Comparative Analysis of Microbial and Mycotoxin Contamination in Korean Traditional Soybean Paste and Soy Sauce Production with and without Starter
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Measuring Aflatoxins and Ochratoxin A in Doenjang and Ganjang
2.2.1. Standards and Reagents
2.2.2. Sample Preparation
2.3. Measuring Microbial Contamination Levels in Doenjang and Ganjang
2.4. Microbial Taxonomic Profiling
3. Results and Discussion
3.1. Monitoring the Risk Factors of Commercial Products according to the Manufacturing Methods
3.1.1. Detection of Aflatoxins and Ochratoxin A in Doenjang and Ganjang
3.1.2. Measuring Microbial Contamination Levels in Doenjang and Ganjang
3.2. Analysis of Risk Factors according to the Production Stages
3.2.1. Detection of Mycotoxins according to the Production Stage
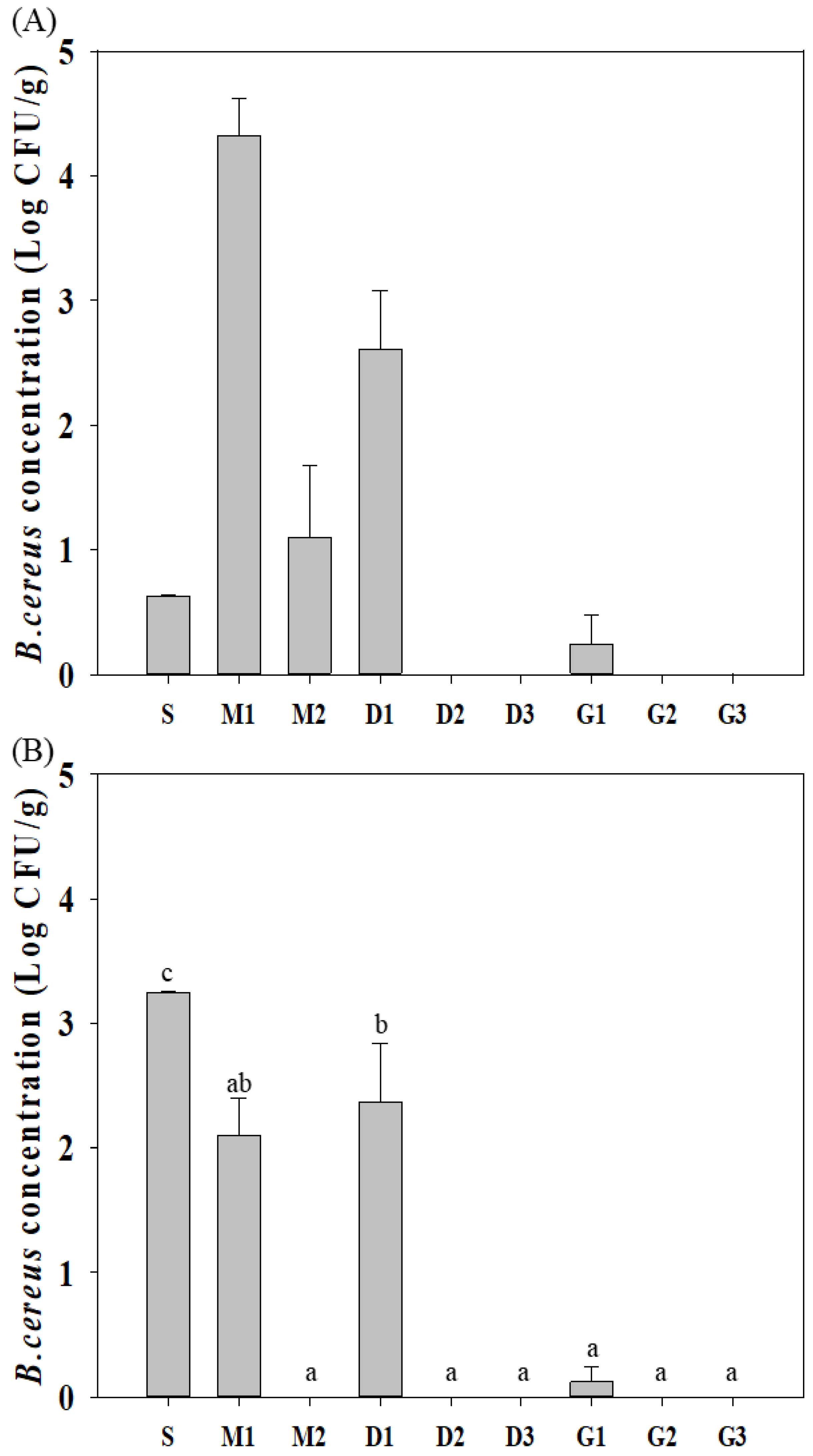
3.2.2. Occurrence of B. cereus according to the Production Stage
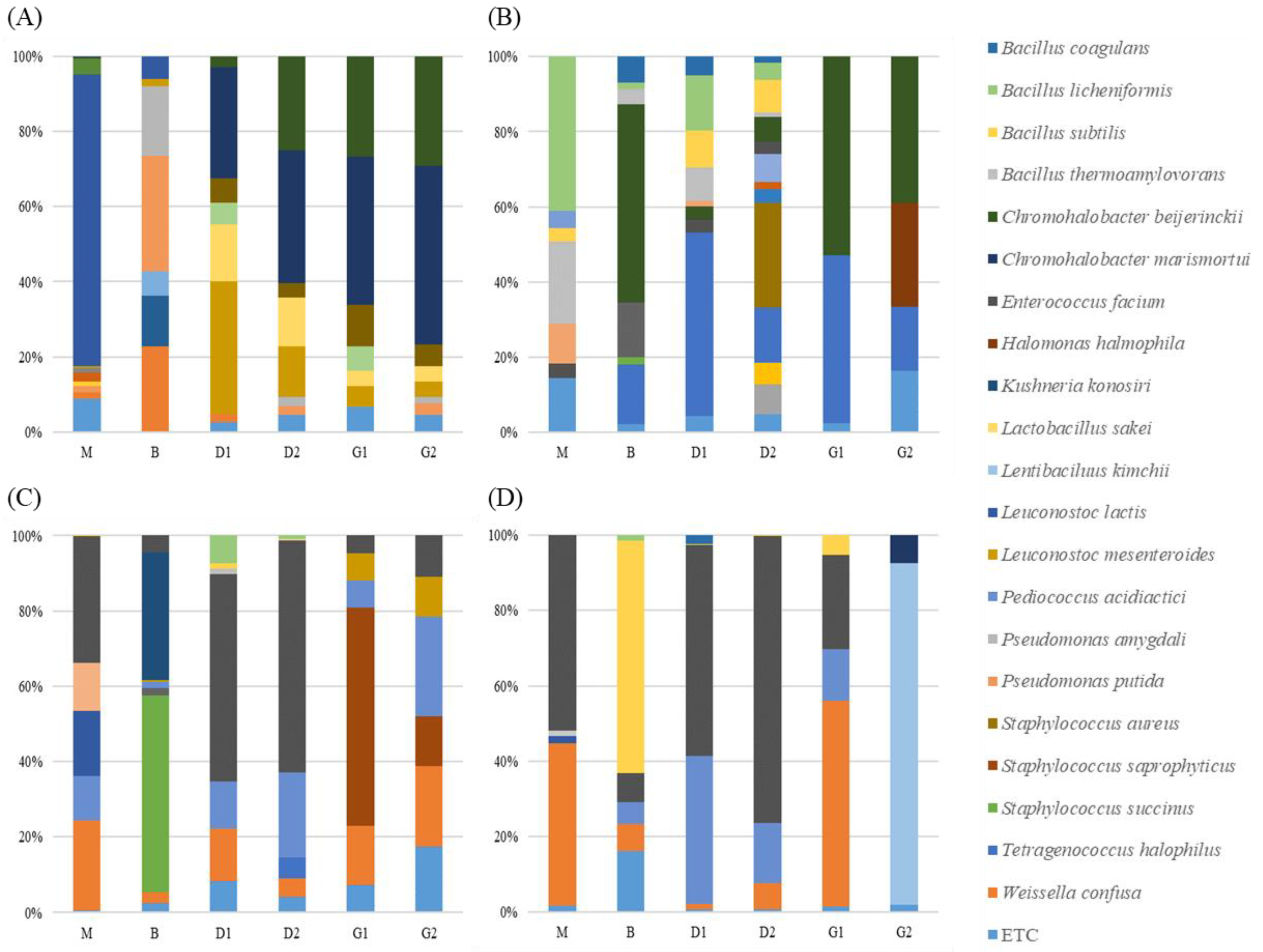
3.2.3. Microbial Taxonomic Profiling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, S.L.; Lee, Y.H.; Chi, H.Y.; Lee, S.J.; Kim, S.J. Diversity in lipid contents and fatty acid composition of soybean seeds cultivated in Korea. Korean J. Crop Sci. 2007, 52, 348–357. [Google Scholar]
- Park, K.Y.; Hwang, K.M.; Jung, K.O.; Lee, K.B. Studies on the Standardization of Doenjang (Korean Soybean Paste) 1. Standardization of Manufacturing Method of Doenjang by Literatures. J. Korean Soc. Food Sci. Nutr. 2002, 31, 343–350. [Google Scholar] [CrossRef]
- Jeon, H.; Lee, S.; Kim, S.; Kim, Y. Quality characteristics of modified doenjang and traditional doenjang. J. Korean Soc. Food Sci. Nutr. 2016, 45, 1001–1009. [Google Scholar] [CrossRef]
- Kim, S.; Park, S.Y.; Hong, S.; Lim, S.D. Quality characteristics of regional traditional and commercial soy sauce (Ganjang). KoreanW J. Food Cook. Sci. 2017, 33, 45–53. [Google Scholar] [CrossRef]
- Lee, H.T.; Kim, J.H.; Lee, S.S. Analysis of microbiological contamination and biogenic amines content in traditional and commercial doenjang. J. Food Hyg. S. Afr. 2009, 24, 102–109. [Google Scholar]
- Seon, Y.K.; Park, J.S.; Yang, E.J. Isolation of microorganism with high protease activity from doenjang and production of doenjang with isolated strain. J. Korean Soc. Food Sci. Nutr. 2021, 50, 79–87. [Google Scholar] [CrossRef]
- Kim, Y.S.; Jeong, D.Y.; Hwang, Y.T.; Uhm, T.B. Bacterial community profiling during the manufacturing process of traditional soybean paste by Pyrosequencing method. Korean J. Microbiol. 2011, 47, 275–280. [Google Scholar]
- Lee, E.J.; Hurh, B.S.; Lee, I. Development of ready-to-use starters for the production of doenjang. Microbiol. Biotechnol. Lett. 2019, 47, 234–241. [Google Scholar] [CrossRef]
- Nyenje, M.E.; Odjadjare, C.E.; Tanih, N.F.; Green, E.; Ndip, R.N. Foodborne pathogens recovered from ready-to-eat foods from roadside cafeterias and retail outlets in Alice, Eastern Cape Province, South Africa: Public health implications. Int. J. Environ. Res. Public Health 2012, 9, 2608–2619. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G.; Roh, W.S. Changes of aflatoxins during the ripening of Korean soy paste and soy sauce and the characteristics of the changes—Part 1. J. Food Hyg. Saf. 1998, 13, 313–317. [Google Scholar]
- Abdin, M.Z.; Ahmad, M.M.; Javed, S. Advances in molecular detection of Aspergillus: An update. Arch. Microbiol. 2010, 192, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.N.; Salleh, B.; Saad, B.; Abbas, H.K.; Abel, C.A.; Shier, W.T. An overview of mycotoxin contamination in foods and its implications for human health. Toxin Rev. 2010, 29, 3–26. [Google Scholar] [CrossRef]
- Murphy, P.A.; Hendrich, S.; Landgren, C.; Bryant, C.M. Food mycotoxins: An update. J. Food Sci. 2006, 71, R51–R65. [Google Scholar] [CrossRef]
- Mousa, W.; Ghazali, F.M.; Jinap, S.; Ghazali, H.M.; Radu, S. Modelling the effect of water activity and temperature on growth rate and aflatoxin production by two isolates of Aspergillus flavus on paddy. J. Appl. Microbiol. 2011, 111, 1262–1274. [Google Scholar] [CrossRef] [PubMed]
- Luh, B.S. Industrial production of soy sauce. J. Ind. Microbiol. 1995, 14, 467–471. [Google Scholar] [CrossRef]
- Nagarajan, V.; Bhat, R.V.; Tulpule, P.G. Aflatoxin production in some varieties of soybeans (Glycine max L.). Experientia 1973, 29, 1302–1303. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.E.; Chung, S.H.; Hong, S.Y. Natural occurrence of aflatoxins and ochratoxin A in meju and soybean paste produced in South Korea. Appl. Biol. Chem. 2019, 62, 65. [Google Scholar] [CrossRef]
- Park, J.W.; Yoo, M.S.; Kuk, J.H.; Ji, Y.; Lee, J.H. Simultaneous determination and Mornitoring of aflatoxin and ochratoxin A in food. J. Food Hyg. S. Afr. 2013, 28, 75–82. [Google Scholar] [CrossRef]

| Sample | Number of Samples | Number of Positive Samples | Range | Mean | ||||
|---|---|---|---|---|---|---|---|---|
| AFB1 (ug/kg) | AFG1 (ug/kg) | Total Afs (ug/kg) | OTA (ug/kg) | Total Afs (ug/kg) | ||||
| Doenjang | Traditional | 24 | 14 | 1.43–8.45 | 2.10–87.67 | 1.64–87.67 | ND | 8.22 |
| Commercial | 24 | 12 | 3.14–16.68 | 0.25–35.75 | 0.25–35.75 | ND | 10.20 | |
| Ganjang | Traditional | 18 | 3 | 3.39–5.07 | ND | 3.14–16.68 | ND | 7.80 |
| Commercial | 17 | 13 | 1.57–14.24 | ND | 1.57–14.24 | ND | 6.57 | |
| Sample | No. of Samples | No. of Positive Samples | Range (LogCFU/g) | Positive Mean (LogCFU/g) | |
|---|---|---|---|---|---|
| Doenjang | Traditional | 24 | 5 | 0–2.49 | 0.58 |
| Commercial | 24 | 3 | 0.81–3.23 | 1.79 | |
| Ganjang | Traditional | 18 | 2 | 0–0.62 | 0.18 |
| Commercial | 17 | 0 | ND | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Jeong, J.; Jang, M.; Kim, J.-C.; Lee, H. Comparative Analysis of Microbial and Mycotoxin Contamination in Korean Traditional Soybean Paste and Soy Sauce Production with and without Starter. Fermentation 2023, 9, 621. https://doi.org/10.3390/fermentation9070621
Kim J, Jeong J, Jang M, Kim J-C, Lee H. Comparative Analysis of Microbial and Mycotoxin Contamination in Korean Traditional Soybean Paste and Soy Sauce Production with and without Starter. Fermentation. 2023; 9(7):621. https://doi.org/10.3390/fermentation9070621
Chicago/Turabian StyleKim, Jinkwi, Jiyoun Jeong, Mi Jang, Jong-Chan Kim, and Heeyoung Lee. 2023. "Comparative Analysis of Microbial and Mycotoxin Contamination in Korean Traditional Soybean Paste and Soy Sauce Production with and without Starter" Fermentation 9, no. 7: 621. https://doi.org/10.3390/fermentation9070621
APA StyleKim, J., Jeong, J., Jang, M., Kim, J.-C., & Lee, H. (2023). Comparative Analysis of Microbial and Mycotoxin Contamination in Korean Traditional Soybean Paste and Soy Sauce Production with and without Starter. Fermentation, 9(7), 621. https://doi.org/10.3390/fermentation9070621





