The Effect of Yeast Inoculation Methods on the Metabolite Composition of Sauvignon Blanc Wines
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Characterization of Sauvignon Blanc Juice
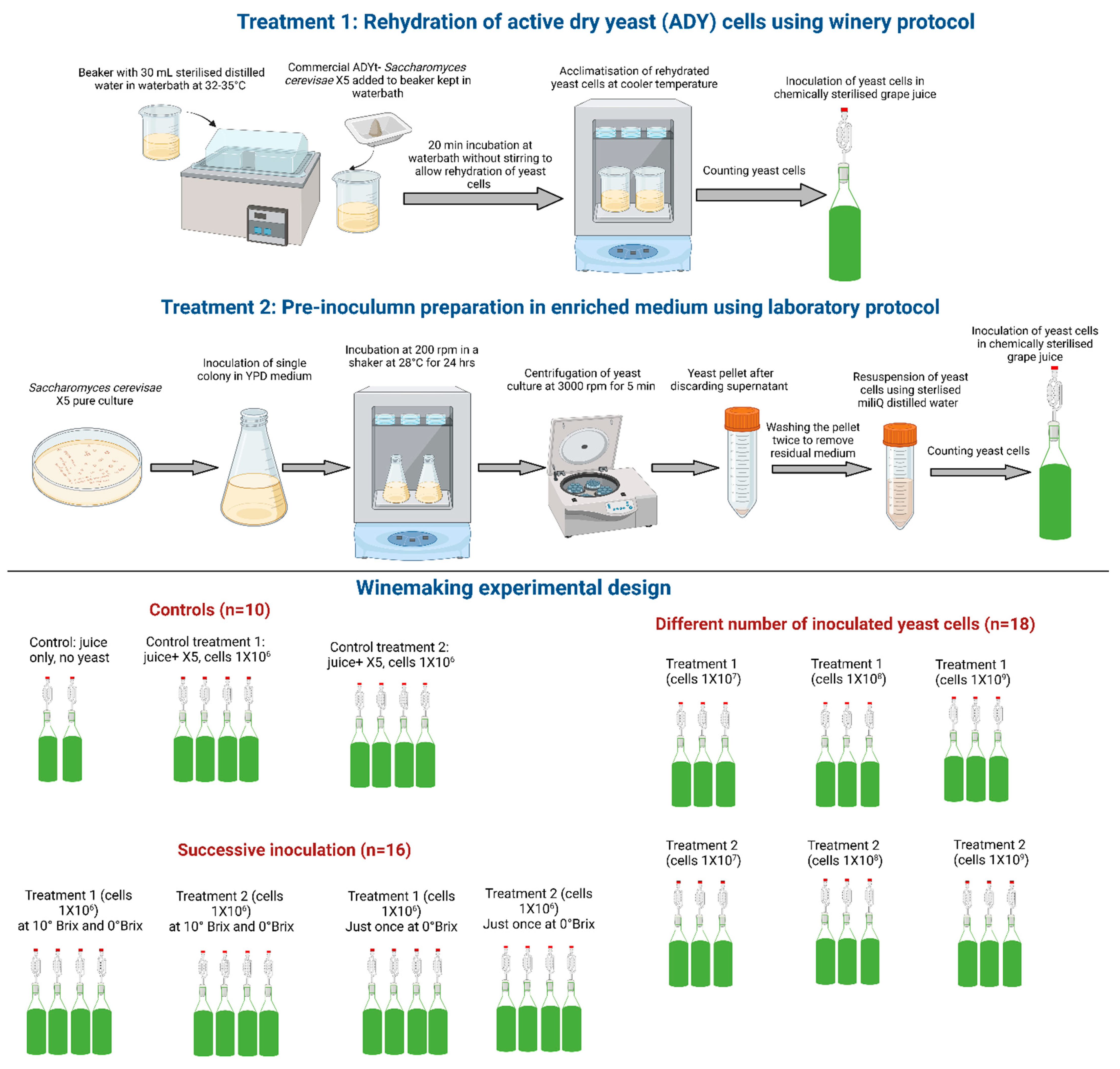
2.2. Different Inoculum Preparation Methods
2.2.1. Rehydration of Commercial ADY (RY)
2.2.2. Preparation of Pre-Inoculum in a Rich Growth Medium (PI)
2.3. Counting of Yeast Cells and Viability Testing
2.4. Experimental Design and Wine Fermentation
2.5. Analysis of the Resulting Wines
2.5.1. Varietal Thiols
2.5.2. Aroma Compounds
2.5.3. Organic Acids
2.5.4. Amino Acids
2.6. Statistical Analysis
3. Results and Discussion
3.1. Fermentation Completion Time Depended on the Type of Inoculation Methods
3.2. No significant Change Was Observed on Different Oenological Properties of the Wines Based on Inoculation Methods
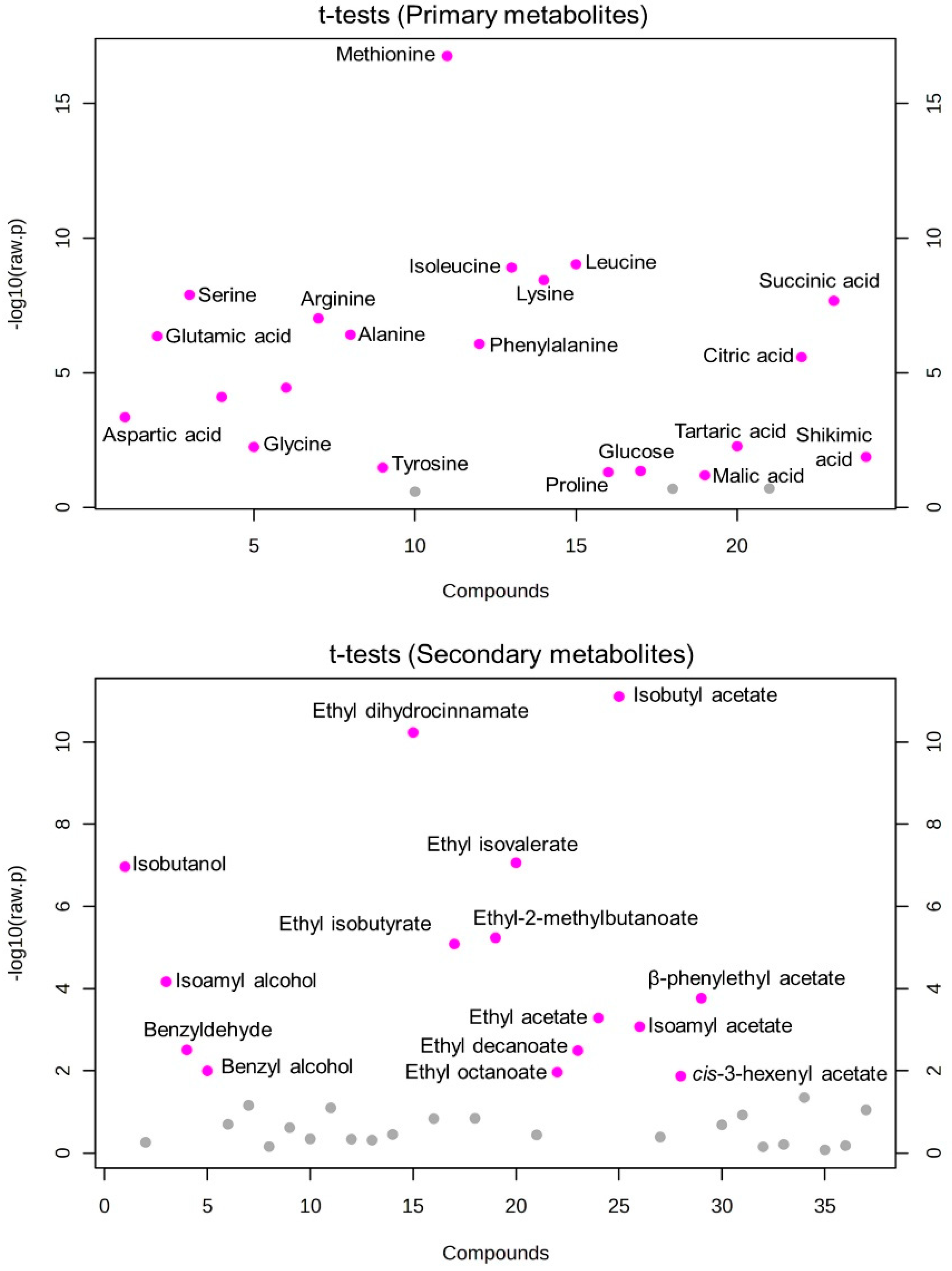
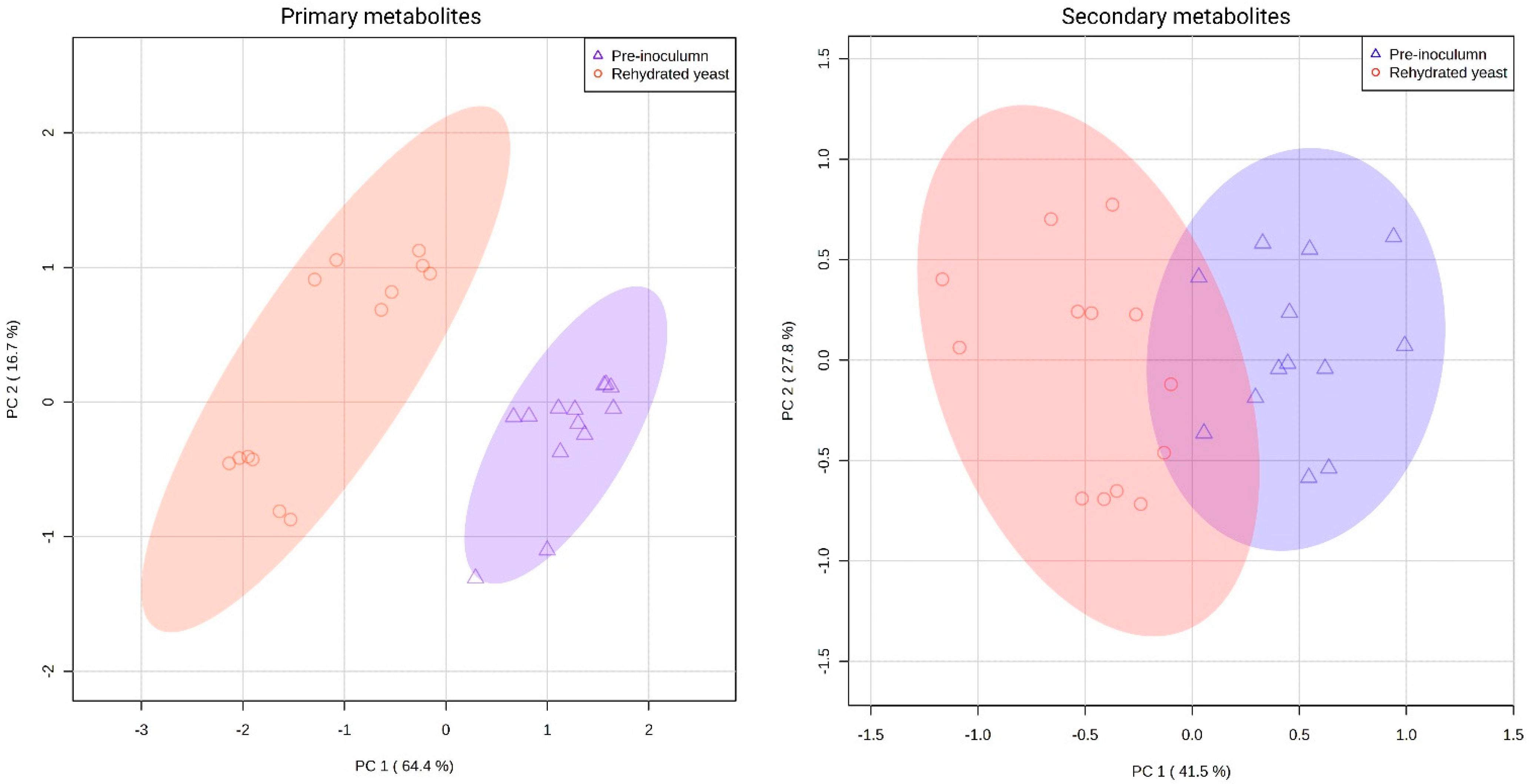
3.3. Inoculation Methods Altered the Overall Metabolite Composition of the Resulting Wines
3.4. Varietal Thiols Are Affected by the Inoculation Methods
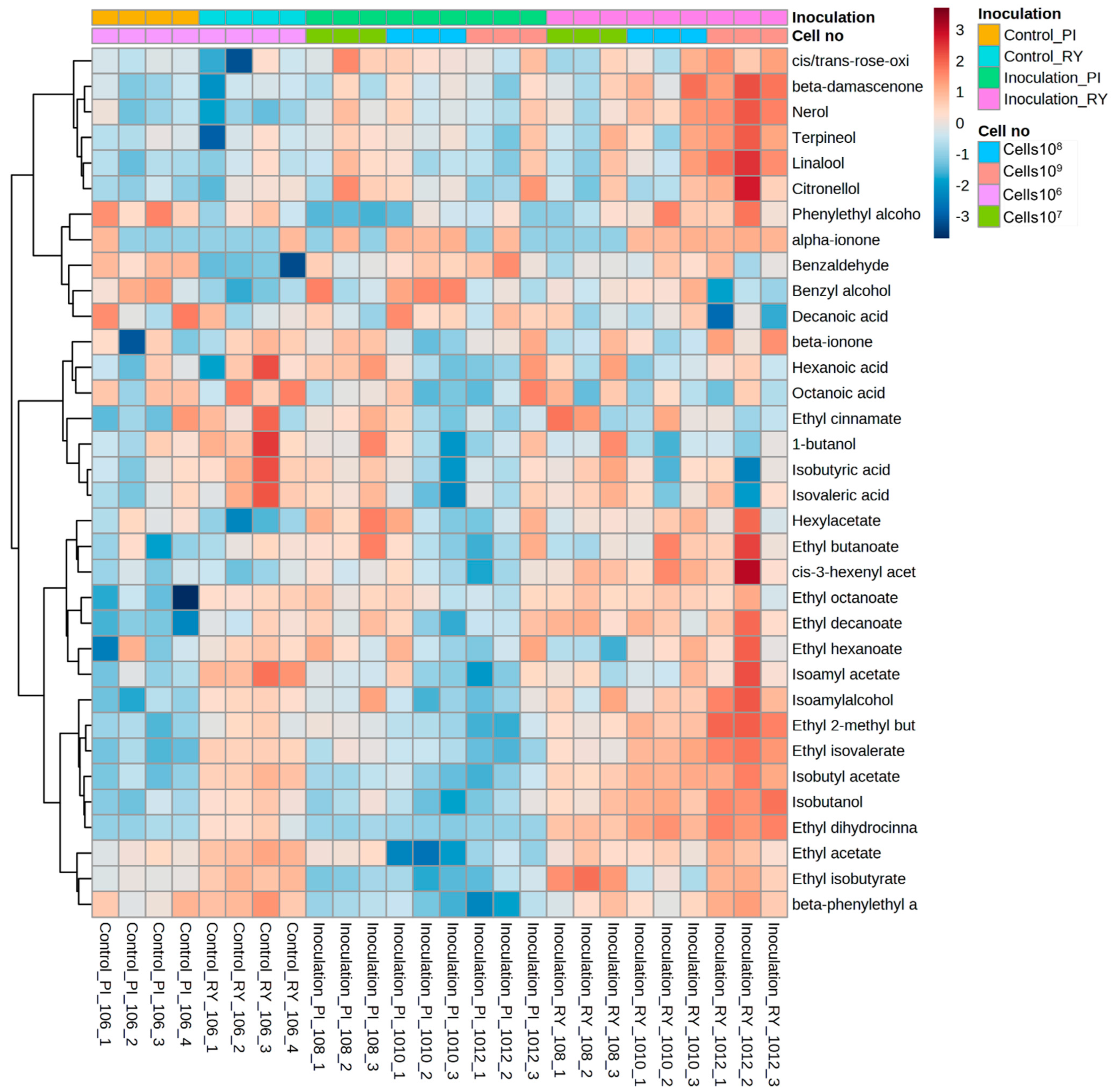
3.5. Interactions between Inoculation Methods and Inoculated Cell Concentrations on Metabolite Composition
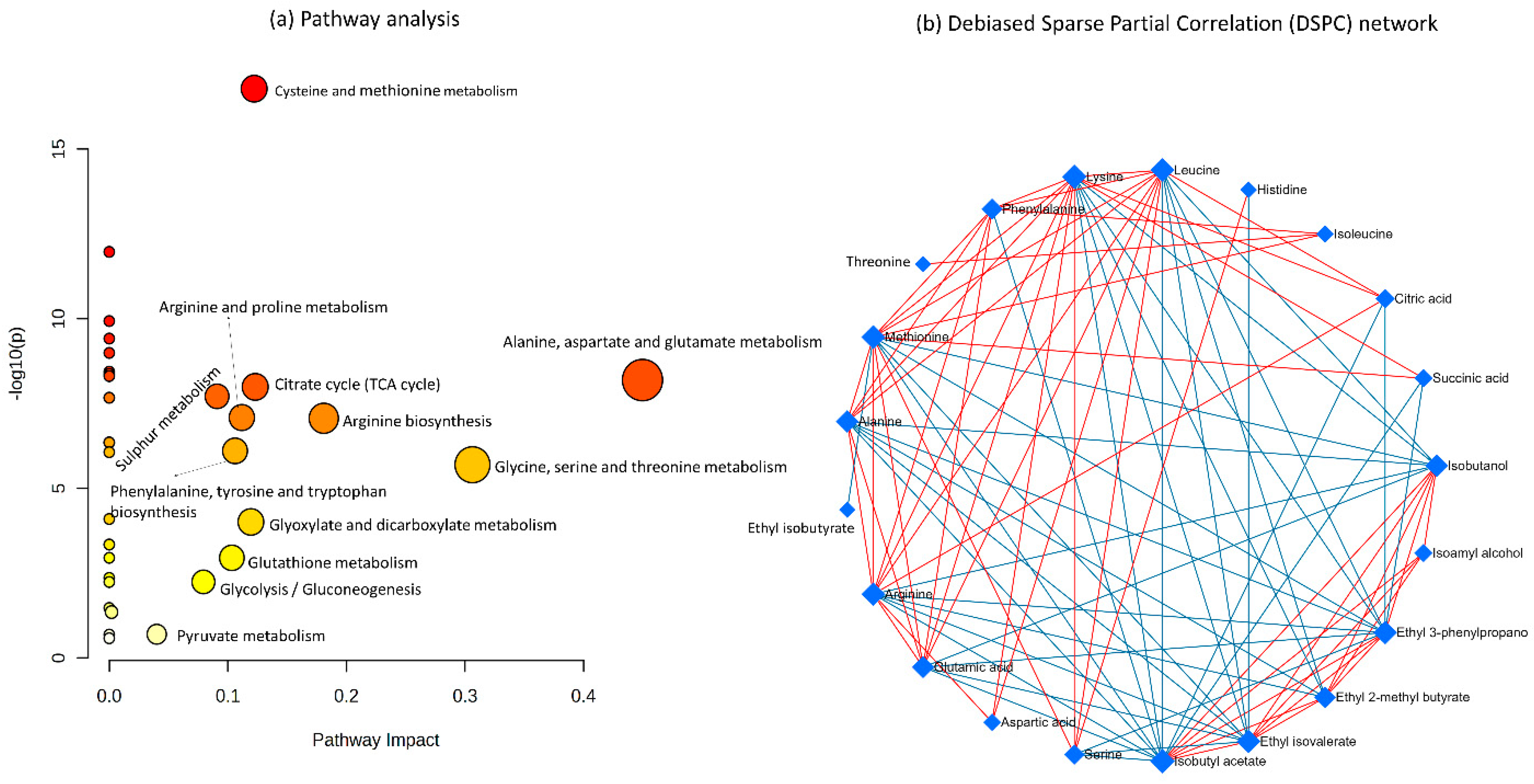
3.6. Pathway Analysis Indicates the Changes in Metabolic Pathways Due to the Differences in Inoculation Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antalick, G.; Perello, M.C.; de Revel, G. Esters in Wines: New Insight through the Establishment of a Database of French Wines. Am. J. Enol. Vitic. 2014, 65, 293–304. [Google Scholar] [CrossRef]
- Hirst, M.B.; Richter, C.L. Review of Aroma Formation through Metabolic Pathways of Saccharomyces cerevisiae in Beverage Fermentations. Am. J. Enol. Vitic. 2016, 67, 361–370. [Google Scholar] [CrossRef]
- Cardona, F.; Carrasco, P.; Perez-Ortin, J.E.; del Olmo, M.L.; Aranda, A. A novel approach for the improvement of stress resistance in wine yeasts. Int. J. Food Microbiol. 2007, 114, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Pizarro, F.; Vargas, F.A.; Agosin, E. A systems biology perspective of wine fermentations. Yeast 2007, 24, 977–991. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Hellin, P.; Ubeda, J.; Briones, A. Improving alcoholic fermentation by activation of Saccharomyces species during the rehydration stage. LWT-Food Sci. Technol. 2013, 50, 126–131. [Google Scholar] [CrossRef]
- Jenkins, D.M.; Powell, C.D.; Fischborn, T.; Smart, K.A. Rehydration of Active Dry Brewing Yeast and its Effect on Cell Viability. J. Inst. Brew. 2011, 117, 377–382. [Google Scholar] [CrossRef]
- Schmidt, S.A.; Henschke, P.A. Production, reactivation and nutrient requirements of active dried yeast in winemaking: Theory and practice. Aust. J. Grape Wine Res. 2015, 21, 651–662. [Google Scholar] [CrossRef]
- Monk, P.R. Rehydration and propagation of active dry wine yeast. Aust. Wine Ind. J. 1986, 1, 35. [Google Scholar]
- Redon, M.; Guillamon, J.M.; Mas, A.; Rozes, N. Effect of active dry wine yeast storage upon viability and lipid composition. World J. Microbiol. Biotechnol. 2008, 24, 2555–2563. [Google Scholar] [CrossRef]
- Kontkanen, D.; Inglis, D.L.; Pickering, G.J.; Reynolds, A. Effect of yeast inoculation rate, acclimatization, and nutrient addition on icewine fermentation. Am. J. Enol. Vitic. 2004, 55, 363–370. [Google Scholar] [CrossRef]
- Diaz-Hellin, P.; Ubeda, J.; Briones, A. Study of two wine strains rehydrated with different activators in sluggish fermentation conditions. Eur. Food Res. Technol. 2013, 237, 547–554. [Google Scholar] [CrossRef]
- Andujar-Ortiz, I.; Chaya, C.; Martin-Alvarez, P.J.; Moreno-Arribas, M.V.; Pozo-Bayon, M.A. Impact of using new commercial glutathione enriched inactive dry yeast oenological preparations on the aroma and sensory properties of wines. Int. J. Food Prop. 2014, 17, 987–1001. [Google Scholar] [CrossRef]
- Novo, M.; Beltran, G.; Rozes, N.; Guillamon, J.M.; Sokol, S.; Leberre, V.; Francois, J.; Mas, A. Early transcriptional response of wine yeast after rehydration: Osmotic shock and metabolic activation. FEMS Yeast Res. 2007, 7, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Winter, G.; Henschke, P.A.; Higgins, V.J.; Ugliano, M.; Curtin, C.D. Effects of rehydration nutrients on H2S metabolism and formation of volatile sulfur compounds by the wine yeast VL3. AMB Express 2011, 1, 11. [Google Scholar] [CrossRef] [PubMed]
- Soubeyrand, V.; Julien, A.; Sablayrolles, J.M. Rehydration protocols for active dry wine yeasts and the search for early indicators of yeast activity. Am. J. Enol. Vitic. 2006, 57, 474–480. [Google Scholar] [CrossRef]
- Vaudano, E.; Noti, O.; Costantini, A.; Garcia-Moruno, E. Effect of additives on the rehydration of Saccharomyces cerevisiae wine strains in active dry form: Influence on viability and performance in the early fermentation phase. J. Inst. Brew. 2014, 120, 71–76. [Google Scholar] [CrossRef]
- Rodriguez-Porrata, B.; Novo, M.; Guillamon, J.; Rozes, N.; Mas, A.; Otero, R.C. Vitality enhancement of the rehydrated active dry wine yeast. Int. J. Food Microbiol. 2008, 126, 116–122. [Google Scholar] [CrossRef]
- Carrau, F.; Medina, K.; Farina, L.; Boido, E.; Dellacassa, E. Effect of Saccharomyces cerevisiae inoculum size on wine fermentation aroma compounds and its relation with assimilable nitrogen content. Int. J. Food Microbiol. 2010, 143, 81–85. [Google Scholar] [CrossRef]
- Erten, H.; Tanguler, H.; Cabaroglu, T.; Canbas, A. The influence of inoculum level on fermentation and flavour compounds of white wines made from cv. Emir. J. Inst. Brew. 2006, 112, 232–236. [Google Scholar] [CrossRef]
- Deed, N.K.; van Vuuren, H.J.J.; Gardner, R.C. Effects of nitrogen catabolite repression and di-ammonium phosphate addition during wine fermentation by a commercial strain of S. cerevisiae. Appl. Microbiol. Biotechnol. 2011, 89, 1537–1549. [Google Scholar] [CrossRef]
- Harsch, M.J.; Benkwitz, F.; Frost, A.; Colonna-Ceccaldi, B.; Gardner, R.C.; Salmon, J.M. New Precursor of 3-Mercaptohexan-1-ol in Grape Juice: Thiol-Forming Potential and Kinetics during Early Stages of Must Fermentation. J. Agric. Food Chem. 2013, 61, 3703–3713. [Google Scholar] [CrossRef] [PubMed]
- Pinu, F.R.; Edwards, P.J.B.; Jouanneau, S.; Kilmartin, P.A.; Gardner, R.C.; Villas-Boas, S.G. Sauvignon blanc metabolomics: Grape juice metabolites affecting the development of varietal thiols and other aroma compounds in wines. Metabolomics 2014, 10, 556–573. [Google Scholar] [CrossRef]
- Shekhawat, K.; Bauer, F.F.; Setati, M.E. Impact of oxygenation on the performance of three non-Saccharomyces yeasts in co-fermentation with Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2017, 101, 2479–2491. [Google Scholar] [CrossRef] [PubMed]
- Ancin-Azpilicueta, C.; Jimenez-Moreno, N.; Moler, J.A.; Nieto-Rojo, R.; Urmeneta, H. Effects of reduced levels of sulfite in wine production using mixtures with lysozyme and dimethyl dicarbonate on levels of volatile and biogenic amines. Food Addit. Contam. Part A-Chem. 2016, 33, 1518–1526. [Google Scholar] [CrossRef] [PubMed]
- Pinu, F.R.; Jouanneau, S.; Nicolau, L.; Gardner, R.C.; Villas-Boas, S.G. Concentrations of the Volatile Thiol 3-Mercaptohexanol in Sauvignon blanc Wines: No Correlation with Juice Precursors. Am. J. Enol. Vitic. 2012, 63, 407–412. [Google Scholar] [CrossRef]
- Iland, P.; Bruer, N.; Wilkes, E. Chemical Analysis of Grapes and Wine: Techniques and Concepts; Patrick Iland Wine Promotions: Adelaide, Australia, 2004. [Google Scholar]
- Pinu, F.R.; Villas-Boas, S.G.; Martin, D. Pre-fermentative supplementation of fatty acids alters the metabolic activity of wine yeasts. Food Res. Int. 2019, 121, 835–844. [Google Scholar] [CrossRef]
- Martin, D.; Grose, C.; Fedrizzi, B.; Stuart, L.; Albright, A.; McLachlan, A. Grape cluster microclimate influences the aroma composition of Sauvignon blanc wine. Food Chem. 2016, 210, 640–647. [Google Scholar] [CrossRef]
- Hansen, E.H.; Nissen, P.; Sommer, P.; Nielsen, J.C.; Arneborg, N. The effect of oxygen on the survival of non-Saccharomyces yeasts during mixed culture fermentations of grape juice with Saccharomyces cerevisiae. J. Appl. Microbiol. 2001, 91, 541–547. [Google Scholar] [CrossRef]
- Pinu, F.R. Sauvignon Blanc Metabolomics: Metabolite Profile Analysis before and after Fermentation. Ph.D. Thesis, University of Auckland, Auckland, New Zealand, 2013. [Google Scholar]
- Green, J.A.; Parr, W.V.; Breitmeyer, J.; Valentin, D.; Sherlock, R. Sensory and chemical characterisation of Sauvignon blanc wine: Influence of source of origin. Food Res. Int. 2011, 44, 2788–2797. [Google Scholar] [CrossRef]
- Herbst-Johnstone, M.; Araujo, L.D.; Allen, T.A.; Logan, G.; Nicolau, L.; Kilmartin, P.A. Effects of Mechanical Harvesting on ‘Sauvignon Blanc’ Aroma; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2013; pp. 179–186. [Google Scholar]
- Shi, S.; Condron, L.; Larsen, S.; Richardson, A.E.; Jones, E.; Jiao, J.; O’Callaghan, M.; Stewart, A. In situ sampling of low molecular weight organic anions from rhizosphere of radiata pine (Pinus radiata) grown in a rhizotron system. Environ. Exp. Bot. 2011, 70, 131–142. [Google Scholar] [CrossRef]
- Henderson, J.W.J.; Brooks, A. Improved Amino Acid Methods using Agilent ZORBAX Eclipse Plus C18 Columns for a Variety of Agilent LC Instrumentation and Separation Goals; Agilent Application Note 5990–4547EN; Agilent Technologies, Inc.: Wilmington, NC, USA, 2010. [Google Scholar]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Duren, W.; Evans, C.R.; Burant, C.F.; Michailidis, G.; Karnovsky, A. Sparse network modeling and metscape-based visualization methods for the analysis of large-scale metabolomics data. Bioinformatics 2017, 33, 1545–1553. [Google Scholar] [CrossRef] [PubMed]
- Pinu, F.R.; Stuart, L.; Grose, C. Effects of Different Yeast Preparation, Inoculation Timing and Ferment Oxygenation Methodologies on Ethanol Yield and Varietal Aroma Compound Formation; A Plant & Food Research Report; New Zealand Winegrowers: Auckland, New Zealand, 2019. [Google Scholar]
- Rollero, S.; Bloem, A.; Camarasa, C.; Sanchez, I.; Ortiz-Julien, A.; Sablayrolles, J.M.; Dequin, S.; Mouret, J.R. Combined effects of nutrients and temperature on the production of fermentative aromas by Saccharomyces cerevisiae during wine fermentation. Appl. Microbiol. Biotechnol. 2015, 99, 2291–2304. [Google Scholar] [CrossRef]
- Pinu, F.R.; Edwards, P.J.B.; Gardner, R.C.; Villas-Boas, S.G. Nitrogen and carbon assimilation by Saccharomyces cerevisiae during Sauvignon blanc juice fermentation. FEMS Yeast Res. 2014, 14, 1206–1222. [Google Scholar] [CrossRef]
- Pinu, F.R.; Tumanov, S.; Grose, C.; Raw, V.; Albright, A.; Stuart, L.; Villas-Boas, S.G.; Martin, D.; Harker, R.; Greven, M. Juice Index: An integrated Sauvignon blanc grape and wine metabolomics database shows mainly seasonal differences. Metabolomics 2019, 15, 3. [Google Scholar] [CrossRef]
- Benkwitz, F.; Takatoshi, T.; Kilmartin, P.A.; Lund, C.; Wohlers, M.; Nicolau, L. Identifying the Chemical Composition Related to the Distinct Aroma Characteristics of New Zealand Sauvignon blanc Wines. Am. J. Enol. Vitic. 2012, 63, 62. [Google Scholar] [CrossRef]
- Pinu, F.R.; Villas-Boas, S.G.; Aggio, R. Analysis of Intracellular Metabolites from Microorganisms: Quenching and Extraction Protocols. Metabolites 2017, 7, 53. [Google Scholar] [CrossRef]
- Drew, S.W.; Demain, A.L. Effect of Primary Metabolites on Secondary Metabolism. Annu. Rev. Microbiol. 1977, 31, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J. Cell factory engineering for improved production of natural products. Nat. Prod. Rep. 2019, 36, 1233–1236. [Google Scholar] [CrossRef] [PubMed]
- Pinu, F.R.; Villas-Boas, S.G. Extracellular Microbial Metabolomics: The State of the Art. Metabolites 2017, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, S.J.; De Leonardis, A.; Lustrato, G.; Testa, B.; Iorizzo, M. Yeast Autolysis in Sparkling Wine Aging: Use of Killer and Sensitive Saccharomyces cerevisiae Strains in Co-Culture. Recent Pat. Biotechnol. 2015, 9, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Tumanov, S.; Pinu, F.R.; Greenwood, D.R.; Villas-Bôas, S.G. Effect of free fatty acids and lipolysis on Sauvignon Blanc fermentation. Aust. J. Grape Wine Res. 2018, 24, 398–405. [Google Scholar] [CrossRef]
- Prusova, B.; Humaj, J.; Sochor, J.; Baron, M. Formation, Losses, Preservation and Recovery of Aroma Compounds in the Winemaking Process. Fermentation 2022, 8, 93. [Google Scholar] [CrossRef]
- Cordente, A.G.; Schmidt, S.; Beltran, G.; Torija, M.J.; Curtin, C.D. Harnessing yeast metabolism of aromatic amino acids for fermented beverage bioflavouring and bioproduction. Appl. Microbiol. Biotechnol. 2019, 103, 4325–4336. [Google Scholar] [CrossRef] [PubMed]
- García-Ríos, E.; Gutiérrez, A.; Salvadó, Z.; Arroyo-López, F.N.; Guillamon, J.M. The Fitness Advantage of Commercial Wine Yeasts in Relation to the Nitrogen Concentration, Temperature, and Ethanol Content under Microvinification Conditions. Appl. Environ. Microbiol. 2014, 80, 704. [Google Scholar] [CrossRef] [PubMed]


| Wine | Inoculated Yeast Cells (cells/mL) | Completion Time | Alcohol (%v/v) | pH | Titratable Acidity (g/L) | Glucose (g/L) | Fructose (g/L) | Total Residual Sugar (g/L) | Phenolics (mg Gallic Acid/L) |
|---|---|---|---|---|---|---|---|---|---|
| Rehydrated ADY | |||||||||
| Control RY | 1 × 106 | 13 | 11.81 (0.01) | 3.20 (0.01) | 8.46 (0.13) | 0.03 (0.01) | 0.60 (0.28) | 0.63 (0.27) | 207.62 (6.50) |
| RY 1 | 1 × 107 | 12 | 11.76 (0.00) | 3.21 (0.01) | 8.41 (0.06) | 0.05 (0.01) | 1.09 (0.03) a | 1.14 (0.03) | 210.41 (3.38) |
| RY2 | 1 × 108 | 11 | 11.65 (0.01) | 3.19 (0.01) | 9.52 (0.17) | 0.00 | 1.62 (0.32) a | 1.62 (0.32) | 201.19 (2.50) |
| RY3 | 1 × 109 | 11 | 11.64 (0.02) | 3.19 (0.01) | 9.51 (0.09) | 0.00 b | 0.87 (0.10) b | 0.87 (0.10) b | 204.95 (1.17) |
| SI RY 1 | 1 × 106, then 1 × 106 at 10 and 0 °Brix | 14 * | 11.86 (0.03) | 3.28 (0.01) | 8.48 (0.04) | 0.00 | 0.04 (0.03) a | 0.04 (0.03) | 207.93 (4.47) |
| SI RY 2 | 1 × 106, then 1 × 106 at 0 °Brix | 14 * | 11.91 (0.00) | 3.24 (0.01) | 8.38 (0.11) | 0.03 (0.04) | 0.02 (0.02) a | 0.05 (0.06) | 211.37 (0.61) |
| Pre-inoculum | |||||||||
| Control PI | 1 × 106 | 17 | 11.91 (0.02) | 3.24 (0.02) | 9.01 (0.16) | 0.05 (0.05) | 0.87 (0.28) | 0.92 (0.28) | 209.82 (3.55) |
| PI 1 | 1 × 107 | 16 | 11.80 (0.08) | 3.20 (0.02) | 8.68 (0.10) | 0.04 (0.01) | 1.73 (0.74) | 1.77 (0.74) | 212.81 (2.83) |
| PI 2 | 1 × 108 | 17 | 11.83 (0.03) | 3.24 (0.02) | 8.56 (0.13) | 0.01 (0.01) | 1.05 (0.37) | 1.06 (0.37) | 209.70 (2.80) |
| PI 3 | 1 × 109 | 16 | 11.79 (0.01) | 3.19 (0.04) | 8.41 (0.06) | 0.08 (0.02) b | 1.54 (0.24) b | 1.62 (0.21) b | 212.64 (4.27) |
| SI PI 1 | 1 × 106, then 1 × 106 at 10 and 0 °Brix | 17 * | 11.90 (0.00) | 3.28 (0.02) | 8.29 (0.04) | 0.02 (0.00) | 0.06 (0.02) a | 0.08 (0.02) | 212.04 (2.73) |
| SI PI 2 | 1 × 106, then 1 × 106 at 0 °Brix | 17 * | 11.92 (0.01) | 3.26 (0.02) | 8.29 (0.10) | 0.02 (0.00) | 0.02 (0.01) a | 0.04 (0.01) | 206.93 (4.61) |
| Wine | Inoculated Yeast Cells (cells/mL) | 3MH (ng/L) | 3MHA (ng/L) | 4MMP (ng/L) |
|---|---|---|---|---|
| Inoculation of rehydrated ADY | ||||
| Control RY | 1 × 106 | 11,498 (2717) | 3242 (843) | 62 (41) |
| RY 1 | 1 × 107 | 12,657 (1414) a | 3197 (250) | 69 (14) |
| RY2 | 1 × 108 | 14,217 (829) b | 3146 (175) | 105 (15) a |
| RY3 | 1 × 109 | 14,066 (742) b | 2943 (77) | 157 (59) b |
| SI RY 1 | 1 × 106, then 1 × 106 at 10 and 0 °Brix | 13,947 (1210) a | 3588 (492) | 29 (5) b |
| SI RY 2 | 1 × 106, then 1 × 106 at 0 °Brix | 14,626 (636) b | 4037 (131) a | 27 (9) b |
| Inoculation of pre-inoculum | ||||
| Control PI | 1 × 106 | 14,382 (802) | 3147 (335) | 20 (8) |
| PI 1 | 1 × 107 | 15,573 (596) a | 3131 (216) | 25 (2) |
| PI 2 | 1 × 108 | 14,502 (2010) | 3095 (301) | 21 (8) |
| PI 3 | 1 × 109 | 15,914 (692) a | 3078 (117) | 38 (8) b |
| SI PI 1 | 1 × 106, then 1 × 106 at 10 and 0 °Brix | 13,879 (2846) | 3091 (522) | 17 (5) |
| SI PI 2 | 1 × 106, then 1 × 106 at 0 °Brix | 15,679 (859) a | 3382 (305) | 24 (4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinu, F.R.; Stuart, L.; Topal, T.; Albright, A.; Martin, D.; Grose, C. The Effect of Yeast Inoculation Methods on the Metabolite Composition of Sauvignon Blanc Wines. Fermentation 2023, 9, 759. https://doi.org/10.3390/fermentation9080759
Pinu FR, Stuart L, Topal T, Albright A, Martin D, Grose C. The Effect of Yeast Inoculation Methods on the Metabolite Composition of Sauvignon Blanc Wines. Fermentation. 2023; 9(8):759. https://doi.org/10.3390/fermentation9080759
Chicago/Turabian StylePinu, Farhana R., Lily Stuart, Taylan Topal, Abby Albright, Damian Martin, and Claire Grose. 2023. "The Effect of Yeast Inoculation Methods on the Metabolite Composition of Sauvignon Blanc Wines" Fermentation 9, no. 8: 759. https://doi.org/10.3390/fermentation9080759
APA StylePinu, F. R., Stuart, L., Topal, T., Albright, A., Martin, D., & Grose, C. (2023). The Effect of Yeast Inoculation Methods on the Metabolite Composition of Sauvignon Blanc Wines. Fermentation, 9(8), 759. https://doi.org/10.3390/fermentation9080759







