Bacillus subtilis Fermentation Augments the Anti-Inflammatory and Skin Moisture Improvement Activities of Tetragonia tetragonoides through the Upregulation of Antioxidant Components
Abstract
:1. Introduction
2. Materials and Methods
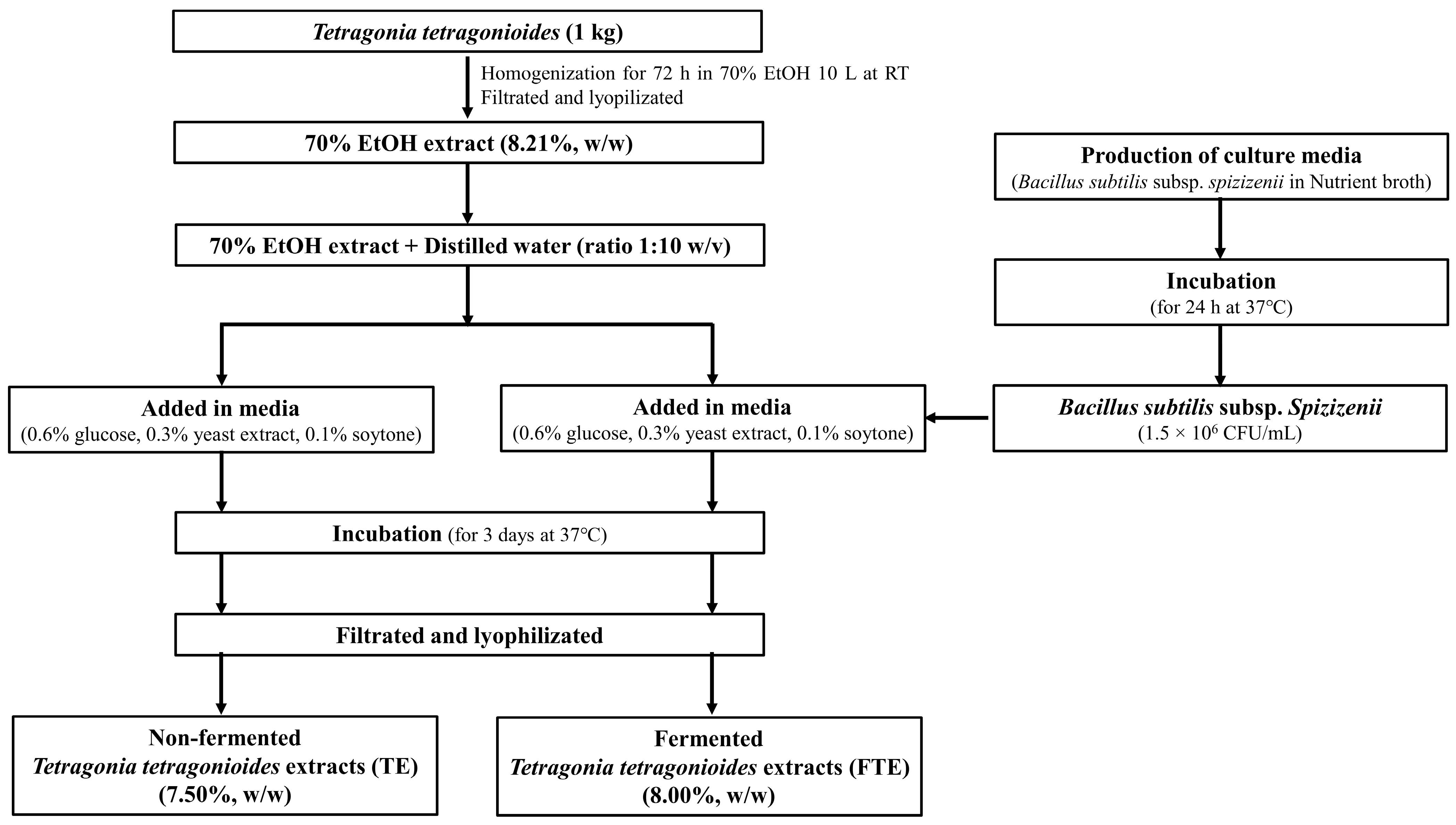
2.1. Preparation of T. tetragonioides 70% EtOH Extracts
2.2. Preparation of TEs and FTEs
2.3. Determination of Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
2.4. High-Performance Liquid Chromatography (HPLC) Analysis
2.5. Free Radical Inhibition Activity
2.6. Cell Culture
2.7. Cell Viability
2.8. Measurement of Nitric Oxide (NO) Inhibition
2.9. Western Blot Analysis
2.10. RNA Isolation and Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
2.11. Statistical Analysis
3. Results
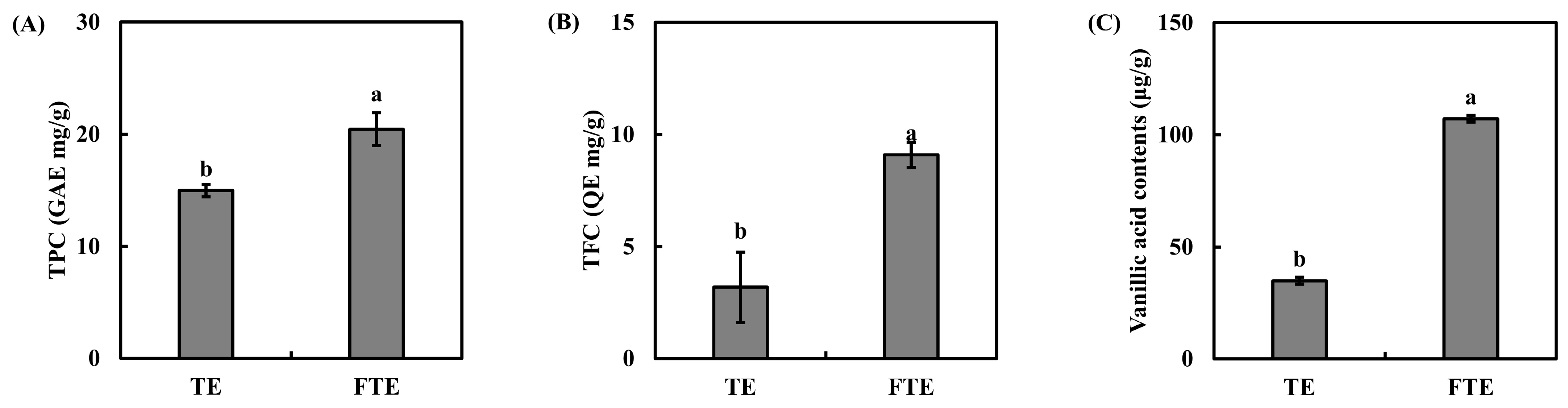
3.1. Total Phenolic, Flavonoid, and Vanillic Acid Contents of TEs and FTEs
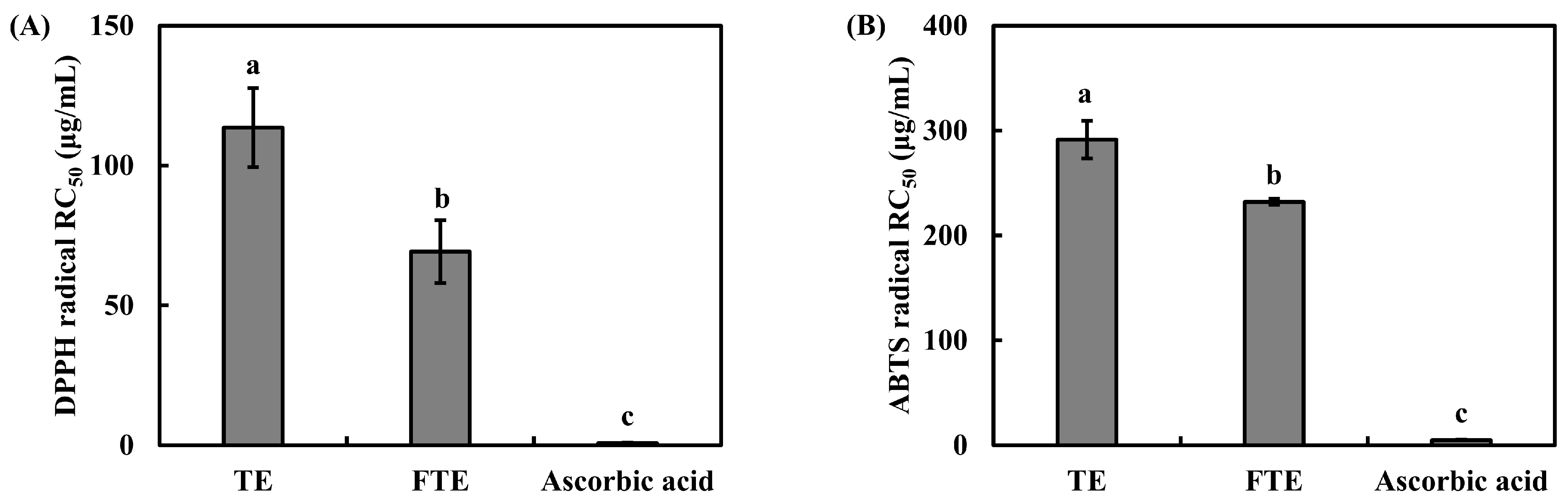
3.2. Antioxidant Activity Analysis
3.3. Effect of TEs and FTEs on NO Production via LPS Stimulation
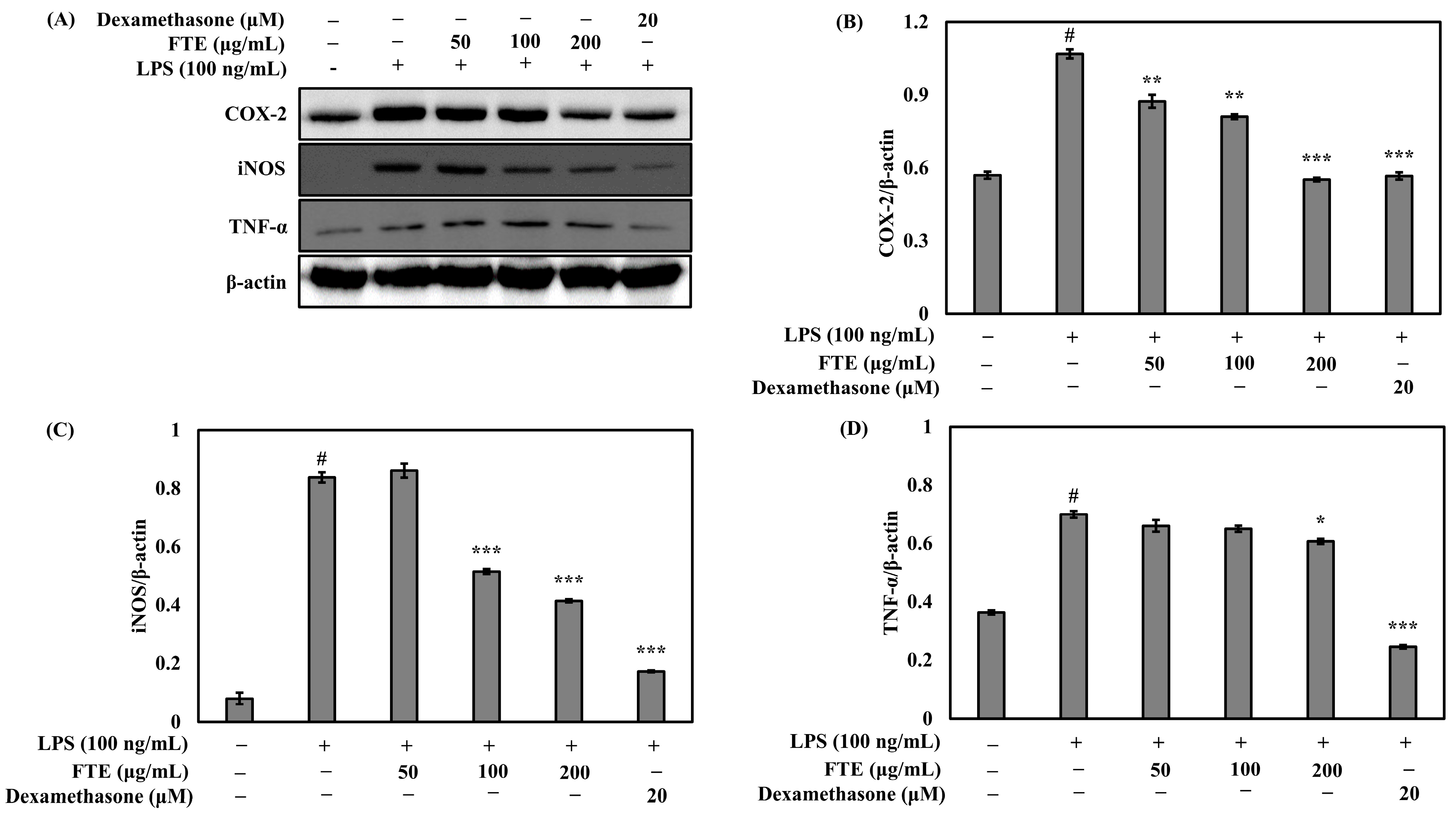
3.4. The FTEs Inhibit LPS-Stimulated COX-2, iNOS, and TNF-α Expression
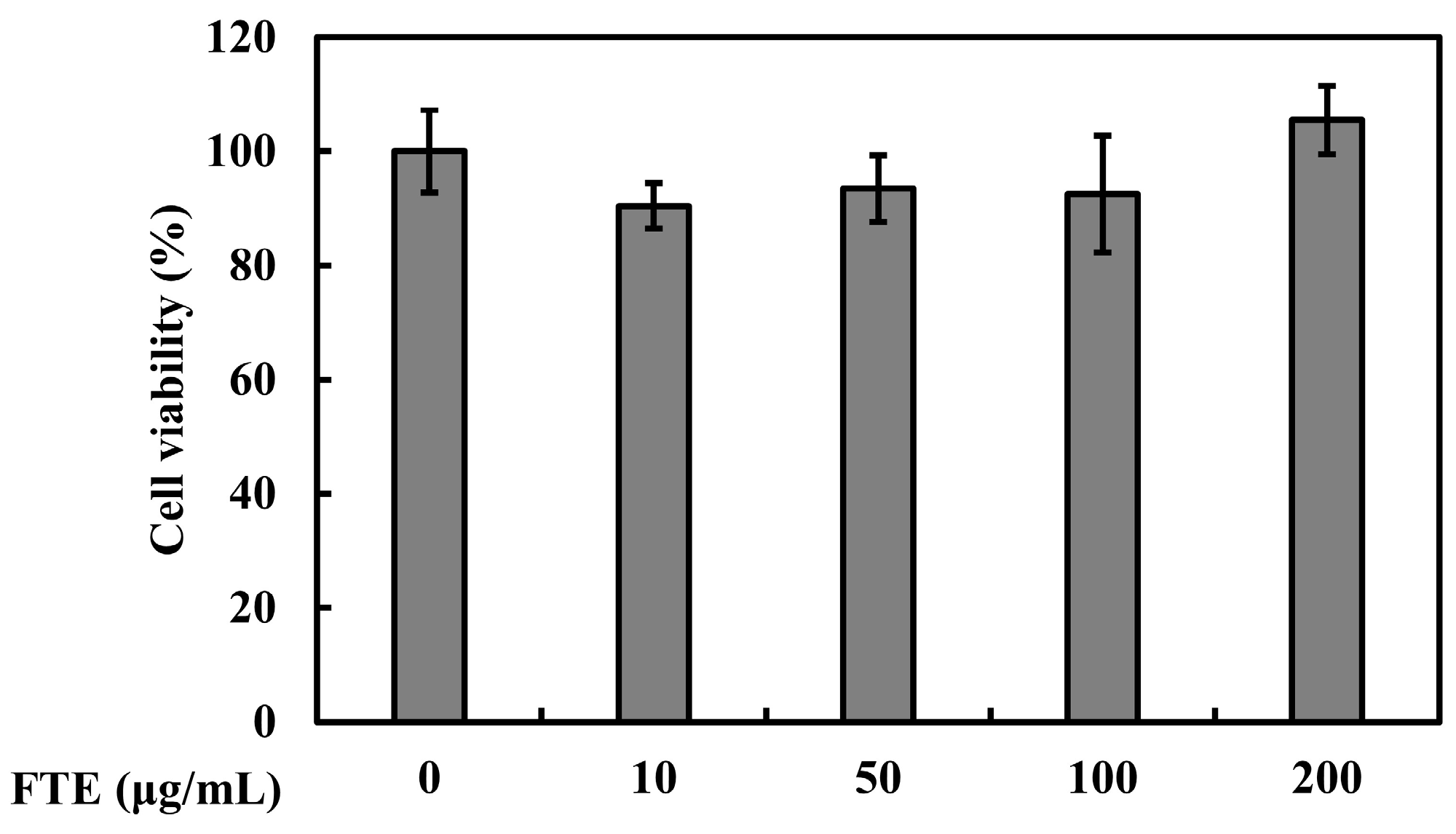
3.5. Effects of FTEs on Cell Viability and Skin Moisture Protection Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rahimuddin, S.A.; Khoja, S.M.; Zuhair, M.M.; Howell, N.K.; Brown, J.E. Inhibition of lipid peroxidantion in UVA-treated skin fibroblasts by luteolin and its glucosides. Eur. J. Lipid Sci. Technol. 2007, 109, 647–655. [Google Scholar] [CrossRef]
- Park, S.N. Skin aging and antioxidants. J. Soc. Cosmet. Sci. Korea 2003, 29, 75–77. [Google Scholar]
- Fantone, J.C.; Ward, P.A. Role of oxygen-derived free radicals and metabolites in leukocyte dependent inflammatory reaction. Ann. J. Path. 1982, 107, 395–418. [Google Scholar]
- Davies, K.J.A. Protein damage and degradation by oxygen radical. J. Biol. Chem. 1987, 262, 9895–9901. [Google Scholar] [CrossRef] [PubMed]
- Park, S.N. Protective effect of isoflavone, genistein from soybean on singlet oxygen induced photohemolysis of human erythrocytes. Korean J. Food Sci. Technol. 2003, 35, 510–518. [Google Scholar]
- Park, S.N. Antioxidative properties of baicalein, component from Scutellaria baicalensis Georgi and its application to cosmetics (I). J. Korean Ind. Eng. Chem. 2003, 14, 657–665. [Google Scholar]
- Kim, N.M.; Koo, B.S.; Lee, S.K.; Hwang, E.I.; So, S.H.; Do, J.H. Effect of Korean red ginseng on collagen biosynthesis and MMP-I activity in human dermal fibroablast. J. Ginseng Res. 2007, 31, 86–92. [Google Scholar]
- Rittié, L.; Fisher, G.J. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002, 1, 705–720. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.J.; Chu, A.W.; Lu, Z.F.; Pan, M.H.; Che, D.F.; Zhou, X.J. Ultraviolet B-induced alterations of the skin barrier and epidermal calcium gradient. Exp. Dermatol. 2007, 16, 985–992. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Dermato-Endocrinol. 2012, 4, 253–258. [Google Scholar] [CrossRef]
- Wlaschek, M.; Tantcheva-Poór, I.; Naderi, L.; Ma, W.; Schneider, L.A.; Razi-Wolf, Z.; Schüller, J.; Scharffetter-Kochanek, K. Solar UV irradiation and dermal photoaging. J. Photochem. Photobiol. B 2001, 63, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Varani, J.; Perone, P.; Fligiel, S.E.G.; Fisher, G.J.; Voorhees, J.J. Inhibition of type I procollagen production in photodamage: Correlation between presence of high molecular weight collagen fragments and reduced procollagen synthesis. J. Investig. Dermatol. 2002, 119, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Takagi, K.; Hira, T.; Suga, T. Two naturally occurring acyclic diterpene and norditerpene aldehydes from Tetragonia tetragonioides. Phytochemistry 1982, 21, 1361–1363. [Google Scholar] [CrossRef]
- Cambie, R.C.; Ferguson, L.R. Potential functional foods in the traditional Maori diet. Mutat. Res. 2003, 523, 109–117. [Google Scholar] [CrossRef]
- Emi, O.; Mikio, Y. The principles of Tetragonia tetragonioides having anti-ulcergenic activity. Isolation and identification of a sterol glucoside mixture (compound A). Yakuguku Zasshi. 1983, 103, 43–48. [Google Scholar]
- Kato, M.; Takeda, T.; Ogihara, Y.; Shimizu, M.; Nomura, T.; Tomita, T. Syudies on the structure of polysaccharide from Tetragonia tetragonoides. I. Chem. Pharm. Bull. 1985, 33, 3675–3680. [Google Scholar] [CrossRef]
- Kemo, M.S.; Bueden, R.S.; Brown, C. A new naturally occurring flavanone from Tetragonia expansa. Phytochemistry 1979, 18, 1765–1766. [Google Scholar]
- Mori, K.; Kinsho, T. Synthesis of anti-ulcergenic cerebroside isolated from Tetragonia tetragonioides. Liebigs. Ann. Chem. 1988, 1988, 807–814. [Google Scholar] [CrossRef]
- Ryu, H.Y.; Bae, K.H.; Kum, E.J.; Park, S.J.; Lee, B.H.; Sohn, H.Y. Evaluation for the Antimicrobial, Antioxidant and Antithrombosis Activity of Natural Spices for Fresh-cut yam. J. Life Sci. 2007, 17, 652–657. [Google Scholar] [CrossRef]
- Park, S.Y.; Bang, M.A.; Oh, B.J.; Park, J.H.; Song, W.S.; Choi, K.M.; Choung, E.S.; Boo, H.O.; Cho, S.S. Fermentation and quality characteristics of Cheonggukjang fermented with Bacillus subtilis BC-P1. Korean J. Microbiol. 2013, 49, 262–269. [Google Scholar] [CrossRef]
- Moon, J.Y.; Kwon, S.W.; Hong, S.B.; Seok, S.J.; Kim, J.S.; Kim, S.J. Characteristics and functional analysis of Bacillus strains from the fermented soybean products, Cheonggukjang. Korean J. Microbiol. 2015, 51, 300–307. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, and [15N] nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Seo, S.H.; Park, S.E.; Kim, E.J.; Oh, D.; Son, H.S. Characterization of Fermented Mulberry Leaf Using Bacillus subtilis. J. Korean Soc. Food Sci. Nutr. 2017, 46, 108–114. [Google Scholar] [CrossRef]
- Kim, Y.S.; Jo, C.; Choi, G.H.; Lee, K.H. Changes of Antioxidative Components and Activity of Fermented Tea during Fermentation Period. J. Korean Soc. Food Sci. Nutr. 2011, 40, 1073–1078. [Google Scholar] [CrossRef]
- Lee, Y.S. Antioxidative and physiological activity of extracts of Angelica dahurica leaves. Korean J. Food Preserv. 2007, 14, 78–86. [Google Scholar]
- Jeong, C.H.; Jang, C.W.; Lee, K.Y.; Kim, I.H.; Shim, K.W. Chemical components and anti-oxidant activities of black currant. Korean J. Food Preserv. 2012, 19, 263–270. [Google Scholar] [CrossRef]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Asp. Med. 2010, 31, 435–445. [Google Scholar] [CrossRef]
- Hur, S.J.; Lee, S.Y.; Kim, Y.C.; Choi, I.; Kim, G.B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Adetuyi, F.O.; Ibrahim, T.A. Effect of Fermentation Time on the Phenolic, Flavonoid and Vitamin C Contents and Antioxidant Activities of Okra (Abelmoschus esculentus) Seeds. Niger. Food J. 2014, 32, 128–137. [Google Scholar] [CrossRef]
- Salau, V.F.; Erukainure, O.L.; Ibeji, C.U.; Olasehinde, T.A.; Koorbanally, N.A.; Islam, M.S. Vanillin and vanillic acid modulate antioxidant defense system via amelioration of metabolic complications linked to Fe2+-induced brain tissues damage. Metab. Brain Dis. 2020, 35, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Jiao, S.; Qin, M.; Hu, W.; Liu, D. Vanillic acid alleviates acute myocardial hypoxia/reoxygenation injury by inhibiting oxidative stress. Oxid. Med. Cell. Longev. 2020, 2019, 8348035. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Yang, M.; Wang, T.; Sun, Z.; Liu, M.; Zhang, J.; Zeng, Q.; Cai, C.; Li, Y. Antibacterial mechanism of vanillic acid on physiological, morphological, and biofilm properties of carbapenem-resistant Enterobacter hormaechei. J. Food Prot. 2020, 83, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Calixto-Campos, C.; Carvalho, T.T.; Hohmann, M.S.N.; Pinho-Ribeiro, F.A.; Fattori, V.; Manchope, M.F.; Zarpelon, A.C.; Baracat, M.M.; Georgetti, S.R.; Casagrande, R.; et al. Vanillic acid inhibits inflammatory pain by inhibiting neutrophil recruitment, oxidative stress, cytokine production, and NFκB activation in mice. J. Nat. Prod. 2015, 78, 1799–1808. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Mustafa, S.; Ismail, A. Effect of lactic fermentation on the antioxidant capacity of Malaysian herbal teas. Int. Food Res. J. 2014, 21, 1483–1488. [Google Scholar]
- Guzik, T.J.; Korbut, R.; Adamek-Guzik, T. Nitric oxide and superoxide in inflammation and immune regulation. J. Physiol. Pharmacol. 2003, 54, 469–487. [Google Scholar]
- Antonopoulou, I.; Varriale, S.; Topakas, E.; Rova, U.; Christakopoulos, P.; Faraco, V. Enzymatic synthesis of bioactive compounds with high potential for cosmeceutical application. Appl. Microbiol. Biotechnol. 2016, 100, 6519–6543. [Google Scholar] [CrossRef]
- Armengot-Carbo, M.; Hernández-Martín, Á.; Torrelo, A. The role of filaggrin in the skin barrier and disease development. Actas Dermosifiliogr. 2015, 106, 86–95. [Google Scholar] [CrossRef]
- Jeong, D.O.; Lee, J.S.; Park, S.H.; Kim, Y.A.; Park, B.J.; Oh, J.S.; Sung, G.H.; Aravinthan, A.; Kim, J.H.; Kang, H.H.; et al. Antiphotoaging and antimelanogenic effects of penthorum chinense pursh ethanol extract due to antioxidant- and autophagy-inducing properties. Oxid. Med. Cell. Longev. 2019, 2019, 9679731. [Google Scholar] [CrossRef] [PubMed]
- Turino, G.M.; Cantor, J.O. Hyaluronan in respiratory injury and repair. Am. J. Respir. Crit. Care Med. 2003, 167, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Prehm, P. Hyaluronate is synthesized at plasma membranes. Biochem. J. 1984, 220, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Itano, N.; Kimata, K. Mammalian hyaluronan synthases. IUBMB Life 2002, 54, 195–199. [Google Scholar] [CrossRef] [PubMed]


| Instrument | Conditions | ||
|---|---|---|---|
| Column | Agilent ZORBAX SB-C18 (4.6 mm × 250 mm, 5.0 µm, Agilent) | ||
| Column temp. | 27 °C | ||
| Mobile phase (Gradient) | Time (min) | A (1) | B (2) |
| 0 | 100 | 0 | |
| 45 | 100 | 0 | |
| 55 | 60 | 40 | |
| 65 | 20 | 80 | |
| 70 | 100 | 0 | |
| Detector | Shimadzu, SPD-M20A Diode Array Detector (334 nm) | ||
| Flow rate | 1.0 mL/min | ||
| Injection volume | 10 μL | ||
| Run time | 70 min | ||
| Gene | Sequences (5′ to 3′) | |
|---|---|---|
| FLG | F | AGGGAAGATCCAAGAGCCCA |
| R | ACTCTGGATCCCCTACGCTT | |
| HAS-1 | F | CCACCCAGTACAGCGTCAAC |
| R | CATGGTGCTTCTGTCGCTCT | |
| HAS-3 | F | TATACCGCGCGCTCCAA |
| R | GCCACTCCCGGAAGTAAGACT | |
| GAPDH | F | GCACCGTCAAGGCTGAGAAC |
| R | ATGGTGGTGAAGACGCCAGT | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, H.; Hwang, J.-W.; Lee, S.-G. Bacillus subtilis Fermentation Augments the Anti-Inflammatory and Skin Moisture Improvement Activities of Tetragonia tetragonoides through the Upregulation of Antioxidant Components. Fermentation 2023, 9, 800. https://doi.org/10.3390/fermentation9090800
Kang H, Hwang J-W, Lee S-G. Bacillus subtilis Fermentation Augments the Anti-Inflammatory and Skin Moisture Improvement Activities of Tetragonia tetragonoides through the Upregulation of Antioxidant Components. Fermentation. 2023; 9(9):800. https://doi.org/10.3390/fermentation9090800
Chicago/Turabian StyleKang, Hyun, Jin-Woo Hwang, and Sung-Gyu Lee. 2023. "Bacillus subtilis Fermentation Augments the Anti-Inflammatory and Skin Moisture Improvement Activities of Tetragonia tetragonoides through the Upregulation of Antioxidant Components" Fermentation 9, no. 9: 800. https://doi.org/10.3390/fermentation9090800
APA StyleKang, H., Hwang, J.-W., & Lee, S.-G. (2023). Bacillus subtilis Fermentation Augments the Anti-Inflammatory and Skin Moisture Improvement Activities of Tetragonia tetragonoides through the Upregulation of Antioxidant Components. Fermentation, 9(9), 800. https://doi.org/10.3390/fermentation9090800





