pH and Heat Pretreatments with Zero-Valent Iron Addition to Enhance Biogas Production from Cassava Pulp Wastewater: Optimization and Comparison of Mathematical Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Wastewater Sources and Characterizations
2.2. Reactor Design
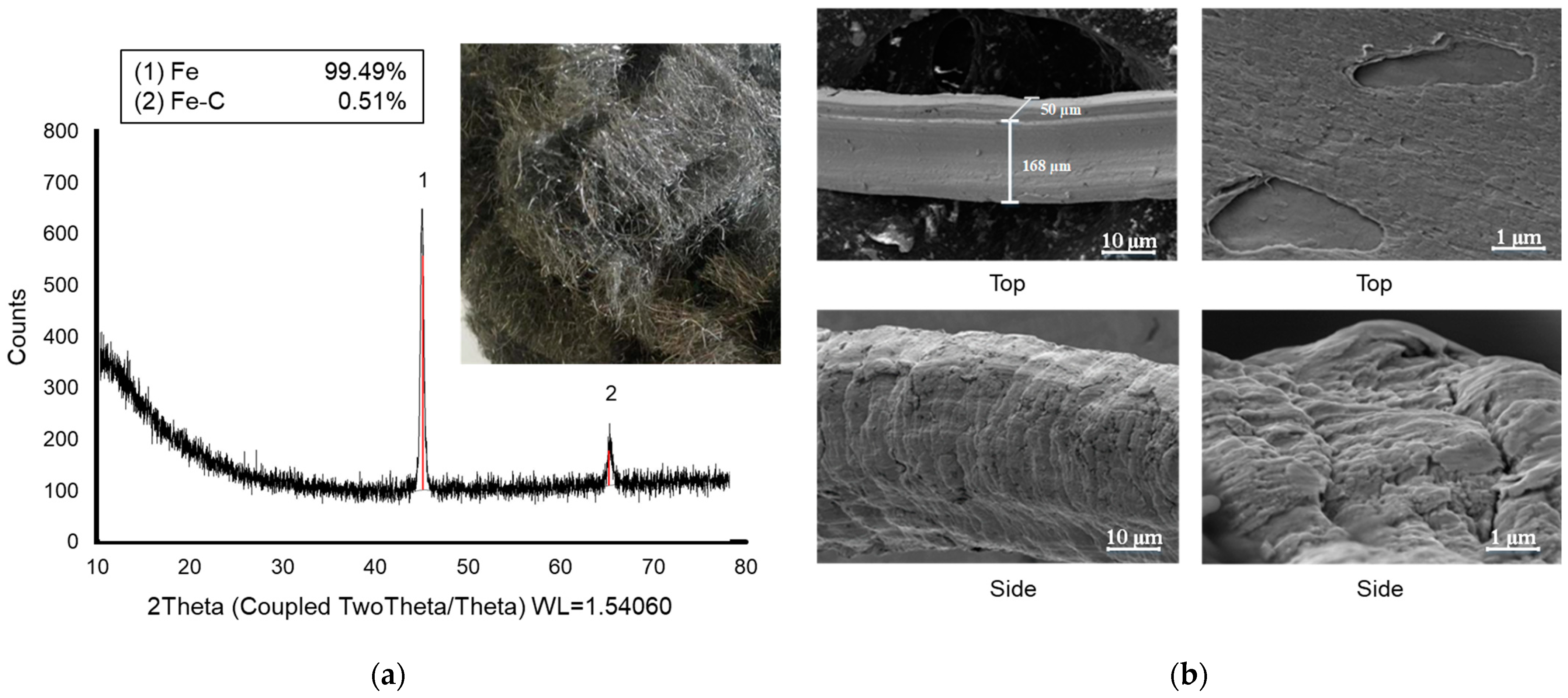
2.3. Zero-Valent Iron Preparation
2.4. Experimental Design
2.5. Analytical Methods
2.6. Mathematical Models and Kinetics
2.7. Data Analysis
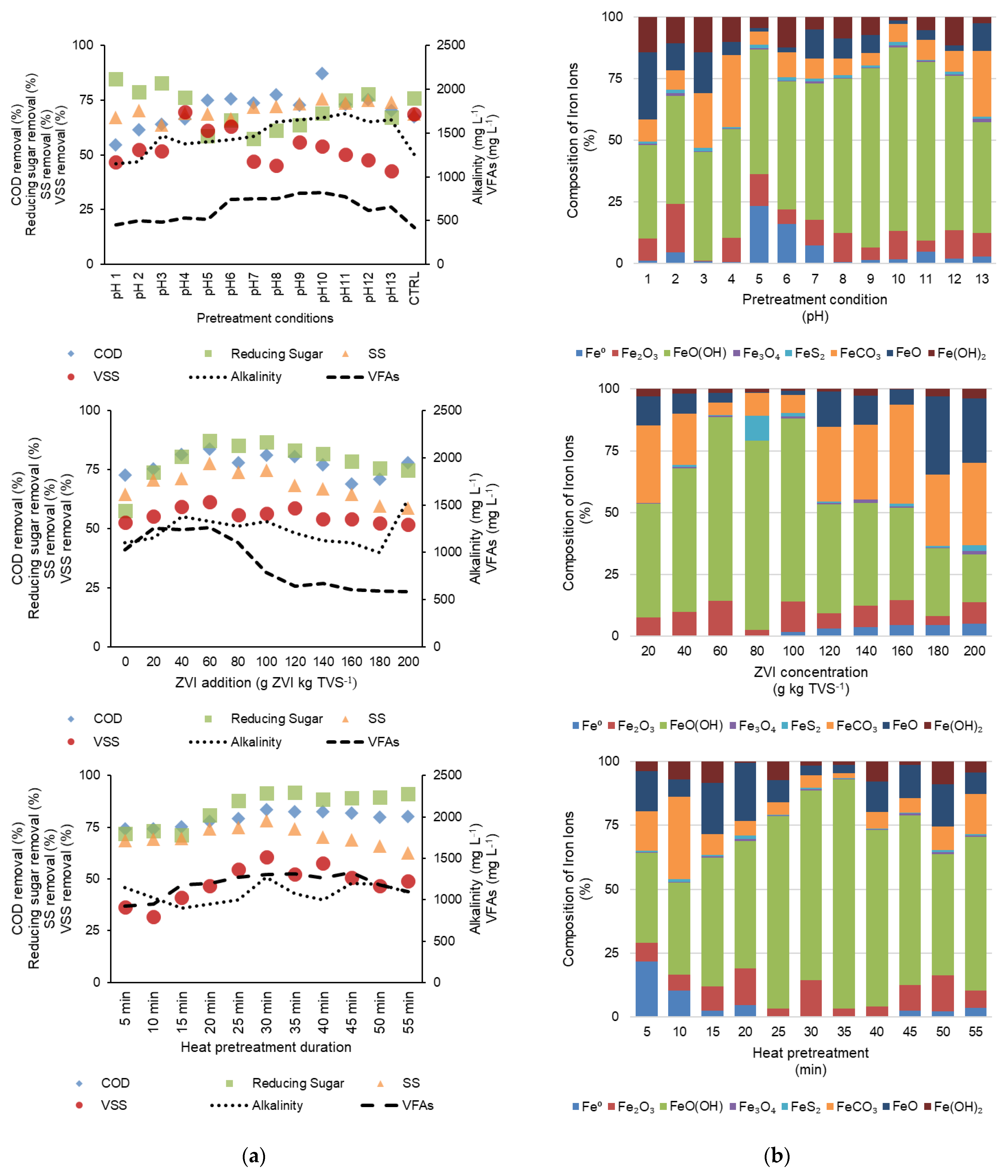
3. Results and Discussion
3.1. ZVI Characterization
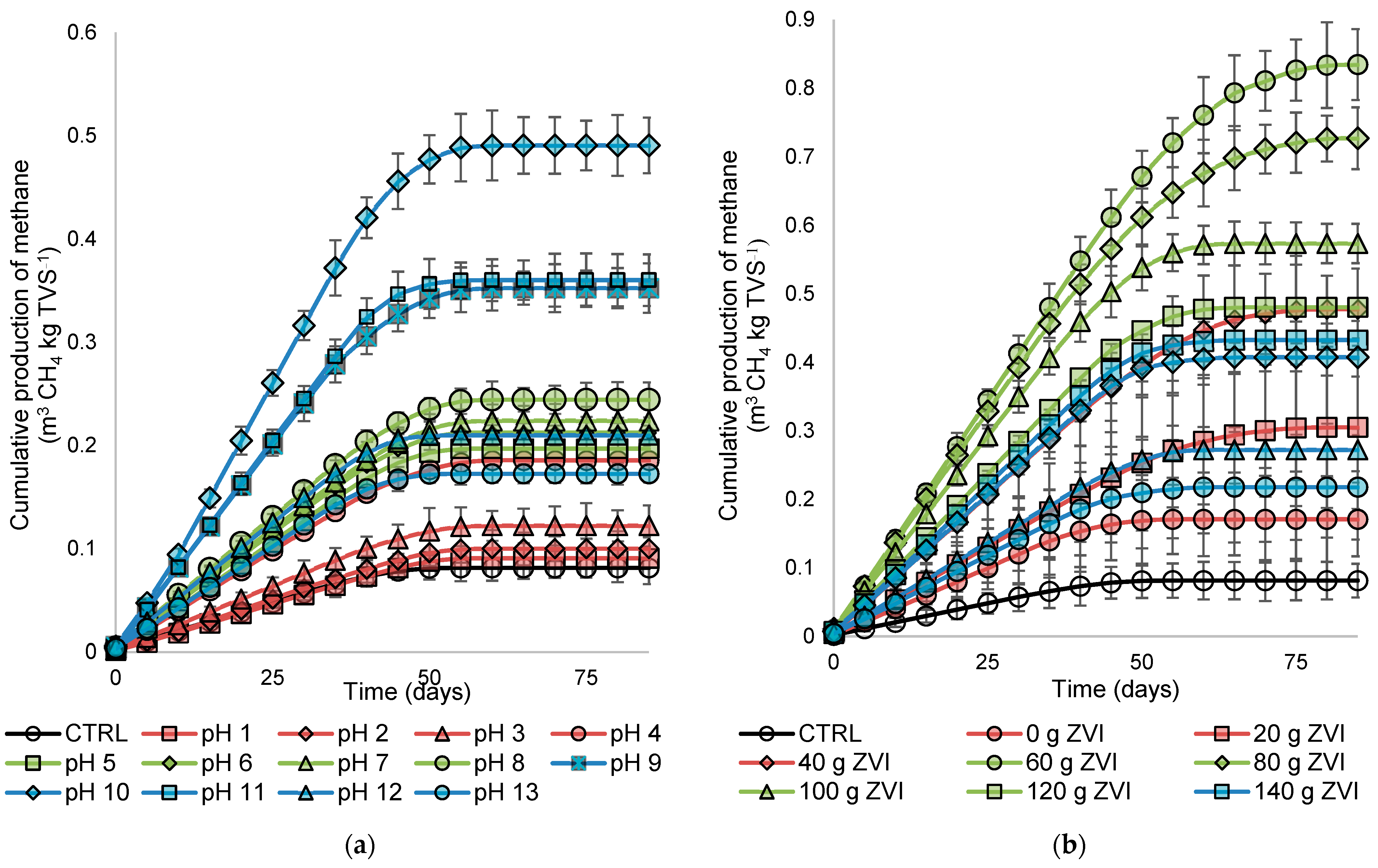
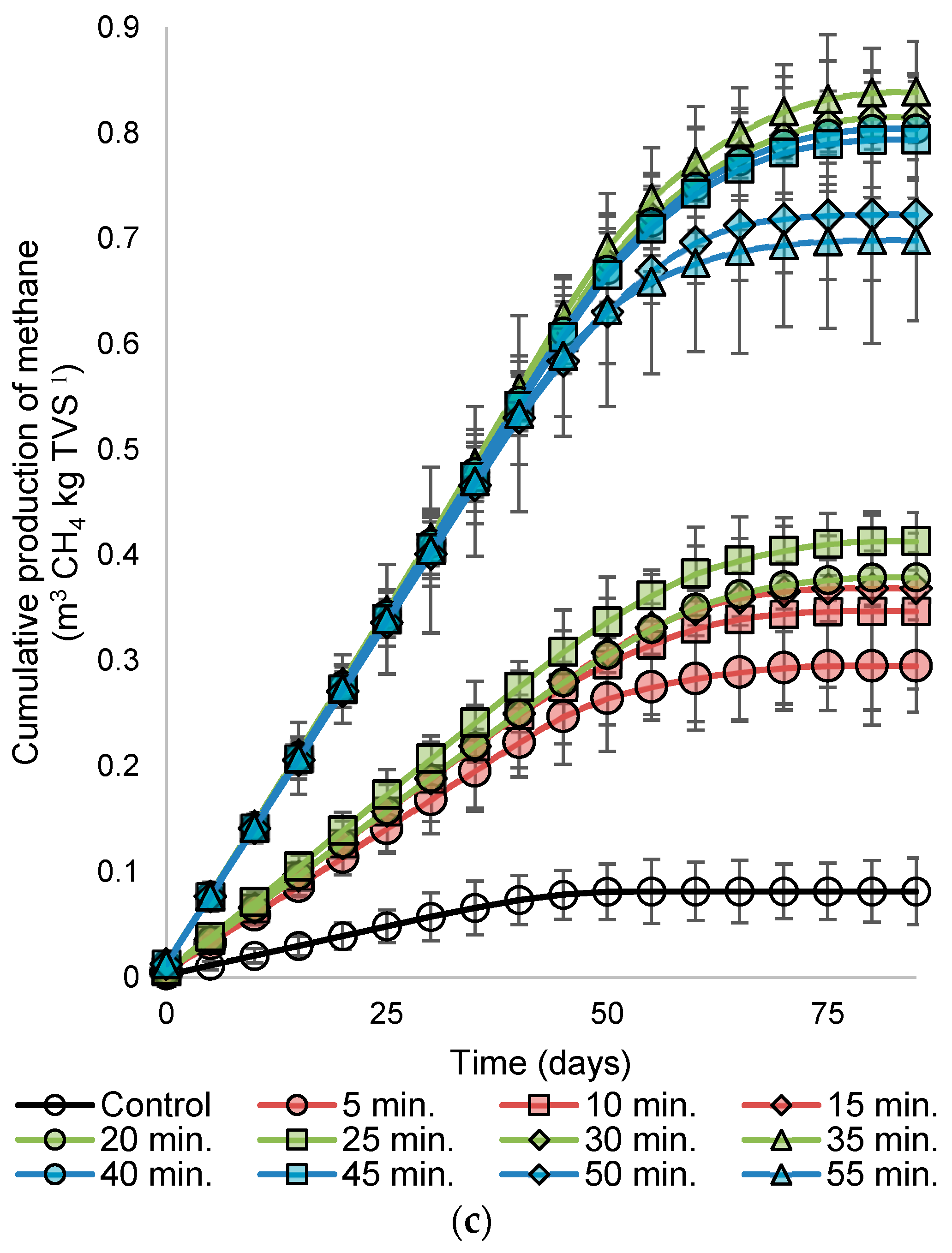
3.2. Biogas Production and Composition
3.3. Substrate and ZVI Utilizations
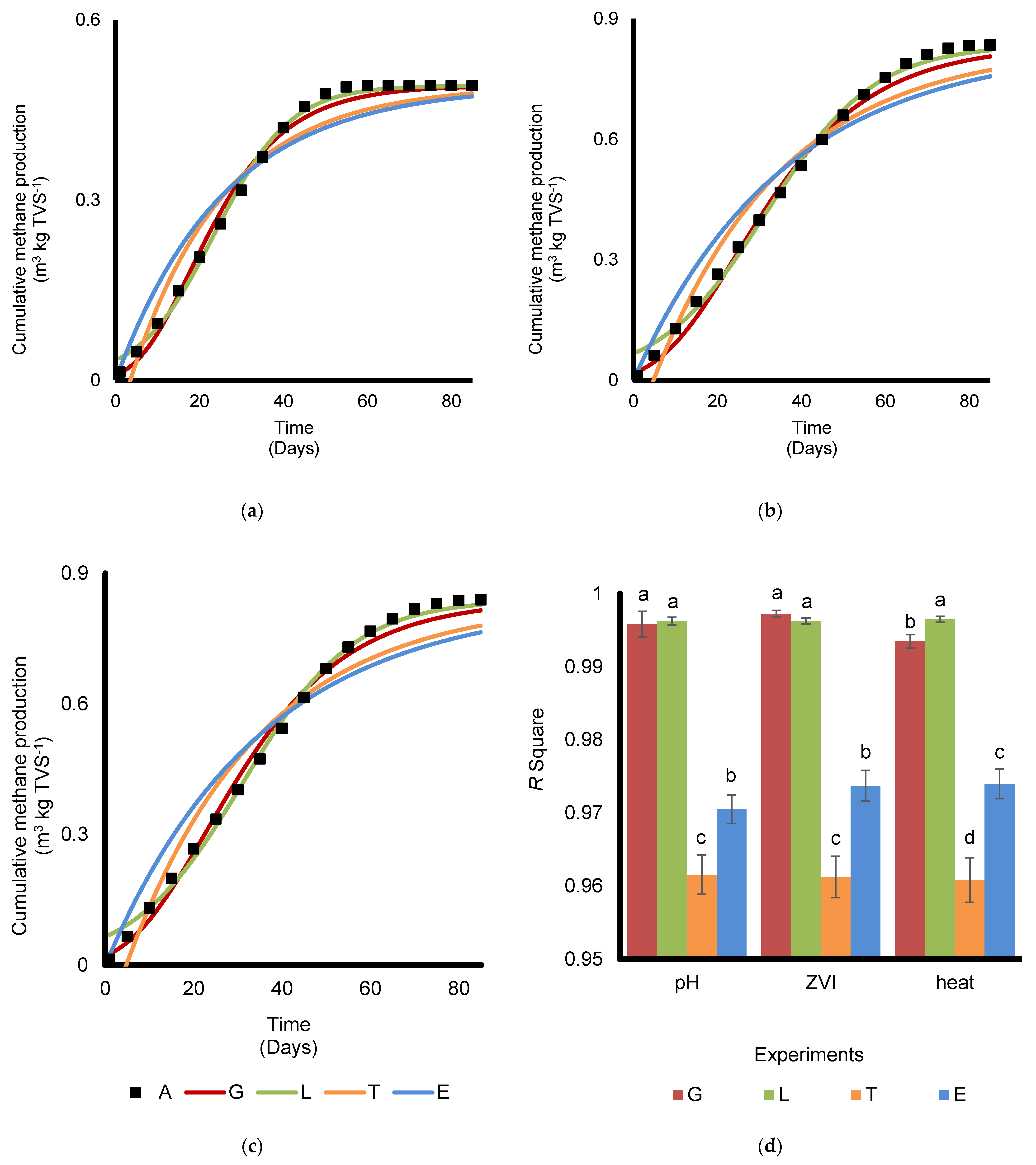
3.4. Models for Biogas Production
- The alkaline condition showed effective performance in the heat pretreatment for cassava pulp wastewater. Although the acid showed higher organic matter removal, the biogas generation was better in the alkaline–heat pretreatment.
- Synthesized ZVI was successfully applied in the AD. It was made from the affordable and abundant raw material iron wool. The Fe0 oxidation reached the end product of FeOOH (Fe3+), which was depicted by the large portion of these iron forms at the end of biogas generation.
- Several models were used to calculate the kinetics parameters of the papers. It can be seen that the Modified Gompertz and Logistic models fit the data more than the Transference Function model, with a slightly better performance of the Logistic model. A correlation between the kinetics parameters from the suitable models was also found. The Rm and maximum biomass potential correlated with the effluent’s FeOOH.
- The optimization using three experiment steps may still be improved to determine the exact time, pH, and ZVI concentrations in other approaches to obtain accurate numbers for each treatment [11,38]. Several previous studies regarding biogas optimization that involved several parameters had used factorial or response surface method experimental designs to obtain more detail levels of pH, durations of heating, and concentrations of ZVI. The mentioned experimental designs can also be explained further using principal component analysis (PCA), where the relationship between each dataset can be observed in detail.
- The claim of correlation of the P and Rm with FeOOH concentration can be further analyzed and tested in numerous experiments. It can also help to better understand the mechanisms and efficiency of the AD system [76].
- SS and VSS removals efficiencies were still moderate. Thus, different processes must be performed before the effluent can be recycled or released into the environment. Several studies have performed better using other substrates [24,45]. In future investigations, the efficiency of removals shall be deliberated in the overall output from the addition of pretreatment and additives.
- The modification of pretreatments and the addition of ZVI may result in switches of the microbial community in the AD system. The switch may also be important to be observed and determined in order to determine the sustainability of the recirculate effluent as seeds with regard to the quality of the generated biogas [5].
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wattanasilp, C.; Songprakorp, R.; Nopharatana, A.; Khompatraporn, C. Techno-Cost-Benefit Analysis of Biogas Production from Industrial Cassava Starch Wastewater in Thailand for Optimal Utilization with Energy Storage. Energies 2021, 14, 416. [Google Scholar] [CrossRef]
- Lin, H.; Borrion, A.; da Fonseca-Zang, W.A.; Zang, J.W.; Leandro, W.M.; Campos, L.C. Life cycle assessment of a biogas system for cassava processing in Brazil to close the loop in the water-waste-energy-food nexus. J. Clean. Prod. 2021, 299, 126861. [Google Scholar] [CrossRef]
- dos Santos, A.L.M.; Castro, A.L.S.; Salomon, K.R.; de Souza, T.S.O.; Vich, D.V. Global research trends on anaerobic digestion and biogas production from cassava wastewater: A bibliometric analysis. J. Chem. Technol. Biotechnol. 2022, 97, 1379–1389. [Google Scholar] [CrossRef]
- Cruz, I.A.; Santos Andrade, L.R.; Bharagava, R.N.; Nadda, A.K.; Bilal, M.; Figueiredo, R.T.; Romanholo Ferreira, L.F. Valorization of cassava residues for biogas production in Brazil based on the circular economy: An updated and comprehensive review. Clean. Eng. Technol. 2021, 4, 100196. [Google Scholar] [CrossRef]
- Mirmohamadsadeghi, S.; Karimi, K.; Azarbaijani, R.; Parsa Yeganeh, L.; Angelidaki, I.; Nizami, A.-S.; Bhat, R.; Dashora, K.; Vijay, V.K.; Aghbashlo, M.; et al. Pretreatment of lignocelluloses for enhanced biogas production: A review on influencing mechanisms and the importance of microbial diversity. Renew. Sustain. Energy Rev. 2021, 135, 110173. [Google Scholar] [CrossRef]
- Gunes, B.; Stokes, J.; Davis, P.; Connolly, C.; Lawler, J. Pre-treatments to enhance biogas yield and quality from anaerobic digestion of whiskey distillery and brewery wastes: A review. Renew. Sustain. Energy Rev. 2019, 113, 109281. [Google Scholar] [CrossRef]
- Zhang, M.; Xie, L.; Yin, Z.; Khanal, S.K.; Zhou, Q. Biorefinery approach for cassava-based industrial wastes: Current status and opportunities. Bioresour. Technol. 2016, 215, 50–62. [Google Scholar] [CrossRef]
- Okudoh, V.; Trois, C.; Workneh, T.; Schmidt, S. The potential of cassava biomass and applicable technologies for sustainable biogas production in South Africa: A review. Renew. Sustain. Energy Rev. 2014, 39, 1035–1052. [Google Scholar] [CrossRef]
- Toreci, I.; Droste, R.L.; Kennedy, K.J. Mesophilic Anaerobic Digestion with High-Temperature Microwave Pretreatment and Importance of Inoculum Acclimation. Water Environ. Res. 2011, 83, 549–559. [Google Scholar] [CrossRef]
- Mañunga, T.; Barrios-Pérez, J.D.; Zaiat, M.; Rodríguez-Victoria, J.A. Evaluation of pretreatment methods and initial pH on mixed inoculum for fermentative hydrogen production from cassava wastewater. Biofuels 2022, 13, 301–308. [Google Scholar] [CrossRef]
- Zhang, Q.; Tang, L.; Zhang, J.; Mao, Z.; Jiang, L. Optimization of thermal-dilute sulfuric acid pretreatment for enhancement of methane production from cassava residues. Bioresour. Technol. 2011, 102, 3958–3965. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.; Zhang, J.; Zhou, J.; Lv, C.; Geng, Z. The byproduct-organic acids strengthened pretreatment of cassava straw: Optimization and kinetic study. Bioresour. Technol. 2019, 290, 121756. [Google Scholar] [CrossRef]
- Lomwongsopon, P.; Aramrueang, N. Mild chemical pretreatment of cassava pulp for enhancing high-load anaerobic digestion. Bioresour. Technol. Rep. 2022, 17, 100896. [Google Scholar] [CrossRef]
- Guo, Q.; Majeed, S.; Xu, R.; Zhang, K.; Kakade, A.; Khan, A.; Hafeez, F.Y.; Mao, C.; Liu, P.; Li, X. Heavy metals interact with the microbial community and affect biogas production in anaerobic digestion: A review. J. Environ. Manag. 2019, 240, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Wandera, S.M.; Qiao, W.; Algapani, D.E.; Bi, S.; Yin, D.; Qi, X.; Liu, Y.; Dach, J.; Dong, R. Searching for possibilities to improve the performance of full scale agricultural biogas plants. Renew. Energy 2018, 116, 720–727. [Google Scholar] [CrossRef]
- Hoelzle, R.D.; Virdis, B.; Batstone, D.J. Regulation mechanisms in mixed and pure culture microbial fermentation. Biotechnol. Bioeng. 2014, 111, 2139–2154. [Google Scholar] [CrossRef]
- Ho, D.; Jensen, P.; Batstone, D. Effects of Temperature and Hydraulic Retention Time on Acetotrophic Pathways and Performance in High-Rate Sludge Digestion. Environ. Sci. Technol. 2014, 48, 6468–6476. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Liu, J.; Ma, C.; Li, Y.-Y.; Zou, L.; Qian, G.; Xu, Z.P. Improving the stability and efficiency of anaerobic digestion of food waste using additives: A critical review. J. Clean. Prod. 2018, 192, 316–326. [Google Scholar] [CrossRef]
- Su, L.; Zhen, G.; Zhang, L.; Zhao, Y.; Niu, D.; Chai, X. The use of the core–shell structure of zero-valent iron nanoparticles (NZVI) for long-term removal of sulphide in sludge during anaerobic digestion. Environ. Sci. Process. Impacts 2015, 17, 2013–2021. [Google Scholar] [CrossRef]
- Wu, D.; Zheng, S.; Ding, A.; Sun, G.; Yang, M. Performance of a zero valent iron-based anaerobic system in swine wastewater treatment. J. Hazard. Mater. 2015, 286, 1–6. [Google Scholar] [CrossRef]
- Zhang, S.; Ren, Y.; Ma, X.; Guan, W.; Gao, M.; Li, Y.-Y.; Wang, Q.; Wu, C. Effect of zero-valent iron addition on the biogas fermentation of food waste after anaerobic preservation. J. Environ. Chem. Eng. 2021, 9, 106013. [Google Scholar] [CrossRef]
- Gundoshmian, T.M.; Ahmadi-Pirlou, M. Increasing biogas and methane yield by adding sewage sludge and zero-valent iron nanoparticles during the single-stage anaerobic digestion with municipal solid waste. Int. J. Energy Res. 2022, 46, 20611–20623. [Google Scholar] [CrossRef]
- Domrongpokkaphan, V.; Phalakornkule, C.; Khemkhao, M. In-situ methane enrichment of biogas from anaerobic digestion of palm oil mill effluent by addition of zero valent iron (ZVI). Int. J. Hydrog. Energy 2021, 46, 30976–30987. [Google Scholar] [CrossRef]
- Ugwu, S.N.; Enweremadu, C.C. Enhancement of biogas production process from biomass wastes using iron-based additives: Types, impacts, and implications. Energy Sources Part A Recovery Util. Environ. Eff. 2022, 44, 4458–4480. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, Y.; Quan, X. Zero-valent iron enhanced methanogenic activity in anaerobic digestion of waste activated sludge after heat and alkali pretreatment. Waste Manag. 2015, 38, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Sarto, S.; Hildayati, R.; Syaichurrozi, I. Effect of chemical pretreatment using sulfuric acid on biogas production from water hyacinth and kinetics. Renew. Energy 2019, 132, 335–350. [Google Scholar] [CrossRef]
- Syaichurrozi, I.; Villta, P.K.; Nabilah, N.; Rusdi, R. Effect of sulfuric acid pretreatment on biogas production from Salvinia molesta. J. Environ. Chem. Eng. 2019, 7, 102857. [Google Scholar] [CrossRef]
- Taherdanak, M.; Zilouei, H.; Karimi, K. The influence of dilute sulfuric acid pretreatment on biogas production from wheat plant. Int. J. Green Energy 2016, 13, 1129–1134. [Google Scholar] [CrossRef]
- Loow, Y.-L.; Wu, T.Y.; Jamaliah, M.J.; Mohammad, A.W.; Teoh, W.H. Typical conversion of lignocellulosic biomass into reducing sugars using dilute acid hydrolysis and alkaline pretreatment. Cellulose 2016, 23, 1491–1520. [Google Scholar] [CrossRef]
- Watthier, E.; Andreani, C.L.; Torres, D.G.B.; Kuczman, O.; Tavares, M.H.F.; Lopes, D.D.; Gomes, S.D. Cassava Wastewater Treatment in Fixed-Bed Reactors: Organic Matter Removal and Biogas Production. Front. Sustain. Food Syst. 2019, 3, 6. [Google Scholar] [CrossRef]
- Costa, R.C.; Ramos, M.D.N.; Fleck, L.; Gomes, S.D.; Aguiar, A. Critical analysis and predictive models using the physicochemical characteristics of cassava processing wastewater generated in Brazil. J. Water Process Eng. 2022, 47, 102629. [Google Scholar] [CrossRef]
- Kong, X.; Niu, J.; Zhang, W.; Liu, J.; Yuan, J.; Li, H.; Yue, X. Mini art review for zero valent iron application in anaerobic digestion and technical bottlenecks. Sci. Total Environ. 2021, 791, 148415. [Google Scholar] [CrossRef]
- Kannah, R.Y.; Kavitha, S.; Rajesh Banu, J.; Yeom, I.T.; Johnson, M. Synergetic effect of combined pretreatment for energy efficient biogas generation. Bioresour. Technol. 2017, 232, 235–246. [Google Scholar] [CrossRef]
- Kouas, M.; Torrijos, M.; Sousbie, P.; Harmand, J.; Sayadi, S. Modeling the anaerobic co-digestion of solid waste: From batch to semi-continuous simulation. Bioresour. Technol. 2019, 274, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Elagroudy, S.; Radwan, A.G.; Banadda, N.; Mostafa, N.G.; Owusu, P.A.; Janajreh, I. Mathematical models comparison of biogas production from anaerobic digestion of microwave pretreated mixed sludge. Renew. Energy 2020, 155, 1009–1020. [Google Scholar] [CrossRef]
- Zaidi, A.A.; Feng, R.; Malik, A.; Khan, S.Z.; Shi, Y.; Bhutta, A.J.; Shah, A.H. Combining Microwave Pretreatment with Iron Oxide Nanoparticles Enhanced Biogas and Hydrogen Yield from Green Algae. Processes 2019, 7, 24. [Google Scholar] [CrossRef]
- Zaidi, A.A.; RuiZhe, F.; Malik, A.; Khan, S.Z.; Bhutta, A.J.; Shi, Y.; Mushtaq, K. Conjoint effect of microwave irradiation and metal nanoparticles on biogas augmentation from anaerobic digestion of green algae. Int. J. Hydrog. Energy 2019, 44, 14661–14670. [Google Scholar] [CrossRef]
- Daiem, M.M.A.; Hatata, A.; Galal, O.H.; Said, N.; Ahmed, D. Prediction of biogas production from anaerobic co-digestion of waste activated sludge and wheat straw using two-dimensional mathematical models and an artificial neural network. Renew. Energy 2021, 178, 226–240. [Google Scholar] [CrossRef]
- Salehi, R.; Yuan, Q.; Chaiprapat, S. Development of Data-Driven Models to Predict Biogas Production from Spent Mushroom Compost. Agriculture 2022, 12, 1090. [Google Scholar] [CrossRef]
- American Public Health, A. APHA standard methods for the examination of water and wastewater. In Standard Methods for the Examination of Water & Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Cremonez, P.A.; Sampaio, S.C.; Teleken, J.G.; Weiser Meier, T.; Dieter, J.; Teleken, J. Influence of inoculum to substrate ratio on the anaerobic digestion of a cassava starch polymer. Ind. Crops Prod. 2019, 141, 111709. [Google Scholar] [CrossRef]
- Lavine, B.K.; Auslander, G.; Ritter, J. Polarographic studies of zero valent iron as a reductant for remediation of nitroaromatics in the environment. Microchem. J. 2001, 70, 69–83. [Google Scholar] [CrossRef]
- Bang, S.; Johnson, M.D.; Korfiatis, G.P.; Meng, X. Chemical reactions between arsenic and zero-valent iron in water. Water Res. 2005, 39, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Zerrouki, S.; Rihani, R.; Lekikot, K.; Ramdhane, I. Enhanced biogas production from anaerobic digestion of wastewater from the fruit juice industry by sonolysis: Experiments and modelling. Water Sci. Technol. 2021, 84, 644–655. [Google Scholar] [CrossRef]
- Das, A.; Mondal, C.; Roy, S. Kinetic Study of Biogas Recovery from Thermo-chemically Pre-treated Rice Husk. Indian Chem. Eng. 2018, 60, 297–313. [Google Scholar] [CrossRef]
- Dong, D.; Kyung Choi, O.; Woo Lee, J. Influence of the continuous addition of zero valent iron (ZVI) and nano-scaled zero valent iron (nZVI) on the anaerobic biomethanation of carbon dioxide. Chem. Eng. J. 2022, 430, 132233. [Google Scholar] [CrossRef]
- Xi, Y.; Mallavarapu, M.; Naidu, R. Reduction and adsorption of Pb2+ in aqueous solution by nano-zero-valent iron—A SEM, TEM and XPS study. Mater. Res. Bull. 2010, 45, 1361–1367. [Google Scholar] [CrossRef]
- Boontian, N.; Phorndon, T.; Piasai, C.; Padri, M. Combination of Alkaline and Heat Pretreatments with Zero-Valent Iron Application in Cassava Pulp and Wastewater for Methane Generation: Development from Batch to Continuous Systems. Fermentation 2023, 9, 108. [Google Scholar] [CrossRef]
- Boontian, N. Effect of Zero Valent Iron (ZVI) in Wastewater Treatment: A Review. Appl. Mech. Mater. 2015, 775, 180–184. [Google Scholar] [CrossRef]
- Ullah, S.; Faiz, P.; Leng, S. Synthesis, Mechanism, and Performance Assessment of Zero-Valent Iron for Metal-Contaminated Water Remediation: A Review. CLEAN–Soil Air Water 2020, 48, 2000080. [Google Scholar] [CrossRef]
- Li, J.; Tabassum, S. Effect of zero-valent iron and iron-carbon particles on the denitrification efficiency of anammox process under gradual cooling conditions. Clean. Eng. Technol. 2022, 8, 100477. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Pretreatment of Lignocellulosic Wastes to Improve Ethanol and Biogas Production: A Review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef] [PubMed]
- Virunanon, C.; Ouephanit, C.; Burapatana, V.; Chulalaksananukul, W. Cassava pulp enzymatic hydrolysis process as a preliminary step in bio-alcohols production from waste starchy resources. J. Clean. Prod. 2013, 39, 273–279. [Google Scholar] [CrossRef]
- Mozhiarasi, V. Overview of pretreatment technologies on vegetable, fruit and flower market wastes disintegration and bioenergy potential: Indian scenario. Chemosphere 2022, 288, 132604. [Google Scholar] [CrossRef] [PubMed]
- Zhen, G.; Lu, X.; Kato, H.; Zhao, Y.; Li, Y.-Y. Overview of pretreatment strategies for enhancing sewage sludge disintegration and subsequent anaerobic digestion: Current advances, full-scale application and future perspectives. Renew. Sustain. Energy Rev. 2017, 69, 559–577. [Google Scholar] [CrossRef]
- Carrere, H.; Antonopoulou, G.; Affes, R.; Passos, F.; Battimelli, A.; Lyberatos, G.; Ferrer, I. Review of feedstock pretreatment strategies for improved anaerobic digestion: From lab-scale research to full-scale application. Bioresour. Technol. 2016, 199, 386–397. [Google Scholar] [CrossRef]
- Puyol, D.; Flores-Alsina, X.; Segura, Y.; Molina, R.; Padrino, B.; Fierro, J.L.G.; Gernaey, K.V.; Melero, J.A.; Martinez, F. Exploring the effects of ZVI addition on resource recovery in the anaerobic digestion process. Chem. Eng. J. 2018, 335, 703–711. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Li, Y.; Quan, X.; Zhao, Z. Comparing the mechanisms of ZVI and Fe3O4 for promoting waste-activated sludge digestion. Water Res. 2018, 144, 126–133. [Google Scholar] [CrossRef]
- Fox, M.H.; Noike, T.; Ohki, T. Alkaline subcritical-water treatment and alkaline heat treatment for the increase in biodegradability of newsprint waste. Water Sci. Technol. 2003, 48, 77–84. [Google Scholar] [CrossRef]
- Şenol, H.; Açıkel, Ü.; Demir, S.; Oda, V. Anaerobic digestion of cattle manure, corn silage and sugar beet pulp mixtures after thermal pretreatment and kinetic modeling study. Fuel 2020, 263, 116651. [Google Scholar] [CrossRef]
- Kor-Bicakci, G.; Ubay-Cokgor, E.; Eskicioglu, C. Effect of dewatered sludge microwave pretreatment temperature and duration on net energy generation and biosolids quality from anaerobic digestion. Energy 2019, 168, 782–795. [Google Scholar] [CrossRef]
- Sumardiono, S.; Budiyono; Mardiani, D.T. The effect of microwave power and heating time pretreatment on biogas production from fresh and dried water hyacinth (Eichhornia crassipes). AIP Conf. Proc. 2015, 1699, 050018. [Google Scholar] [CrossRef]
- Mirmasoumi, S.; Khoshbakhti Saray, R.; Ebrahimi, S. Evaluation of thermal pretreatment and digestion temperature rise in a biogas fueled combined cooling, heat, and power system using exergo-economic analysis. Energy Convers. Manag. 2018, 163, 219–238. [Google Scholar] [CrossRef]
- McVoitte, W.P.A.; Clark, O.G. The effects of temperature and duration of thermal pretreatment on the solid-state anaerobic digestion of dairy cow manure. Heliyon 2019, 5, e02140. [Google Scholar] [CrossRef] [PubMed]
- Shangdiar, S.; Lin, Y.-C.; Ponnusamy, V.K.; Wu, T.-Y. Pretreatment of lignocellulosic biomass from sugar bagasse under microwave assisted dilute acid hydrolysis for biobutanol production. Bioresour. Technol. 2022, 361, 127724. [Google Scholar] [CrossRef]
- Aruwajoye, G.S.; Faloye, F.D.; Kana, E.G. Soaking assisted thermal pretreatment of cassava peels wastes for fermentable sugar production: Process modelling and optimization. Energy Convers. Manag. 2017, 150, 558–566. [Google Scholar] [CrossRef]
- Antonopoulou, G.; Lyberatos, G. Effect of Pretreatment of Sweet Sorghum Biomass on Methane Generation. Waste Biomass Valorization 2013, 4, 583–591. [Google Scholar] [CrossRef]
- Liu, T.; Wu, C.; Wang, Y.; Xue, G.; Zhang, M.; Liu, C.; Zheng, Y. Enhanced Deep Utilization of Low-Organic Content Sludge by Processing Time-Extended Low-Temperature Thermal Pretreatment. ACS Omega 2021, 6, 28946–28954. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Q.; Wang, D.; Yang, Q.; Wu, Y.; Li, Y.; Fu, Q.; Yang, F.; Liu, Y.; Ni, B.-J.; et al. Thermal-alkaline pretreatment of polyacrylamide flocculated waste activated sludge: Process optimization and effects on anaerobic digestion and polyacrylamide degradation. Bioresour. Technol. 2019, 281, 158–167. [Google Scholar] [CrossRef]
- Wyman, C.E.; Dale, B.E.; Elander, R.T.; Holtzapple, M.; Ladisch, M.R.; Lee, Y.Y. Coordinated development of leading biomass pretreatment technologies. Bioresour. Technol. 2005, 96, 1959–1966. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, Y.; Quan, X.; Chen, S. Enhanced anaerobic digestion of waste activated sludge digestion by the addition of zero valent iron. Water Res. 2014, 52, 242–250. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Quan, X.; Li, Y.; Zhao, Z.; Meng, X.; Chen, S. Optimization of anaerobic acidogenesis by adding Fe0 powder to enhance anaerobic wastewater treatment. Chem. Eng. J. 2012, 192, 179–185. [Google Scholar] [CrossRef]
- Zhang, J.; Qu, Y.; Qi, Q.; Zhang, P.; Zhang, Y.; Tong, Y.W.; He, Y. The bio-chemical cycle of iron and the function induced by ZVI addition in anaerobic digestion: A review. Water Res. 2020, 186, 116405. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Li, D.; Yang, X.; Xing, W.; Li, J.; Zhang, Q. Biological denitrification process based on the Fe(0)–carbon micro-electrolysis for simultaneous ammonia and nitrate removal from low organic carbon water under a microaerobic condition. Bioresour. Technol. 2016, 219, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Honetschlägerová, L.; Škarohlíd, R.; Martinec, M.; Šír, M.; Luciano, V. Interactions of nanoscale zero valent iron and iron reducing bacteria in remediation of trichloroethene. Int. Biodeterior. Biodegrad. 2018, 127, 241–246. [Google Scholar] [CrossRef]
- Kumar, N.; Auffan, M.; Gattacceca, J.; Rose, J.; Olivi, L.; Borschneck, D.; Kvapil, P.; Jublot, M.; Kaifas, D.; Malleret, L.; et al. Molecular Insights of Oxidation Process of Iron Nanoparticles: Spectroscopic, Magnetic, and Microscopic Evidence. Environ. Sci. Technol. 2014, 48, 13888–13894. [Google Scholar] [CrossRef]
- Ren, X.; Chen, Y.; Guo, L.; She, Z.; Gao, M.; Zhao, Y.; Shao, M. The influence of Fe2+, Fe3+ and magnet powder (Fe3O4) on aerobic granulation and their mechanisms. Ecotoxicol. Environ. Saf. 2018, 164, 1–11. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, Z.; Yang, Y.; Zhang, Y. Dual roles of zero-valent iron in dry anaerobic digestion: Enhancing interspecies hydrogen transfer and direct interspecies electron transfer. Waste Manag. 2020, 118, 481–490. [Google Scholar] [CrossRef]
- Wang, C.; Wei, W.; Dai, X.; Ni, B.-J. Zero valent iron greatly improves sludge destruction and nitrogen removal in aerobic sludge digestion. Chem. Eng. J. 2022, 433, 134459. [Google Scholar] [CrossRef]
- Li, L.; Kong, X.; Yang, F.; Li, D.; Yuan, Z.; Sun, Y. Biogas Production Potential and Kinetics of Microwave and Conventional Thermal Pretreatment of Grass. Appl. Biochem. Biotechnol. 2012, 166, 1183–1191. [Google Scholar] [CrossRef]
- Pavlostathis, S.G.; Giraldo-Gomez, E. Kinetics of anaerobic treatment: A critical review. Crit. Rev. Environ. Control 1991, 21, 411–490. [Google Scholar] [CrossRef]
- Donoso-Bravo, A.; Pérez-Elvira, S.I.; Fdz-Polanco, F. Application of simplified models for anaerobic biodegradability tests. Evaluation of pre-treatment processes. Chem. Eng. J. 2010, 160, 607–614. [Google Scholar] [CrossRef]
- Rajput, A.A.; Zeshan; Visvanathan, C. Effect of thermal pretreatment on chemical composition, physical structure and biogas production kinetics of wheat straw. J. Environ. Manag. 2018, 221, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, T.A.M.; Mohanty, M.K.; Sahoo, P.K.; Behera, D. Metal nanoparticle mixtures to improve the biogas yield of cattle manure. Biomass Convers. Biorefinery 2023, 13, 2243–2254. [Google Scholar] [CrossRef]
- Deepanraj, B.; Sivasubramanian, V.; Jayaraj, S. Kinetic study on the effect of temperature on biogas production using a lab scale batch reactor. Ecotoxicol. Environ. Saf. 2015, 121, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Pardilhó, S.; Pires, J.C.; Boaventura, R.; Almeida, M.; Maia Dias, J. Biogas production from residual marine macroalgae biomass: Kinetic modelling approach. Bioresour. Technol. 2022, 359, 127473. [Google Scholar] [CrossRef]
- Ap, Y.; Farghali, M.; Mohamed, I.M.A.; Iwasaki, M.; Tangtaweewipat, S.; Ihara, I.; Sakai, R.; Umetsu, K. Potential of biogas production from the anaerobic digestion of Sargassum fulvellum macroalgae: Influences of mechanical, chemical, and biological pretreatments. Biochem. Eng. J. 2021, 175, 108140. [Google Scholar] [CrossRef]
- Nielfa, A.; Cano, R.; Fdz-Polanco, M. Theoretical methane production generated by the co-digestion of organic fraction municipal solid waste and biological sludge. Biotechnol. Rep. 2015, 5, 14–21. [Google Scholar] [CrossRef]
- Patowary, D.; Baruah, D.C. Effect of combined chemical and thermal pretreatments on biogas production from lignocellulosic biomasses. Ind. Crops Prod. 2018, 124, 735–746. [Google Scholar] [CrossRef]
- David, R.J.; Helbling Damian, E.; Lee Tae, K.; Park, J.; Fenner, K.; Kohler Hans-Peter, E.; Ackermann, M. Association of Biodiversity with the Rates of Micropollutant Biotransformations among Full-Scale Wastewater Treatment Plant Communities. Appl. Environ. Microbiol. 2015, 81, 666–675. [Google Scholar] [CrossRef]
- Wu, P.; Li, L.; Jiang, J.; Sun, Y.; Yuan, Z.; Feng, X.; Guo, Y. Effects of fermentative and non-fermentative additives on silage quality and anaerobic digestion performance of Pennisetum purpureum. Bioresour. Technol. 2020, 297, 122425. [Google Scholar] [CrossRef]


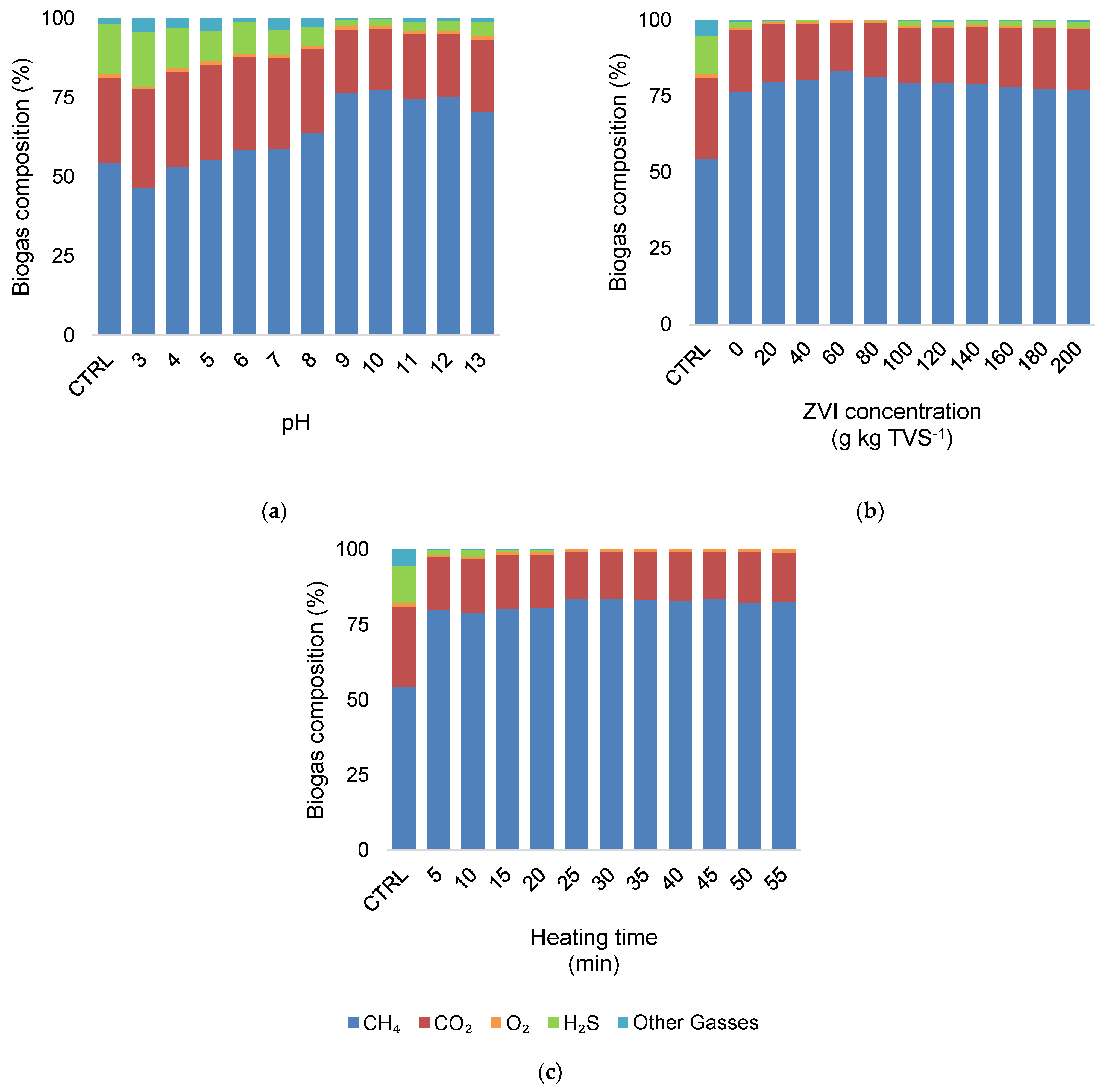
| Wastewater | |
| Parameters | Mean ± sd |
| pH | 4.23 ± 0.24 |
| BOD (mg·L−1) | 42,000 ± 6879 |
| COD (mg·L−1) | 55,400 ± 6116 |
| VFA (mg·CH3COOH·L−1) | 161.67 ± 25 |
| Alkalinity (mg·CaCO3·L−1) | 336.67 ± 26 |
| TP (mg·L−1) | 70.86 ± 12 |
| Orto-P (mg·L−1) | 54.90 ± 2.89 |
| TKN (mg·L−1) | 393.52 ± 63.08 |
| TS (%) | 23.73 ± 3.90 |
| TVS (%) | 79.56 ± 10.41 |
| Seed | |
| Parameters | Mean ± sd |
| pH | 4.13 ± 0.65 |
| BOD (mg·L−1) | 42,000 ± 3927 |
| TS (mg·L−1) | 40,465 ± 1889 |
| TVS (mg·L−1) | 38,336 ± 6574 |
| VSS (mg·L−1) | 37,936 ± 2814 |
| VFA (mg·CH3COOH·L−1) | 140 ± 7.59 |
| Alkalinity | 716 ± 47.90 |
| Models | Parameters | Exp. I (pH 10) | Exp. II (60 g·ZVI·kg·TVS−1) | Exp. III (35 min Heating) |
|---|---|---|---|---|
| Modified Gomperzt | P | 0.491 | 0.834 | 0.839 |
| Rm | 0.014 | 0.017 | 0.018 | |
| λ | 5.011 | 6.199 | 5.352 | |
| R2 | 0.997 | 0.997 | 0.993 | |
| Logistic Function | P | 0.491 | 0.834 | 0.839 |
| Rm | 0.014 | 0.016 | 0.017 | |
| λ | 5.644 | 5.857 | 6.056 | |
| R2 | 0.997 | 0.996 | 0.997 | |
| Transference Function | P | 0.491 | 0.834 | 0.839 |
| Rm | 0.022 | 0.027 | 0.028 | |
| λ | 3.503 | 4.771 | 4.744 | |
| R2 | 0.959 | 0.959 | 0.958 | |
| Exponential Function | P | 0.491 | 0.834 | 0.839 |
| k | 0.039 | 0.028 | 0.028 | |
| R2 | 0.969 | 0.973 | 0.972 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boontian, N.; Yingchon, U.; Padri, M. pH and Heat Pretreatments with Zero-Valent Iron Addition to Enhance Biogas Production from Cassava Pulp Wastewater: Optimization and Comparison of Mathematical Models. Fermentation 2023, 9, 622. https://doi.org/10.3390/fermentation9070622
Boontian N, Yingchon U, Padri M. pH and Heat Pretreatments with Zero-Valent Iron Addition to Enhance Biogas Production from Cassava Pulp Wastewater: Optimization and Comparison of Mathematical Models. Fermentation. 2023; 9(7):622. https://doi.org/10.3390/fermentation9070622
Chicago/Turabian StyleBoontian, Nittaya, Usa Yingchon, and Mohamad Padri. 2023. "pH and Heat Pretreatments with Zero-Valent Iron Addition to Enhance Biogas Production from Cassava Pulp Wastewater: Optimization and Comparison of Mathematical Models" Fermentation 9, no. 7: 622. https://doi.org/10.3390/fermentation9070622
APA StyleBoontian, N., Yingchon, U., & Padri, M. (2023). pH and Heat Pretreatments with Zero-Valent Iron Addition to Enhance Biogas Production from Cassava Pulp Wastewater: Optimization and Comparison of Mathematical Models. Fermentation, 9(7), 622. https://doi.org/10.3390/fermentation9070622





