Abstract
A randomized, double-blind, placebo-controlled trial was designed to assess the efficacy of the parabiotic Bifidobacterium breve IDCC 4401, named BBR 4401, for lowering cholesterol levels. The 66 subjects (per protocol set, n = 60) with low-density lipoprotein-cholesterol (LDL-C) levels between 100 mg/dL and 150 mg/dL were enrolled after a 4-week run-in period (e.g., no probiotics, low cholesterol diet and no food affecting lipid profiles). The two groups were prescribed 1 × 1010 (low-dose) and 1 × 1011 CFU (high-dose), whereas the placebo group was prescribed 97% (w/w) of maltodextrin for 4 weeks. The compliance rates exceeded 97% in the subjects who completed the study. Comparison of the mean changes from baseline between the placebo group and test groups after the 12 weeks of BBR 4401 consumption showed a statistically significant reduction in LDL-C (up to −10.8%, p-value = 0.008) and apolipoproteinB (up to −8.1%, p-value = 0.008). Meanwhile, there were no clinically significant changes in vital signs, clinical pathology tests or electrocardiograms and no significant adverse events were reported during the study period. Concerning bowel habits, the consumption of BBR 4401 alleviated defecation strain, distension and watery feces in the high-dose group. Thus, BBR 4401 may be a safe and functional food for adults with moderate hypercholesterolemia.
1. Introduction
Cholesterol is a ubiquitous component of the body and its main function is to regulate membrane integrity, fluidity and microstructure. It also plays an important role as a precursor of steroid hormones, bile acids and vitamin D [1,2]. Although cholesterol deficiency, called “hypolipidemia” (i.e., a very low level of HDL and LDL), rarely causes serious illness [3], excessive cholesterol accumulation, called “hyperlipidemia”, is considered a higher risk factor for cardiovascular disease and chronic kidney disease [4]. Typically, cholesterol levels are balanced by regulating its influx, efflux and synthesis in enterocytes and hepatocytes [5]. However, cholesterol accumulation increases ROS levels in the mitochondria of hepatocytes owing to mGSH depletion and causes the secretion of TGF-β in Kupffer cells, resulting in inflammation. Subsequently, increased TGF-β is sensitized by stellate cells, which induces liver fibrogenesis [6]. With regard to cardiovascular disease, excessive cholesterol (i.e., LDL-C, low-density lipoprotein cholesterol) frequently causes artery-clogging plaque in the blood [7]. Although chemical-based therapeutic drugs (e.g., statins, fibrates and niacin) have been developed to lower cholesterol levels, their long-term adverse effects include liver enzyme abnormalities, increased blood glucose levels and muscle pain [8,9]. Thus, alternatives (e.g., food supplements) with lower costs and fewer adverse events are being considered.
Recently, probiotics have been introduced as cholesterol-lowering agents [10], which are live microorganisms that confer health benefits when administered in sufficient amounts [11]. The mechanisms underlying cholesterol-lowering by probiotics are briefly explained by (1) BSH (bile salt hydrolase) activity of probiotics (e.g., the genus Lactobacillus) for inhibition of readsorption of free bile acid (insoluble form), resulting in the synthesis of bile acids from cholesterol in the liver; (2) the cell wall of probiotics capable of incorporating cholesterol, causing depletion of cholesteryl ester from LDL particles; and (3) enzymatic conversion of cholesterol into coprostanol (excretable form) by probiotics. Based on these mechanisms, early studies have suggested that the cholesterol-lowering effect of probiotics could be due to the components of probiotic bacteria. Thereafter, studies of cell walls of probiotics (or dead cells) concerning cholesterol-lowering effects have been reported [12,13,14].
Indeed, components of probiotics and their metabolites have been proven to exert beneficial effects in many other fields, such as gut health, immunity, obesity and skin health [15,16]. In 2022, a postbiotic was newly defined as “a preparation of inanimate microorganisms and/or their components that confers a health benefit on the host [17]”. The inanimate form of probiotics is now called “parabiotics,” long recognized as “dead cells” or “cell walls”. Parabiotics are advantageous because they have direct and specific mechanisms of action and better interactions with pattern recognition receptors [18]. Previously, we selected the parabiotic BBR 4401 for its potential to improve hypercholesterolemia based on the following criteria: (1) cholesterol-scavenging ability; (2) serum and hepatic lipid profiles; and (3) fecal bile acid profiles in a hypercholesterolemia-induced rat model [19,20]. Next, we further proved that BBR 4401 improved dyslipidemia in rat models by showing (1) increased bile acid content in feces; (2) increased expression levels of LDL-receptor, bile acyl-CoA synthetase and CYP7α1 in the liver; and (3) decreased apoA and apoB contents in blood and liver, respectively [19,21]. Consequently, we demonstrated that the cholesterol-lowering cascade is triggered by the cohesion of bile acids and cholesterol with BBR 4401.
We conducted a randomized, double-blind, placebo-controlled clinical trial to evaluate the cholesterol-lowering efficacy of BBR 4401 in adults with moderate hypercholesterolemia. Sixty-one subjects were prescribed low- and high-dose BBR 4401 (1 × 1010 and 10 × 1011 CFU of parabiotics, respectively) for 12 weeks. Blood lipid and apolipoprotein profiles were measured. Furthermore, adverse events and bowel habit changes caused by BBR 4401 consumption were investigated. Therefore, this study has significant implications that might lead to the further development of parabiotics for health benefits.
2. Materials and Methods
2.1. Process of Manufacturing for Investigation Product
For seed culture, 1% (v/v) of glycerol stock of Bifidobacterium breve IDCC 4401 was suspended and statistically incubated in De Man, Rogosa and Sharpe (MRS) broth at 37 °C in a 40 L fermenter for 18 h. An amount of 10% (v/v) of the seed culture was transferred into a commercial medium (data not shown) and incubated in a 400 L fermenter with 30 rpm for 10 h. The main culture was performed with 2% of preculture under the conditions mentioned for 18 h in a 16 kL fermenter (Figure 1). The biomass and culture media were separated by continuous centrifugation at a constant rate of 8 kL/h and 8000 rpm, yielding 290 kg of B. breve IDCC 4401 with an approx. 52% (w/w) moisture content. Next, the obtained cells were treated at 80 °C for 8 h to collect heat-killed and concentrated cells. The heat-killed cells were mixed with 10% (v/v) maltodextrin of total volume in a 1 kL mixer and freeze-dried at a temperature range of −80–25 °C under less than −300 mmHg. Finally, the heat-killed cells were grilled, finely ground and sieved to obtain <50 μm particles. The particles were then encapsulated and the compositions of the investigational and placebo products are listed in Table S1.
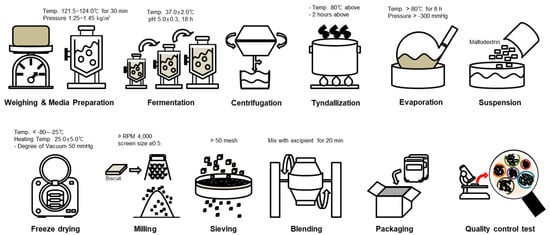
Figure 1.
The manufacturing process for investigational products.
2.2. Subjects and Study Design
This double-blind, parallel-group, placebo-controlled, randomized trial was conducted between May 2018 and October 2019 at the Seoul National University Hospital, Seoul, Korea. The participants were informed of the purpose, expected effects, unexpected side effect(s) and study method before giving their consent. The hospital recruited the subjects via advertisements (e.g., subway stations), and those who consented to participation and signed a written informed consent form were included in this study. Individuals qualified for randomization met the following criteria: (1) age group ≥ 20 years; (2) LDL-cholesterol group ≥ 100 mg/dL and group ≥ 150 mg/dL; and (3) not using drugs or health-functional foods that influence lipid metabolism within one month before the screening visit. The exclusion criteria were as follows: individuals with (1) cardiovascular, renal or hepatic disease and diabetes (fasting glucose ≥ 180 mg/dL), hypertension, endocrine disorders, autoimmune disease, gastrointestinal disorder or cancer; (2) hypertriglyceridemia (≥500 mg/dL); (3) hypersensitivity associated with probiotics; (4) alcohol abuse, pregnancy or breastfeeding; and (5) participation in other studies within one month. Eligibility criteria were reviewed at −4 weeks. After a 4-week run-in period, individuals following low total fat, saturated fat and cholesterol diets were enrolled before the randomization process.
The trial consisted of two phases: a 4-week baseline run-in observation period (weeks −4 to 0); a 12-week intervention period (weeks 0 to 12) (Figure 2). All participants completed a series of questionnaires [22,23] and the Schedule for data collection are summarized in Table 1.
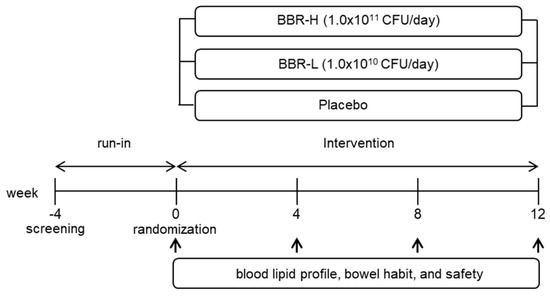
Figure 2.
A flow diagram illustrating the study design.

Table 1.
Schedule for data collection.
All participants were enrolled based on the inclusion and exclusion criteria and randomly divided into 3 parallel groups of 22 people using a “computer generated random list” with a blocked randomization method (Figure 3). After allocation, six participants were excluded because of withdrawal of consent or participation in another clinical trial. Participants were prescribed a low dose of B. breve IDCC 4401 parabiotics (BBR-L, 1.0 × 1010 CFU/day), a high dose of parabiotics (BBR-H, 1.0 × 1011 CFU/day) and placebo products. Postentry visits were conducted at the end of weeks 4, 8 and 12. Moreover, compliance conformed to the percentage of remnants of investigational products returned. The full analysis set was defined as 61 participants who had visited at week 4, while the per-protocol set was defined as 60 participants who completed the clinical test. The safety set was defined as 66 participants who had consumed investigation products at least 1 time after randomization.
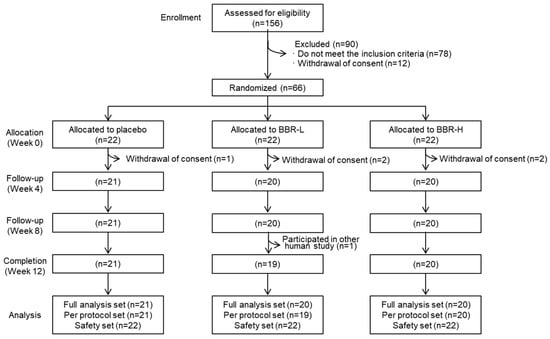
Figure 3.
Consort diagram for the flow of subjects throughout the study. BBR-L, low dose of B. breve IDCC 4401 parabiotics (1.0 × 1010 CFU/day); BBR-H, high dose of B. breve IDCC 4401 parabiotics (1.0 × 1011 CFU/day).
2.3. Statistical Analysis
All the statistical analyses were performed using SAS (version 9.4, SAS Institute Inc., Cary, NC, USA). One-way analysis of variance (ANOVA), chi-square test, McNemar’s test and Fisher’s exact test were performed to compare the significance, relationship and differences between (or within) continuous and categorical independent variables. Compliance of the groups was analyzed using ANOVA. A linear mixed-effects model was used to analyze the continuous variables of dietary intake, physical activity, functionality (i.e., lipid and apolipoprotein profiles) and safety. Furthermore, McNemar’s and Fisher’s exact tests (or Chi-square tests) were used to compare values from blood and urine samples and adverse events within (independent of each group) and between groups, respectively. Statistical significance was determined when the p-value was <0.05.
2.4. Metabolites Analysis
Lyophilized B. breve IDCC 4401 parabiotic (0.1 g) was suspended in 1 mL of ice-cold methanol and sonicated for 1 h, whereas 20 mL of the culture medium (control) was diluted in 1 mL of ice-cold methanol and sonicated for 1 h, followed by concentration in a vacuum concentrator. Two hundred microliters of supernatants were collected and concentrated using a vacuum concentrator, followed by centrifugation at 13,000× g for 10 min at 4 °C. The samples were derivatized by the addition of 50 μL of 20 mg/mL methoxyamine hydrochloride in pyridine (Sigma-Aldrich, Burlington, MA, USA) and incubated at 30 °C for 90 min. Then, the samples were added with 50 uL of N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA; Sigma-Aldrich) and further incubated at 30 °C for 90 min. A mixture of alkane standards (Sigma-Aldrich) and fluoranthene (Sigma-Aldrich) was used as the retention index and internal standard, respectively. The GC-MS analysis was performed using a Thermo Trace 1030 GC-MS (Trace 1030) (ThermoFisher, Waltham, MA, USA) coupled with an ISQ LT single quadrupole mass spectrometer (ThermoFisher Scientific, Waltham, MA, USA). The derivatized sample (0.1 μL) was injected into the GC system equipped with a DB-5MS capillary column (60 m length, 0.25 mm ID, 0.25 μm film thickness) (Agilent, Santa Clara, CA, USA) at 300 °C in a 1:20 split ratio. Metabolites were separated using a helium flow of 1.5 mL using the following oven temperature program: 50 °C for 2 min, 50 °C to 180 °C at a rate of 5 °C/min, 180 °C for 8 min, 180 °C to 210 °C at a rate of 2.5 °C/min, 210 °C to 320 °C at a rate of 5 °C/min and 320 °C for 20 min. Mass spectra were acquired in the mass range at 35–650 m/z in electron impact ionization mode (70 eV) and ion source temperature of 270 °C. MS-DIAL software (version 4.92) was used for the deconvolution of mass spectrometry (MS) data and identification of metabolites. The latest FienhnLib RI Libraries provided by MS-DIAL were used to identify metabolites by matching MS peaks. Hierarchical cluster analysis (HCA) and volcano plots were generated using MetaboAnalyst version 5.0.
3. Results and Discussion
3.1. Lipids and Apolipoprotein Profiles
The baseline characteristics of the study participants are presented in Table S2. The number of participants in the placebo, BBR-L (low-dose) and BBR-H (high-dose) groups were 22, 22 and 22, respectively, and the ages were 52.1 ± 2.9, 51.5 ± 1.6 and 50.8 ± 2.5 years, respectively. LDL-cholesterol was 124.7 ± 2.0 mg/dL, 131.1 ± 2.8 mg/dL and 127.4 ± 3.0 mg/dL, respectively, and total cholesterol was 201.0 ± 2.4 mg/dL, 212.8 ± 4.2 mg/dL and 207.8 ± 5.7 mg/dL, respectively (Table S2). There were no significant differences in any baseline characteristics between the groups. The compliance, including taking investigational products and following a diet, was measured at >97% in all 3 groups after 12 weeks (Table S3). Furthermore, dietary intake, including carbohydrates, protein, fat, fatty acids, cholesterol and physical activity, are shown in Table S4. None of the variables differed significantly between groups. Higher compliance (i.e., >80%) in clinical trials generally indicates that the primary assessment variable to determine the efficacy, reproducibility and transparency of an investigational product is reliable [24]. Based on insignificant baseline characteristics and high compliance, it was implied that this study is conducted under systematic control by investigators and that the primary variable of BBR 4401 is reliable in the analysis stage.
With regard to lipid profiles, LDL-cholesterol was significantly decreased compared to the placebo group in the low- and high-dose groups at weeks 4–12 (Figure 4). More specifically, 8.1% and 10.8% of LDL-cholesterol were decreased compared to the placebo group at 12 weeks. As expected, apoB showed a significant decrease in both the low- and high-dose groups after 12 weeks (Figure 5). A clinical study exploring the cholesterol-lowering effects of Lactobacillus paracasei N1115 in adults with dyslipidemia also showed a decrease in LDL-cholesterol and apoB [25]. In this study, the significant decrease in LDL-cholesterol and apoB clearly supports the cholesterol-lowering effect of BBR 4401. Statins, a hyperlipidemia drug, can reduce LDL-cholesterol levels by 15% to 20% [26,27]. Based on an approximately 10% reduction in LDL-cholesterol, it can be suggested that BBR 4401 should be a functional food to reduce blood cholesterol levels.
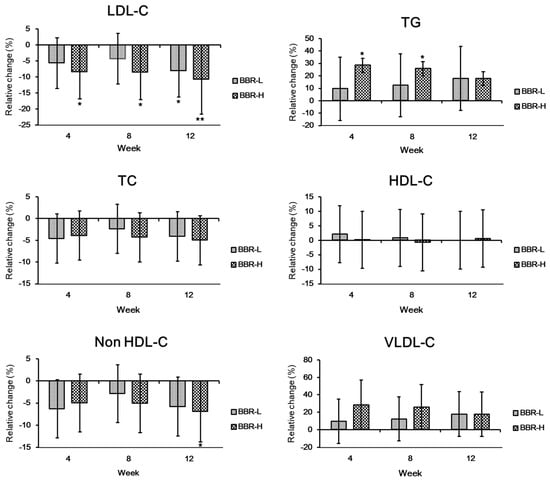
Figure 4.
Changes in lipid profile by BBR 4401. Data were expressed as mean ± standard deviation. Significant differences compared to the placebo group are indicated as * (p < 0.05) and ** (p < 0.01). BBR-L, low dose of B. breve IDCC 4401 parabiotics (1.0 × 1010 CFU/day); BBR-H, high dose of B. breve IDCC 4401 parabiotics (1.0 × 1011 CFU/day). Abbreviations: LDC-C, low-density lipoprotein-cholesterol; TG, triglyceride; TC, total cholesterol; HDL-C, high-density lipoprotein-cholesterol; VLDL-C, very-low-density lipoprotein-cholesterol.
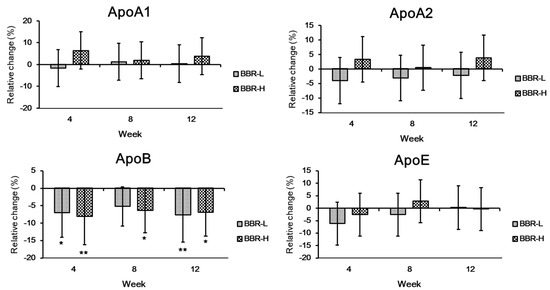
Figure 5.
Changes in apolipoprotein profile by BBR 4401. Data were expressed as mean ± standard deviation. Significant differences compared to the placebo group are indicated as * (p < 0.05) and ** (p < 0.01). Abbreviations: Apo, apolipoprotein.
In contrast, triglyceride (TG) increased in the high-dose group at 4 and 8 weeks (Figure 4), whereas non-HDL-cholesterol decreased only at 12 weeks. Given that the normal range of TG in the blood is less than 150 mg/dL [28], the increased concentration of TG, 128.7 mg/dL, in the high-dose group would not be an issue. Additionally, to decrease blood TG levels, a reduction in the non-HDL-cholesterol level is recommended by Adult Treatment Panel III guidelines of the National Cholesterol Education Program (NCEP) [29]. Considering that trends in TG level deescalated from 0 to 12 weeks (Figure 4), BBR 4401 consumption might be suggested for more than 12 weeks, with a significant decrease in TG levels expected.
Typically, VLDL-cholesterol comprises abundant TG and its secretion from the liver into the blood increases blood TG levels [30]. Although overall TG and VLDL-cholesterol showed a slight increase compared to the placebo group (p > 0.05), both TG and VLDL-cholesterol tended to decrease in the high-dose group during the prescription. Thus, it can be assumed that the liver regulates the LDL-cholesterol level, which senses TG and VLDL-cholesterol [31]. Meanwhile, ratios of TC/HDL-c, TG/HDL-c, non-HDL-c/HDL-c and LDL-c/ApoB did not significantly decrease, whereas those of LDL-c/HDL-c and ApoB/ApoA1 were significantly decreased by 12.2% (p = 0.018) and 11.2% (p = 0.029) at 4 weeks and 12 weeks, respectively, only in the high-dose group.
3.2. Metabolites Analysis
To elucidate the mechanism underlying the cholesterol-lowering effects of BBR 4401, a metabolite analysis was performed using BBR 4401 and its culture medium (negative control). A total of 108 metabolites were identified, including amino acids, sugars, organic acids, fatty acids and polyamines. The metabolic profiles of BBR 4401 and the negative control were clearly separated using hierarchical clustering analysis (Figure 6A). Among the 108 metabolites, 86 were significantly decreased, while 14 were significantly increased in BBR 4401 compared to those in the control. The metabolites with increased metabolites were 2-hydroxyhexanoic acid, 2-methyl-nonadecane, α-lactose, D-3-phenyllactic acid,-hydroxybutyric acid, D-xylulose, galactitol, lactic acid, l-ascorbic acid, L-iditol, ribitol, shikimic acid and threitol (Figure 6B). Meanwhile, no distinct clues were found in the decreased metabolites regarding the cholesterol-lowering effect of BBR 4401.
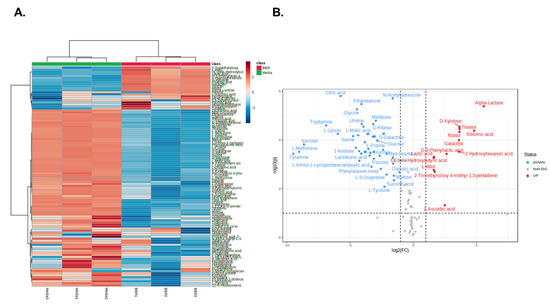
Figure 6.
Comparison of metabolites profiling of BBR 4401 and its culture medium. HCA (A) and a volcano plot (B) for significantly altered metabolites in BBR 4401.
L-ascorbic acid supplementation lowers the plasma cholesterol concentration in hypercholesterolemic adults with low vitamin C and its recommended concentration is at least 500 mg/day for 3–24 weeks [32]. Although the role of L-ascorbic acid is still arguable, its cholesterol-lowering effects might be due to its antioxidant properties, which are involved in essential metabolic pathways [33]. In previous study, the metabolomic analysis indicated that shikimic acid levels dramatically increased following statin administration in adults with hypercholesterolemia [34]. Shikimic acid is an enterobacteria-derived precursor of aromatic and indole-containing amino acids [35], implying that cholesterol reduction is associated with gut microbiome modulation. Furthermore, shikimic acid is reported to suppress lipid accumulation in 3T3L-1, HepG2 and Huh7 and down-regulate mRNA expression of lipogenesis-related genes such as SREBP-1c, LXR-α and FAS in HepG2 cells [36]. Previously, mice were fed either the standard diet or a high-fat diet (HFD) with or without dark-phase restricted feeding (12 h) in a spontaneous metastasis model of Lewis lung carcinoma (LLC) [37]. As expected, the number and size of lung metastases were significantly greater in the HFD group than in the standard diet group. However, time-restricted feeding (TRF) significantly attenuates high-fat diet-enhanced spontaneous metastasis. Through blood analysis, it was found that HFD-induced glucose, proinflammatory cytokines and angiogenic factors were significantly decreased in the TRF group. Furthermore, 2-hydroxyhexanoic acid significantly increased metabolite in BBR 4401, which also increased in the time-restricted feeding (TRF) group with the HFD diet. Thus, BBR 4401 containing 2-hydroxyhexanoic acid might play an important role in obesity-associated symptoms along with a cholesterol-lowering effect.
3.3. Changes in Bowel Habits
Considering that (1) BBR 4401 hydrolyzes conjugated bile, promoting de novo synthesis of bile acids from cholesterol and (2) BBR 4401 interacts with cholesterol physiochemically, resulting in the excretion of cholesterol into feces [21], BBR 4401 may induce adverse events such as indigestion and colic [38]. Thus, we investigated whether BBR 4401, originating from the Bifidobacterium breve 4401 probiotic, would improve gut health using a bowel activity questionnaire including abdominal pain, discomfort, distention, incomplete evacuation and the Bristol Stool chart [22,23]. The results showed that defecation strain, distension and runny feces significantly improved after a 12-week prescription of BBR 4401 in the low- and high-dose groups, except for the defecation strain (only in the high-dose group) (Figure 7). This result may be explained by the beneficial effects of the heat-killed cells and their components. For example, heat-killed Lactobacillus rhamnosus GG exhibited anti-inflammatory effects in a rat model, and heat-killed Lactobacillus brevis SBC8803 ameliorated intestinal injury by enhancing the intestinal barrier in a colitis mouse model [39,40]. Furthermore, the membrane surface proteins of Lactiplantibacillus plantarum 423 exert antiadhesion effects against pathogens in Caco-2 cells [41].
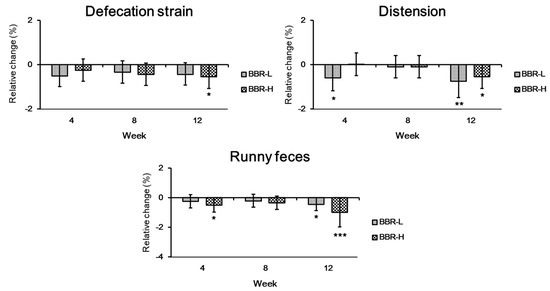
Figure 7.
Changes in bowel habits by BBR 4401. Data were expressed as mean ± standard deviation. Significant differences compared to the placebo group are indicated as * (p < 0.05), ** (p < 0.01) and *** (p < 0.001). BBR-L, low dose of B. breve IDCC 4401 parabiotics (1.0 × 1010 CFU/day); BBR-H, high dose of B. breve IDCC 4401 parabiotics (1.0 × 1011 CFU/day).
Regarding safety, there were no clinically significant changes in vital signs, clinical pathology tests or electrocardiograms in either the low- or high-dose groups and no significant adverse reactions were reported (Table S5); thus, BBR 4401 was regarded as a safe food supplement.
4. Conclusions
The relationship between the homeostasis of gut microbiota and human health becomes more important according to published articles in the last decades. In this study, BBR 4401 (parabiotic of Bifidobacterium breve IDCC 4401) exerted a cholesterol-lowering effect in adults with moderate hypercholesterolemia as well as an improvement in bowel activity. Thus, these results suggested BBR 4401 as a multifunctional food supplement for human health.
Supplementary Materials
The following supporting information can be downloaded from: https://www.mdpi.com/article/10.3390/fermentation9080766/s1, Table S1: Composition of BBR 4401 and placebo products; Table S1: Baseline Characteristics; Table S3: Compliance; Table S4: Dietary intake and physical activity; Table S5: Adverse events.
Author Contributions
M.K.; Y.-J.K.; H.-J.K.: Conceptualization, M.K.; M.L.; H.K.; J.Y.: Writing Original Draft, M.K.; M.L.; M.-G.K.; H.K.; B.C.; S.K.; W.-Y.B.: Methodology, Visualization, Investigation, Data curation, J.Y.; Y.-J.K.; H.-J.K.: Writing—review and editing, Y.-J.K.; H.-J.K.: Supervision; Project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ildong Pharmaceutical Co., Ltd., Seoul, Republic of Korea.
Institutional Review Board Statement
This study was approved by the Institutional Review Board of Seoul National University College of Medicine-Seoul National University Hospital (SNUCM-SNUH IRB, approval number: H-1802-104-925) and registered at the CRIS (Clinical Research Information Service) under registration number KCT003040.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
Mincheol Kim and Min-Goo Kim served as a senior and a junior researcher in Ildong Pharmaceutical, respectively.
References
- Paukner, K.; Králová Lesná, I.; Poledne, R. Cholesterol in the cell membrane-an emerging player in atherogenesis. Int. J. Mol. Sci. 2022, 23, 533. [Google Scholar] [CrossRef] [PubMed]
- Craig, M.; Yarrarapu, S.N.S.; Dimri, M. Biochemistry, Cholesterol. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Elmehdawi, R.R. Hypolipidemia: A word of caution. Libyan J. Med. 2008, 3, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Sahadevan, M.; Kasiske, B.L. Hyperlipidemia in kidney disease: Causes and consequences. Curr. Opin. Nephrol. Hypertens. 2002, 11, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.W.; Zheng, L.; Wang, Q. Regulation of cholesterol homeostasis by liver X receptors. Clin. Chim. Acta 2010, 411, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Gambino, R.; Cassader, M. Cholesterol metabolism and the pathogenesis of non-alcoholic steatohepatitis. Prog. Lipid Res. 2013, 52, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Nair, P. Brown and Goldstein: The cholesterol chronicles. Proc. Natl Acad. Sci. USA 2013, 110, 14829–14832. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R. Cholesterol lowering drugs. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Boyce, A., Blackman, M.R., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Kalra, S., Kaltsas, G., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2020. [Google Scholar]
- Taher, T.H.; Dzavik, V.; Reteff, E.M.; Pearson, G.J.; Woloschuk, B.L.; Francis, G.A. Tolerability of statin-fibrate and statin-niacin combination therapy in dyslipidemic patients at high risk for cardiovascular events. Am. J. Cardiol. 2002, 89, 390–394. [Google Scholar] [CrossRef]
- Jones, M.L.; Tomaro-Duchesneau, C.; Martoni, C.J.; Prakash, S. Cholesterol lowering with bile salt hydrolase-active probiotic bacteria, mechanism of action, clinical evidence, and future direction for heart health applications. Expert Opin. Biol. Ther. 2013, 13, 631–642. [Google Scholar] [CrossRef]
- Williams, N.T. Probiotics. Am. J. Health Syst. Pharm. 2010, 67, 449–458. [Google Scholar] [CrossRef]
- Ishimwe, N.; Daliri, E.B.; Lee, B.H.; Fang, F.; Du, G. The perspective on cholesterol-lowering mechanisms of probiotics. Mol. Nutr. Food Res. 2015, 59, 94–105. [Google Scholar] [CrossRef]
- Ooi, L.G.; Liong, M.T. Cholesterol-lowering effects of probiotics and prebiotics: A review of in vivo and in vitro findings. Int. J. Mol. Sci. 2010, 11, 2499–2522. [Google Scholar] [CrossRef] [PubMed]
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-parabiotics: The new horizons in microbial biotherapy and functional foods. Microb. Cell Fact. 2020, 19, 168. [Google Scholar] [CrossRef]
- Park, D.H.; Kim, J.W.; Park, H.J.; Hahm, D.H. Comparative analysis of the microbiome across the gut–skin axis in atopic dermatitis. Int. J. Mol. Sci. 2021, 22, 4228. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Kim, G.; Noh, M.G.; Park, J.H.; Jang, M.; Fang, S.; Park, H. Lactobacillus fermentum promotes adipose tissue oxidative phosphorylation to protect against diet-induced obesity. Exp. Mol. Med. 2020, 52, 1574–1586. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, R.A.; Reale, A.; Mazzeo, M.F.; Morandi, S.; Silvetti, T.; Brasca, M. Paraprobiotics: A new perspective for functional foods and nutraceuticals. Nutrients 2021, 13, 1225. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.C.; Kim, M.G. The mechanism of blood cholesterol-lowering action of parabiotics. Food Suppl. Biomater. Health 2023, 3, e1. [Google Scholar] [CrossRef]
- Kim, M.G.; Lee, D.G.; Kim, T.Y. Development of heat-killed Bifidobacterium breve IDCC 4401 with the potential to improve hypercholesterolemia in rats. Korean J. Microbiol. 2020, 56, 307–314. [Google Scholar]
- Joo, I.-H.; Kim, M.-G.; Park, J.-M.; Min, G.-Y.; Han, S.-H.; Lee, S.-B.; Sim, B.-Y.; Kim, J.-H.; Kim, D.-H. Bifidobacterium breve strain IDCC 4401 improves dyslipidemia in rat model. Int. J. Clin. Exp. Med. 2020, 13, 4137–4144. [Google Scholar]
- Zhuang, X.; Tian, Z.; Li, L.; Zeng, Z.; Chen, M.; Xiong, L. Fecal microbiota alternations associated with diarrhea-predominant irritable bowel syndrome. Front. Microbiol. 2018, 9, 1600. [Google Scholar] [CrossRef]
- O’Donnell, L.J.; Virjee, J.; Heaton, K.W. Detection of pseudodiarrhoea by simple clinical assessment of intestinal transit rate. BMJ 1990, 300, 439–440. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-J.; Na, H.-O.; Kim, Y.-I.; Kim, K.-S. The factors affecting subject’s compliance in a clinical study. Transl. Clin. Pharmacol. 2009, 17, 61–71. [Google Scholar] [CrossRef]
- Jiang, H.; Tan, S.; Ning, K.; Li, H.; Zhao, W.; Zhao, A.; Zhu, H.; Wang, S.; Wang, P.; Zhang, Y. Effects of Lactobacillus paracasei N1115 on dyslipidaemia: A randomized controlled study. J. Funct. Foods 2022, 89, 104956. [Google Scholar] [CrossRef]
- Zhou, Z.; Rahme, E.; Pilote, L. Are statins created equal? Evidence from randomized trials of pravastatin, simvastatin, and atorvastatin for cardiovascular disease prevention. Am. Heart J. 2006, 151, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Weng, T.C.; Yang, Y.H.K.; Lin, S.J.; Tai, S.H. A systematic review and meta-analysis on the therapeutic equivalence of statins. J. Clin. Pharm. Ther. 2010, 35, 139–151. [Google Scholar] [CrossRef]
- Kelly, M.S.; Beavers, C.; Bucheit, J.D.; Sisson, E.M.; Dixon, D.L. Pharmacologic approaches for the management of patients with moderately elevated triglycerides (150–499 mg/dl). J. Clin. Lipidol. 2017, 11, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Capuzzi, D.M.; Morgan, J.M.; Weiss, R.J.; Chitra, R.R.; Hutchinson, H.G.; Cressman, M.D. Beneficial effects of rosuvastatin alone and in combination with extended-release niacin in patients with a combined hyperlipidemia and low high-density lipoprotein cholesterol levels. Am. J. Cardiol. 2003, 91, 1304–1310. [Google Scholar] [CrossRef]
- Feingold, K.R.; Grunfeld, C. Introduction to lipids and lipoproteins. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Boyce, A., Blackman, M.R., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Kalra, S., Kaltsas, G., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2018. [Google Scholar]
- Chen, W.; Guo, J.X.; Chang, P. The effect of taurine on cholesterol metabolism. Mol. Nutr. Food Res. 2012, 56, 681–690. [Google Scholar] [CrossRef]
- McRae, M.P. Vitamin C supplementation lowers serum low-density lipoprotein cholesterol and triglycerides: A meta-analysis of 13 randomized controlled trials. J. Chiropr. Med. 2008, 7, 48–58. [Google Scholar] [CrossRef]
- Dludla, P.V.; Nkambule, B.B.; Nyambuya, T.M.; Ziqubu, K.; Mabhida, S.E.; Mxinwa, V.; Mokgalaboni, K.; Ndevahoma, F.; Hanser, S.; Mazibuko-Mbeje, S.E.; et al. Vitamin C intake potentially lowers total cholesterol to improve endothelial function in diabetic patients at increased risk of cardiovascular disease: A systematic review of randomized controlled trials. Front. Nutr. 2022, 9, 1011002. [Google Scholar] [CrossRef]
- Trupp, M.; Zhu, H.; Wikoff, W.R.; Baillie, R.A.; Zeng, Z.B.; Karp, P.D.; Fiehn, O.; Krauss, R.M.; Kaddurah-Daouk, R. Metabolomics reveals amino acids contribute to variation in response to simvastatin treatment. PLoS ONE 2012, 7, e38386. [Google Scholar] [CrossRef] [PubMed]
- Catalina, D.; Quiroz, D.; Carmona, B.; Bolívar, F.; Escalante, A. Current perspectives on applications of shikimic and aminoshikimic acids in pharmaceutical chemistry. Res. Rep. Med. Chem. 2014, 4, 35–46. [Google Scholar]
- Kim, M.J.; Sim, D.Y.; Lee, H.M.; Lee, H.J.; Kim, S.H. Hypolipogenic effect of shikimic acid via inhibition of MID1IP1 and phosphorylation of AMPK/ACC. Int. J. Mol. Sci. 2019, 20, 582. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Rust, B.; Picklo, M.J. Plasma metabolomics changes in mice with time restricted feeding-attenuated pulmonary metastasis of Lewis lung carcinoma. Anticancer Res. 2020, 40, 1833–1841. [Google Scholar] [CrossRef] [PubMed]
- Aldridge, M.A.; Ito, M.K. Colesevelam hydrochloride: A novel bile acid-binding resin. Ann. Pharmacother. 2001, 35, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Ueno, N.; Fujiya, M.; Segawa, S.; Nata, T.; Moriichi, K.; Tanabe, H.; Mizukami, Y.; Kobayashi, N.; Ito, K.; Kohgo, Y. Heat-killed body of Lactobacillus brevis SBC8803 ameliorates intestinal injury in a murine model of colitis by enhancing the intestinal barrier function. Inflamm. Bowel Dis. 2011, 17, 2235–2250. [Google Scholar] [CrossRef] [PubMed]
- Ramiah, K.; van Reenen, C.A.; Dicks, L.M. Surface-bound proteins of Lactobacillus plantarum 423 that contribute to adhesion of Caco-2 cells and their role in competitive exclusion and displacement of Clostridium sporogenes and Enterococcus faecalis. Res. Microbiol. 2008, 159, 470–475. [Google Scholar] [CrossRef]
- Li, N.; Russell, W.M.; Douglas-Escobar, M.; Hauser, N.; Lopez, M.; Neu, J. Live and heat-killed Lactobacillus rhamnosus GG: Effects on proinflammatory and anti-inflammatory cytokines/chemokines in gastrostomy-fed infant rats. Pediatr. Res. 2009, 66, 203–207. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).