Lactic Acid Production Using Sugarcane Juice as an Alternative Substrate and Purification through Ion-Exchange Resins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Microorganism
2.3. LA Production Using Sugarcane Juice as Substrate
2.3.1. Inoculum
2.3.2. LA Production Kinetics
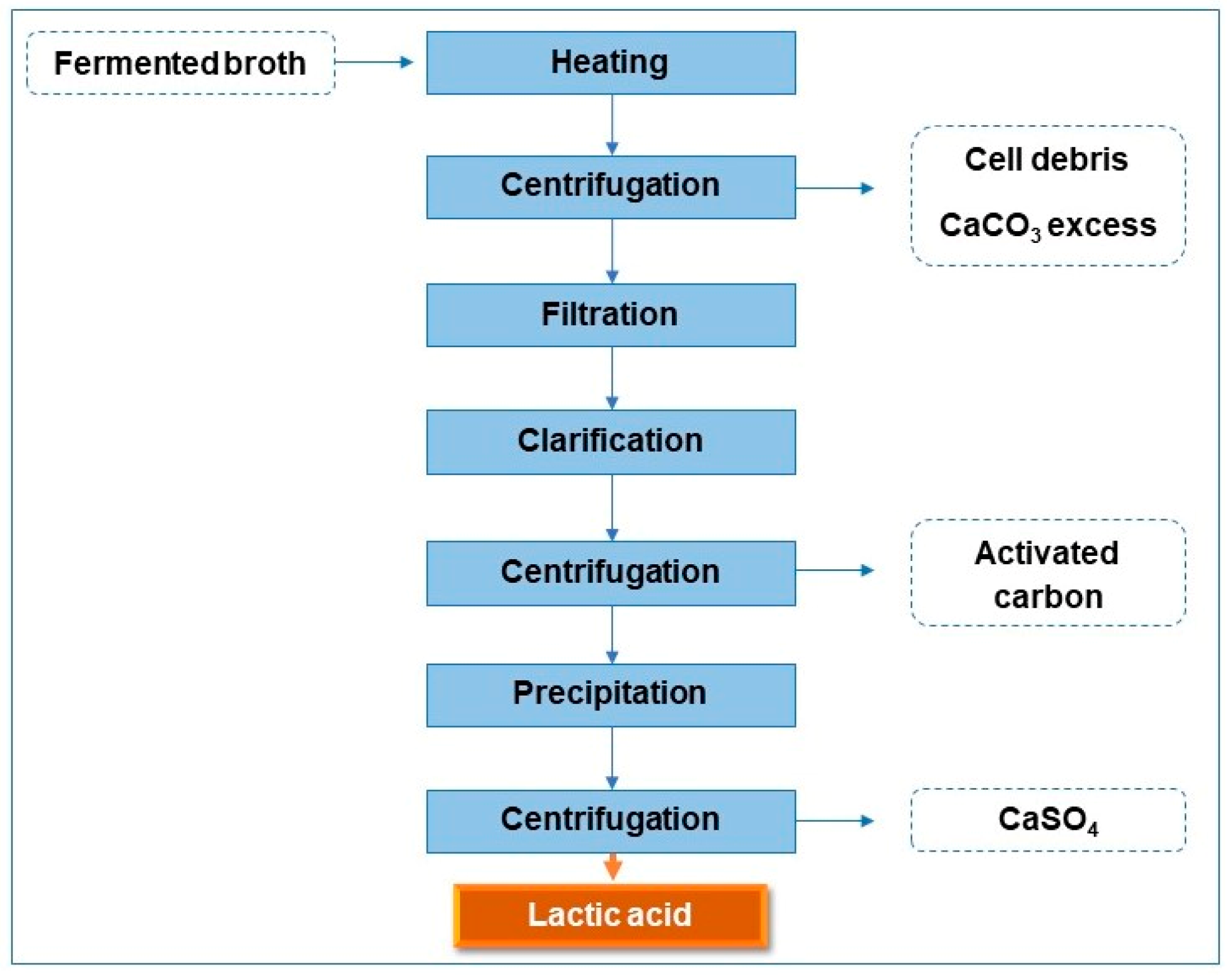
2.4. Conventional LA Recovery
2.5. LA Purification through Ion-Exchange Resins
2.5.1. Resin Activation
2.5.2. LA Purification Using Cationic and Anionic resins (Glass Column)
2.5.3. LA Purification Using Cationic and Anionic Resins Simultaneously (Stirred Tank)
2.6. Analytical Methods
2.7. Statistics
3. Results and Discussion
3.1. LA Production Kinetics
3.2. LA Recovery
3.3. LA Purification through Ion-Exchange Resins
3.3.1. LA Purification Using Cationic and Anionic resins (Glass Column)
3.3.2. LA Purification Using Cationic and Anionic Resins Simultaneously (Stirred Tank)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klotz, S.; Kuenza, A.; Prüßea, U. Nutritional requirements and the impact of yeast extract on the D-lactic acid production by Sporolactobacillus inulinus. Green Chem. 2017, 19, 4633–4641. [Google Scholar] [CrossRef]
- Martinez, F.A.C.; Balciunas, E.M.; Salgado, J.M.; González, J.M.D.; Converti, A.; Oliveira, R.P.S. Lactic acid properties, applications and production: A review. Trends Food Sci. Technol. 2013, 30, 70–83. [Google Scholar] [CrossRef]
- Awasthi, D.; Wang, L.; Rhee, M.S.; Wang, Q.; Chauliac, D.; Ingram, L.O.; Shanmugam, K.T. Metabolic engineering of Bacillus subtilis for production of D-lactic acid. Biotechnol. Bioeng. 2018, 115, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Alexandri, M.; Schneider, R.; Mehlmann, K.; Venus, J. Recent Advances in D-Lactic Acid Production from Renewable Resources: Case Studies on Agro-Industrial Waste Streams. Food Technol. Biotechnol. 2019, 57, 293–304. [Google Scholar] [CrossRef]
- Chandrapala, J.; Duke, M.C.; Gray, S.R.; Weeks, M.; Palmer, M.; Vasiljevic, T. Strategies for maximizing removal of lactic acid from acid whey—Addressing the un-processability issue. Sep. Purif. Technol. 2017, 172, 489–497. [Google Scholar] [CrossRef]
- Orrego, D.; Zapata-Zapata, A.D.; Kim, D. Optimization and Scale-Up of Coffee Mucilage Fermentation for Ethanol Production. Energies 2018, 11, 786. [Google Scholar] [CrossRef]
- CONAB (Companhia Nacional de Abastecimento). Histórico Mensal da Cana-de-Açúcar; CONAB: Brasília, Brazil, 2023. Available online: https://www.conab.gov.br/info-agro/analises-do-mercado-agropecuario-e-extrativista/analises-do-mercado/historico-mensal-de-cana-de-acucar (accessed on 17 August 2023).
- Marasinghege, C.; Broadfoot, R.; Bottle, S.; Bartley, J.; Doherty, W.O.S.; Rackemann, D.W. Investigation on the effect of the heating surface temperature of 1st evaporator on sucrose loss and the degradation of sugarcane juice constituents. J. Food Eng. 2022, 329, 111074. [Google Scholar] [CrossRef]
- Panigrahi, C.; Mishra, H.N.; De, S. Combined ultrafiltration and ozone processing of sugarcane juice: Quantitative assessment of polyphenols, investigation of storage effects by multivariate techniques and shelf-life prediction. Food Chem. Adv. 2023, 2, 100214. [Google Scholar] [CrossRef]
- Tarafdar, A.; Kumar, Y.; Kaur, B.P.; Badgujar, P.C. High-pressure microfluidization of sugarcane juice: Effect on total phenols, total flavonoids, antioxidant activity, and microbiological quality. J. Food Process. Preserv. 2021, 45, e15428. [Google Scholar] [CrossRef]
- Geremias-Andrade, I.M.; Rocheto, A.C.; Gallo, F.A.; Petrus, R.R. The shelf life of standardized sugarcane juice stored under refrigeration. Food Sci. Technol. 2020, 40, 95–101. [Google Scholar] [CrossRef]
- Papadimitriou, K.; Alegría, Á.; Bron, P.A.; de Angelis, M.; Gobbetti, M.; Kleerebezem, M.; Lemos, J.A.; Linares, D.M.; Ross, P.; Stanton, C.; et al. Stress Physiology of Lactic Acid Bacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 837–890. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, W.; Gu, X.; Guo, Z.; Song, J.; Zhu, D.; Liu, Y.; Liu, Y.; Xue, G.; Li, X.; et al. Stabilizing lactate production through repeated batch fermentation of food waste and waste activated sludge. Bioresour. Technol. 2020, 300, 122709. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sadiq, S.; Zhang, W.; Chen, Y.; Xu, X.; Abbas, A.; Chen, S.; Zhang, R.; Xue, G.; Sobotka, D.; et al. Salinity enhances high optically active L-lactate production from co-fermentation of food waste and waste activated sludge: Unveiling the response of microbial community shift and functional profiling. Bioresour. Technol. 2021, 319, 124124. [Google Scholar] [CrossRef]
- Othman, M.; Ariff, A.B.; Kapri, M.R.; Rios-Solis, L.; Halim, M. Growth Enhancement of Probiotic Pediococcus acidilactici by Extractive Fermentation of Lactic Acid Exploiting Anion-Exchange Resin. Front. Microbiol. 2018, 29, 2554. [Google Scholar] [CrossRef] [PubMed]
- Luongo, V.; Palma, A.; Rene, E.R.; Fontana, A.; Pirozzi, F.; Esposito, G.; Lens, P.N.L. Lactic acid recovery from a model of Thermotoga neapolitana fermentation broth using ion exchange resins in batch and fixed-bed reactors. Sep. Sci. Technol. 2019, 54, 1008–1025. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Poulopoulos, S.G. Adsorption and Ion Exchange. In Adsorption, Ion Exchange and Catalysis: Design of Operations and Environmental Applications, 1st ed.; Inglezakis, V.J., Poulopoulos, S.G., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2006; pp. 243–353. [Google Scholar]
- Harrison, R.G.; Todd, P.W.; Rudge, S.R.; Petrides, D.P. Bioseparations Science and Engineering, 2nd ed.; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Oliveira, J.; Vandenberghe, L.P.S.; Oliveira, P.Z.; Mello, A.F.M.; Rodrigues, C.; Nigam, P.S.; Faraco, V.; Soccol, C.R. Bioconversion of potato-processing wastes into an industrially-important chemical lactic acid. Bioresour. Technol. Rep. 2021, 15, 100698. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Karp, S.G.; Igashiyama, A.H.; Siqueira, P.F.; Carvalho, J.C.; Vandenberghe, L.P.S.; Thomaz-Soccol, V.; Coral, J.; Tholozan, J.L.; Pandey, A.; Soccol, C.R. Application of the biorefinery concept to produce L-lactic acid from the soybean vinasse at laboratory and pilot scale. Bioresour. Technol. 2011, 102, 1765–1772. [Google Scholar] [CrossRef]
- Bernardo, M.P.; Coelho, L.F.; Sass, D.C.; Contiero, J. L-(+)-Lactic acid production by Lactobacillus rhamnosus B103 from dairy industry waste. Braz. J. Microbiol. 2016, 47, 640–646. [Google Scholar] [CrossRef]
- Wang, J.; Huang, J.; Laffend, H.; Jiang, S.; Zhang, J.; Ning, Y.; Fang, M.; Liu, S. Optimization of immobilized Lactobacillus pentosus cell fermentation for lactic acid production. Bioresour. Bioprocess. 2020, 7, 15. [Google Scholar] [CrossRef]
- Paulova, L.; Chmelik, J.; Branska, B.; Patakova, P.; Drahokoupil, M.; Melzoch, K. Comparison of Lactic Acid Production by L. casei in Batch, Fed-batch and Continuous Cultivation, Testing the use of Feather Hydrolysate as a Complex Nitrogen Source. Braz. Arch. Biol. Technol. 2020, 63, e20190151. [Google Scholar] [CrossRef]
- de la Torre, I.; Acedos, M.G.; Ladero, M.; Santos, V.E. On the use of resting L. delbrueckii spp. delbrueckii cells for D-lactic acid production from orange peel wastes hydrolysates. Biochem. Eng. J. 2019, 145, 162–169. [Google Scholar] [CrossRef]
- Cirlini, M.; Ricci, A.; Galaverna, G.; Lazzi, C. Application of lactic acid fermentation to elderberry juice: Changes in acidic and glucidic fractions. LWT 2020, 118, 108779. [Google Scholar] [CrossRef]
- Vidra, A.; Németh, Á.; Salgó, A. Factors Affecting Precipitation of Calcium Lactate from Fermentation Broths and from Aqueous Solution. Period. Polytech. Chem. Eng. 2019, 63, 533–540. [Google Scholar] [CrossRef]
- Bishai, M.; De, S.; Adhikari, B.; Banerjee, R. A platform technology of recovery of lactic acid from a fermentation broth of novel substrate Zizyphus oenophlia. 3 Biotech 2015, 5, 455–463. [Google Scholar] [CrossRef]
- Msuya, N.; Minja, R.J.A.; Katima, J.H.Y.; Masanja, E.; Temu, A.K. Separation and Purification of Lactic Acid from Sisal Wastes. Am. J. Chem. 2018, 8, 13–18. [Google Scholar] [CrossRef]
- Arcanjo, M.R.A.; Fernandes, F.A.N.; Silva, I.J. Separation of Lactic Acid Produced by Hydrothermal Conversion of Glycerol Using Ion-Exchange Chromatography. Adsorpt. Sci. Technol. 2015, 33, 139–151. [Google Scholar] [CrossRef]
- Zaini, N.A.M.; Chatzifragkou, A.; Tverezovskiy, V.; Charalampopoulos, D. Purification and polymerisation of microbial D-lactic acid from DDGS hydrolysates fermentation. Biochem. Eng. J. 2019, 150, 107265. [Google Scholar] [CrossRef]
- Ahmad, A.; Othman, I.; Taher, H.; Banat, F. Lactic acid recovery from date pulp waste fermentation broth by ions exchange resins. Environ. Technol. Innov. 2021, 22, 101438. [Google Scholar] [CrossRef]
- Vecino, X.; Reig, M.; Valderrama, C.; Cortina, J.L. Ion-Exchange Technology for Lactic Acid Recovery in Downstream Processing: Equilibrium and Kinetic Parameters. Water 2021, 13, 1572. [Google Scholar] [CrossRef]
- Pleissner, D.; Schneider, R.; Venus, J.; Koch, T. Separation of lactic acid and recovery of salt-ions from fermentation broth. J. Chem. Technol. Biotechnol. 2017, 92, 504–511. [Google Scholar] [CrossRef]

| Properties | Amberlite IR 120 | Amberlite IRA 67 | Purolite C10SH | Purolite A847S |
|---|---|---|---|---|
| Description | Strong cationic | Weak anionic | Strong acid cationic | Weak basic anionic |
| T max. | 121 °C | ≤60 °C | 120 °C | 40 °C |
| Matrix | Styrene divinylbenzene (gel) | Acrylic (gel) | Polystyrene/divinylbenzene | Polyacrylic gel/divinylbenzene |
| Functional group | Sulfonic acid | Polyamine | Sulfonic acid | Tertiary amine |
| Particle size | 620–830 μm | 500–750 μm | 425–1200 µm | 425–1200 µm |
| Humidity | 53–58% | ~60% | 54–59% | 56–62% |
| Time (h) | pH | TS (g/L) | LA (g/L) | Productivity (g/L∙h) | YP/S | LA Mass Recovery |
|---|---|---|---|---|---|---|
| 0 | 6.53 | 230.67 ± 0.00 | – | – | – | – |
| 24 | 6.52 | 146.62 ± 0.00 | 73.49 ± 50.78 | 3.06 | 0.28 | 28% |
| 48 | 6.41 | 136.37 ± 0.00 | 71.73 ± 5.89 | 1.49 | 0.18 | 30% |
| 72 | 6.33 | 161.15 ± 0.00 | 79.16 ± 7.42 | 1.10 | 0.29 | 33% |
| 96 | 6.00 | 156.02 ± 0.00 | 113.74 ± 29.53 | 1.18 | 0.34 | 49% |
| Resins | pH | LA (g/L) | LA (g/L)—wH2O |
|---|---|---|---|
| Purolite | |||
| C10SH | 1–1 | 53.92 ± 5.10 | 15.78 ± 1.12 |
| A847S | 1–6 | 36.35 ± 2.85 | 18.42 ± 2.44 |
| Amberlite | |||
| IR 120 | 1–1 | 34.98 ± 3.40 | 20.83 ± 3.10 |
| IRA 67 | 1–8 | 62.24 ± 5.40 | 14.97 ± 1.88 |
| Resin | Recovery | Efficiency | Adsorption Capacity (mg/mL) |
|---|---|---|---|
| Amberlite IR 120 | 19% | 75.86% | 165 |
| Amberlite IRA 67 | 35% | 57.50% | 32.7 |
| Purolite C10SH | 30% | 62.79% | 136 |
| Purolite A847S | 20% | 74.91% | 21 |
| Resins | LA (g/L) | pH | LA (g/L)—wH2O | Recovery |
|---|---|---|---|---|
| Purolite | ||||
| C10SH and A847S | 144.91–42.38 | 1–4 | 32.15 | 23% |
| Amberlite | ||||
| IR 120 and IRA 67 | 144.91–189.11 | 1–3 | 24.05 | 95% |
| Products | Price * (USD) | Total (USD) | |
|---|---|---|---|
| Activated carbon (1 kg) | 75.60 | Conventional recovery | 225.30 |
| H2SO4 4 M (1 L) | 149.70 | ||
| HCl 1 N (1 L) | 51.86 | Solutions for resins | 94.66 |
| NaOH 1 N (1 L) | 42.80 | ||
| Amberlite IR 120 (500 g) | 71.20 | Amberlite resins | 161.50 |
| Amberlite IRA 67 (1 kg) | 90.30 | Resins + solutions | 256.16 |
| Purolite C10SH (1 L) | 86.64 | Purolite resins | 173.28 |
| Purolite A847S (1 L) | 86.64 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, P.Z.; Vandenberghe, L.P.d.S.; Soccol, C.R. Lactic Acid Production Using Sugarcane Juice as an Alternative Substrate and Purification through Ion-Exchange Resins. Fermentation 2023, 9, 879. https://doi.org/10.3390/fermentation9100879
de Oliveira PZ, Vandenberghe LPdS, Soccol CR. Lactic Acid Production Using Sugarcane Juice as an Alternative Substrate and Purification through Ion-Exchange Resins. Fermentation. 2023; 9(10):879. https://doi.org/10.3390/fermentation9100879
Chicago/Turabian Stylede Oliveira, Priscilla Zwiercheczewski, Luciana Porto de Souza Vandenberghe, and Carlos Ricardo Soccol. 2023. "Lactic Acid Production Using Sugarcane Juice as an Alternative Substrate and Purification through Ion-Exchange Resins" Fermentation 9, no. 10: 879. https://doi.org/10.3390/fermentation9100879
APA Stylede Oliveira, P. Z., Vandenberghe, L. P. d. S., & Soccol, C. R. (2023). Lactic Acid Production Using Sugarcane Juice as an Alternative Substrate and Purification through Ion-Exchange Resins. Fermentation, 9(10), 879. https://doi.org/10.3390/fermentation9100879







