Abstract
In order to address human zinc deficiency, fortifying staple foods with zinc is a safe and cost-effective solution. To ensure the nutritional properties and quality of a final product, zinc tolerance of the microorganisms involved in the fermentation is necessary. Bread, which is widely consumed, occupies a substantial place in many people’s diets, and is often based on a sourdough making process; thus, it might be an important headlining product. This study investigated the zinc tolerance of yeasts and lactic acid bacteria that are specifically suited to produce sourdough bread made with cricket powder hydrolysate and wheat flour. Amongst the yeasts, Kazachstania servazzii KAZ2 and Kazachstania unispora FM2 were only slightly affected in regard to cell growth and colony-forming ability when cultured in YPD broth spiked with 0.5 or 1 mM ZnSO4, respectively. Yarrowia lipolytica RO25 showed a higher tolerance for up to 2.5 mM zinc (ZnSO4). All the yeast strains were capable of accumulating zinc in the range between 200 and 400 fg/cell. The heterofermentative lactic acid bacterium Fructilactobacillus sanfranciscensis DG1 appeared to have a moderate zinc requirement and was homeostatically balanced, even under a high 20 mM extracellular ZnSO4 load. A better understanding of zinc homeostasis in yeast and lactic acid bacteria for food industry applications may lead to improvements in zinc fortification, which might contribute to diminishing Zn deficiencies, especially in vulnerable population groups.
1. Introduction
More than one billion people worldwide (17% of the world’s population) suffer from zinc deficiency. In particular, people in Asia, Sub-Saharan Africa, and Central America were identified as being at the highest risk of inadequate zinc intake, based on the low zinc content in their regional nutrition []. Additionally, in Western countries, a considerable percentage of the population, mostly the elderly, is at risk of zinc deficiency, due to inadequate dietary intake and reduced absorption of this essential micronutrient [,]. Even moderate zinc deficiency can have substantial consequences; an impaired immune system and the resulting increased susceptibility to infections are of particular concern. Zinc deficiency is considered as a possible risk factor for increased susceptibility to and severe progression of COVID-19 []. People in the Global South are most affected by zinc deficiency-related problems such as diarrhea and increased mortality rates [,,]. The prevention or reversal of a zinc deficit can be achieved by dietary diversification, the consumption of (bio)fortified foods, as well as the consumption of zinc supplements [,]. Potentially suitable staple foods for fortification with zinc are milk and milk powder, grains or flours thereof, and cereal products in general (wheat, rice, maize) [,,,]. The fortification of bread and other bakery goods with zinc salts offers the possibility of achieving a more favorable zinc/phytate ratio, and, thus, producing high-quality products with good zinc bioavailability for human consumption [,,]. The use of sourdough to improve the texture and palatability of baked goods is becoming increasingly important and highly appreciated by consumers []. The process of sourdough fermentation is one of the oldest methods of leavening bread. Through the fermentation process, lactic acid bacteria (LAB) and yeasts turn grain flour into attractive, tasty, and more digestible bread. [,,]. Moreover, longer fermentation processes in sourdough making even increase the degradation of phytic acid [,,,]. This is suggested to further improve the bioavailability of trace elements [,].
In addition to wheat flour, other raw materials are nowadays being discussed and investigated for their use in the production of sourdough-based bakery goods, e.g., hydrolyzed insect powder [,]. The fortification of insect-blended wheat flour-based products with zinc could be a useful and safe intervention, particularly for the most zinc-deficient populations in Sub-Saharan Africa and South Asia, where entomophagy is a long-standing tradition [,]. Micronutrient-enriched insect-based food products could also provide new market opportunities in the Western world, and promote the acceptance of insect-based foods in societies that are currently more reluctant [,]. However, fortification has to be integrated into the production process without interfering, for example, with fermentation during sourdough making. In this case, the tolerance of the microorganisms toward the compound of interest has to be tested. Despite Saccharomyces cerevisiae’s dominance in industrial fermentation, other nonconventional yeasts have also emerged as valuable contributors [,,,]. Recent studies, performed by Rossi et al. [,], have highlighted the use of Yarrowia lipolytica RO25 cricket-based hydrolysate as an ingredient for sourdough breads implementing Kazachstania unispora (basionym: Saccharomyces unisporus), Kazachstania servazzii (basionym: Saccharomyces servazzii), and the heterofermentative LAB Fructilactobacillus sanfranciscensis (formerly Lactobacillus sanfranciscensis) as fermentative organisms. In light of the potential health and economic benefits of micronutrient fortifications of wheat bakery products valorized with insect powders, this study examined how zinc affects these four microorganisms in order to gain an understanding of their tolerance and accumulation of zinc.
2. Materials and Methods
2.1. Materials
Agar bacteriological (Oxoid Deutschland GmbH, Wesel, Germany), 4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (Carl Roth GmbH + Co. KG, Karlsruhe, Germany), hydrogen peroxide (30%, ultrapure; Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany), MRS (Oxoid Deutschland GmbH, Wesel, Germany) with 2% w/v maltose (mMRS) broth, NaCl (Carl Roth GmbH + Co. KG, Karlsruhe, Germany), nitric acid (67%, ultrapure) (Carl Roth GmbH + Co. KG, Karlsruhe, Germany), TraceCERT®/transition metal mix for ICP-MS (100 mg/L each: V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Ag, and Cd in 2% nitric acid) (Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany), TraceCERT®/Rhodium standard for ICP-MS (1000 mg/L Rh in hydrochloric acid) (Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany), Yeast Extract–Peptone–Dextrose (YPD) Broth (Oxoid Deutschland GmbH, Wesel, Germany), and ZnSO4·7H2O (Sigma-Aldrich, Steinheim, Germany) were used.
2.2. Preparation of Zinc-Enriched Microorganisms
Microorganisms used in the study were the yeast strains Yarrowia lipolytica RO25, Kazachstania unispora FM2, Kazachstania servazzii KAZ2, and the heterofermentative LAB Fructilactobacillus sanfranciscensis DG1 (obtained from the strain collection of the Department of Agricultural and Food Sciences, University of Bologna, Bologna, Italy) []. Yarrowia lipolytica RO25 was regularly cultivated in YPD broth at 25 °C with agitation for 48 h. The two yeast strains of Kazachstania (FM2, KAZ2) were subcultured in YPD broth at 30 °C. Fructilactobacillus sanfranciscensis DG1 was grown in MRS broth +2% w/v maltose (mMRS) at 30 °C for 24 h. Using these pre-cultures, the microbial strains were inoculated with a 1:100 dilution either in YPD for the yeasts or mMRS for the LAB. Microorganisms were incubated at the appropriate temperature and conditions, until the beginning of the logarithmic phase was reached. Subsequently, defined amounts of ZnSO4 (0.5–10 mM for yeasts and 5–20 mM for the LAB) were added and the cultivation continued until the stationary phase was reached. For each strain, a control sample was prepared with the addition of water instead of ZnSO4. At the end of the incubation, aliquots of cells were taken for the determination of the colony-forming ability and zinc quantification. The remaining cell suspension was collected in 50 mL tubes and cells were separated by centrifugation (3000× g for 5 min at room temperature) and freeze-dried.
2.3. OD600 Assay
Biomass accumulation was monitored by OD600 measurements []. For each sample, 1.5 mL was transferred into a cuvette and the OD was measured at 600 nm using a UV/vis spectrometer (Spectronic Unicam UV1, Thermo Fisher Scientific Inc., Waltham, MA, USA).
2.4. Determination of Cell Viability via Colony-Forming Units
The number of colony-forming units (cfu) was determined using a traditional plate counting method []. Samples were subjected to decimal dilutions in physiologic saline solution. Then, 100 µL of the appropriate dilution was plated on YPD-agar for the yeasts or mMRS-agar for LAB. Plates inoculated with Yarrowia lipolytica RO25 were aerobically incubated at 25 °C for 48–72 h, while the other plates were incubated at 30 °C for 24–48 h before counting cfu.
2.5. Determination of Cellular Zinc Concentration
To determine the concentration of zinc in the cells, fresh biomass was centrifuged (3000× g for 5 min at room temperature), supernatant was discarded, and cells were rinsed twice with HEPES buffer (50 mM HEPES in bidistilled water adjusted to pH 6.5 with sodium hydroxide solution; depleted of multivalent cations by Chelex® 100 resin pretreatment according to Alker et al. (2019)) []. Cell pellets were dissolved in 500 µL of a 1:1 mixture of ultrapure HNO3 (65%) and H2O2 and dried twice at 95 °C overnight using a thermoshaker. Following dilution in 0.65 % HNO3 containing 5 μg/L rhodium, samples were analyzed on an Elan DRC II inductively coupled plasma-mass spectrometer (ICP-MS; PerkinElmer Inc., Rodgau, Germany) []. The experimental conditions for the ICP-MS measurement are listed in Table 1.

Table 1.
Experimental conditions for ICP-MS measurements.
2.6. FTIR Analysis
Infrared spectra of the dried yeast and bacteria samples were measured with a Bruker Tensor II spectrometer (Bruker Optic GmbH, Karlsruhe, Germany) equipped with a liquid nitrogen-cooled mercury cadmium telluride detector. The samples were measured in absorption mode with a Platinum ATR Diamond cell (Bruker Optic GmbH, Karlsruhe, Germany) against the ambient air as a background. Each spectrum resulted from an average of 120 scans recorded with a resolution of 2 cm−1 from 4000–800 cm−1 at 20 °C. Spectra were off-set-corrected in the region of 3800–4000 cm−1 using the software provided with the spectrometer (OPUS 7.5) [].
2.7. Statistical Analyses
The statistical significance of the experimental results was calculated using GraphPad prism software (version 8.0, GraphPad Software, San Diego, CA, USA). All experiments were conducted in at least four independent replicates as indicated in the figure legends.
3. Results
The first experiments based on OD600 cell density measurements focused on recording the yeast and LAB growth in batch culture. Using this information, the time of zinc addition during the early logarithmic phase and the duration of fermentation were determined for the follow-up studies (Figure 1). The heterofermentative LAB Fructilactobacillus sanfranciscensis DG1 was treated with ZnSO4 seven hours after starting the fermentation, and it grew for an additional 17 h. Kazachstania unispora FM2 cultures received Zn treatment from 4 h after starting the fermentation until 29 h, while Kazachstania servazzii KAZ2 was incubated between 5 and 32 h. The incubation of Yarrowia lipolytica RO25 with ZnSO4 lasted from 8 to 30 h.
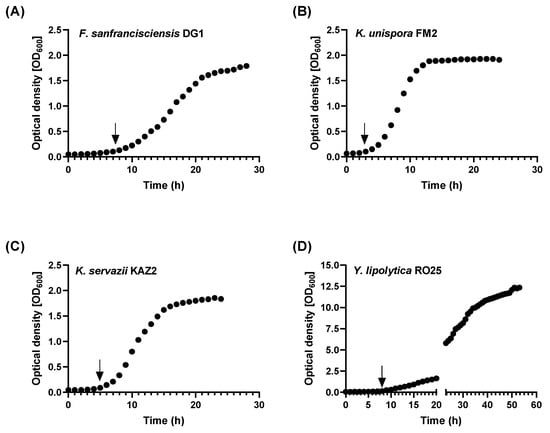
Figure 1.
Growth curves of the lactic acid bacteria and yeasts. The microorganisms (A) Fructilactobacillus sanfranciscensis DG1, (B) Kazachstania unispora FM2, (C) Kazachstania servazzii KAZ2 and (D) Yarrowia lipolytica RO25 were grown in their respective incubation media without additional ZnSO4 supply. Growth was monitored over the incubation time by measuring OD600. Based on these experiments, the transition from resting to logarithmic growth phase was determined (marked with arrows), at which zinc was added in the subsequent experiments.
Different zinc concentrations between 0 and 20 mM were then applied in order to investigate the effects of zinc on the growth performance of the four microorganisms, analyzing biomass accumulation via OD600 measurements (Figure 2) and the enumeration of colony-forming units (Figure 3). For the yeast strain Kazachstania servazzii KAZ2, a statistically significant reduction in the final OD600 was observed when culturing in YPD containing additional 0.5 mM ZnSO4 (~20% reduced biomass). Increasing to 2.5 mM ZnSO4, the final OD600 absorbance of the yeast suspensions was reduced by about 75% compared to control yeasts (Figure 2). Still, the OD600 value was statistically higher than before zinc addition, suggesting ongoing yeast proliferation (Supplementary Table S1). The other Saccharomyces strain Kazachstania unispora FM2 was slightly better at tolerating zinc; OD600 values were almost comparable to controls in the presence of 1 mM ZnSO4. When adding 2.5 mM zinc into YPD broth, a 50% reduction in biomass outcome was obtained (Figure 2). Yarrowia lipolytica RO25 tolerated zinc treatment even better, with growth inhibition starting at 5 mM ZnSO4 (Figure 2).
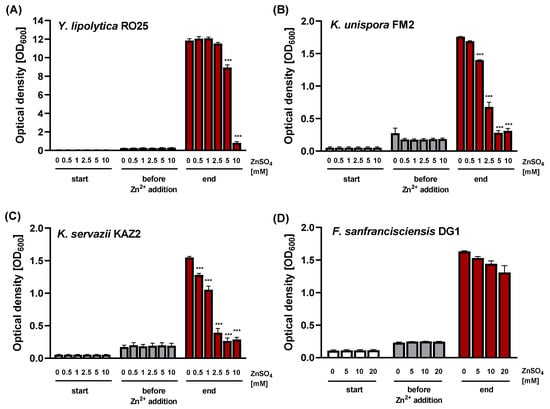
Figure 2.
Growth of yeast and lactic acid bacteria analyzed by OD600 measurement. The microorganisms were transferred from a pre-culture into the respective incubation medium. At the beginning of the log growth phase, defined amounts of ZnSO4 were added and the cultivation continued until the stationary phase was reached. The growth of (A) Yarrowia lipolytica RO25, (B) Kazachstania unispora FM2, (C) Kazachstania servazzii KAZ2 and (D) Fructilactobacillus sanfranciscensis DG1 was analyzed by measuring the optical density at 600 nm. Data are shown as means ± SEM of four independent replicates. Significant differences from 0 mM ZnSO4 at the respective time point are indicated *** p < 0.001; two-way ANOVA with Tukey’s multiple comparison test).
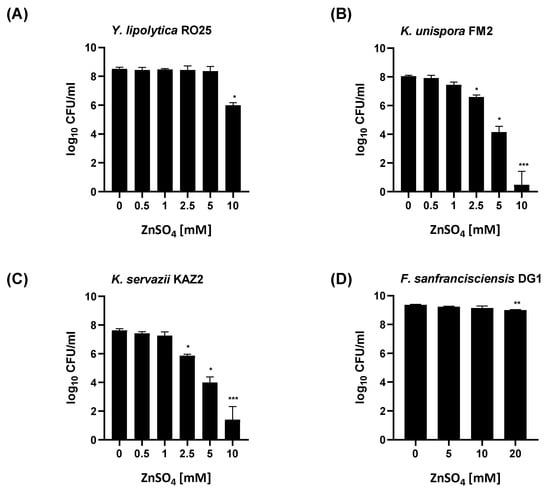
Figure 3.
Viability of yeast and lactic acid bacteria following fermentation in Zn-enriched media analyzed by colony formation. An appropriate dilution of the microorganisms at the end of the enrichment procedure was plated on YPD or mMRS to allow the growth of yeasts and LAB colonies (A) Yarrowia lipolytica RO25, (B) Kazachstania unispora FM2, (C) Kazachstania servazzii KAZ2 and (D) Fructilactobacillus sanfranciscensis DG1, respectively. Data are shown as means ± SEM of four independent replicates. Significant differences to 0 mM ZnSO4 are indicated (* p < 0.05, ** p < 0.01, *** p < 0.001; one-way ANOVA with Kruskal–Wallis).
A further increase in the extracellular zinc led to increased cytotoxicity in all three yeasts, as reduced viability was observed based on their cell density measurements (Figure 2) as well as colony-forming ability (Figure 3).
The LAB strain Fructilactobacillus sanfranciscensis DG1 proved to be much more resistant toward ZnSO4 with no changes in OD600 measurements and only minor effects in colony-forming ability with up to 20 mM zinc addition (Figure 2 and Figure 3).
Upon the completion of fermentation, the zinc content of all samples with a significant amount of cell yield was determined. The zinc levels in yeast cells grown in standard media ranged from 3.6 fg/cell to 8.0 fg/cell (equivalent to 3.3 to 7.8 × 107 atoms of zinc per cell) (Figure 4A). This value clearly exceeds the zinc content previously determined in yeast cells grown with the minimally required amount of zinc (~1 × 107 Zn atoms/ Saccharomyces cerevisiae cell; []) and is consistent with the good propagation of either Yarrowia lipolytica RO25, Kazachstania unispora FM2, or Kazachstania servazzii KAZ2, when grown in YPD broth (Figure 1 and Figure 2). Adding non-cytotoxic amounts of ZnSO4 to the media enhanced the zinc content in all of the three yeast strain cells with similar relative enrichment quota compared to basal zinc (Figure 4A,B). In the presence of an additional 1 mM ZnSO4 in the YPD medium, the cellular zinc content of Kazachstania unispora FM2 and Kazachstania servazzii KAZ2 increased to values up to 180–200 fg/cell. Yarrowia lipolytica RO25 was slightly less responsive; an equivalent zinc treatment led to ~75 fg zinc/ cell. A zinc surplus of 5 mM resulted in an 80-fold increase in zinc content compared to control cells grown in YPD (~400 fg zinc/Yarrowia lipolytica RO25 cell) (Figure 4A,B).
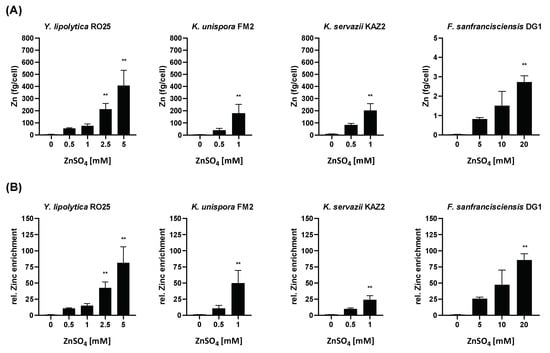
Figure 4.
Zinc quantification in yeast and lactic acid bacteria biomass following fermentation in Zn-enriched media. The zinc content of the microbial biomass at the end of the enrichment procedure was measured by ICP-MS. Cellular zinc levels (A) and relative zinc enrichment compared to cells incubated in basal medium (B) are shown. Data are shown as means ± SEM of four independent replicates. Significant differences from 0 mM ZnSO4 are indicated (** p < 0.01; one-way ANOVA with Kruskal–Wallis).
Regarding their total zinc content, Fructilactobacillus sanfranciscensis DG1 was one hundred times lower in basal zinc than the yeasts after incubation in the standard mMRS medium (approximately 0.032 fg Zn/cell). Even after fermentation at comparable zinc excess (5 mM ZnSO4), Yarrowia lipolytica RO25 and Fructilactobacillus sanfranciscensis DG1 differed by a factor of 150 (Figure 4A).
Potential changes in the general (bio)chemical composition of yeasts and LAB after incubation in zinc-enriched media were studied by FTIR of the cells. Figure 5 shows the total mid-IR range (4000–800 cm−1). Irrespective of zinc treatment, spectra were relatively similar in the range of 3100–2800 cm−1 and 1700–1500 cm−1, which were dominated by bands assigned to the stretching vibrations of membrane fatty acids and to the amide I and II bands of proteins or peptides. Minor changes in the spectra were observed in the 1200–900 cm−1 region. Band changes in this region were assigned to C–O/C–O–C vibrations of polysaccharides, nucleic acids, phospholipids, and pyrophosphates. The biosorption or intracellular binding of zinc in microorganisms is largely mediated by negatively charged molecules such as carboxyl, (poly)phosphates, or hydroxyl groups.
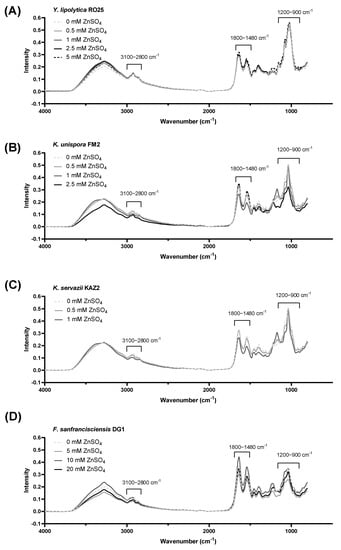
Figure 5.
Fourier-transform infrared spectroscopy (FTIR) spectra of yeast and lactic acid bacteria fermented in Zn-enrichment media. Infrared spectra of dried yeast material measured in absorption mode against ambient air as background, recorded from 4000–800 cm−1 at 20 °C (N = 120 scans). (A) Yarrowia lipolytica RO25, (B) Kazachstania unispora FM2, (C) Kazachstania servazzii KAZ2 and (D) Fructilactobacillus sanfranciscensis DG1. Spectra are shown after offset-correction in the region of 2010–2000 cm−1. Interpretation of the spectra is based on the references [,,].
4. Discussion
Zinc is a trace element essential for numerous physiological functions in humans such as growth, immune defense, reproduction, cognition, collagen synthesis, bone mineralization, and neurobehavioral development [,]. As the human body has a limited zinc-storing capacity, zinc deficiency can develop rapidly when zinc intake is low. Several intervention strategies can be used to treat zinc deficiency. Postharvest food fortification with zinc salts is a cost-effective and sustainable option for increasing zinc intake, with a positive impact on selected indicators of zinc status [,,]. Due to their widespread consumption and their inherent high nutritional value, wheat flour and wheat-based bakery goods are preferred candidates for zinc (bio)fortification [,,]. The WHO has already given recommendations for zinc concentrations and compounds to be used for wheat flour fortification (30–100 ppm Zn; considering the phytate extraction quota and the estimated per capita availability of flour) [,]. The Global Fortification Data Exchange (GFDx) database provides an overview of the fortification status for countries with mandatory and voluntary zinc fortification standards with regard to wheat flour (fortification level 15–101 ppm Zn for wheat flour) []. Considering the imminent depletion of sustainable resources, insect flour is expected to play an increasingly important role as a food ingredient in the near future [,]. Although insects have an abundance of minerals and trace elements, their gastrointestinal solubility is limited. This makes (bio)fortification a good option for improving their micronutrient value []. A crucial topic in the preparation of zinc-fortified insect-powder-blended bakery goods, such as cricket powder hydrolysate valorized sourdough bread [], is the effect of zinc on yeasts and LAB added as starter or adjunctive cultures. Knowledge about the effects of this microelement on non-conventional yeasts and LAB, which might become relevant in the production of (sourdough-based) bakery goods [], is not yet comprehensive.
Amongst the three yeast strains studied here, Yarrowia lipolytica RO25 was slightly more zinc tolerable with unrestricted growth up to 2.5 mM ZnSO4 addition. This observation is consistent with reports for marine Yarrowia lipolytica strains under heavy metal stress [,]. Zinc tolerance and enrichment have so far mainly been studied in Saccharomyces cerevisiae and Saccharomyces pastorianus strains, which have zinc tolerance and an enrichment quota of the same magnitude as the yeasts in the present study []. A few studies have examined the zinc tolerance of yeast strains derived from environmental samples. García-Béjar et al. (2020) conducted a comprehensive screening of 213 wild yeast strains from various sources, including flowers, animals, water, and soils from the food industry environment, to assess zinc biodetoxification and resistance. The results revealed significant differences depending on the yeast genus or species surveyed []. According to De Nicola et al. (2009), Distiller’s M-type Saccharomyces cerevisae yeasts (Kerry Biosciences Ltd., Menstrie, Scotland) were found to have a basal content of 15 fg Zn/cell after incubation in YPM, which increased to 200 fg zinc/cell following 100 µM ZnSO4 treatment []. For Saccharomyces cerevisiae laboratory strain DY1457, zinc levels of 3.7 fg/cell were determined after 24 h incubation in SD media (Zn level in SD medium ~2.5 µM). A further increase in extracellular zinc supply was accompanied by an increase in cell-associated zinc (~ 84 fg Zn/cell upon 1 mM ZnCl2 treatment) []. Yet, extreme zinc surplus (4 mM ZnCl2) led to the collapse of zinc homeostasis and reduced the cell growth of Saccharomyces cerevisiae DY1457 [,]. For another laboratory strain Saccharomyces cerevisiae EB83, Garca-Béjar et al. (2020) determined a zinc content of ~30 pg/cell after incubation in YPD broth supplemented with 382 µM ZnSO4, with most of the zinc detected inside the cells (80%). Existing data regarding the compartmentation of zinc in Yarrowia lipolytica cells indicate that significant amounts of zinc are associated with cell wall components, in addition to zinc internalization []. Intracellular bio-enrichment, by now best investigated for Saccharomyces cerevisiae and Schizosaccharomyces pombe, involves various transporters and trafficking components. This includes membrane transport proteins of the solute carrier family (SLC)39 (Zrt- and Irt-like protein (ZIP)), a family responsible for the transport of zinc from the extracellular environment or intracellular membrane compartments into the cytosol. Zinc transport across the plasma membrane involves (at least in Saccharomyces cerevisiae) two systems: the high affinity Zrt1 (the apparent Km for zinc ions is 10 nM) and Zrt2 (apparent Km ∼100 nM) transporters. In Saccharomyces cerevisiae cells exposed to high zinc concentrations, regulated endocytosis of these plasma membrane proteins prevents intracellular zinc overaccumulation []. As counterparts, CDF transporters (Cation Diffusion Facilitator) move cytosolic zinc into organelles to be stored for an interim period or to be secreted. By sequestering excess zinc into the vacuole, the Saccharomyces cerevisiae ZRC1 vacuolar zinc transporter helps to handle a so called “cellular zinc shock” [,,]. Vacuolar zinc accumulation in yeast is largely dependent on zinc-binding ligands. Polyphosphate, characterized by long chains of phosphate groups, accumulates in large amounts within the yeast vacuole and binds zinc with high affinity. Furthermore, organic anions such as glutamate and citrate are considered to contribute to temporary zinc storage in the vacuoles [,,]. Aside from that, cytoplasmic proteins and peptides (metallothionein, glutathione, phytochelatins) also contribute to zinc storage in yeast cells [,,,]. However, these mechanisms have limitations to their capacity. Zinc dyshomeostasis due to extracellular zinc excess or disturbed cellular zinc distribution is toxic and impedes yeast growth and metabolism [,].
Similar to yeasts, LAB developed mechanisms to tolerate zinc by preventing its toxicity. Several lactobacilli and bifidobacteria have been reported to resist various metal ions at different concentration levels; some of them exhibited the capacity to tolerate up to 100–500 mM extracellular zinc [,]. To resist zinc toxicity, LAB can induce the expression of zinc efflux systems most likely encoded by czcD or cadA genes [,]. Moreover, they limit the amount of zinc available through surface biosorption and the extracellular formation of ZnO nanoparticles [,]. Fructilactobacillus sanfranciscensis DG1’s tolerance toward zinc may be due to one of these mechanisms or even a combination thereof. When being fermented in mMRS broth, Fructilactobacillus sanfranciscensis DG1 contains approximately 0.032 fg Zn/cell (~300.000 atoms of zinc per cell), which is very close to the experimentally determined “minimum zinc quota” in prokaryotes (105 atoms of zinc per Escherichia coli cell). According to the available evidence, the minimum level of zinc is likely to be determined by the number of zinc binding sites that are present on proteins essential for optimal cell function []. According to our data, Fructilactobacillus sanfranciscensis DG1 appears to have a moderate zinc requirement and to be homeostatically balanced despite a high extracellular zinc load.
Although the conditions under which the yeasts and bacteria exposed in the tests carried out here differ from those under which sourdough bread is made, it seems likely that the addition of ZnSO4 in the above-mentioned concentration range for wheat flour fortification (approximately 0.18–0.6 mM Zn; assuming a density of sourdough bread of ~0.4 []) should not negatively affect the growth and metabolic activity of the microorganisms. A thorough understanding of the role of zinc in the reprogramming of metabolic networks in conventional and biotechnologically improved yeast and lactic acid bacteria is crucial for the breeding of robust strains and their suitability for the production of trace element-enriched goods.
5. Conclusions
Food fortification has become a recognized measure to improve the micronutrient status of large populations in recent years. Cereal flours and bakery products are good candidates for zinc fortification. A future application of this technology to other sustainable resources would be of substantial benefit from a trace element supply perspective. The results regarding the zinc tolerance and zinc enrichment capacities of the three yeast strains Yarrowia lipolytica RO25, Kazachstania unispora FM2, Kazakhstania servazzii KAZ2, and the heterofermentative lactic acid bacterium Fructilactobacillus sanfranciscensis DG1 presented here provide valuable information and will be used soon to create zinc-enriched bakery goods with the inclusion of hydrolyzed cricket flour as a protein-rich nutritional resource. Besides providing micronutrients, zinc-enriched microorganisms may also improve health through their effects on human probiotics—either provided as part of baked goods or as supplements []. Bread is one of the most widely eaten staple foods in the world. Both traditional and modern aspects of bakery production offer a wide range of opportunities to integrate nutritional and health benefits. In low- and middle-income countries, where cheap staple foods are prevalent and dietary diversity is limited, the consumption of zinc-fortified (homemade) bakery goods can be a solution to address trace element deficiencies. Over the past few years, functional fortified foods have become increasingly popular in Western countries. There is great potential for financial growth in this market, particularly since the effect is also considered to be scientifically proven.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/fermentation9060521/s1, Table S1: Results of a two-way ANOVA on growth of yeast and lactic acid bacteria during the Zn-enrichment procedure analyzed by OD600 measurement.
Author Contributions
Conceptualization: C.K. and O.S.; data curation: C.K. and S.R. (Samantha Rossi); formal analysis: C.K., S.R. (Samantha Rossi), M.M. and H.K.; investigation: C.K., M.M. and S.R. (Samantha Rossi); project administration: C.K.; resources: C.K., M.M., O.S., S.R. (Sascha Rohn), and H.H.; supervision: C.K., O.S., F.P. and R.L.; writing—original draft: C.K.; writing—review and editing: C.K., S.R. (Samantha Rossi), M.M., H.K., S.R. (Sascha Rohn), F.P., O.S., R.L. and H.H. All authors have read and agreed to the published version of the manuscript.
Funding
We acknowledge support by the German Research Foundation and the Open Access Publication Fund of TU Berlin.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to thank Julia Keitel, Silvia Heim, and Timo Neubert for excellent technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ohanenye, I.C.; Emenike, C.U.; Mensi, A.; Medina-Godoy, S.; Jin, J.; Ahmed, T.; Sun, X.; Udenigwe, C.C. Food fortification technologies: Influence on iron, zinc and vitamin A bioavailability and potential implications on micronutrient deficiency in sub-Saharan Africa. Sci. Afr. 2021, 11, e00667. [Google Scholar] [CrossRef]
- Wessells, K.R.; Brown, K.H. Estimating the Global Prevalence of Zinc Deficiency: Results Based on Zinc Availability in National Food Supplies and the Prevalence of Stunting. PLoS ONE 2012, 7, e50568. [Google Scholar] [CrossRef]
- Beal, T.; Massiot, E.; Arsenault, J.E.; Smith, M.R.; Hijmans, R.J. Global trends in dietary micronutrient supplies and estimated prevalence of inadequate intakes. PLoS ONE 2017, 12, e0175554. [Google Scholar] [CrossRef]
- Wessels, I.; Rolles, B.; Slusarenko, A.J.; Rink, L. Zinc deficiency as a possible risk factor for increased susceptibility and severe progression of Corona Virus Disease 19. Br. J. Nutr. 2022, 127, 214–232. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Discovery of Human Zinc Deficiency: Its Impact on Human Health and Disease. Adv. Nutr. Int. Rev. J. 2013, 4, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Lessons Learned from Experimental Human Model of Zinc Deficiency. J. Immunol. Res. 2020, 2020, 9207279. [Google Scholar] [CrossRef] [PubMed]
- Maares, M.; Haase, H. A Guide to Human Zinc Absorption: General Overview and Recent Advances of In Vitro Intestinal Models. Nutrients 2020, 12, 762. [Google Scholar] [CrossRef] [PubMed]
- Roohani, N.; Hurrell, R.; Kelishadi, R.; Schulin, R. Zinc and its importance for human health: An integrative review. J. Res. Med. Sci. 2013, 18, 144–157. [Google Scholar] [PubMed]
- Gupta, S.; Brazier, A.K.M.; Lowe, N.M. Zinc deficiency in low- and middle-income countries: Prevalence and approaches for mitigation. J. Hum. Nutr. Diet. 2020, 33, 624–643. [Google Scholar] [CrossRef]
- Shah, D.; Sachdev, H.S.; Gera, T.; De-Regil, L.M.; Peña-Rosas, J.P. Fortification of staple foods with zinc for improving zinc status and other health outcomes in the general population. Cochrane Database Syst. Rev. 2016, 2016, CD010697. [Google Scholar] [CrossRef]
- Gomes, M.J.C.; Martino, H.S.D.; Tako, E. Zinc-biofortified staple food crops to improve zinc status in humans: A systematic review. Crit. Rev. Food Sci. Nutr. 2021, 60, 1–13. [Google Scholar] [CrossRef]
- Tsang, B.L.; Holsted, E.; McDonald, C.M.; Brown, K.H.; Black, R.; Mbuya, M.N.N.; Grant, F.; A Rowe, L.; Manger, M.S. Effects of Foods Fortified with Zinc, Alone or Cofortified with Multiple Micronutrients, on Health and Functional Outcomes: A Systematic Review and Meta-Analysis. Adv. Nutr. Int. Rev. J. 2021, 12, 1821–1837. [Google Scholar] [CrossRef]
- Hall, A.G.; King, J.C. Zinc Fortification: Current Trends and Strategies. Nutrients 2022, 14, 3895. [Google Scholar] [CrossRef] [PubMed]
- de Romaña, D.L.; Lönnerdal, B.; Brown, K.H. Absorption of zinc from wheat products fortified with iron and either zinc sulfate or zinc oxide. Am. J. Clin. Nutr. 2003, 78, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Das, J.K.; Kumar, R.; Salam, R.A.; Bhutta, Z.A. Systematic Review of Zinc Fortification Trials. Ann. Nutr. Metab. 2013, 62, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Anjum, F.M.; Randhawa, M.A.; Farooq, U.; Akhtar, S.; Sultan, M.T. Effect of multiple fortification on the bioavailability of minerals in wheat meal bread. J. Food Sci. Technol. 2012, 49, 737–744. [Google Scholar] [CrossRef]
- Garnweidner-Holme, L.; Hallquist, M.; Watters, S.I.; Gjøvik, M.; Frederiksen, M.P.; Jonassen, S.; Ravnanger, I.; Henriksen, C.; Myhrstad, M.; Telle-Hansen, V. Attitudes towards and experiences with sourdough and baker’s yeast bread amongst par ticipants in a randomised controlled trial: A qualitative study. Food Nutr. Res. 2022, 66, 66. [Google Scholar] [CrossRef] [PubMed]
- Graça, C.; Lima, A.; Raymundo, A.; Sousa, I. Sourdough Fermentation as a Tool to Improve the Nutritional and Health-Promoting Properties of Its Derived-Products. Fermentation 2021, 7, 246. [Google Scholar] [CrossRef]
- Fernández-Peláez, J.; Paesani, C.; Gómez, M. Sourdough Technology as a Tool for the Development of Healthier Grain-Based Products: An Update. Agronomy 2020, 10, 1962. [Google Scholar] [CrossRef]
- Gobbetti, M.; De Angelis, M.; Di Cagno, R.; Calasso, M.; Archetti, G.; Rizzello, C.G. Novel insights on the functional/nutritional features of the sourdough fermentation. Int. J. Food Microbiol. 2019, 302, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Leenhardt, F.; Levrat-Verny, M.-A.; Chanliaud, E.; Rémésy, C. Moderate Decrease of pH by Sourdough Fermentation Is Sufficient To Reduce Phytate Content of Whole Wheat Flour through Endogenous Phytase Activity. J. Agric. Food Chem. 2005, 53, 98–102. [Google Scholar] [CrossRef]
- Yildirim, R.M.; Arici, M. Effect of the fermentation temperature on the degradation of phytic acid in whole-wheat sourdough bread. LWT 2019, 112, 108224. [Google Scholar] [CrossRef]
- Canesin, M.R.; Cazarin, C.B.B. Nutritional quality and nutrient bioaccessibility in sourdough bread. Curr. Opin. Food Sci. 2021, 40, 81–86. [Google Scholar] [CrossRef]
- Gobbetti, M.; Rizzello, C.G.; Di Cagno, R.; De Angelis, M. How the sourdough may affect the functional features of leavened baked goods. Food Microbiol. 2014, 37, 30–40. [Google Scholar] [CrossRef]
- Lopez, H.W.; Duclos, V.; Coudray, C.; Krespine, V.; Feillet-Coudray, C.; Messager, A.; Demigné, C.; Rémésy, C. Making bread with sourdough improves mineral bioavailability from reconstituted whole wheat flour in rats. Nutrition 2003, 19, 524–530. [Google Scholar] [CrossRef]
- Najafi, M.A.; Rezaei, K.; Safari, M.; Razavi, S.H. Use of sourdough to reduce phytic acid and improve zinc bioavailability of a traditional flat bread (sangak) from Iran. Food Sci. Biotechnol. 2012, 21, 51–57. [Google Scholar] [CrossRef]
- Nissen, L.; Samaei, S.P.; Babini, E.; Gianotti, A. Gluten free sourdough bread enriched with cricket flour for protein fortification: Antioxidant improvement and Volatilome characterization. Food Chem. 2020, 333, 127410. [Google Scholar] [CrossRef] [PubMed]
- Vasilica, B.; Chiș, M.S.; Alexa, E.; Pop, C.; Păucean, A.; Man, S.; Igual, M.; Haydee, K.M.; Dalma, K.E.; Stănilă, S.; et al. The Impact of Insect Flour on Sourdough Fermentation-Fatty Acids, Amino-Acids, Minerals and Volatile Profile. Insects 2022, 13, 576. [Google Scholar] [CrossRef] [PubMed]
- Hlongwane, Z.T.; Slotow, R.; Munyai, T.C. Indigenous Knowledge about Consumption of Edible Insects in South Africa. Insects 2021, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Stull, V.J.; A Patz, J.; Myers, S.S. Nutritional and environmental benefits of increasing insect consumption in Africa and Asia. Environ. Res. Lett. 2021, 16, 065001. [Google Scholar] [CrossRef]
- Svanberg, I.; Berggren, Å. Insects as past and future food in entomophobic Europe. Food Cult. Soc. 2021, 24, 624–638. [Google Scholar] [CrossRef]
- Kröger, T.; Dupont, J.; Büsing, L.; Fiebelkorn, F. Acceptance of Insect-Based Food Products in Western Societies: A Systematic Review. Front. Nutr. 2022, 8, 759885. [Google Scholar] [CrossRef]
- Steensels, J.; Snoek, T.; Meersman, E.; Nicolino, M.P.; Voordeckers, K.; Verstrepen, K.J. Improving industrial yeast strains: Exploiting natural and artificial diversity. FEMS Microbiol. Rev. 2014, 38, 947–995. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Semumu, T.; Gamero, A. Non-Conventional Yeasts as Alternatives in Modern Baking for Improved Performance and Aroma Enhancement. Fermentation 2021, 7, 102. [Google Scholar] [CrossRef]
- Landis, E.A.; Oliverio, A.M.; McKenney, E.A.; Nichols, L.M.; Kfoury, N.; Biango-Daniels, M.; Shell, L.; Madden, A.; Shapiro, L.; Sakunala, S.; et al. The diversity and function of sourdough starter microbiomes. elife 2021, 10, e61644. [Google Scholar] [CrossRef] [PubMed]
- Wittwer, A.; Howell, K. Rising stars in the bakery: Novel yeasts for modern bread. Microbiol. Aust. 2022, 43, 75–78. [Google Scholar] [CrossRef]
- Rossi, S.; Parrotta, L.; Del Duca, S.; Rosa, M.D.; Patrignani, F.; Schluter, O.; Lanciotti, R. Effect of Yarrowia lipolytica RO25 cricket-based hydrolysates on sourdough quality parameters. LWT 2021, 148, 111760. [Google Scholar] [CrossRef]
- Rossi, S.; Parrotta, L.; Gottardi, D.; Glicerina, V.; Del Duca, S.; Rosa, M.D.; Patrignani, F.; Schlüter, O.; Lanciotti, R. Unravelling the potential of cricket-based hydrolysed sourdough on the quality of an innovative bakery product. J. Insects Food Feed. 2022, 8, 921–935. [Google Scholar] [CrossRef]
- Haase, H.; Jordan, L.; Keitel, L.; Keil, C.; Mahltig, B. Comparison of methods for determining the effectiveness of antibacterial functionalized textiles. PLoS ONE 2017, 12, e0188304. [Google Scholar] [CrossRef]
- Gottardi, D.; Ciccone, M.; Siroli, L.; Lanciotti, R.; Patrignani, F. Use of Yarrowia lipolytica to Obtain Fish Waste Functional Hydrolysates Rich in Flavoring Compounds. Fermentation 2022, 8, 708. [Google Scholar] [CrossRef]
- Alker, W.; Schwerdtle, T.; Schomburg, L.; Haase, H. A Zinpyr-1-based Fluorimetric Microassay for Free Zinc in Human Serum. Int. J. Mol. Sci. 2019, 20, 4006. [Google Scholar] [CrossRef]
- Maares, M.; Keil, C.; Pallasdies, L.; Schmacht, M.; Senz, M.; Nissen, J.; Kieserling, H.; Drusch, S.; Haase, H. Zinc availability from zinc-enriched yeast studied with an in vitro digestion/Caco-2 cell culture model. J. Trace Elements Med. Biol. 2022, 71, 126934. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Weisenhorn, E.; MacDiarmid, C.W.; Andreini, C.; Bucci, M.; Taggart, J.; Banci, L.; Russell, J.; Coon, J.J.; Eide, D.J. The cellular economy of the Saccharomyces cerevisiae zinc proteome. Metallomics 2018, 10, 1755–1776. [Google Scholar] [CrossRef]
- Santivarangkna, C.; Wenning, M.; Foerst, P.; Kulozik, U. Damage of cell envelope of Lactobacillus helveticus during vacuum drying. J. Appl. Microbiol. 2007, 102, 748–756. [Google Scholar] [CrossRef]
- Mihoubi, W.; Sahli, E.; Gargouri, A.; Amiel, C. FTIR spectroscopy of whole cells for the monitoring of yeast apoptosis mediated by p53 over-expression and its suppression by Nigella sativa extracts. PLoS ONE 2017, 12, e0180680. [Google Scholar] [CrossRef]
- Erenstein, O.; Jaleta, M.; Mottaleb, K.A.; Sonder, K.; Donovan, J.; Braun, H.-J. Global Trends in Wheat Production, Consumption and Trade. In Wheat Improvement; Reynolds, M.P., Braun, H.-J., Eds.; Springer: Cham, Switzerland, 2022; pp. 47–66. [Google Scholar] [CrossRef]
- Chen, X.-P.; Zhang, Y.-Q.; Tong, Y.-P.; Xue, Y.-F.; Liu, D.-Y.; Zhang, W.; Deng, Y.; Meng, Q.-F.; Yue, S.-C.; Yan, P.; et al. Harvesting more grain zinc of wheat for human health. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Wang, M.; Kong, F.; Liu, R.; Fan, Q.; Zhang, X. Zinc in Wheat Grain, Processing, and Food. Front. Nutr. 2020, 7, 124. [Google Scholar] [CrossRef]
- WHO Guideline: Fortification of Maize Flour and Corn Meal with Vitamins and Minerals; World Health Organization: Geneva, Switzerland, 2016.
- Guideline: Fortification of Wheat Flour with Vitamins and Minerals as a Public Health Strategy; World Health Organization: Geneva, Switzerland, 2022.
- GFDX. Global Fortification Data Exchange. Nutrient Levels in Fortification Standards (Mid-Range or Average). Available online: https://fortificationdata.org/map-nutrient-levels-in-fortification-standards/ (accessed on 21 January 2023).
- Borges, M.M.; da Costa, D.V.; Trombete, F.M.; Câmara, A.K.F.I. Edible insects as a sustainable alternative to food products: An insight into quality aspects of reformulated bakery and meat products. Curr. Opin. Food Sci. 2022, 46, 100864. [Google Scholar] [CrossRef]
- Kröncke, N.; Grebenteuch, S.; Keil, C.; Demtröder, S.; Kroh, L.; Thünemann, A.F.; Benning, R.; Haase, H. Effect of Different Drying Methods on Nutrient Quality of the Yellow Mealworm (Tenebrio molitor L.). Insects 2019, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Bankar, A.; Zinjarde, S.; Telmore, A.; Walke, A.; Ravikumar, A. Morphological response of Yarrowia lipolytica under stress of heavy metals. Can. J. Microbiol. 2018, 64, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Bankar, A.; Zinjarde, S.; Shinde, M.; Gopalghare, G.; Ravikumar, A. Heavy metal tolerance in marine strains of Yarrowia lipolytica. Extremophiles 2018, 22, 617–628. [Google Scholar] [CrossRef] [PubMed]
- García-Béjar, B.; Arévalo-Villena, M.; Guisantes-Batan, E.; Rodríguez-Flores, J.; Briones, A. Study of the bioremediatory capacity of wild yeasts. Sci. Rep. 2020, 10, 11265. [Google Scholar] [CrossRef] [PubMed]
- de Nicola, R.; Hall, N.; Melville, S.G.; Walker, G.M. Influence of Zinc on Distiller’s Yeast: Cellular Accumulation of Zinc and Impact on Spirit Congeners. J. Inst. Brew. 2009, 115, 265–271. [Google Scholar] [CrossRef]
- Simm, C.; Lahner, B.; Salt, D.; LeFurgey, A.; Ingram, P.; Yandell, B.; Eide, D.J. Saccharomyces cerevisiae Vacuole in Zinc Storage and Intracellular Zinc Distribution. Eukaryot. Cell 2007, 6, 1166–1177. [Google Scholar] [CrossRef]
- MacDiarmid, C.W.; Milanick, M.A.; Eide, D.J. Induction of the ZRC1 Metal Tolerance Gene in Zinc-limited Yeast Confers Resistance to Zinc Shock. J. Biol. Chem. 2003, 278, 15065–15072. [Google Scholar] [CrossRef]
- Strouhal, M.; Kizek, R.; Vacek, J.; Trnková, L.; Němec, M. Electrochemical study of heavy metals and metallothionein in yeast Yarrowia lipolytica. Bioelectrochemistry 2003, 60, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Eide, D.J. Transcription factors and transporters in zinc homeostasis: Lessons learned from fungi. Crit. Rev. Biochem. Mol. Biol. 2020, 55, 88–110. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Dziuba, N.; Lindahl, P.A. Isolated Saccharomyces cerevisiae vacuoles contain low-molecular-mass transition-metal polyphosphate complexes. Metallomics 2019, 11, 1298–1309. [Google Scholar] [CrossRef]
- Krężel, A.; Maret, W. The biological inorganic chemistry of zinc ions. Arch. Biochem. Biophys. 2016, 611, 3–19. [Google Scholar] [CrossRef]
- Sun, J.; Xu, S.; Du, Y.; Yu, K.; Jiang, Y.; Weng, H.; Yuan, W. Accumulation and Enrichment of Trace Elements by Yeast Cells and Their Applications: A Critical Review. Microorganisms 2022, 10, 1746. [Google Scholar] [CrossRef]
- Bird, A.J.; Wilson, S. Zinc homeostasis in the secretory pathway in yeast. Curr. Opin. Chem. Biol. 2020, 55, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Li, R.; Huang, Y. Zinc homeostasis in Schizosaccharomyces pombe. Arch. Microbiol. 2023, 205, 126. [Google Scholar] [CrossRef]
- Zhao, Y.-Y.; Cao, C.-L.; Liu, Y.-L.; Wang, J.; Li, J.; Li, S.-Y.; Deng, Y. Identification of the Genetic Requirements for Zinc Tolerance and Toxicity in Saccharomyces cerevisiae. G3 Genes|Genomes|Genetics 2020, 10, 479–488. [Google Scholar] [CrossRef]
- Leonardi, A.; Zanoni, S.; De Lucia, M.; Amaretti, A.; Raimondi, S.; Rossi, M. Zinc Uptake by Lactic Acid Bacteria. ISRN Biotechnol. 2013, 2013, 312917. [Google Scholar] [CrossRef]
- Yusof, H.M.; Mohamad, R.; Zaidan, U.H.; Rahman, N.A. Sustainable microbial cell nanofactory for zinc oxide nanoparticles production by zinc-tolerant probiotic Lactobacillus plantarum strain TA4. Microb. Cell Factories 2020, 19, 10. [Google Scholar] [CrossRef]
- Papadimitriou, K.; Alegría, Á.; Bron, P.A.; de Angelis, M.; Gobbetti, M.; Kleerebezem, M.; Lemos, J.A.; Linares, D.M.; Ross, P.; Stanton, C.; et al. Stress Physiology of Lactic Acid Bacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 837–890. [Google Scholar] [CrossRef] [PubMed]
- Huynh, U.; Qiao, M.; King, J.; Trinh, B.; Valdez, J.; Haq, M.; Zastrow, M.L. Differential Effects of Transition Metals on Growth and Metal Uptake for Two Distinct Lactobacillus Species. Microbiol. Spectr. 2022, 10, e0100621. [Google Scholar] [CrossRef]
- Mrvčić, J.; Stanzer, D.; Šolić, E.; Stehlik-Tomas, V. Interaction of lactic acid bacteria with metal ions: Opportunities for improving food safety and quality. World J. Microbiol. Biotechnol. 2012, 28, 2771–2782. [Google Scholar] [CrossRef] [PubMed]
- Clément, H.; Prost, C.; Chiron, H.; Bonnand-Ducasse, M.; Della Valle, G.; Courcoux, P.; Onno, B. The effect of organic wheat flour by-products on sourdough performances assessed by a multi-criteria approach. Food Res. Int. 2018, 106, 974–981. [Google Scholar] [CrossRef]
- Meng, Y.; Liang, Z.; Yi, M.; Tan, Y.; Li, Z.; Du, P.; Li, A.; Li, C.; Liu, L. Enrichment of zinc in Lactobacillus plantarum DNZ-4: Impact on its characteristics, metabolites and antioxidant activity. LWT 2022, 153, 112462. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).