The Human Body as an Ethanol-Producing Bioreactor—The Forensic Impacts
Abstract
1. Introduction
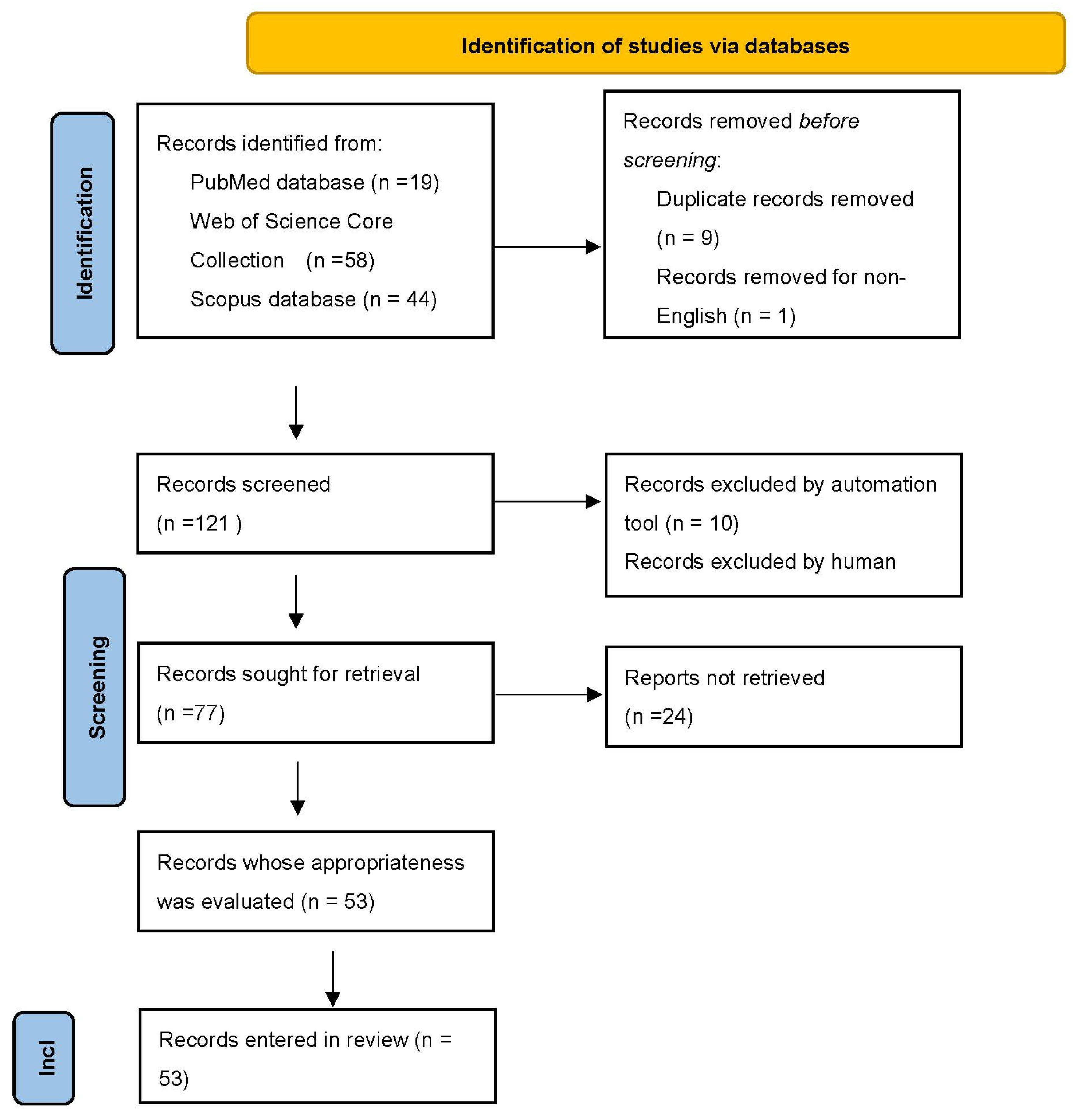
2. A Systematic Review
2.1. Blood Ethanol Levels
2.2. The Argument for the Hypothetical Endogenous Origin of Alcohol
2.3. Non-Alcoholic Food-Derived Ethanol
2.4. Gut–Liver–Brain Axis and Forensic Alcohol Determination
2.5. Genetics
2.6. Forensic Determination of Alcohol Concentration
Postmortem Diffusion of Ethanol vs. Postmortem Microbiome Activity
2.7. Forensic Determination of Alcohol Concentration in Society 5.0
2.8. Comprehensive Evaluation
| Genera of Causative Microorganisms |
|---|
| Saccharomyces spp. |
| Candida spp. |
| Klebsiella spp. (pneumoniae) |
| Escherichia spp. |
| Streptococcus spp. |
| Bacteroides spp. |
| Bifidobacterium spp. |
| Clostridium spp. |
| Pseudomonas spp. |
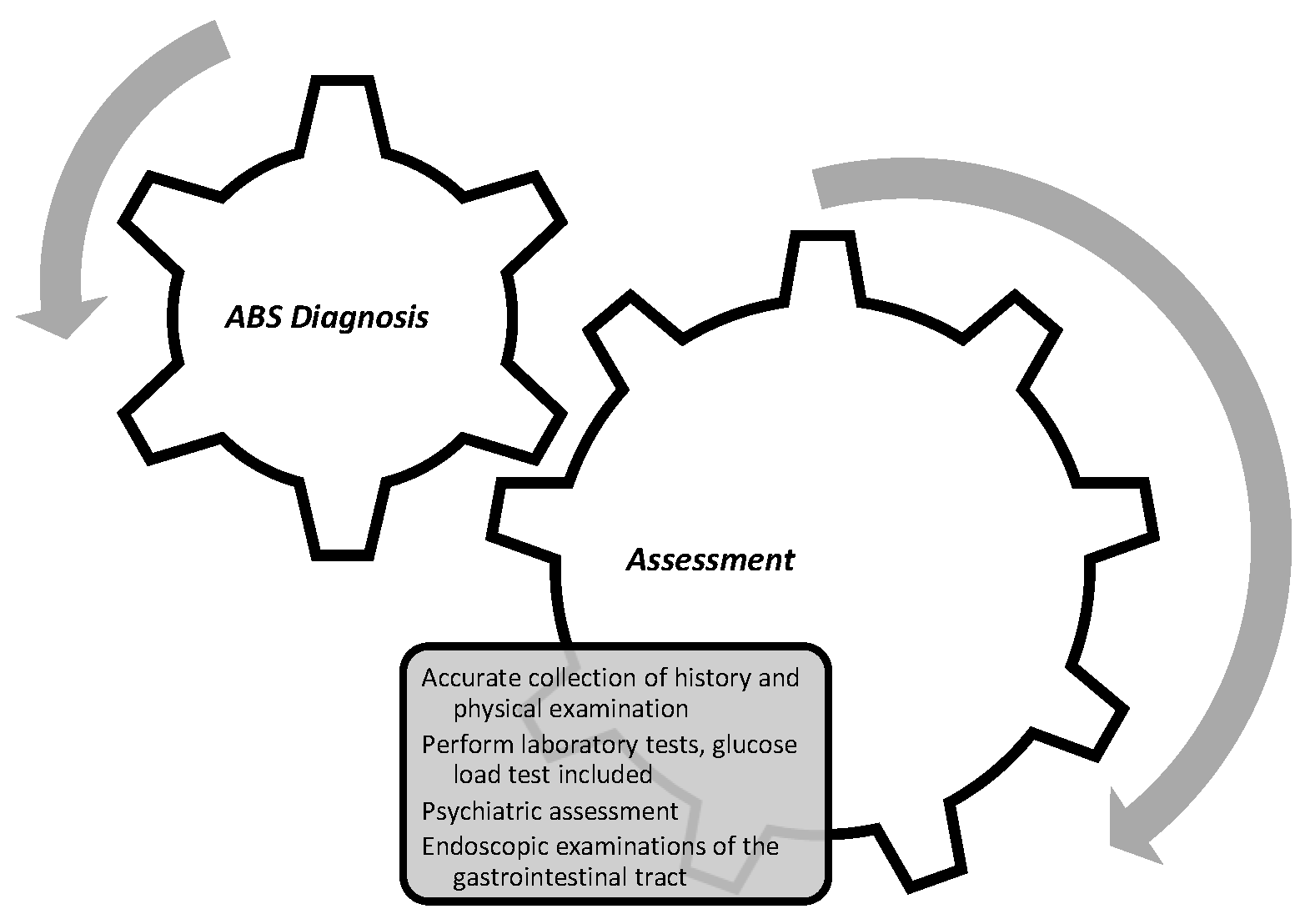
2.9. Treatment Options
2.10. Fecal Microbiota Transplantation
3. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmid, A.; Kreidl, E.; Bertschinger, M.; Vetsch, P. Benchtop Bioreactors in Mammalian Cell Culture: Overview and Guidelines. Methods Mol. Biol. 2022, 2436, 1–15. [Google Scholar] [CrossRef]
- Seydi, M.; Boogar, I.R.; Talepasand, S. The Predictive Role of Gender, Age, and Personality Organization in Risky Driving; Eliva Press: Chișinău, Moldova, 2022. [Google Scholar]
- Akbaba, M. A medicolegal approach to the very rare Auto-Brewery (endogenous alcohol fermentation) syndrome. Traffic Inj. Prev. 2020, 21, 295–297. [Google Scholar] [CrossRef]
- Al-Awadhi, A.; Wasfi, I.A.; Al Reyami, F.; Al-Hatali, Z. Autobrewing revisited: Endogenous concentrations of blood ethanol in residents of the United Arab Emirates. Sci. Justice J. Forensic Sci. Soc. 2004, 44, 149–152. [Google Scholar] [CrossRef]
- Akhavan, B.J.; Ostrosky-Zeichner, L.; Thomas, E.J. Drunk without Drinking: A Case of Auto-Brewery Syndrome. ACG Case Rep. J. 2019, 6, e00208. [Google Scholar] [CrossRef]
- Dinis-Oliveira, R.J. The Auto-Brewery Syndrome: A Perfect Metabolic “Storm” with Clinical and Forensic Implications. J. Clin. Med. 2021, 10, 4637. [Google Scholar] [CrossRef]
- Chen, G.; Shi, F.; Yin, W.; Guo, Y.; Liu, A.; Shuai, J.; Sun, J. Gut microbiota dysbiosis: The potential mechanisms by which alcohol disrupts gut and brain functions. Front. Microbiol. 2022, 13, 916765. [Google Scholar] [CrossRef]
- Bayoumy, A.B.; Mulder, C.J.J.; Mol, J.J.; Tushuizen, M.E. Gut fermentation syndrome: A systematic review of case reports. United Eur. Gastroenterol. J. 2021, 9, 332–342. [Google Scholar] [CrossRef]
- Jones, A.W. Alcohol, its analysis in blood and breath for forensic purposes, impairment effects, and acute toxicity. Wiley Interdiscip. Rev. Forensic Sci. 2019, 1, e1353. [Google Scholar] [CrossRef]
- Smedra, A.; Trzmielak, M.; Goralska, K.; Dzikowiec, M.; Brzezianska-Lasota, E.; Berent, J. Oral form of auto-brewery syndrome. J. Forensic Leg. Med. 2022, 87, 102333. [Google Scholar] [CrossRef]
- Paramsothy, J.; Gutlapalli, S.D.; Ganipineni, V.D.P.; Okorie, I.J.; Ugwendum, D.; Piccione, G.; Ducey, J.; Kouyate, G.; Onana, A.; Emmer, L.; et al. Understanding Auto-Brewery Syndrome in 2023: A Clinical and Comprehensive Review of a Rare Medical Condition. Cureus 2023, 15, e37678. [Google Scholar] [CrossRef]
- Brick, J.; Bennett, W. Alcohol Calculations in Emergency and Forensic Medicine. J. Addict Med. Ther. Sci. 2017, 3, 024–029. [Google Scholar] [CrossRef]
- Ostrovsky Yu, M. Endogenous ethanol--its metabolic, behavioral and biomedical significance. Alcohol 1986, 3, 239–247. [Google Scholar] [CrossRef]
- Simic, M.; Ajdukovic, N.; Veselinovic, I.; Mitrovic, M.; Djurendic-Brenesel, M. Endogenous ethanol production in patients with diabetes mellitus as a medicolegal problem. Forensic Sci. Int. 2012, 216, 97–100. [Google Scholar] [CrossRef]
- Jones, A. Biochemical and physiological research on the disposition and fate of ethanol in the body. In Medico Legal Aspects of Alcohol, 5th ed.; Lawyers and Judges Publishing Company: Tucson, AZ, USA, 2008. [Google Scholar]
- Jones, A.W. Driving under the influence of alcohol. Handb. Forensic Med. 2022, 3, 1387–1408. [Google Scholar]
- Cederbaum, A.I. Alcohol metabolism. Clin. Liver Dis. 2012, 16, 667–685. [Google Scholar] [CrossRef]
- Riccardi, G.; Giosue, A.; Calabrese, I.; Vaccaro, O. Dietary recommendations for prevention of atherosclerosis. Cardiovasc. Res. 2022, 118, 1188–1204. [Google Scholar] [CrossRef]
- Kiely, L.J.; Hickey, R.M. Characterization and Analysis of Food-Sourced Carbohydrates. Methods Mol. Biol. 2022, 2370, 67–95. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Hafez, E.M.; Hamad, M.A.; Fouad, M.; Abdel-Lateff, A. Auto-brewery syndrome: Ethanol pseudo-toxicity in diabetic and hepatic patients. Hum. Exp. Toxicol. 2017, 36, 445–450. [Google Scholar] [CrossRef]
- Lester, D. The concentration of apparent endogenous ethanol. Q. J. Stud. Alcohol. 1962, 23, 17–25. [Google Scholar] [CrossRef]
- Tameez Ud Din, A.; Alam, F.; Tameez-Ud-Din, A.; Chaudhary, F.M.D. Auto-Brewery Syndrome: A Clinical Dilemma. Cureus 2020, 12, e10983. [Google Scholar] [CrossRef]
- Malik, F.; Wickremesinghe, P.; Saverimuttu, J. Case report and literature review of auto-brewery syndrome: Probably an underdiagnosed medical condition. BMJ Open Gastroenterol. 2019, 6, e000325. [Google Scholar] [CrossRef]
- Nambiema, A.; Sembajwe, G.; Lam, J.; Woodruff, T.; Mandrioli, D.; Chartres, N.; Fadel, M.; Le Guillou, A.; Valter, R.; Deguigne, M.; et al. A Protocol for the Use of Case Reports/Studies and Case Series in Systematic Reviews for Clinical Toxicology. Front. Med. 2021, 8, 708380. [Google Scholar] [CrossRef]
- Ragab, A.R.; Al-Mazroua, M.K.; Afify, M.M.; Al Saeed, I.; Katbai, C. Endogenous ethanol production levels in Saudi Arabia residents. J. Alcohol. Drug Depend. 2015, 3, 211. [Google Scholar] [CrossRef]
- Logan, B.K.; Jones, A.W. Endogenous ethanol ‘auto-brewery syndrome’ as a drunk-driving defence challenge. Med. Sci. Law 2000, 40, 206–215. [Google Scholar] [CrossRef]
- Beitel, G.A.; Sharp, M.C.; Glauz, W.D. Probability of arrest while driving under the influence of alcohol. Inj. Prev. 2000, 6, 158–161. [Google Scholar] [CrossRef]
- Jamai, L.; Ettayebi, K.; El Yamani, J.; Ettayebi, M. Production of ethanol from starch by free and immobilized Candida tropicalis in the presence of alpha-amylase. Bioresour. Technol. 2007, 98, 2765–2770. [Google Scholar] [CrossRef]
- Aruna, A.; Nagavalli, M.; Girijashankar, V.; Ponamgi, S.P.; Swathisree, V.; Rao, L.V. Direct bioethanol production by amylolytic yeast Candida albicans. Lett. Appl. Microbiol. 2015, 60, 229–236. [Google Scholar] [CrossRef]
- Roerecke, M.; Vafaei, A.; Hasan, O.S.M.; Chrystoja, B.R.; Cruz, M.; Lee, R.; Neuman, M.G.; Rehm, J. Alcohol Consumption and Risk of Liver Cirrhosis: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2019, 114, 1574–1586. [Google Scholar] [CrossRef]
- Kovačić, Z.; Nestić, M.; Stemberga, V.; Bosnar, A.; Petrovečki, M.; Sutlović, D. Reliability of breath alcohol testing with Dräger Alcotest 7410Plus analyzer in a court process. Medica Jadertina 2008, 38, 47–51. [Google Scholar]
- Painter, K.; Cordell, B.; Sticco, K. Auto-Brewery Syndrome (Gut Fermentation); StatPearls Publishing LLC: Treasure Island, FL, USA, 2018. [Google Scholar]
- Malik, F.; Wickremesinghe, P.; Saleem, A. Auto-Brewery Syndrome: A Schematic for Diagnosis and Appropriate Treatment. Pract. Gastroenterol. 2021, 45, 10–20. [Google Scholar]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Verges, M.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- Riccio, P.; Rossano, R. The human gut microbiota is neither an organ nor a commensal. FEBS Lett. 2020, 594, 3262–3271. [Google Scholar] [CrossRef]
- Wang, L.; Hao, J.; Wang, C.; Li, Y.; Yang, Q. Carbohydrate-to-protein ratio regulates hydrolysis and acidogenesis processes during volatile fatty acids production. Bioresour. Technol. 2022, 355, 127266. [Google Scholar] [CrossRef]
- Niemelä, O.; Aalto, M.; Bloigu, A.; Bloigu, R.; Halkola, A.S.; Laatikainen, T. Alcohol Drinking Patterns and Laboratory Indices of Health: Does Type of Alcohol Preferred Make a Difference? Nutrients 2022, 14, 4529. [Google Scholar] [CrossRef]
- Center, N.T.L. Challenges and Defenses III. Responses to Common. Challenges and Defenses in Impaired Driving Case. Available online: https://www.tampermonkey.net/changelog.php?version=4.19.0&ext=dhdg&updated=true&old=4.18.1 (accessed on 19 December 2022).
- Rajeswari, S.; Baskaran, D.; Saravanan, P.; Rajasimman, M.; Rajamohan, N.; Vasseghian, Y. Production of ethanol from biomass—Recent research, scientometric review and future perspectives. Fuel 2022, 317, 123448. [Google Scholar] [CrossRef]
- Chen, W.H.; Biswas, P.P.; Ong, H.C.; Hoang, A.T.; Nguyen, T.B.; Dong, C.D. A critical and systematic review of sustainable hydrogen production from ethanol/bioethanol: Steam reforming, partial oxidation, and autothermal reforming. Fuel 2023, 333, 126526. [Google Scholar] [CrossRef]
- Ladkin, R.G.; Davies, J.N. Rupture of the stomach in an African child. Br. Med. J. 1948, 1, 644. [Google Scholar] [CrossRef]
- LaHood, A.J.; Kok, S.J. Ethanol Toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Mukamal, K.J.; Rimm, E.B. Alcohol’s effects on the risk for coronary heart disease. Alcohol Res. Health 2001, 25, 255. [Google Scholar]
- Centers for Disease Control and Prevention. Drinking Too Much Alcohol Can Harm Your Health. Learn the Facts. Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/alcohol/fact-sheets/alcohol-use.htm (accessed on 2 July 2023).
- Crowe, S.E. Food Allergy vs. Food Intolerance in Patients with Irritable Bowel Syndrome. Gastroenterol. Hepatol. 2019, 15, 38–40. [Google Scholar]
- Takahashi, G.; Hoshikawa, K.; Kan, S.; Akimaru, R.; Kodama, Y.; Sato, T.; Kakisaka, K.; Yamada, Y. Auto-brewery syndrome caused by oral fungi and periodontal disease bacteria. Acute Med. Surg. 2021, 8, e652. [Google Scholar] [CrossRef]
- Cordell, B.J.; Kanodia, A.; Miller, G.K. Case-Control Research Study of Auto-Brewery Syndrome. Glob. Adv. Health Med. 2019, 8, 2164956119837566. [Google Scholar] [CrossRef] [PubMed]
- Saverimuttu, J.; Malik, F.; Arulthasan, M.; Wickremesinghe, P. A Case of Auto-brewery Syndrome Treated with Micafungin. Cureus 2019, 11, e5904. [Google Scholar] [CrossRef]
- Kruckenberg, K.M.; DiMartini, A.F.; Rymer, J.A.; Pasculle, A.W.; Tamama, K. Urinary Auto-brewery Syndrome: A Case Report. Ann. Intern. Med. 2020, 172, 702–704. [Google Scholar] [CrossRef]
- Cordell, B.; Kanodia, A. Auto-brewery as an emerging syndrome: Three representative case studies. J. Clin. Med. Case Rep. 2015, 2, 5. [Google Scholar]
- Cox, W.M.; Klinger, E. Alcohol and Its Effects on the Body. In Why People Drink; How People Change; Springer: Berlin/Heidelberg, Germany, 2022; pp. 25–38. [Google Scholar]
- Bortoleto, G.G.; Gomes, W.P.C. Determination of ethanol in low-alcohol fermented beverages. Rev. Sítio Novo 2022, 6, 105–111. [Google Scholar] [CrossRef]
- Gürler, M.; Martz, W.; Taştekin, B.; Najafova, T.; Dettmeyer, R.B. Estimates of Non-Alcoholic Food-Derived Ethanol and Methanol Exposure in Human. J. Anal. Toxicol. 2020, 46, 200–211. [Google Scholar] [CrossRef]
- Gibney, M.J.; O’Sullivan, A.; Flynn, A.; Walton, J.; Daniel, H.; Manios, Y.; Martinez, A.; Saris, W.H.M.; Gibney, E.R.; Uzhova, I. Analysis of the National Adult Nutrition Survey (Ireland) and the Food4Me Nutrition Survey Databases to Explore the Development of Food Labelling Portion Sizes for the European Union. Nutrients 2018, 11, 6. [Google Scholar] [CrossRef]
- Lutmer, B.; Zurfluh, C.; Long, C. Potential effect of alcohol content in energy drinks on breath alcohol testing. J. Anal. Toxicol. 2009, 33, 167–169. [Google Scholar] [CrossRef]
- Ariffin, H.; Chong, X.Q.; Chong, P.N.; Okechukwu, P.N. Is the consumption of energy drink beneficial or detrimental to health: A comprehensive review? Bull. Natl. Res. Cent. 2022, 46, 163. [Google Scholar] [CrossRef]
- Türkuçar, S.A.; Dolu, Ö.F.; Alevci, A.; Burnaz, N.A.; Karaçelık, A.A.; Doğan, H.; Küçük, M. Ethanol levels of the non-alcoholic beverages sold in markets in türkiye. Gümüşhane Üniv. Sağlık Bilim. Derg. 2017, 6. [Google Scholar]
- Gorgus, E.; Hittinger, M.; Schrenk, D. Estimates of Ethanol Exposure in Children from Food not Labeled as Alcohol-Containing. J. Anal. Toxicol. 2016, 40, 537–542. [Google Scholar] [CrossRef]
- Yuan, J.; Chen, C.; Cui, J.; Lu, J.; Yan, C.; Wei, X.; Zhao, X.; Li, N.; Li, S.; Xue, G.; et al. Fatty Liver Disease Caused by High-Alcohol-Producing Klebsiella pneumoniae. Cell Metab. 2019, 30, 675–688.e7. [Google Scholar] [CrossRef]
- Li, N.N.; Li, W.; Feng, J.X.; Zhang, W.W.; Zhang, R.; Du, S.H.; Liu, S.Y.; Xue, G.H.; Yan, C.; Cui, J.H.; et al. High alcohol-producing Klebsiella pneumoniae causes fatty liver disease through 2,3-butanediol fermentation pathway in vivo. Gut Microbes 2021, 13, 1979883. [Google Scholar] [CrossRef] [PubMed]
- Welch, B.T.; Coelho Prabhu, N.; Walkoff, L.; Trenkner, S.W. Auto-brewery Syndrome in the Setting of Long-standing Crohn’s Disease: A Case Report and Review of the Literature. J. Crohns Colitis 2016, 10, 1448–1450. [Google Scholar] [CrossRef] [PubMed]
- Dahshan, A.; Donovan, K. Auto-brewery syndrome in a child with short gut syndrome: Case report and review of the literature. J. Pediatr. Gastroenterol. Nutr. 2001, 33, 214–215. [Google Scholar] [CrossRef]
- Ding, J.H.; Jin, Z.; Yang, X.X.; Lou, J.; Shan, W.X.; Hu, Y.X.; Du, Q.; Liao, Q.S.; Xie, R.; Xu, J.Y. Role of gut microbiota via the gut-liver-brain axis in digestive diseases. World J. Gastroenterol. 2020, 26, 6141–6162. [Google Scholar] [CrossRef]
- Gupta, H.; Suk, K.T.; Kim, D.J. Gut Microbiota at the Intersection of Alcohol, Brain, and the Liver. J. Clin. Med. 2021, 10, 541. [Google Scholar] [CrossRef]
- Eaton, K.K.; Howard, M.A. Fungal-type Dysbiosis of the Gut: The Occurrence of Fungal Diseases and the Response to Challenge with Yeasty and Mould-containing Foods. J. Nutr. Environ. Med. 1998, 8, 247–255. [Google Scholar] [CrossRef]
- Cornick, S.; Tawiah, A.; Chadee, K. Roles and regulation of the mucus barrier in the gut. Tissue Barriers 2015, 3, e982426. [Google Scholar] [CrossRef] [PubMed]
- Cordell, B.; Kanodia, A.; Miller, G.K. Factors in an Auto-Brewery Syndrome group compared to an American Gut Project group: A case-control study. F1000Research 2021, 10, 457. [Google Scholar] [CrossRef]
- Vandekerckhove, E.; Janssens, F.; Tate, D.; De Looze, D. Treatment of Gut Fermentation Syndrome with Fecal Microbiota Transplantation. Ann. Intern. Med. 2020, 173, 855. [Google Scholar] [CrossRef]
- Arbanas, S.; Stemberga, V.; Štifter, S.; Šoša, I.; Cuculić, D. Medicolegal application of a simple histopathological analysis. Med. Flum. Med. Flum. 2018, 54, 182–188. [Google Scholar] [CrossRef]
- Edenberg, H.J. The genetics of alcohol metabolism: Role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Res. Health 2007, 30, 5–13. [Google Scholar]
- Ehlers, C.L.; Liang, T.; Gizer, I.R. ADH and ALDH polymorphisms and alcohol dependence in Mexican and Native Americans. Am. J. Drug Alcohol Abus. 2012, 38, 389–394. [Google Scholar] [CrossRef]
- Ushida, Y.; Talalay, P. Sulforaphane accelerates acetaldehyde metabolism by inducing aldehyde dehydrogenases: Relevance to ethanol intolerance. Alcohol Alcohol. 2013, 48, 526–534. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C. Glu504Lys Single Nucleotide Polymorphism of Aldehyde Dehydrogenase 2 Gene and the Risk of Human Diseases. BioMed Res. Int. 2015, 2015, 174050. [Google Scholar] [CrossRef]
- Kumar Jain, S.; Sedha, S.; Mishra, M. Genetic Polymorphism and Alcohol Metabolism. In The Recent Topics in Genetic Polymorphisms; IntechOpen: London, UK, 2020. [Google Scholar]
- Suo, C.; Yang, Y.; Yuan, Z.; Zhang, T.; Yang, X.; Qing, T.; Gao, P.; Shi, L.; Fan, M.; Cheng, H.; et al. Alcohol Intake Interacts with Functional Genetic Polymorphisms of Aldehyde Dehydrogenase (ALDH2) and Alcohol Dehydrogenase (ADH) to Increase Esophageal Squamous Cell Cancer Risk. J. Thorac. Oncol. 2019, 14, 712–725. [Google Scholar] [CrossRef]
- Kashyap, P.C.; Marcobal, A.; Ursell, L.K.; Smits, S.A.; Sonnenburg, E.D.; Costello, E.K.; Higginbottom, S.K.; Domino, S.E.; Holmes, S.P.; Relman, D.A.; et al. Genetically dictated change in host mucus carbohydrate landscape exerts a diet-dependent effect on the gut microbiota. Proc. Natl. Acad. Sci. USA 2013, 110, 17059–17064. [Google Scholar] [CrossRef]
- Chun, H.J.; Poklis, J.L.; Poklis, A.; Wolf, C.E. Development and Validation of a Method for Alcohol Analysis in Brain Tissue by Headspace Gas Chromatography with Flame Ionization Detector. J. Anal. Toxicol. 2016, 40, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Mihretu, L.D.; Gebru, A.G.; Mekonnen, K.N.; Asgedom, A.G.; Desta, Y.H. Determination of ethanol in blood using headspace gas chromatography with flameionization detector (HS-GC-FID): Validation of a method. Cogent Chem. 2020, 6, 1760187. [Google Scholar] [CrossRef]
- Fischer, I.; Milton, C.; Wallace, H. Toxicity testing is evolving! Toxicol. Res. 2020, 9, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Issa, S.Y. Forensic Toxicology. In Poisoning in the Modern World—New Tricks for an Old Dog? IntechOpen: London, UK, 2019. [Google Scholar]
- Kwon, Y.; Cho, Y.S.; Lee, Y.M.; Kim, S.J.; Bae, J.; Jeong, S.J. Changes to Gut Microbiota Following Systemic Antibiotic Administration in Infants. Antibiotics 2022, 11, 470. [Google Scholar] [CrossRef]
- Maskell, P.D.; Jones, A.W.; Heymsfield, S.B.; Shapses, S.; Johnston, A. Total body water is the preferred method to use in forensic blood-alcohol calculations rather than ethanol’s volume of distribution. Forensic Sci. Int. 2020, 316, 110532. [Google Scholar] [CrossRef]
- Maskell, P.D.; Cooper, G.A.A. The Contribution of Body Mass and Volume of Distribution to the Estimated Uncertainty Associated with the Widmark Equation. J. Forensic Sci. 2020, 65, 1676–1684. [Google Scholar] [CrossRef]
- Maskell, P.D.; Korb, A.S. Revised equations allowing the estimation of the uncertainty associated with the Total Body Water version of the Widmark equation. J. Forensic Sci. 2022, 67, 358–362. [Google Scholar] [CrossRef]
- Cermák, M. Forensic Analysis and a New Investigation. J. Forensic Identif. 2022, 72, 245. [Google Scholar]
- Boumba, V.A. Modeling Postmortem Ethanol Production/Insights into the Origin of Higher Alcohols. Molecules 2022, 27, 700. [Google Scholar] [CrossRef]
- Elmsjo, A.; Vikingsson, S.; Soderberg, C.; Kugelberg, F.C.; Green, H. Post-Mortem Metabolomics: A Novel Approach in Clinical Biomarker Discovery and a Potential Tool in Death Investigations. Chem. Res. Toxicol. 2021, 34, 1496–1502. [Google Scholar] [CrossRef]
- Kugelberg, F.C.; Jones, A.W. Interpreting results of ethanol analysis in postmortem specimens: A review of the literature. Forensic Sci. Int. 2007, 165, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Marti, V.; Augsburger, M.; Widmer, C.; Lardi, C. Significant postmortem diffusion of ethanol: A case report. Forensic Sci. Int. 2021, 328, 111046. [Google Scholar] [CrossRef] [PubMed]
- Ziavrou, K.; Boumba, V.A.; Vougiouklakis, T.G. Insights into the Origin of Postmortem Ethanol. Int. J. Toxicol. 2005, 24, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Drummer, O.H.; Kennedy, B.; Bugeja, L.; Ibrahim, J.E.; Ozanne-Smith, J. Interpretation of postmortem forensic toxicology results for injury prevention research. Inj. Prev. 2013, 19, 284–289. [Google Scholar] [CrossRef]
- Kennedy, M. Interpreting postmortem drug analysis and redistribution in determining cause of death: A review. Pathol. Lab. Med. Int. 2015, 7, 55. [Google Scholar] [CrossRef]
- Singer, P.P.; Jones, G.R. Very unusual ethanol distribution in a fatality. J. Anal. Toxicol. 1997, 21, 506–508. [Google Scholar] [CrossRef]
- Pélissier-Alicot, A.-L.; Fornaris, M.; Bartoli, C.; Piercecchi-Marti, M.-D.; Sanvoisin, A.; Leonetti, G. An unusual case of post-mortem redistribution of ethanol. Forensic Sci. Int. 2005, 150, 81–83. [Google Scholar] [CrossRef]
- Sastre, C.; Bartoli, C.; Baillif-Couniou, V.; Leonetti, G.; Pelissier-Alicot, A.-L. Post Mortem Redistribution of Drugs: Current State of Knowledge. Curr. Pharm. Des. 2017, 23, 5530–5541. [Google Scholar] [CrossRef]
- Fernández-Solà, J. The Effects of Ethanol on the Heart: Alcoholic Cardiomyopathy. Nutrients 2020, 12, 572. [Google Scholar] [CrossRef]
- Furumiya, J.; Nishimura, H.; Nakanishi, A.; Hashimoto, Y. Postmortem endogenous ethanol production and diffusion from the lung due to aspiration of wood chip dust in the work place. Leg. Med. 2011, 13, 210–212. [Google Scholar] [CrossRef]
- Savini, F.; Tartaglia, A.; Coccia, L.; Palestini, D.; D’Ovidio, C.; de Grazia, U.; Merone, G.M.; Bassotti, E.; Locatelli, M. Ethanol Determination in Post-Mortem Samples: Correlation between Blood and Vitreous Humor Concentration. Molecules 2020, 25, 2724. [Google Scholar] [CrossRef] [PubMed]
- Meijnikman, A.S.; Davids, M.; Herrema, H.; Aydin, O.; Tremaroli, V.; Rios-Morales, M.; Levels, H.; Bruin, S.; de Brauw, M.; Verheij, J. Microbiome-derived ethanol in nonalcoholic fatty liver disease. Nat. Med. 2022, 28, 2100–2106. [Google Scholar] [CrossRef] [PubMed]
- Al-Asmari, A.I.; Altowairgi, M.M.; Al-Amoudi, D.H. Effects of postmortem interval, putrefaction, diabetes, and location of death on the analysis of ethyl glucuronide and ethyl sulfate as ethanol biomarkers of antemortem alcohol consumption. Forensic Sci. Int. 2022, 335, 111280. [Google Scholar] [CrossRef]
- Hayashi, H.; Sasajima, H.; Takayanagi, Y.; Kanamaru, H. International standardization for smarter society in the field of measurement, control and automation. In Proceedings of the 2017 56th Annual Conference of the Society of Instrument and Control Engineers of Japan (SICE), Kanazawa, Japan, 19–22 September 2017; pp. 263–266. [Google Scholar]
- Carayannis, E.G.; Morawska-Jancelewicz, J. The Futures of Europe: Society 5.0 and Industry 5.0 as Driving Forces of Future Universities. J. Knowl. Econ. 2022, 13, 3445–3471. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, X.; Cao, X.; Huang, C.; Liu, E.; Qian, S.; Liu, X.; Wu, Y.; Dong, F.; Qiu, C.W.; et al. Artificial intelligence: A powerful paradigm for scientific research. Innovation 2021, 2, 100179. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Byrne, R.; Schneider, G.; Yang, S. Concepts of Artificial Intelligence for Computer-Assisted Drug Discovery. Chem. Rev. 2019, 119, 10520–10594. [Google Scholar] [CrossRef] [PubMed]
- Sergi, C.M. Digital pathology: The time is now to bridge the gap between medicine and technological singularity. In Interactive Multimedia-Multimedia Production and Digital Storytelling; IntechOpen: London, UK, 2019. [Google Scholar]
- Jaillant, L.; Caputo, A. Unlocking digital archives: Cross-disciplinary perspectives on AI and born-digital data. AI Soc. 2022, 37, 823–835. [Google Scholar] [CrossRef]
- Ries, T.; Palkó, G. Born-digital archives. Int. J. Digit. Humanit. 2019, 1, 1–11. [Google Scholar] [CrossRef]
- Ahmed Alaa El-Din, E. Artificial intelligence in forensic science: Invasion or revolution? Egypt. Soc. Clin. Toxicol. J. 2022, 10, 20–32. [Google Scholar] [CrossRef]
- González-Domínguez, R.; García-Barrera, T.; Gómez-Ariza, J.L. Metabolite profiling for the identification of altered metabolic pathways in Alzheimer’s disease. J. Pharm. Biomed. Anal. 2015, 107, 75–81. [Google Scholar] [CrossRef]
- Martins, C.; Dreij, K.; Costa, P.M. The State-of-the Art of Environmental Toxicogenomics: Challenges and Perspectives of “Omics” Approaches Directed to Toxicant Mixtures. Int. J. Environ. Res. Public Health 2019, 16, 4718. [Google Scholar] [CrossRef] [PubMed]
- Quireyns, M.; Boogaerts, T.; Van Wichelen, N.; Covaci, A.; Van Nuijs, A.L.N. State-of-the-art analytical approaches and strategies to assess disposal of drugs for wastewater-based epidemiology. Wiley Interdiscip. Rev. Forensic Sci. 2022, 5, e1469. [Google Scholar] [CrossRef]
- Pélissier-Alicot, A.-L. Synthetic cannabinoids: State-of-the-art with a focus on fertility and development. In Cannabis and the Developing Brain; Elsevier: Amsterdam, The Netherlands, 2022; pp. 243–258. [Google Scholar]
- Aggarwal, K.; Mijwil, M.M.; Al-Mistarehi, A.-H.; Alomari, S.; Gök, M.; Alaabdin, A.M.Z.; Abdulrhman, S.H. Has the Future Started? The Current Growth of Artificial Intelligence, Machine Learning, and Deep Learning. Iraqi J. Comput. Sci. Math. 2022, 3, 115–123. [Google Scholar]
- Svensson, A.M.; Jotterand, F. Doctor ex machina: A critical assessment of the use of artificial intelligence in health care. J. Med. Philos. A Forum Bioeth. Philos. Med. 2022, 47, 155–178. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M.; the QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Pantic, I.; Paunovic, J.; Cumic, J.; Valjarevic, S.; Petroianu, G.A.; Corridon, P.R. Artificial neural networks in contemporary toxicology research. Chem. Biol. Interact. 2023, 369, 110269. [Google Scholar] [CrossRef] [PubMed]
- Mehrvar, S.; Himmel, L.E.; Babburi, P.; Goldberg, A.L.; Guffroy, M.; Janardhan, K.; Krempley, A.L.; Bawa, B. Deep Learning Approaches and Applications in Toxicologic Histopathology: Current Status and Future Perspectives. J. Pathol. Inform. 2021, 12, 42. [Google Scholar] [CrossRef]
- Raju, B.; Jumah, F.; Ashraf, O.; Narayan, V.; Gupta, G.; Sun, H.; Hilden, P.; Nanda, A. Big data, machine learning, and artificial intelligence: A field guide for neurosurgeons. J. Neurosurg. 2020, 1, 1–11. [Google Scholar] [CrossRef]
- Katz, P.S. Expert Robot: Using Artificial Intelligence to Assist Judges in Admitting Scientific Expert Testimony. Albany Law J. Sci. Technol. 2014, 24, 1. [Google Scholar]
- Whitford, A.B.; Yates, J.; Burchfield, A.; Anastasopoulos, J.L.; Anderson, D.M. The Adoption of Robotics by Government Agencies: Evidence from Crime Labs. Public Adm. Rev. 2020, 80, 976–988. [Google Scholar] [CrossRef]
- Eaton, K.K. Gut fermentation: A reappraisal of an old clinical condition with diagnostic tests and management: Discussion paper. J. R. Soc. Med. 1991, 84, 669–671. [Google Scholar] [CrossRef]
- Piovezani Ramos, G.; Kane, S. Alcohol Use in Patients with Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2021, 17, 211–225. [Google Scholar]
- Tomlin, J.; Lega, I.; Braun, P.; Kennedy, H.G.; Herrando, V.T.; Barroso, R.; Castelletti, L.; Mirabella, F.; Scarpa, F.; Völlm, B. Forensic mental health in Europe: Some key figures. Soc. Psychiatry Psychiatr. Epidemiol. 2021, 56, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Calcan, A.; Holmes, M. Locked down and drinking? Are more people self-identifying as having mental health difficulties alongside their drinking via an online platform? Adv. Dual Diagn. 2021, 14, 159–166. [Google Scholar] [CrossRef]
- Smędra, A.; Trzmielak, M.; Góralska, K.; Dzikowiec, M.; Wochna, K.; Brzeziańska-Lasota, E.; Berent, J. Can negative results of 40 g glucose load test exclude auto-brewery syndrome? Comment to: “Auto-brewery syndrome caused by oral fungi and periodontal disease bacteria” by Takahashi et al. Acute Med Surg. 2021 May 3; 8: e652. Acute Med. Surg. 2022, 9, e757. [Google Scholar] [CrossRef] [PubMed]
- Mbaye, B.; Borentain, P.; Magdy Wasfy, R.; Alou, M.T.; Armstrong, N.; Mottola, G.; Meddeb, L.; Ranque, S.; Gerolami, R.; Million, M.; et al. Endogenous Ethanol and Triglyceride Production by Gut Pichia kudriavzevii, Candida albicans and Candida glabrata Yeasts in Non-Alcoholic Steatohepatitis. Cells 2022, 11, 3390. [Google Scholar] [CrossRef]
- Benede-Ubieto, R.; Estevez-Vazquez, O.; Ramadori, P.; Cubero, F.J.; Nevzorova, Y.A. Guidelines and Considerations for Metabolic Tolerance Tests in Mice. Diabetes Metab. Syndr. Obes. 2020, 13, 439–450. [Google Scholar] [CrossRef]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef]
- Nishiwaki, H.; Ueyama, J.; Kashihara, K.; Ito, M.; Hamaguchi, T.; Maeda, T.; Tsuboi, Y.; Katsuno, M.; Hirayama, M.; Ohno, K. Gut microbiota in dementia with Lewy bodies. NPJ Park. Dis. 2022, 8, 169. [Google Scholar] [CrossRef]
- Grigoryan, Z.; Shen, M.J.; Twardus, S.W.; Beuttler, M.M.; Chen, L.A.; Bateman-House, A. Fecal microbiota transplantation: Uses, questions, and ethics. Med. Microecol. 2020, 6, 100027. [Google Scholar] [CrossRef]
- Nigam, M.; Panwar, A.S.; Singh, R.K. Orchestrating the fecal microbiota transplantation: Current technological advancements and potential biomedical application. Front. Med. Technol. 2022, 4, 961569. [Google Scholar] [CrossRef] [PubMed]
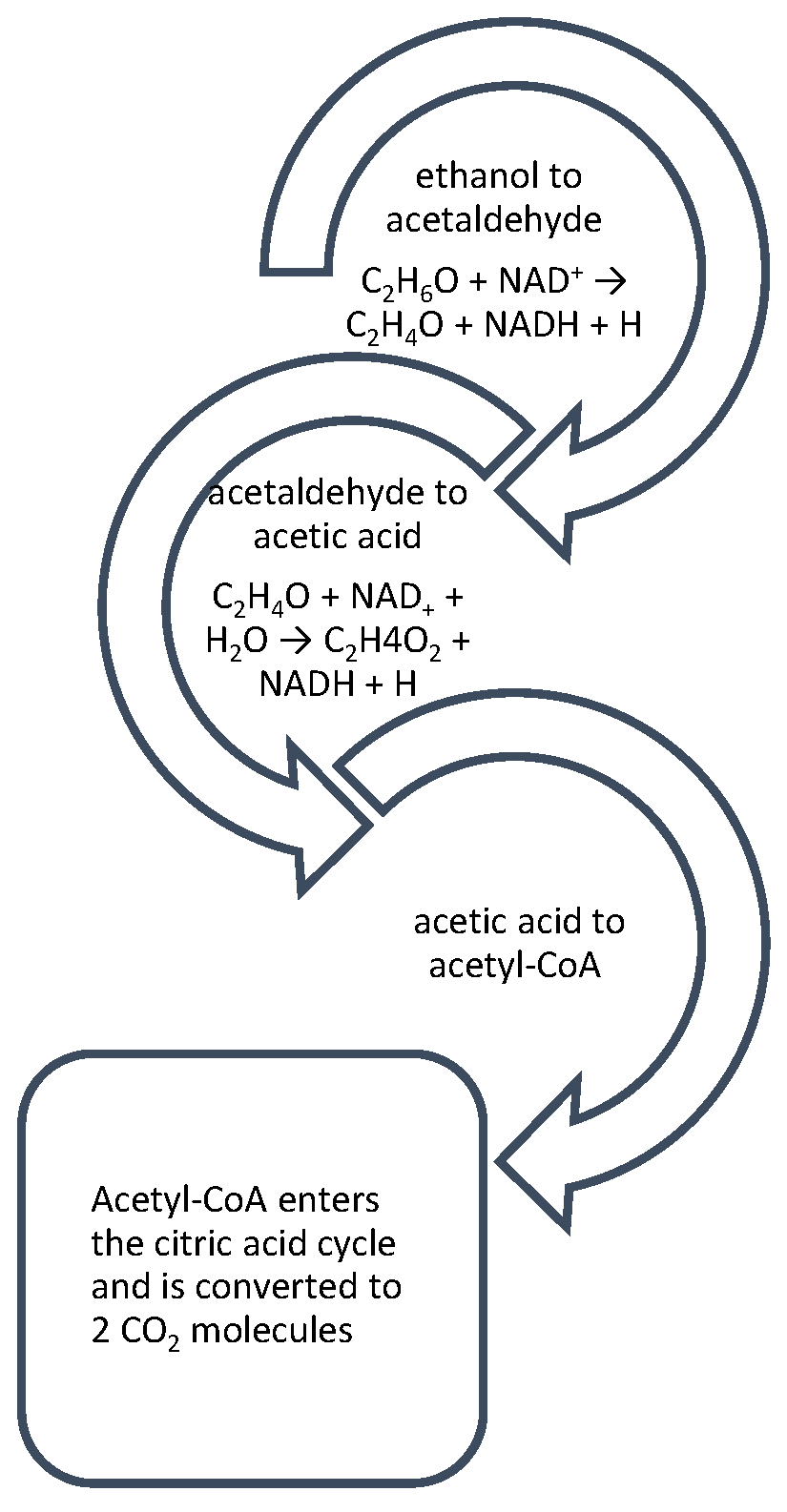
| Systems | Symptoms |
|---|---|
| General | Unexplained intoxication, Glassy eyes, Smell of alcohol in breath, Chronic fatigue. |
| Nervous System | Memory loss, Mental status changes, Recurrent seizures, Slurred speech, Incoherent speech, Difficulty in articulation, Blurred vision, Dizziness, Disorientation, Ataxia |
| Gastrointestinal System | Bloating, Belching, Nausea, Vomiting. |
| Musculoskeletal System | Poor coordination, frequent falls, Stumbling gait. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šoša, I. The Human Body as an Ethanol-Producing Bioreactor—The Forensic Impacts. Fermentation 2023, 9, 738. https://doi.org/10.3390/fermentation9080738
Šoša I. The Human Body as an Ethanol-Producing Bioreactor—The Forensic Impacts. Fermentation. 2023; 9(8):738. https://doi.org/10.3390/fermentation9080738
Chicago/Turabian StyleŠoša, Ivan. 2023. "The Human Body as an Ethanol-Producing Bioreactor—The Forensic Impacts" Fermentation 9, no. 8: 738. https://doi.org/10.3390/fermentation9080738
APA StyleŠoša, I. (2023). The Human Body as an Ethanol-Producing Bioreactor—The Forensic Impacts. Fermentation, 9(8), 738. https://doi.org/10.3390/fermentation9080738






