained using organic solvents. Additionally, to enhance the biological functionalities of the hot water-extracted MC, the extract was fermented with probiotics, specifically Leuconostoc mesenteroides MKSR. Recent research has shown that L. mesenteroides MKSR could potentially modulate glucose metabolism by inhibiting α-glucosidase activity and cholesterol metabolism through bile salt hydrolase activity in test tubes [26]. Furthermore, hot water MC extracts fermented with L. mesenteroides MKSR have remarkably increased antioxidative activity, as evidenced by their increased ferric-reducing antioxidant power, DPPH radical scavenging, and superoxide dismutase-like activity. Hot water MC extracts fermented with L. mesenteroides MKSR may generate functional metabolites from either MC or L. mesenteroides MKSR [27].
We had previously reported on the potential beneficial functionalities of hot water MC extracts fermented with L. mesenteroides MKSR in test tubes [27]. In this study, we aimed to investigate whether dietary intervention with either hot water MC extract or hot water MC extract fermented with L. mesenteroides MKSR could prevent high-fat, high- cholesterol-induced MetS-related disorders, such as weight gain, insulin resistance, and dyslipidemia, in experimental mice.
2. Materials and Methods
2.1. Mice Experiments and Diets
A total of 60 male C57BL/6 mice aged 4 weeks were purchased from DooYeol Biotech (Seoul, Republic of Korea) and housed in shoebox cages under controlled husbandry conditions (temperature 22–24 °C, relative humidity 55–65%, 12 h light/dark cycle). Experimental mice were acclimatized for a week and then randomly assigned into 6 different dietary intervention groups (n = 10 per group): normal diet (ND), high-fat and high-cholesterol diet (HFCD), HFCD mixed with 1% MC (HFCD + 1M), HFCD mixed with 4% MC (HFCD + 4M), HFCD mixed with 1% fermented MC (HFCD + 1F), and HFCD mixed with 4% fermented MC (HFCD + 4F). Given that both MC and FMC are rich dietary sources of fiber, we replaced the fiber portion of either the ND or HFCD with MC and FMC (Table 1). The fermented MC was prepared according to the method reported by Yoon and Kim [28]. Briefly, a probiotic strain, Leuconostoc mesenteroides MKSR KCTC 15665P (6.7 log CFU·mL−1), was inoculated into the sterilized MC extract in the fermentation medium containing 394 mmol of sucrose, 143 mmol of maltose, 5 g·L−1 of yeast extract, 5 g·L−1 of peptone, 20 g·L−1 of K2HPO4, 0.2 g·L−1 of MgSO4, 0.13 g·L−1 of CaCl2·2H2O, 0.01 g·L−1 of FeH14O11S, 0.01 g·L−1 of MnSO4·H2O, and 0.1 g·L−1 of NaCl. The mixture was left to ferment at 30 °C for 24 h. Experimental mice had access to the designated diet and water ad libitum for 12 weeks, and their body weight was measured every week. Experimental mice fasted for 12 h before sacrifice via thoracotomy after CO2 narcosis. Whole blood was collected via cardiac puncture, allowed to clot at room temperature for 30 min, and then centrifuged (LaboGene, Lynge, Denmark) at 1000× g at 4 °C for 15 min to separate the serum [29]. Liver and WATs, including epididymal adipose tissue (EAT), retroperitoneal adipose tissue (RAT), mesenteric adipose tissue (MAT), and perirenal adipose tissue (PAT), were collected and weighed [30]. Feces were collected from the cecum and transferred to 1.5-mL centrifuge tubes (SPL Life Sciences, Pocheon, Republic of Korea). These tissue, serum, and feces samples were stored at −80 °C until further analysis [31].

Table 1.
Composition of experimental diet.
2.2. Oral Glucose Tolerance Test (OGTT) and Insulin Tolerance Test (ITT)
After 9 weeks of dietary intervention, an OGTT was performed on the experimental mice following a 12 h fast. Mice were orally administered a glucose solution (Sigma, St. Louis, MO, USA) at a dose of 2 g/kg body weight (1 g/kg body weight as previously described) [32]. Blood glucose levels were then measured from the tail vein 0, 15, 30, 60, and 120 min after the oral glucose challenge using a blood glucose meter (Accu-Chek, Seoul, Republic of Korea). After 12 weeks of dietary intervention, an ITT was performed using the prescribed method [33] on the experimental animals following a 12 h fast. Mice were intraperitoneally injected with insulin (2 U/kg body weight; Thermo Fisher Scientific, Waltham, MA, USA). Subsequently, blood glucose levels were measured from the tail vein 0, 30, 60, and 90 min after the insulin injection using a blood glucose meter.
2.3. Biochemical Analysis in Serum, Liver and Feces
Lipid levels such as triglyceride (TG), total cholesterol (TC), and high-density lipoprotein cholesterol (HDL-C) in serum, liver, and feces were measured using commercial kits purchased from (Asanphram Co., Seoul, Republic of Korea) according to the manufacturer’s instructions. Briefly, the sample was mixed with enzyme kits and incubated for 5–10 min at 37 °C water bath. Then, absorbance of the reaction products was measured at 500 nm for TC and HDL-C and 550 nm for TG using a spectrophotometer. Lipid components of the liver and feces were analyzed after extraction using a chloroform/methanol solution (1:2, v/v) and centrifugation at 1800× g for 5 min [34].
Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities in serum were determined according to the manufacturer’s instructions using commercial kits purchased from (Asanphram Co., Seoul, Republic of Korea). Briefly, substrate solution was mixed with a serum sample, incubated at 37 °C for 30 min for the ALT reaction or 60 min for AST, and mixed with color reagent. The reaction was terminated by adding 0.4N NaOH, and absorbance of the reaction product was measured at 505 nm using a spectrophotometer.
2.4. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR) Analysis
Total RNA was extracted using the NucleoZOL reagent (Macherey-Nagel, GmbH & Co. KG, Düren, Germany). Total RNA (1 μg) was reverse transcribed to cDNA using a reverse transcription kit (Toyobo: FSQ-201, Osaka, Japan) following manufacturer’s instructions. The resulting cDNA was amplified with iQ™ SYBR® Green Supermix (Bio-Rad Laboratories, Hercules, CA, USA) using the CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA) [35]. The relative gene expression was normalized to that of Gapdh, which did not significantly vary with dietary intervention. The average fold changes in mRNA expression were calculated using the 2−∆∆Ct analytical method [36]. The forward and reverse PCR primer sequences used in this study are available in Table 2 [37].

Table 2.
qRT-PCR primer sequences (5′ to 3′).
2.5. Statistical Analysis
All experimental data are expressed as means ± standard deviations. Statistical significance was analyzed using one-way analysis of variance, followed by Duncan’s post-hoc test. Statistical significance was set at p < 0.05, and means without a common letter were considered significantly different. All statistical analyses were performed using XLSTAT 2012 (Addinsoft Inc., Paris, France), and figures were created using GraphPad Prism 5 software (GraphPad Software, San Diego, CA, USA).
3. Results
3.1. Effect of the Dietary Momordica charantia on Body and Organ Weight
Dietary intervention with MC prevented high fat-induced body weight and WAT gain. Consumption of a high-fat diet significantly increased the final body weight by 31.9% after a 12-week intervention compared to the consumption of an ND (Figure 1a). However, MC decreased the final body weight by 7.12%, 8.81%, 1.17%, and 6.73% in the HFCD + 1M, HFCD + 4M, HFCD + 1F, and HFCD + 4F groups, respectively, compared to the HFCD group (Figure 1b). Among the dietary interventions with MC, only HFCD + 4M promoted a significant decrease in final body weight, whereas other interventions did not (Figure 1c,d). Regardless of dietary intervention with MC, a high-fat diet triggered hepatomegaly, unlike the HFCD. However, MC treatment did not prevent ectopic fat accumulation in the liver induced by the consumption of a high-fat diet (Figure 2a). Intriguingly, dietary consumption of 4% MC significantly prevented high fat-induced WAT expansion in the experimental mice (Figure 2b). A 12-week dietary intervention with a high-fat diet increased WAT by 46.2% compared to that with an ND. Neither 1% MC nor FMC prevented high fat-induced WAT expansion. Interestingly, however, 4% FMC significantly prevented high fat-induced WAT mass gain by 25.7%. Regardless of the anatomical location of the WAT, consumption of a high-fat diet significantly increased WAT mass compared to that of an ND. High-fat diet consumption elevated EAT, RAT, MAT, and PAT 3.8, 3.5, 2.3, and 2.6-fold, respectively, compared to ND consumption (Figure 2c–f). Interestingly, the HFCD + 4F group had a 29.3% and 28.6% lower EAT and RAT mass than did the ND group, respectively. In the case of MAT, the HFCD + 4M significantly attenuated WAT mass by 33.3% compared to the HFCD.
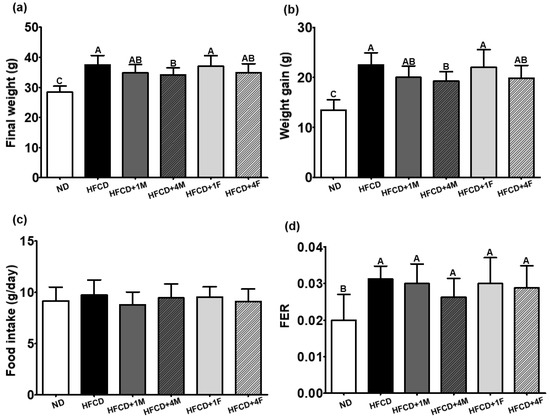
Figure 1.
Effect of the dietary Momordica charantia on body weight, food intake, and FER. Mice were divided into six groups (n = 10): normal diet (ND), high-fat and high-cholesterol diet (HFCD), HFCD mixed with 1% MC (HFCD + 1M), HFCD mixed with 4% MC (HFCD + 4M), HFCD mixed with 1% fermented MC (HFCD + 1F), and HFCD mixed with 4% fermented MC (HFCD + 4F). (a) Final body weight; (b) weight gain (the final body weight—the initial body weight); (c) food intake during experimental period; (d) food efficiency ratio (FER = [weight gain (g/day)]/[food intake (g/day)]. Different capital letters of the labels on the bar are significantly different among the groups (p < 0.05).
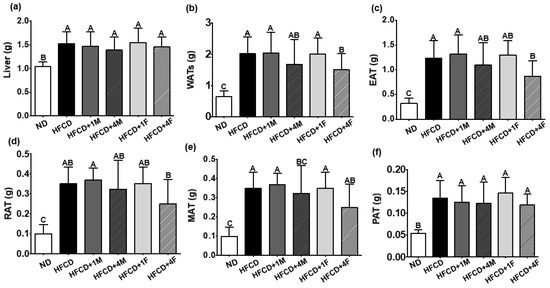
Figure 2.
Effect of the dietary MC on organ weight. Mice were divided into six groups: normal diet (ND), high-fat and high-cholesterol diet (HFCD), HFCD mixed with 1% MC (HFCD + 1M), HFCD mixed with 4% MC (HFCD + 4M), HFCD mixed with 1% fermented MC (HFCD + 1F), and HFCD mixed with 4% fermented MC (HFCD + 4F). (a) Liver weight; (b) white adipose tissues (WATs) weight; (c) epididymal adipose tissue (EAT); (d) retroperitoneal adipose tissue (RAT); (e) mesenteric adipose tissue (MAT); (f) perirenal adipose tissue (PAT). Values are means ± standard deviation, (n = 10). Different capital letters of the labels on the bar are significantly different among the groups (p < 0.05).
3.2. Effect of Dietary MC on Oral Glucose Tolerance and ITT Results
During the 9th and 11th weeks of the dietary intervention with MC, OGTT and ITT were performed to assess whether experimental mice consuming MC or FMC showed improved cellular glucose utilization, resulting in decreased body and WAT weight (Figure 1a and Figure 2b). In the ND and HFCD groups, blood glucose levels peaked 15 min after the glucose challenge. However, the groups receiving MC intervention showed a delay in reaching peak blood glucose levels (i.e., peak levels were reached 30 min after OGTT) (Figure 3a). Interestingly, the HFCD + 1M, HFCD + 1F, and HFCD + 4F groups exhibited significantly lower blood glucose levels after the OGTT than did the HFCD group. The calculated area under the curve (AUC) values after the OGTT were overall higher in the HFCD groups than in the ND group; however, the AUC value in the HFCD + 4F group was lower, similar to that in the ND group (Figure 3b).
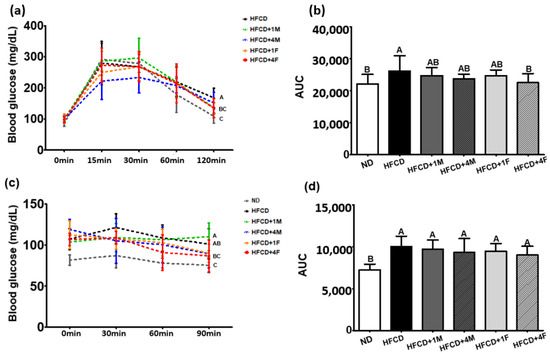
Figure 3.
Effect of dietary MC on the oral glucose tolerance test (OGTT) and insulin tolerance test (ITT). Mice were divided into six groups: normal diet (ND), high-fat and high-cholesterol diet (HFCD), HFCD mixed with 1% MC (HFCD + 1M), HFCD mixed with 4% MC (HFCD + 4M), HFCD mixed with 1% fermented MC (HFCD + 1F), and HFCD mixed with 4% fermented MC (HFCD + 4F). (a) Glucose curve after OGTT; (b) the area under the curve (AUC) after OGTT; (c) blood glucose curve after ITT; (d) the area under the curve (AUC) after ITT. Values are means ± standard deviation, (n = 10). Different capital letters of the labels next to the dot at 120 min or 90 min and on the bar are significantly different among the groups (p < 0.05).
Given that the dietary intervention with MC remarkably attenuated body weight gain and improved insulin sensitivity as seen in the OGTT, we also conducted an ITT after the 11th week of dietary intervention (Figure 3c). Regardless of the dietary interventions, blood glucose levels peaked 30 min after the insulin treatment. Notably, the HFCD + 1M, HFCD + 1F, and HFCD + 4F groups showed a significant decrease in blood glucose levels after the ITT compared to the HFCD group, similar to the findings from the OGTT. However, no significant differences in the calculated AUC values were observed after the ITT (Figure 3d).
3.3. Effect of Dietary MC on Hepatic Functional Indices
Enzymatic activities of ALT and AST in the serum are commonly used indicators to assess normal liver function [32]. Compared to the ND group, the HFCD group exhibited a significant increase in serum ALT and AST activities by approximately 2.33- and 1.25-fold, respectively (Figure 4). Treatment with all doses of MC (HFCD + 1M, HFCD + 4M, HFCD + 1F, and HFCD + 4F) promoted a decrease in serum ALT activities by 11.6%, 26.6%, 31.0%, and 44.2%, respectively. Notably, fermented MC resulted in lower serum ALT activities than did non-fermented MC, and significant reductions were observed only in the HFCD + 4F group. Similarly, all MC-treated groups tended to have reduced serum AST activities, ranging from 3.8% to 12.5%. However, no significant differences were observed.
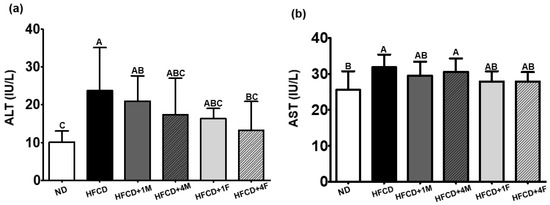
Figure 4.
Effect of dietary MC on hepatic functional indices. Mice were divided into six groups: normal diet (ND), high-fat and high-cholesterol diet (HFCD), HFCD mixed with 1% MC (HFCD + 1M), HFCD mixed with 4% MC (HFCD + 4M), HFCD mixed with 1% fermented MC (HFCD + 1F), and HFCD mixed with 4% fermented MC (HFCD + 4F). (a) Alanine aminotransferase (ALT); (b) aspartate aminotransferase (AST). Values are means ± standard deviation (n = 10). Different capital letters of the labels on the bar are significantly different among the groups (p < 0.05).
3.4. Effect of the Dietary MC on Serum Lipid Profiles and Cardiovascular Parameters
To investigate the lipid-lowering effects of MC consumption in mice fed a high-fat diet, we assessed the serum lipid profiles associated with the risk of cardiovascular disease. As hypothesized, the overall HFCD + 1M, HFCD + 4M, HFCD + 1F, and HFCD + 4F groups showed a 24.1%, 15.5%, 12.3%, and 23.2% lower serum TG level than did the HFCD group, respectively (Figure 5a), showing a similar pattern to that for the reduction in WAT (Figure 2b). Among the four MC-treated groups, only the HFCD + 1M and HFCD + 4F groups showed a significant decrease in serum TG levels. However, regardless of type, such as HDL-C, and LDL-C, the MC-treated groups showed no difference in blood cholesterol levels when compared to the HFCD group (Figure 5c,d).
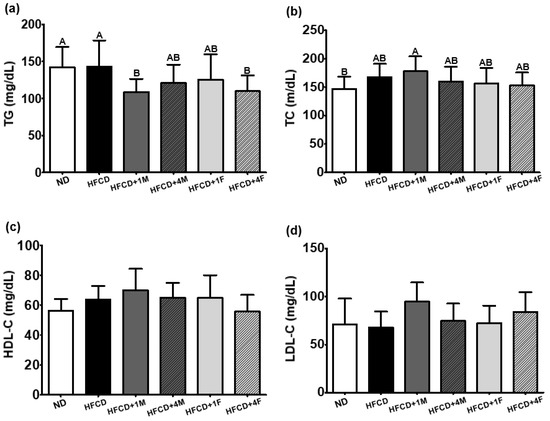
Figure 5.
Effect of dietary MC on serum lipid profiles. Mice were divided into six groups: normal diet (ND), high-fat and high-cholesterol diet (HFCD), HFCD mixed with 1% MC (HFCD + 1M), HFCD mixed with 4% MC (HFCD + 4M), HFCD mixed with 1% fermented MC (HFCD + 1F), and HFCD mixed with 4% fermented MC (HFCD + 4F). (a) Triglyceride (TG); (b) total cholesterol (TC); (c) high-density lipoprotein cholesterol (HDL-C); (d) low-density lipoprotein cholesterol (LDL-C = [total cholesterol-HDL-C-[triglyceride/5]). Values are means ± standard deviation (n = 10). Different capital letters of the labels on the bar are significantly different among the groups (p < 0.05).
3.5. Effect of the Dietary MC on Hepatic and Fecal Lipid Profiles
The enterohepatic circulation of serum TG and cholesterol is a complex process in which cholesterol is absorbed in the small intestine, transported to the liver, and subsequently reabsorbed back into the intestine, influencing blood lipid levels. As shown in Figure 5, MC significantly prevented the increase in serum TG levels but did not significantly affect serum cholesterol levels. This prompted us to question whether MC influences the enterohepatic circulation of lipids. To address this added research question, we measured hepatic and fecal lipid levels. Accordingly, we found that all groups consuming a high-fat diet, regardless of MC consumption, showed elevated hepatic TG accumulation (Figure 6a). Interestingly, however, the HFCD + 4F group showed significant alterations in both hepatic and fecal TC levels (Figure 6b). Specifically, compared to the HFCD group, the HFCD + 4M and HFCD + 4F groups exhibited a 26.4% and 21.5% decrease in hepatic TC accumulation, respectively. Additionally, fecal cholesterol efflux significantly increased, reaching levels approximately 2.7-fold higher than those in the ND group. Notably, all groups receiving fermented MC (HFCD + 1F and HFCD + 4F) demonstrated a 1.54- and 2.51-fold increase in fecal cholesterol efflux, respectively, compared to the HFCD group (Figure 6c). Furthermore, the HFCD + 4F group exhibited a greater increase in fecal cholesterol efflux than did the other groups, indicating the potential role of MC consumption in preventing enterohepatic cholesterol circulation.
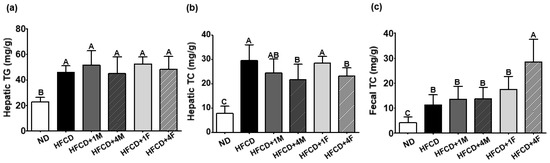
Figure 6.
Effect of the dietary MC on hepatic and fecal lipid profiles. Mice were divided into six groups: normal diet (ND), high-fat and high-cholesterol diet (HFCD), HFCD mixed with 1% MC (HFCD + 1M), HFCD mixed with 4% MC (HFCD + 4M), HFCD mixed with 1% fermented MC (HFCD + 1F), and HFCD mixed with 4% fermented MC (HFCD + 4F). (a) Hepatic triglyceride (TG); (b) hepatic total cholesterol (TC); (c) fecal total cholesterol (TC). Values are means ± standard deviation (n = 10). Different capital letters of the labels on the bar are significantly different among the groups (p < 0.05).
3.6. Effect of the Dietary MC on Metabolic mRNA Expressions in the Liver
To explain the possible working molecular mechanism by which MC ameliorates blood TG and hepatic TC, we measured the cholesterol metabolism and anabolic, catabolic, and inflammatory mRNA expression levels in the liver. The 3-hydrozy-3methylglutaryl-coenzyme A reductase (Hmgcr) is a rate-limiting enzyme involved in the mevalonate pathway by converting 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) to mevalonic acid, which is responsible for cholesterol synthesis [38,39]. Therefore, Hmgcr inhibition is the specific target of statins, which are widely prescribed to lower cholesterol levels in patients with hypercholesterolemia [40,41]. In our experimental setting, HFCD inherently attenuated hepatic Hmgcr mRNA expression due to excess dietary fat consumption; however, HFCD + 4F elevated hepatic Hmgcr mRNA expression by approximately 3.90-fold compared to the HFCD (Figure 7a). A significant elevation in hepatic Hmgcr mRNA expression may reflect high fecal cholesterol efflux (Figure 6c). Another important molecular regulator of hepatic cholesterol metabolism is the low-density lipoprotein receptor (Ldlr). The main function of the hepatic Ldlr is binding and internalizing LDL particles from the bloodstream to the liver [42]. Hepatic Ldlr mRNA expression had a tendency to increase in the overall HFCD-fed groups (Figure 7b). Interestingly, groups treated with 4% MC (HFCD + 4M and HFCD + 4F) exhibited a 2.30-fold greater elevation in hepatic Ldlr mRNA expression than did the HFCD group; however, no significant differences were observed.
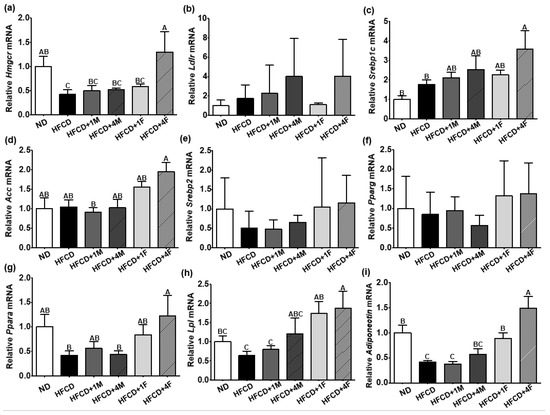
Figure 7.
Effect of dietary MC on metabolic mRNA expressions in the liver. Mice were divided into six groups: normal diet (ND), high-fat and high-cholesterol diet (HFCD), HFCD mixed with 1% MC (HFCD + 1M), HFCD mixed with 4% MC (HFCD + 4M), HFCD mixed with 1% fermented MC (HFCD + 1F), and HFCD mixed with 4% fermented MC (HFCD + 4F). (a) Relative 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase (Hmgcr) mRNA expression; (b) relative low-density lipoprotein receptor (Ldlr) mRNA expression; (c) relative sterol regulatory element-binding protein (Srebp1c) mRNA expression; (d) relative acetyl-CoA carboxylase (Acc) mRNA expression; (e) relative Srebp2 mRNA expression; (f) relative peroxisome proliferator-activated receptor gamma (Pparg) mRNA expression; (g) relative Ppara mRNA expression; (h) relative lipoprotein lipase (Lpl) mRNA expression; (i) relative adiponectin mRNA expression. Values are means ± standard deviation (n = 10). Different capital letters of the labels on the bar are significantly different among the groups (p < 0.05).
Our experimental protocol observed increased cholesterol efflux and decreased hepatic TC content in the HFCD + 4F group (Figure 6). To investigate the underlying molecular mechanisms responsible for these changes, we examined the expression of hepatic mRNA involved in anabolic and catabolic processes. Despite the significant reduction in hepatic TC content in the HFCD + 4F group, we found an increase in adipogenic mRNA expression in the liver. Specifically, the expression of sterol regulatory element-binding protein (Srebp1c), a key factor in hepatic lipogenesis [43], and acetyl-CoA carboxylase (Acc), an enzyme involved in the production of malonyl-CoA from acetyl-CoA [44], were remarkably increased in the HFCD + 4F group compared to the control group, with fold increases of approximately 2.00- and 1.87-fold, respectively (Figure 7c,d). Additionally, other adipogenic transcription factors in the liver, namely Srebp2 and peroxisome proliferator-activated receptor gamma (Pparg), were increased by approximately 2.32- and 1.61-fold in the HFCD + 4F group, respectively, although this increase was not statistically significant (Figure 7e,f).
Despite the reduced hepatic TG accumulation, the HFCD + 4F group showed increased expression of de novo adipogenic transcription factors compared to the control group. Therefore, we logically hypothesized that HFCD + 4F may induce beta-oxidation in the liver. Ppara, a transcription factor that stimulates hepatic beta-oxidation and promotes the uptake, utilization, and catabolism of free fatty acids [45,46], was 58% lower in the control group than in the normal diet group (Figure 7g). However, the HFCD + 4F group exhibited a significant 2.90-fold increase in hepatic Ppara mRNA expression compared to the control group. Alongside the induction of Ppara, Lpl, an enzyme involved in TG hydrolysis and receptor-mediated lipoprotein uptake [47,48], was also 2.94-fold higher in the HFCD + 4F group than in the control group (Figure 7g,h).
Adiponectin is an adipokine that regulates glucose metabolism and β-oxidation in adipocytes. Lower levels of adiponectin have been strongly associated with obesity [49] and diabetes [50] given its regulatory role in metabolism. However, higher levels of adiponectin may attenuate low-grade chronic inflammatory responses induced by MetS [51]. As mentioned earlier, the HFCD + 4F significantly altered the expression of various metabolic mRNAs in the liver. Therefore, we logically hypothesized that HFCD + 4F would also affect hepatic adiponectin mRNA expression. As expected, HFCD significantly reduced hepatic adiponectin mRNA expression by 59%, and MC did not prevent this reduction (Figure 7i). However, FMC remarkably prevented the high-fat diet-induced reduction in hepatic adiponectin mRNA expression. Consequently, the HFCD + 1F and HFCD + 4F promoted significantly higher hepatic adiponectin mRNA expression, with 2.2- and 3.6-fold increases, respectively, than did the HFCD. The increase in the expression of hepatic adiponectin has been negatively associated with pro-inflammatory responses [52], given that MetS is considered a manifestation of low-grade inflammation [51]. Therefore, we further analyzed the expressions of hepatic inflammatory mRNA, such as Tnfa and Il-6; however, we did not observe any significant differences among the dietary interventions.
4. Discussion
High dietary fat intake promotes excessive calorie consumption [53], causing WAT or ectopic fat accumulation in the liver and muscles [54,55], ultimately increasing the risk of obesity and metabolic complications [56]. Obesity has been strongly associated with various metabolic complications, including diabetes [57], heart disease [58], hypertension [59], and dyslipidemia [60]. Reducing dietary fat is an important dietary intervention considering that total dietary fat intake has been considered a significant predictor of obesity due to its high energy content [61,62]. Current research focuses on not only cutting back on high-calorie fat consumption, but also consuming functional nutraceutical components to prevent metabolic complications induced by high-fat diets [63,64]. The current study investigated the potential of MC as a nutraceutical candidate to modulate high-fat diet-induced metabolic complications in experimental animals [16,65]. MC was extracted using hot water to avoid the use of organic solvents, after which the extracted MC was treated with probiotics, specifically L. mesenteroides MKSR. This probiotic strain has been reported for its various biological functionalities, including α-glucosidase inhibitory, bile salt hydrolase, and radical scavenging activities [26].
In the present study, the experimental animals were assigned into six different dietary intervention groups: ND, HFCD, HFCD + 1M, HFCD + 4M, HFCD + 1F, and HFCD + 4F. Except for the ND group, all groups were fed a high-fat diet in which a certain percentage of the energy was derived from fat. To examine the specific biological effects of MC and FMC, we incorporated MC and FMC into the diet by substituting them for the fiber portion as cellulose rather than providing them as separate supplements, as commonly practiced in animal experiments [66,67,68]. After 12 weeks of dietary intervention, the HFCD + 4M group showed a decrease in final body weight, delta body weight (excluding WATs), and hepatic TC levels compared to the HFCD group. Interestingly, consumption of MC fermented with L. mesenteroides MKSR significantly reduced WAT weights (EAT and RAT), serum ALT activity, and TG levels. It also lowered hepatic total cholesterol content, improved glucose clearance during OGTT and ITT, and increased fecal cholesterol efflux. Furthermore, the fermented MC groups showed significantly higher hepatic Hmgcr, Srebp1c, Lpl, Ppara, and adiponectin mRNA expression than did the HFCD group. These findings indicate that fermentation with L. mesenteroides MKSR enhances the biological activities that prevent metabolic complications induced by a high-fat diet.
Charantin, which gives MC its bitter taste, is widely recognized as one of the key bioactive compounds responsible for the potential beneficial health effects of MC against metabolic complications [22,69]. Charantin is a mixture of two steroidal glycosides that may enhance insulin sensitivity by increasing insulin secretion from the pancreatic beta cells [22,70], as well as sensitizing gastric glucose absorption [71] and peripheral glucose uptake [72]. However, extracting charantin from MC requires the use of organic solvents, such as methanol, ethanol, and/or chloroform, considering the lipophilic nature of charantin [65,73]. The potential use of organic solvents has raised concerns among potential customers seeking natural ingredients to prevent or mitigate metabolic complications through the supplementation of nutraceuticals [23,24,25]. An alternative extraction method that avoids the use of organic solvents is water extraction; unfortunately, water extraction may not be a viable method for obtaining charantin from MC. However, it should be noted that charantin in MC may not be the sole factor responsible for mitigating metabolic complications by enhancing insulin sensitivity. Other water extracts of MC, which likely do not contain charantin, have shown decreased insulin resistance in animal experiments (refer to Table 3). Notably, hot water-extracted MC has been found to increase insulin sensitivity in experimental models of both type 1 and type 2 diabetes. Moreover, other studies have shown that water-extracted MC had the ability to attenuate hyperglycemia in animal models of type 1 diabetes induced by streptozotocin or alloxan, which destroy pancreatic beta cells [74,75]. Additionally, MC partially prevented metabolic complications in animal models of type 2 diabetes induced by high-fat diet consumption [66,76].
Regardless of the type of diabetes, MC extract has shown the ability to mitigate metabolic complications by preventing dyslipidemia [77] and WAT weight gain [72], as well as altering the microbiome caused by hyperglycemia [66]. Furthermore, the aforementioned peer-reviewed animal study used water-extracted MC, suggesting that the mitigating effects of MC supplementation on metabolic complications may be entirely independent of charantin. Unless we consider the beneficial biological effects of charantin, fermented fiber may potentially explain the beneficial effects of MC in our animal experiment. Fiber is categorized as either water-soluble or insoluble [78]. Water-soluble fibers, such as pectins, gums, and mucilages, dissolve in water and form a gel-like substance, which can help lower blood glucose and cholesterol levels [79,80]. In contrast, cellulose, hemicellulose, and lignin are major examples of insoluble fiber found in MC, which can enhance insulin sensitivity and promote bowel movement [81,82]. Considering that MC is a rich source of insoluble fiber, its ability to enhance insulin sensitivity, lower lipids, and increase cholesterol efflux in our experiments may partly be attributed to its fiber content. However, the amount of dietary fiber had been made consistent across all groups, and the amount of food consumed did not vary between different interventions. Therefore, the amount of fiber in the HFCD + 4F may not sufficiently explain its ability to mitigate the high-fat diet-induced metabolic complications better than the other interventions. We may also attribute the beneficial effects of MC to its large amount of fiber.
Given that MC contains other bioactive compounds, the results obtained from studying the efficacy of charantin by extracting only charantin may be insufficient. Hence, it is not enough to characterize charantin as the representative physiologically active substance of MC. Wang et al. [17], who studied mice with type 1 and type 2 diabetes, showed that an extract from the Taiwanese M. charantia, which is rich in charantin, increased insulin sensitivity instead of providing protecting against β-cell dysfunction. As shown in Table 3, previous research conducted animal experiments by inducing both type 1 and 2 diabetes through the injection of STZ or alloxan. Our experimental model was intended to examine type 2 diabetes by inducing MetS with a high-fat diet. Notably, we found that the consumption of MC in the absence of charantin improved insulin sensitivity. Fermentation may increase potency to increase insulin sensitivity exclusively from charantin from MC. In our previous test tube experiment, fermentation with L. mesenteroides MKSR significantly inhibited α-amylase activity [27]. Additionally, fermentation with Lactobacillus plantarum in MC (medium of cultivation) reduced α-amylase activity [83]. The suppression of α-amylase activity by MC fermentation with microorganisms occurred independently of the biological effects of charantin resulting from the use of hot water extraction. Therefore, the improvement in insulin sensitivity observed in this experiment with MC fermentation using L. mesenteroides MKSR may be attributed to the independent biological effects of charantin.
After eliminating common denominators in both MC and FMC to determine their beneficial effects against high-fat diet-induced metabolic complications, the one remaining variable was probiotics—L. mesenteroides MKSR—which we used to ferment MC. In our previous study, wherein MC was fermented with MKSR and compared with MC, we confirmed that considerable amounts of lactic acid, mannitol, dextran, and oligosaccharides were present in MC fermented with MKSR [27]. Our experiment showed that these produced compounds may trigger superior effects in mitigating metabolic complications than would other dietary interventions. In particular, highly water-soluble dextran and oligosaccharides in MKSR-MC may have acted as prebiotics and promoted the production of short-chain fatty acids (SCFAs), such as butyrate, acetate, and propionate [84]. SCFAs provide energy to the cells of the colon, promote a healthy gut environment, and have various positive effects on metabolism and overall health [85]. As suggested by our findings, extra consumption of either dextran or oligosaccharides lowered blood cholesterol levels and alteration of colonic microbiome [86,87]. Furthermore, the consumption of oligosaccharides has demonstrated cholesterol-lowering effects in high-fat-inducible animal experimental settings [88,89].
To explain how HFCD + 4F facilitates the efflux of cholesterol from the enterohepatic circulation, we examined the mRNA expressions relevant to cholesterol and lipid metabolism using qRT-PCR. Surprisingly, we observed a significant increase in Hmgcr, a key rate-limiting enzyme that converts HMG-CoA to mevalonic acid. In the pharmacological aspect, targeting HMG-CoA reductase (e.g., with statins) is a crucial approach in mitigating dyslipidemia and preventing cholesterol synthesis [38,39]. However, increasing cholesterol efflux to fecal matter through the consumption of natural compounds tends to upregulate HMG-CoA reductase expression as a compensatory mechanism against decreasing cholesterol levels [90]. Additionally, HFCD + 4F increased both anabolic (Srebp1c) and catabolic (Lpl, Ppara, and adiponectin) mRNA expressions related to lipid metabolism in the liver. This elevation of catabolic reactions in hepatic ectopic fat induced by a high-fat diet likely occurs through increased β-oxidation. Meanwhile, HFCD + 4F increased de novo mRNA expression of the adipogenic Srebp1c, which may indicate an increase in hepatic TG synthesis from catabolized free fatty acids originating from WATs (EAT and RAT; Figure 2). Adiponectin plays an anti-inflammatory role in WATs; thus, we anticipated that HFCD + 4F would attenuate hepatic pro-inflammatory responses. However, throughout our experimentation, we did not observe any significant differences in the expression of inflammatory mRNA.
The current study showed that FMC treatment significantly prevented high-fat diet-induced MetS. Our study is a novel endeavor that investigates the effects of dietary intervention with fermented MC in a murine model while precisely controlling the variables and matching dietary fiber contents across all dietary groups. To date, multiple studies have indicated the beneficial effects of MC against MetS [14,15,16,17,18,19]. However, limited information has been available on which fermented MCs are suitable for potential clinical trials. Therefore, our experimental results strongly suggest that fermented MC may have the potential to modulate lipid and cholesterol metabolism. Although our animal experimental protocol lasted for 12 weeks, we may consider extending the duration of dietary intervention to better observe the effects of fermented MC. In the liver, both de novo adipogenesis and β-oxidation were elevated; therefore, long-term observations may provide a vivid understanding on how fermented MC prevents high-fat diet-induced MetS. In potential future studies, we aim to determine the factor that primarily contributes to the beneficial effects of FMC given that the exact working mechanism is currently unknown. We also plan to explore the potential chemical compounds generated from fermented MC. Additionally, we will investigate the potential probiotic effects of fermented MC by analyzing the microbiome.

Table 3.
Effects of MC in animal models with metabolic disease.
Table 3.
Effects of MC in animal models with metabolic disease.
| Strain | Inducer | Treatment | Biological Markers | Ref. |
|---|---|---|---|---|
| Wistar Rats | STZ (30 mg/kg) after HFD for 8 weeks | MC (water extract, fermented by Lactobacillus plantarum), 10 mL/kg of BW for 4 weeks | BW, FBG, serum insulin ↓ HOMA-IR ↓ Serum TC, TG, LDL-C levels ↓ Serum HDL-C ↑ Enhance oxidative stress (SOD ↑, CAT ↑, MDA ↓) Improve gut microbiome | [75] |
| C57BL/6J | HFD | MC (methanol extract), 0.2–1.0 g/kg BW for 4 weeks | BW ↓ Lpl mRNA expression in adipose tissue ↑ Blood glucose level ↓ Serum TG, TC, insulin levels ↓ Epididymal WAT Pparg mRNA expression ↑ Hepatic Ppara mRNA expression ↑ | [91] |
| Sparague-Dawley | HFD | MC powder, 300 mg/kg BW for 8 weeks | Serum TG, TC ↓ HOMA-IR ↓ TNF-α, IL-6, MCP-1 IL-10 ↑ Improve gut microbiome | [66] |
| Sparague-Dawley | STZ (50 mg/kg) | MC (water extract), 1.5 g/kg BW for 28 days | Serum TC, TG, LDL ↓ Serum HDL ↑ MDA ↓, NO ↑ e NOS expression ↑ | [68] |
| Albino rats | STZ (45 mg/kg) | MC (diluted with distilled water), 10 mL/kg BW for 21 days | Serum glucose, TG, TC ↓ Serum HDL, insulin ↑ Serum TAOC ↑ Pancreatic GSH ↑, MDA ↓ | [67] |
↑, increased; ↓, decreased; HFD, high-fat diet; STZ, streptozotocin; BW, body weight; MC, Momordica charantia; FBG, fasting blood glucose; HOMA-IR, homeostatic model assessment of insulin resistance; TG, triglyceride; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SOD, superoxide dismutase; CAT, catalase; MDA, malondialdehyde; Lpl, lipoprotein lipase; WAT, white adipose tissue; Pparg, peroxisome proliferator-activated receptor gamma; Ppara, peroxisome proliferator-activated receptor alpha; TNF-α, tumor necrosis factor-α; IL-6, interlukin-6; MCP-1, monocyte chemotactic protein-1; IL-10, interlukin-6; NO, nitric oxide; e NOS, endothelial nitric oxide synthase; TAOC, total antioxidant capacity; GSH, reduced glutathione.
5. Conclusions
The current study investigated the potential of non- and fermented MC as a nutraceutical candidate to modulate high-fat diet-induced metabolic complications in C57BL/6 mice. The findings suggest that L. mesenteroides MKSR-fermented MC has beneficial effects in mitigating metabolic complications associated with a high-fat diet. The study demonstrated that consumption of fermented MC reduced WAT weights, serum ALT activity, and TG levels. It also lowered hepatic total cholesterol content, improved glucose clearance, and increased fecal cholesterol efflux. Additionally, the fermented MC groups showed increased mRNA expression of key genes involved in cholesterol and lipid metabolism. Although charantin has been recognized as a key bioactive compound in MC, our result suggests that the beneficial effects of MC may not solely rely on charantin. Other water extracts of MC have shown decreased insulin resistance, and the fermentation process itself may enhance the physiological activities of MC. The findings of this study support the potential use of fermented MC as a dietary intervention to prevent and mitigate metabolic complications associated with high-fat diets. Further research is needed to better understand the specific mechanisms of action and to explore the potential chemical compounds and probiotic effects of fermented MC. Longer-term studies are also warranted to evaluate the sustained effects of fermented MC and its potential for clinical applications.
Author Contributions
Conceptualization, M.K. and H.J.; methodology, J.-H.H. and H.M.; validation, H.M., J.-H.H., I.K. and M.K.; formal analysis, H.M., J.-H.H., J.L., H.J., D.K. (Dain Kwon), M.C. and D.K. (Dahyun Kang); data curation, H.M., J.-H.H., I.K. and M.K.; writing—original draft preparation, H.M. and J.-H.H.; writing—review and editing, I.K. and M.K.; visualization, H.M. and J.-H.H.; supervision, M.K.; project administration, M.K.; funding acquisition, I.K. and M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research Foundation of Korea, 2020R1I1A3072127, and Ministry of Small and Medium-sized Enterprises and Startups of Korea: S3093086.
Institutional Review Board Statement
The study was approved by the Institutional Animal Care and Use Committee of Dankook University (protocol code DKU-21-036).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kobayashi, M.; Ogawa, S.; Tayama, J.; Sagara, I.; Takeoka, A.; Bernick, P.; Kawano, T.; Abiru, N.; Hayashida, M.; Shirabe, S. Intra-abdominal fat accumulation is an important predictor of metabolic syndrome in young adults. J. Med. 2020, 99, e22202. [Google Scholar] [CrossRef]
- Regufe, V.M.; Pinto, C.M.; Perez, P.M. Metabolic syndrome in type 2 diabetic patients: A review of current evidence. Porto Biomed. J. 2020, 5, e101. [Google Scholar] [CrossRef]
- Silveira Rossi, J.L.; Barbalho, S.M.; Reverete de Araujo, R.; Bechara, M.D.; Sloan, K.P.; Sloan, L.A. Metabolic syndrome and cardiovascular diseases: Going beyond traditional risk factors. Diabetes Metab. Res. Rev. 2022, 38, e3502. [Google Scholar] [CrossRef]
- Yasmin, I.; Khan, W.A.; Naz, S.; Iqbal, M.W.; Awuchi, C.G.; Egbuna, C.; Hassan, S.; Uche, C.Z. Etiology of obesity, cancer, and diabetes. Etiology of Obesity, Cancer, and Diabetes. In Dietary phytochemiclas; Egbuna, C., Hassan, S., Eds.; Springer: Cham, Switzerland, 2021; pp. 1–27. [Google Scholar] [CrossRef]
- Tanase, D.M.; Gosav, E.M.; Neculae, E.; Costea, C.F.; Ciocoiu, M.; Hurjui, L.L.; Tarniceriu, C.C.; Maranduca, M.A.; Lacatusu, C.M.; Floria, M.; et al. Role of gut microbiota on onset and progression of microvascular complications of type 2 diabetes (T2DM). Nutrients 2020, 12, 3719. [Google Scholar] [CrossRef]
- Rodríguez-Correa, E.; González-Pérez, I.; Clavel-Pérez, P.I.; Contreras-Vargas, Y.; Carvajal, K. Biochemical and nutritional overview of diet-induced metabolic syndrome models in rats: What is the best choice? Nutr. Diabetes 2020, 10, 24. [Google Scholar] [CrossRef]
- Hugo, M.; Mehsen-Cetre, N.; Pierreisnard, A.; Schaeverbeke, T.; Gin, H.; Rigalleau, V. Energy expenditure and nutritional complications of metabolic syndrome and rheumatoid cachexia in rheumatoid arthritis: An observational study using calorimetry and actimetry. J. Rheumatol. 2016, 55, 1202–1209. [Google Scholar] [CrossRef]
- Ussar, S.; Griffin, N.W.; Bezy, O.; Bry, L.; Gordon, J.I.; Ronald, C.; Correspondence, K. Interactions between gut microbiota, host genetics and diet modulate the predisposition to obesity and metabolic syndrome. Cell Metab. 2015, 22, 516–530. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Barrea, L.; Savastano, S.; Colao, A. Nutritional recommendations for COVID-19 quarantine. Eur. J. Clin. Nutr. 2020, 74, 850–851. [Google Scholar] [CrossRef]
- Aghili, S.M.M.; Ebrahimpur, M.; Arjmand, B.; Shadman, Z.; Pejman Sani, M.; Qorbani, M.; Larijani, B.; Payab, M. Obesity in COVID-19 era, implications for mechanisms, comorbidities, and prognosis: A review and meta-analysis. Int. J. Obes. 2021, 45, 998–1016. [Google Scholar] [CrossRef]
- Pinheiro, M.M.; Wilson, T. Dietary fat: The good, the bad, and the ugly. Nutr. Res. Pract. 2017, 241–247. [Google Scholar] [CrossRef]
- Kuipers, E.N.; Held, N.M.; Panhuis, W.I.H.; Modder, M.; Ruppert, P.M.M.; Kersten, S.; Kooijman, S.; Guigas, B.; Houtkooper, R.H.; Rensen, P.C.N.; et al. A single day of high-fat diet feeding induces lipid accumulation and insulin resistance in brown adipose tissue in mice. Am. J. Physiol. Metab. Endocrinol. 2019, 317, 820–830. [Google Scholar] [CrossRef]
- Bordoni, A.; Boesch, C.; Malpuech-Brugère, C.; Orfila, C.; Tomás-Cobos, L. The role of bioactives in energy metabolism and metabolic syndrome. Proc. Nutr. Soc. 2019, 78, 340–350. [Google Scholar] [CrossRef]
- Park, S.; Yeo, S.; Lee, Y.; Jeong, Y.; Kim, M. Inhibitory activities of digestive enzymes and antioxidant activities of fermented beverages using Momordica Charantia L. J. Korean Soc. Food Sci. Nutr. 2017, 46, 1308–1315. [Google Scholar]
- Samadov, B.S. The chemical composition of the medicinal plant Momordica Charantia L. used in folk medicine. J. Math. Chem. 2022, 6, 36–51. [Google Scholar]
- Bortolotti, M.; Mercatelli, D.; Polito, L. Momordica charantia, a nutraceutical approach for inflammatory related diseases. Front. Pharmacol. 2019, 10, 486. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Kan, W.-C.; Cheng, T.-J.; Yu, S.-H.; Chang, L.-H.; Chuu, J.-J. Differential anti-diabetic effects and mechanism of action of charantin-rich extract of Taiwanese Momordica charantia between type 1 and type 2 diabetic mice. Food Chem. Toxicol. 2014, 69, 347–356. [Google Scholar] [CrossRef]
- Massounga Bora, A.F.M.; Li, X.; Liu, L. Physicochemical and functional characterization of newly designed biopolymeric-based encapsulates with probiotic culture and charantin. Foods 2021, 10, 2677. [Google Scholar] [CrossRef]
- Shim, J.; Kim, J.G.; Lim, E.Y.; Kim, Y.T. Charantin relieves pain by inhibiting pro-inflammatory cytokine induction. Pharmacogn. Mag. 2020, 16, 282–287. [Google Scholar]
- Litwiniuk, A.; Bik, W.; Kalisz, M.; Baranowska-Bik, A. Inflammasome NLRP3 potentially links obesity-associated low-grade systemic inflammation and insulin resistance with Alzheimer’s disease. Int. J. Mol. Sci. 2021, 22, 5603. [Google Scholar] [CrossRef]
- Varghese, J.F.; Patel, R.; Yadav, U. Novel insights in the metabolic syndrome-induced oxidative stress and inflammation-mediated atherosclerosis. Curr. Cardiol. Rev. 2018, 14, 4–14. [Google Scholar] [CrossRef]
- Desai, S.; Tatke, P. Charantin: An important lead compound from Momordica charantia for the treatment of diabetes. J. Pharmacogn. Phytochem. 2015, 3, 163–166. [Google Scholar]
- Adeoti, I.A.; Hawboldt, K. A review of lipid extraction from fish processing by-product for use as a biofuel. Biomass Bioenergy 2014, 63, 330–340. [Google Scholar] [CrossRef]
- Joshi, D.R.; Adhikari, N. An overview on common organic solvents and their toxicity. J. Pharm. Res. Int. 2019, 28, 1–18. [Google Scholar] [CrossRef]
- Cunha, S.C.; Fernandes, J.O. Extraction techniques with deep eutectic solvents. TrAC Trends Anal. Chem. 2018, 105, 225–239. [Google Scholar] [CrossRef]
- Lee, S.; Kim, M. Leuconostoc mesenteroides MKSR isolated from kimchi possesses α-glucosidase inhibitory activity, antioxidant activity, and cholesterol-lowering effects. LWT 2019, 116, 108570. [Google Scholar] [CrossRef]
- Kim, J.; Yu, S.; Jeong, Y.; Kim, M. Enhancement of bioactive properties in Momordica charantia by Leuconostoc fermentation. Fermentation 2023, 9, 523. [Google Scholar] [CrossRef]
- Yoon, J.; Kim, M. In vitro evaluation of antidiabetic, antidementia, and antioxidant activity of Artemisia capillaris fermented by Leuconostoc spp. LWT 2022, 172, 114163. [Google Scholar] [CrossRef]
- Jeong, S.; Bae, S.; Yu, D.; Yang, H.-S.; Yang, M.-J.; Lee, J.-H.; Ha, J.-H. Dietary intervention with quercetin attenuates diesel exhaust particle-instilled pulmonary inflammation and behavioral abnormalities in mice. J. Med. Food 2023, 26, 93–103. [Google Scholar] [CrossRef]
- Zheng, J.; Lee, J.; Byun, J.; Yu, D.; Ha, J.-H. Partial replacement of high-fat diet with n-3 PUFAs enhanced beef tallow attenuates dyslipidemia and endoplasmic reticulum stress in tunicamycin-injected rats. Front. Nutr. 2023, 10, 1155436. [Google Scholar] [CrossRef]
- Son, H.-K.; Xiang, H.; Park, S.; Lee, J.; Lee, J.-J.; Jung, S.; Ha, J.-H. Partial replacement of dietary fat with polyunsaturated fatty acids attenuates the lipopolysaccharide-induced hepatic inflammation in Sprague-Dawley rats fed a high-fat diet. Int. J. Environ. Res. Public Health 2021, 18, 10986. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.J.; Lee, J.; Lee, J.K.; Byun, J.; Kim, I.; Ha, J.H. Lowering n-6/n-3 ratio as an important dietary intervention to prevent LPS-inducible dyslipidemia and hepatic abnormalities in ob/ob mice. Int. J. Mol. Sci. 2022, 23, 6384. [Google Scholar] [CrossRef] [PubMed]
- Son, H.K.; Kim, B.H.; Lee, J.; Park, S.; Oh, C.B.; Jung, S.; Lee, J.K.; Ha, J.H. Partial replacement of dietary fat with krill oil or coconut oil alleviates dyslipidemia by partly modulating lipid metabolism in lipopolysaccharide-injected rats on a high-fat di-et. Int. J. Environ. Res. Public Health 2022, 19, 843. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.-J.; Shin, H.-W.; Jung, S.; Ha, J.-H. Effect of soybean and soybean koji on obesity and dyslipidemia in rats fed a high-fat diet: A comparative study. Int. J. Environ. Res. Public Health 2021, 18, 6032. [Google Scholar] [CrossRef]
- Jeong, S.; Bae, S.; Shin, E.-C.; Lee, J.-H.; Ha, J.-H. Ellagic acid prevents particulate matter-induced pulmonary inflammation and hyperactivity in mice: A pilot study. Int. J. Environ. Res. Public Health 2023, 20, 4523. [Google Scholar] [CrossRef]
- Jeong, S.; Shin, E.-C.; Lee, J.-H.; Ha, J.-H. Particulate matter elevates ocular inflammation and endoplasmic reticulum stress in human retinal pigmented epithelium cells. Int. J. Environ. Res. Public Health 2023, 20, 4766. [Google Scholar] [CrossRef]
- Guo, W.; Zhu, S.; Li, S.; Feng, Y.; Wu, H.; Zeng, M. Microalgae polysaccharides ameliorates obesity in association with modu-lation of lipid metabolism and gut microbiota in high-fat-diet fed C57BL/6 mice. Int. J. Biol. Macromol. 2021, 182, 1371–1383. [Google Scholar] [CrossRef]
- Tan, J.M.; Cook, E.C.; van den Berg, M.; Scheij, S.; Zelcer, N.; Loregger, A. Differential use of E2 ubiquitin conjugating enzymes for regulated degradation of the rate-limiting enzymes HMGCR and SQLE in cholesterol biosynthesis. Atherosclerosis 2019, 281, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Guerra, B.; Recio, C.; Aranda-Tavío, H.; Guerra-Rodríguez, M.; García-Castellano, J.M.; Fernández-Pérez, L. The mevalonate pathway, a metabolic target in cancer therapy. Front. Oncol. 2021, 11, 626971. [Google Scholar] [CrossRef]
- Würtz, P.; Wang, Q.; Soininen, P.; Kangas, A.J.; Fatemifar, G.; Tynkkynen, T.; Tiainen, M.; Perola, M.; Tillin, T.; Hughes, A.D.; et al. Metabolomic profiling of statin use and genetic inhibition of HMG-CoA reductase. J. Am. Coll. Cardiol. 2019, 67, 1200–1210. [Google Scholar] [CrossRef]
- Bhattarai, A.K.; Acharya, A.; Karki, P.K. Use of statins as lipid lowering agent in hypercholesterolemia in a tertiary care hos-pital: A descriptive cross-sectional study. JNMA J. Nepal Med. Assoc. 2020, 58, 1031. [Google Scholar] [PubMed]
- Klein-Szanto, A.J.; Bassi, D.E. Keep recycling going: New approaches to reduce LDL-C. Biochem. Pharmacol. 2019, 164, 336–341. [Google Scholar] [CrossRef]
- Ferré, P.; Phan, F.; Foufelle, F. SREBP-1c and lipogenesis in the liver: An update. Biochem. J. 2021, 478, 3723–3739. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, W.; Li, S.; Guo, D.; He, J.; Wang, Y. Acetyl-CoA carboxylases and diseases. Front. Oncol. 2022, 12, 836058. [Google Scholar] [CrossRef]
- Souza-Mello, V. Peroxisome proliferator-activated receptors as targets to treat non-alcoholic fatty liver disease. World J. Hepatol. 2015, 7, 1012. [Google Scholar] [CrossRef] [PubMed]
- Montaigne, D.; Butruille, L.; Staels, B. PPAR control of metabolism and cardiovascular functions. Nat. Rev. Cardiol. 2021, 18, 809–823. [Google Scholar] [CrossRef] [PubMed]
- He, P.-P.; Jiang, T.; OuYang, X.-P.; Liang, Y.-Q.; Zou, J.-Q.; Wang, Y.; Shen, Q.-Q.; Liao, L.; Zheng, X.-L. Lipoprotein lipase: Biosynthesis, regulatory factors, and its role in atherosclerosis and other diseases. Clin. Chim. Acta 2018, 480, 126–137. [Google Scholar] [CrossRef]
- Hayne, C.K.; Lafferty, M.J.; Eglinger, B.J.; Kane, J.P.; Neher, S.B. Biochemical analysis of the lipoprotein lipase truncation variant, LPLS447X, reveals increased lipoprotein uptake. Biochemistry 2017, 56, 525–533. [Google Scholar] [CrossRef]
- Nagaraju, G.P.; Rajitha, B.; Aliya, S.; Kotipatruni, R.P.; Madanraj, A.S.; Hammond, A.; Park, D.; Chigurupati, S.; Alam, A.; Pattnaik, S. The role of adiponectin in obesity-associated female-specific carcinogenesis. Cytokine Growth Factor Rev. 2016, 31, 37–48. [Google Scholar] [CrossRef]
- Howlader, M.; Sultana, M.I.; Akter, F.; Hossain, M.M. Adiponectin gene polymorphisms associated with diabetes mellitus: A descriptive review. Heliyon 2021, 7, e07851. [Google Scholar] [CrossRef]
- Guarner, V.; Rubio-Ruiz, M.E. Low-grade systemic inflammation connects aging, metabolic syndrome and cardiovascular disease. Aging Dis. 2015, 40, 99–106. [Google Scholar]
- Tong, H.V.; Luu, N.K.; Son, H.A.; Hoan, N.V.; Hung, T.T.; Velavan, T.P.; Toan, N.L. Adiponectin and pro-inflammatory cytokines are modulated in Vietnamese patients with type 2 diabetes mellitus. J. Diabetes Investig. 2017, 8, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Burchfield, J.G.; Kebede, M.A.; Meoli, C.C.; Stöckli, J.; Whitworth, P.T.; Wright, A.L.; Hoffman, N.J.; Minard, A.Y.; Ma, X.; Krycer, J.R.; et al. High dietary fat and sucrose result in an extensive and time-dependent deterioration in health of multiple physiological systems in mice. J. Biol. Chem. 2018, 293, 5731–5745. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. Adipose tissue and metabolic syndrome: Too much, too little or neither. Eur. J. Clin. Investig. 2015, 45, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Trouwborst, I.; Bowser, S.M.; Goossens, G.H.; Blaak, E.E. Ectopic fat accumulation in distinct insulin resistant phenotypes; targets for personalized nutritional interventions. Front. Nutr. 2018, 5, 77. [Google Scholar] [CrossRef]
- Ferrara, D.; Montecucco, F.; Dallegri, F.; Carbone, F. Impact of different ectopic fat depots on cardiovascular and metabolic diseases. J. Cell. Physiol. 2019, 234, 21630–21641. [Google Scholar] [CrossRef]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose tissue dysfunction as determinant of obesity-associated metabolic complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef]
- Andonian, C.; Langer, F.; Beckmann, J.; Bischoff, G.; Ewert, P.; Freilinger, S.; Kaemmerer, H.; Oberhoffer, R.; Pieper, L.; Neidenbach, R.C. Overweight and obesity: An emerging problem in patients with congenital heart disease. Cardiovasc. Diagn. Ther. 2019, 9, S360. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.Z.; Lu, W.; Zong, X.F.; Ruan, H.Y.; Liu, Y. Obesity and hypertension. Exp. Ther. Med. 2016, 12, 2395–2399. [Google Scholar] [CrossRef]
- Vekic, J.; Zeljkovic, A.; Stefanovic, A.; Jelic-Ivanovic, Z.; Spasojevic-Kalimanovska, V. Obesity and dyslipidemia. Metabolism 2019, 92, 71–81. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.; Zhang, B.; Popkin, B.M.; Du, S. Elevated fat intake increases body weight and the risk of overweight and obesity among Chinese adults: 1991–2015 Trends. Nutrients 2020, 12, 3272. [Google Scholar] [CrossRef] [PubMed]
- Swarnamali, H.; Jayawardena, R.; Chourdakis, M.; Ranasinghe, P. Is the proportion of per capita fat supply associated with the prevalence of overweight and obesity? an ecological analysis. BMC Nutr. 2020, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y. Optimal diet strategies for weight loss and weight loss maintenance. J. Obes. Metab. Syndr. 2021, 30, 20. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidi, M.; Koutelidakis, A.E. Functional foods and bioactive compounds: A review of its possible role on weight management and obesity’s metabolic consequences. Medicines 2019, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Hazra, P.; Hazra, S.; Acharya, B.; Dutta, S.; Saha, S.; Mahapatra, P.; Pradeepkumar, P.; Pal, H.; Chattopadhyay, A.; Chakraborty, I.; et al. Diversity of nutrient and nutraceutical contents in the fruits and its relationship to morphological traits in bitter gourd (Momordica charantia L.). Sci. Hortic. 2022, 305, 111414. [Google Scholar] [CrossRef]
- Bai, J.; Zhu, Y.; Dong, Y. Response of gut microbiota and inflammatory status to bitter melon (Momordica charantia L.) in high fat diet induced obese rats. J. Ethnopharmacol. 2016, 194, 717–726. [Google Scholar] [CrossRef]
- Mahmoud, M.F.; El Ashry, F.E.Z.Z.; El Maraghy, N.N.; Fahmy, A. Studies on the antidiabetic activities of Momordica charantia fruit juice in streptozotocin-induced diabetic rats. Pharm. Biol. 2017, 55, 758–765. [Google Scholar] [CrossRef]
- Abas, R.; Othman, F.; Thent, Z.C. Effect of Momordica charantia fruit extract on vascular complication in type 1 diabetic rats. EXCLI J. 2015, 14, 179. [Google Scholar] [CrossRef]
- Gayathry, K.S.; John, J.A. A comprehensive review on bitter gourd (Momordica charantia L.) as a gold mine of functional bio-active components for therapeutic foods. Food Prod. Process. Nutr. 2022, 4, 10. [Google Scholar] [CrossRef]
- Saeed, F.; Afzaal, M.; Niaz, B.; Arshad, M.U.; Tufail, T.; Hussain, M.B.; Javed, A. Bitter melon (Momordica charantia): A natural healthy vegetable. Int. J. Food Prop. 2018, 21, 1270–1290. [Google Scholar] [CrossRef]
- Prabhakar, P.K.; Doble, M. Mechanism of action of natural products used in the treatment of diabetes mellitus. Chin. J. Integr. Med. 2011, 17, 563–574. [Google Scholar] [CrossRef]
- Fan, M.; Kim, E.-K.; Choi, Y.-J.; Tang, Y.; Moon, S.-H. The role of Momordica charantia in resisting obesity. Int. J. Environ. Res. Public Health 2019, 16, 3251. [Google Scholar] [CrossRef] [PubMed]
- Saeed, F.; Sultan, M.T.; Riaz, A.; Ahmed, S.; Bigiu, N.; Amarowicz, R.; Manea, R. Bitter Melon (Momordica charantia L.) Fruit bioactives charantin and vicine potential for diabetes prophylaxis and treatment. Plants 2021, 10, 730. [Google Scholar] [CrossRef]
- Xu, X.; Shan, B.; Liao, C.-H.; Xie, J.-H.; Wen, P.-W.; Shi, J.-Y. Anti-diabetic properties of Momordica charantia L. polysaccharide in alloxan-induced diabetic mice. Int. J. Biol. Macromol. 2015, 81, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wen, J.-J.; Hu, J.-L.; Nie, Q.-X.; Chen, H.-H.; Xiong, T.; Nie, S.-P.; Xie, M.-Y. Fermented Momordica charantia L. juice modulates hyperglycemia, lipid profile, and gut microbiota in type 2 diabetic rats. Food Res. Int. 2019, 121, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Bai, J.; Zhang, Y.; Xiao, X.; Dong, Y. Effects of bitter melon (Momordica charantia L.) on the gut microbiota in high fat diet and low dose streptozocin-induced rats. Int. J. Food Sci. Nutr. 2016, 67, 686–695. [Google Scholar] [CrossRef]
- Poovitha, S.; Parani, M. Protein extracts from Momordica charantia var. charantia and M. charantia var. muricata show anti-lipidemic and antioxidant properties in experimental type 2 diabetic rats. J. Food Biochem. 2020, 44, e13370. [Google Scholar]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary fibre in foods: A review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar] [CrossRef]
- Guan, Z.-W.; Yu, E.-Z.; Feng, Q. Soluble dietary fiber, one of the most important nutrients for the gut microbiota. Molecules 2021, 26, 6802. [Google Scholar] [CrossRef]
- Li, L.; Pan, M.; Pan, S.; Li, W.; Zhong, Y.; Hu, J.; Nie, S. Effects of insoluble and soluble fibers isolated from barley on blood glucose, serum lipids, liver function and caecal short-chain fatty acids in type 2 diabetic and normal rats. Food Chem. Toxicol. 2020, 135, 110937. [Google Scholar] [CrossRef]
- Khan, A.; Raghunathan, V.; Singaravelu, D.L.; Sanjay, M.R.; Siengchin, S.; Jawaid, M.; Alamry, K.A.; Asiri, A.M. Extraction and characterization of cellulose fibers from the stem of Momordica Charantia. J. Nat. Fibers 2022, 19, 2232–2242. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, L.; Gutin, B.; Zhu, H. Total, insoluble, and soluble dietary fiber intake and insulin resistance and blood pressure in adolescents. Eur. J. Clin. Nutr. 2019, 73, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.-H.; Jaiswal, R.; Peng, Y.-Y.; Liu, T.-H. Improving bioactivities of Momordica charantia broth through fermentation using mixed cultures of Lactobacillus plantarum, Gluconacetobacter sp. and Saccharomyces cerevisiae. Process. Biochem. 2022, 117, 142–152. [Google Scholar] [CrossRef]
- Koirala, P.; Costantini, A.; Maina, H.N.; Rizzello, C.G.; Verni, M.; Beni, V.D.; Coda, R. Fermented brewers’ spent grain con-taining dextran and oligosaccharides as ingredient for composite wheat bread and its impact on gut metabolome in vitro. Fermentation 2022, 8, 487. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Rahim, M.A.; Saeed, F.; Khalid, W.; Hussain, M.; Anjum, F.M. Functional and nutraceutical properties of fructo-oligosaccharides derivatives: A review. Int. J. Food Prop. 2021, 24, 1588–1602. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, S.; Liu, F.; Zhang, P.; Muhammad, Z.; Pan, S. Role of the gut microbiota and their metabolites in modulating the cholesterol-lowering effects of citrus pectin oligosaccharides in C57BL/6 Mice. J. Agric. Food Chem. 2019, 67, 11922–11930. [Google Scholar] [CrossRef]
- Wang, J.; He, W.; Yang, D.; Cao, H.; Bai, Y.; Guo, J.; Su, Z. Beneficial metabolic effects of chitosan and chitosan oligosaccharide on epididymal WAT browning and thermogenesis in obese Rats. Molecules 2019, 24, 4455. [Google Scholar] [CrossRef]
- Tao, W.; Sun, W.; Liu, L.; Wang, G.; Xiao, Z.; Pei, X.; Wang, M. Chitosan oligosaccharide attenuates nonalcoholic fatty liver disease induced by high fat diet through reducing lipid accumulation, inflammation and oxidative stress in C57BL/6 Mice. Mar. Drugs 2019, 17, 645. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Lee, J.-J.; Kim, Y.-K.; Lee, Y.; Ha, J.-H. Stachys sieboldii Miq. root attenuates weight gain and dyslipidemia in rats on a high-fat and high-cholesterol diet. Nutrients 2020, 12, 2063. [Google Scholar] [CrossRef]
- Shih, C.-C.; Lin, C.-H.; Lin, W.-L. Effects of Momordica charantia on insulin resistance and visceral obesity in mice on high-fat diet. Diabetes Res. Clin. Pract. 2008, 81, 134–143. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).