Abstract
In the present study, a wild-type Lacticaseibacillus paracasei SP5 (L. paracasei SP5) potential probiotic strain (previously isolated from kefir grains) was applied for the 1-day fermentation of an apple–orange–carrot mixed juice. After the fermentation, the mixed juice was refrigerated in cold storage at 4 °C, and the microbiological stability, characterization of volatiles, physicochemical properties (pH, total titratable acidity (TTA), residual sugar content and organic acids content), the sensorial validation (aroma, taste and overall acceptability) of the juice, and the viability of the potential probiotic strain were analyzed. The stored juice exhibited zero colonies of yeasts/fungi and simultaneously the viability of L. paracasei SP5 was retained to 8.28 Log CFU/mL, even after the 4th week of cold storage. The pH values ranged from 3.80 to 3.35 and the TTA values ranged from 0.1344% to 0.1844% lactic acid for the unfermented juice up until the 4th week of cold storage. Furthermore, the organic acids content consisted mostly of lactic acid (4.6 to 9.1 g/L), while malic acid (3.7 to 1.5 g/L), acetic acid (0.6 g/L) and propionic acid (0.3 g/L) were detected only after the 4th week of cold storage. Residual sugar content ranged from the initial value of 122.2 g/L and 106.6 g/L at the end of cold storage. As far as the volatiles’ characterization is concerned: 9 esters, 2 organic acids, 12 alcohols, 3 aldehydes, 1 ketone, 6 terpenes and 4 sesquiterpenes (37 in total) were detected in the unfermented mixed juice and 33 compounds in the fermented juice after 4 weeks of cold storage. The sensorial properties (aroma, taste and overall acceptability) of the fermented mixed juice samples were positively influenced. Consequently, L. paracasei SP5 potential probiotic strain may be applied for the production of probiotic mixed juices, with satisfying viability, volatile profile and organoleptic results.
1. Introduction
Functional foods have been gaining attention during the last decade from the food industry, research and consumers’ preference [1]. The relevant scientific findings, which accompany their consumption on a daily basis, have established them as disease-preventing factors in consumers’ minds [2,3]. Functional foods may contain bioactive compounds or/and probiotic microorganisms. Probiotics are live organisms that may enhance their host’s health when administered in adequate quantities (106–107 CFU/mL) [4].
The intestinal microflora equilibrium, the level of serum cholesterol, the empow-erment of the immune system, plus the antimutagenic and antidiarrheal properties, are only some of the beneficial effects produced by the consumption of probiotics [1]. The main vehicles for probiotic delivery are dairy products [5,6]. Nevertheless, dairy products have drawbacks for their consumption by several categories of consumers. Reasons for this may be lactose intolerance, vegetarianism, allergies, etc. [7].
Thus, alternative substrates for probiotic delivery have been examined in non-dairy products. The development of innovative fruit and vegetable probiotic beverages has increased in order to meet this demand [8].
Moreover, fruit and vegetable juices are a rich source of vitamins, minerals and antioxidant compounds that, at the same time, provide a good alternative environment for probiotic growth [8]. Many fruits and vegetables or their combinations have been examined as possible substrates for probiotic bacteria. Among others, pineapple [9], grape [10], pomegranate [11], carrot [5], sweet potato [12], and beet [6] are some of the fruit and vegetable juices examined for their impact on probiotic bacteria growth.
Fermentation as a traditional practice of sensorial improvement has been applied to many food matrices for centuries. Additionally, the extension of storage period and the enhancement of nutritional properties of fermented foods should be taken into consideration [13]. During fermentation, several microbial and enzymatic alterations occur provoking changes in the constituents of food materials. Strains belonging to Bacillus spp. and the lactic acid bacteria family have been used for this purpose with very impressive and encouraging results.
Mixed juices of fruits and/or vegetables have gained attention mostly because of their ability to achieve a pleasant appearance and natural taste. Moreover, the juice ratio is crucial for the content of bioactive compounds (carotenoids, vitamins, polyphenols, flavonols, anthocyanins) and dietary fiber [13]. Physicochemical properties are influenced as well. For example, the final pH value determines the stability of bioactive compounds and the survivability of the probiotic strains if the juice is fermented [14]. Several studies concerning mixed juices have been carried out to examine their technological and nutritional properties. In one study [15], the mixed juice of peach, apple and carrot was examined with very satisfying results, concerning the fact that the antioxidant activity of mixed juices increased due to alterations of the physico-chemical attributes and bioactive compounds through high pressure homogenization and water-soluble pectin characteristics.
Apples (Malus domestica) are one of the most well-known and consumed fruits worldwide. Their trees grow in temperate and tropical climates [16], and belong to the ‘Rosaceae’ family and ‘Maloideae’ subfamily. Furthermore, apple juice usually undergoes alcoholic fermentation, mainly for the production of vinegar, cider and apple wine [17]. Nowadays, lactic acid fermentation is applied to apple juice solely or in combination with other fruits and/or vegetables for the production of fermented beverages [11]. Additionally, oranges (Citrus nobilis L.) and their juice are also well known and consumed in the global market for its rich content in bioactive compounds, ascorbic acid, flavonoid and carotenoid content [18]. Orange juice has been examined for its properties after alcoholic and lactic acid fermentation, providing promising results [19]. Carrot (Daucus carota L.) is one of the most commonly consumed root vegetables. It has high carotenoid, phenolic and vitamin content, and thus, exhibits antioxidant, anti-aging and eye-sight protective activity [20]. Despite the fact that carrot is a major vegetable source of provitamin A, its terpene flavor makes its sensorial acceptance by some consumer groups problematic (especially children and young people) [21]. The probiotic delivery of lactobacilli strains in non-dairy matrices and the production of beverages with high consumer acceptability is a new challenge emerging in the food industry and in research [22].
The specific Lactobacillus strain has previously been added to pomegranate juice for fermentation at different pH levels with very satisfying results as far as the production of a beverage with high phenolic content is concerned [14]. While other studies have already evaluated the viability of probiotics in fruit and vegetable juices, there is a lack of studies with the application of L. paracasei in this particular fruit and vegetable combination, apple–orange–carrot, which is known to be globally commercialized.
Specifically, in the present study, a wild type potential probiotic, L. paracasei SP5 strain, previously isolated from kefir grains, was added to orange–apple–carrot juice for fermentation. Thus, the goal of our study was to assess the probiotic potential of L. paracasei SP5 in the aforementioned substrate after 24 h of fermentation and 28 days of cold storage. The effect of fermentation on volatiles of the juice was examined with headspace solid-phase microextraction and gas chromatography–mass spectrometry (HS-SPME GC–MS) analysis. The differences in sugar and acid content before and after fermentation were detected with high-performance liquid chromatography (HPLC) analysis, while the microbiological stability of the fermented juice was evaluated during cold storage (4 °C) over a 4-week period. Finally, an organoleptic evaluation of the fermented juice was performed testing aroma, taste and overall ac-ceptability.
2. Materials and Methods
2.1. Microorganism
The potential probiotic strain L. paracasei SP5 was previously isolated from kefir grains in our laboratory [14]. It was characterized for its probiotic attributes and was grown under anaerobic conditions at 37 °C for 48 h in MRS broth.
2.2. Apple-Orange-Carrot Juice Fermentation
Fresh apples, oranges and carrots were purchased from the local market of Nea Orestiada (Evros, Greece) and used for the preparation of the juice. The seeds and cavity tissues were removed from the oranges and apples, and the carrots were carefully peeled. The juice was prepared in an apple-to-orange-to-carrot ratio of 5:3:2 by volume. Juices were extracted with the aid of a Philips Viva Collection juicer. After that, the juices were filtered through a cheesecloth. Finally, the fresh juice was divided into quantities of 100 mL inside Erlenmeyer flasks and pasteurized at 80 °C for 15 min [23]. The pasteurized juice without the addition of L. paracasei SP5 was designed as a con-trol.
An amount of 1 g of wet biomass L. paracasei SP5 was added to each flask (in trip-licate) and left to ferment for 24 h at 30 °C. The cell density was 109 CFU/mL. Then, the flasks were kept for 4 weeks at 4 °C.
2.3. pH and Total Titratable Acidity (TTA)
A digital pH meter (Milwaukee Instruments, Martini, MI, USA) was used for pH determination. Total acidity was expressed as a percent of lactic acid and was determined by titrating juice samples (10 mL) with 0.1 N NaOH with phenolphthalein indicator to the endpoint (pH 8.2 ± 0.1) in triplicate [24].
2.4. HPLC Analysis
Concentration of residual sugars and organic acids (citric, malic, lactic, acetic and propionic acid) was determined by HPLC on a Shimadzu chromatography system (Shimadzu Corp., Duisburg, Germany) using standard curves prepared with standard solutions (R2 ≥ 0.99), as recently described [25]. Briefly, juice samples (20 μL) were directly injected into the Nucleogel ION 300 OA column (Macherey-Nagel, Germany) after double filtration with 0.22 μm filters. H2SO4 solution (0.049 g/L) was used as mo-bile phase at 0.3 mL/min. The detector cell temperature was set at 60 °C and the oven temperature at 85 °C.
2.5. Microbiological ANALYSIS
Microorganisms responsible for the spoilage of juice (mostly yeasts and molds) were detected during fermentation and refrigerated storage. In addition, the microbial counts of lactic acid bacteria (LAB) were screened in triplicate before and after 24 h fermentation and every week during refrigerated storage. Therefore, representative 10 mL from each juice sample were blended with 90 mL of sterilized 1/4 Ringers solution (Sigma-Aldrich) and subjected to serial dilutions.
The following tests were performed: (i) lactobacilli [Gram (+), catalase (−)] on acidified MRS agar (Oxoid Ltd., Basingstoke, Hampshire, UK) at 37 °C for 48 h anaerobically (Anaerobic jar, Anerocult C, Merck, Rahway, New Jersey, USA), and (ii) yeasts and molds on malt agar (Oxoid Ltd., Basingstoke, Hampshire, UK) (pH was adjusted to 4.5 by sterile solution of 10% lactic acid) at 30 °C for 48 h. All incubations were further extended up to 120 h, but no extra colonies were observed. Gram staining and catalase tests were performed for LAB confirmation. Results are presented as log of mean colony-forming units (CFU) per mL of apple-orange-carrot juice.
2.6. Volatiles Analysis by HS-SPME GC-MS
Juice samples collected before/after fermentation (timepoints: 0 d and 1st d) and after storage (4th week) were analyzed for minor volatiles by using HS-SPME GC–MS [6890N GC, 5973 NetworkedMS MSD, HP-5MS column (30 m, 0.25 mm i.d., 0.25 μm film thickness), Agilent Technologies, Santa Clara, CA, USA] and semi-quantified, as previously described [26]. Initially, each sample (10 mL) was sealed in a 20 mL head-space vial along with NaCl (3 g) and an internal standard (4-methyl-2-pentanol); an SPME fiber [50/30 mm divinylbenzene/carboxen on polydimethylsiloxane (Supelco, Bellefonte, PA, USA)] was used for volatile absorption at 60 °C for 45 min. The injector temperature was set at 240 °C and the oven temperature was programmed at 35 °C for 6 min, raised to 60 °C with a rate of 2 °C/min, held constant for 5 min, then raised to 200 °C with a rate of 5 °C and finally raised to 250 °C with a rate of 25 °C/min, held constant for 6 min. The carrier gas was helium (1.8 mL/min). Volatile compound peaks were identified by comparing their retention times and mass spectra to NBS75K and Wiley275 reference libraries, to in-house reference standard libraries, and by determining Kovats’ retention indexes (KI) and comparing them to the available literature. Semi-quantification was performed by dividing each peak’s area with the peak area of the internal standard, and this ratio was multiplied by its initial concentration (expressed as mg/L). The peak areas were measured from a full scan chromatograph using the total ion current (TIC).
2.7. Sensory Evaluation
Sensory evaluation of the fermented juice samples during storage (4 °C/28 days) was employed regarding the aroma, flavor and overall acceptability in comparison with commercial apple–orange–carrot juice, by 25 untrained panelists after fermentation and after the 1st, 2nd, 3rd and 4th week of cold storage (4 °C). Prior to sample evaluation, all panelists participated in orientation sessions to familiarize with the scale at-tributes of the juice using an intensity scale.
Each sample was coded by a different 3-digit number and was served in a randomized order. The panel was asked to evaluate based on a 0–10 preference scale (0 as unacceptable and 10 as excellent) according to Plessas et al. [27].
2.8. Statistical Analysis
Bacterial counts were logarithmically transformed and presented as Log CFU/g. Analysis of variance with Dunnett’s post hoc application along with variance check at a significance level of 95% was applied. All analyses were performed with SPSS v25 (IBM Corp., Armonk, NY, USA). Comparison of the differences of the means of each attribute at different time intervals during the sensorial evaluation was accomplished by using analysis of variance with Tukey’s post hoc test.
3. Results
3.1. Microbiological Analyses
Microbial counts of lactic acid bacteria and yeasts/fungi were recorded after fermentation and during storage period. The results are presented in Table 1, along with the statistical significance from the comparison (ANOVA with Dunnett’s post hoc test).

Table 1.
Bacterial counts (mean Log CFU/mL ± SD of three samples) of the apple-orange-carrot juice samples during 24 h of fermentation and refrigerated storage at 4 °C.
According to the results, cell viability of the potential probiotic L. paracasei SP5 strain was maintained at high levels during all refrigerated storage periods (above 8 Log CFU/mL). Therefore, it can be stated that the viability value was over the limit of 6–7 Log CFU/mL. which is necessary for probiotic strains [28]. Yeasts and fungi were not detected at any studied time periods of fermentation and storage. Regarding the unfermented juice, yeasts/fungi were presented during the 14th, 21st and 28th day of storage, while in the fermented juice samples yeasts/fungi were not detected at any time periods.
3.2. pH and TTA
The measured pH values and TTA values of the fermented juices during cold storage, are presented in the following diagrams in the Figure 1a and Figure 1b respectively.
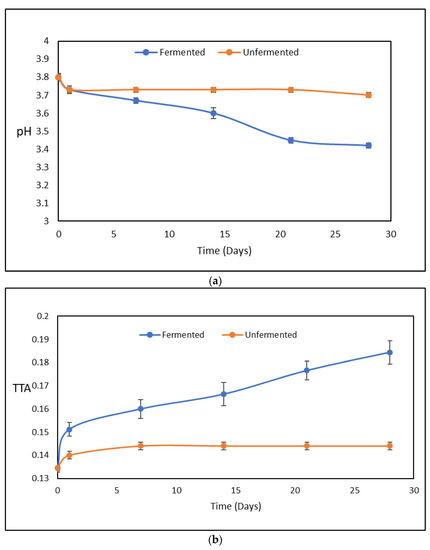
Figure 1.
(a) pH values and (b) total titratable acidity (TTA) values of the fermented and unfermented juices during cold storage.
As can be observed, the pH and the TTA of the juices were measured after the 1st day of fermentation and 4th week of cold storage. After fermentation, there was a decline in pH from 3.80 to 3.73 and a respective raise in TTA from 0.1344% to 0.1512% lactic acid. After the 1st day fermentation period, the decline in pH continued to 3.35 and the respective TTA to 0.1844% lactic acid. The small decrease in pH values and acidity have been observed and explained by other researchers who have stated that fermented juices by LAB exhibit buffering activities [29].
3.3. Residual Sugar and Organic Acids Analysis
The residual sugars and the organic acids were detected after 24 h fermentation and during 4 weeks of refrigerated storage with the use of HPLC analysis. For the purpose of comparison, the same parameters were monitored for non- fermented juice.
The results of the analysis of sugars and organic acids in orange, apple and carrot juice samples before, after fermentation and during refrigerated storage are shown in Table 2. Residual sugars (glucose and fructose) levels decreased while the levels of lactic acid increased. At this point, it should be underlined that the concentration of lactic acid at the beginning of fermentation slightly increased, mainly due to the adaptation of the used LAB in the matrix of the juices, as other researchers have noted. At the same time, a low rate of malolactic fermentation was recorded [25,30]. This phenomenon is not strange, considering that it is possible for the applied LAB, due to adaptation conditions at the beginning of concentration, to select carbon source from malic acid in order to begin the fermentation. At all the other time periods (after the 1st week) malic acid was determined at the same levels, with no further degradation. In addition, the constant and unchanged levels of lactic acid concentration for all the studied time periods regarding the unfermented juice further justifies that the potential probiotic strain of L. paracasei SP5 was effective for lactic acid fermentation of apple–orange–carrot juice. The initial value of lactic acid was 3.6 g/L and after 4 weeks of storage reached its maximum value (9.1 g/L). The concentration values of malic acid (3.7 g/L) slightly decreased during fermentation (3.1 g/L) and after the 1st week of refrigerated storage dramatically dropped to 1.8g/L, and finally, after the 4th week of storage reached 1.5 g/L, as a result of malic–lactic fermentation. Acetic and propionic acids were detected only after the 3rd week of storage, 0.6 g/L and 0.3 g/L, respectively. Additionally, ethanol was not detected at all.

Table 2.
Analysis of sugars and organic acids in orange, apple and carrot juice in control samples (0 d), before fermentation (unfermented UF samples), after fermentation (fermented samples F) (30 °C for 1 d) and during storage at 4 °C for unfermented and fermented samples for 4 weeks.
3.4. Volatiles Analysis
In Table 3, the composition of volatile compounds that were detected in the fermented and the unfermented apple-orange-carrot juice at 0 and 1st d of fermentation is exhibited. The same composition was determined for the 4th week of refrigerated storage.

Table 3.
Volatile compounds identified in the fermented apple-orange-carrot juice at 0 d, 1st d of fermentation and during 4 weeks of cold storage at 4 °C.
Esters, alcohols, organic acids, aldehydes, terpenes and sesquiterpenes are the predominant compounds identified. In particular, 9 esters, 12 alcohols, 2 organic acids, 3 aldehydes, 1 ketone, 6 terpenoids and 4 sesquiterpenes were detected in the fermented apple-orange-carrot juice. All these compounds have been previously characterized in similar and in other fermented by LAB fruit and/or vegetable juices or beverages [11]. D-limonene was the most abundant aromatic component in both fermented (60 mg/L) and unfermented juice (175 mg/L), followed by 4-ethyl-benzaldehyde (9 mg/L in the unfermented juice) and linalool (4.6 mg/L and 4.5 mg/ L in unfermented and fermented juice respectively). A-terpineol, b-myrcene and valencene were the next in concentration compounds detected in both unfermented (3.7, 2.2 and 2.2 mg/L respectively) and fermented juice (4.2, 0.6 and 1.1 mg/L respectively).
3.5. Sensorial Evaluation of Apple-Orange-Carrot Juice Samples
A sensorial evaluation of apple–orange–carrot juice samples was performed (Table 4). The samples were analyzed for their aroma, taste and overall acceptability by 25 untrained panelists before and after 24 h of fermentation and after the 1st, 2nd, 3rd and 4th week of refrigerated storage.

Table 4.
Sensorial features of apple-orange-carrot juice samples during 24 h of fermentation and 4 weeks of storage at 4 °C.
The results regarding the sensorial evaluation of the unfermented and fermented juice included aroma, taste and overall acceptability. In terms of aroma, the fermented juice managed higher scores (8.9) compared to the unfermented by the panelists, even after the 4th week of refrigerated storage. As far as the taste of the examined samples is concerned, no statistically significant differences were observed during the whole ex-perimental period between the fermented and the unfermented juices; nevertheless, the fresh juice sample achieved the highest score (9) among evaluators. Finally, the fermented juice achieved a higher score in overall acceptability until the 3rd week of storage (8.1, 7.3 and 7.5, respectively) and with no statistical difference score during the last week of storage (6.5). Additionally, it should be highlighted that in this final parameter, fresh juice also scored the higher value (8.7) according to the panelists’ preferences.
4. Discussion
From the measurement of pH values during 24 h fermentation of orange–apple–carrot juice with the L. paracasei SP5 strain and its decreasing trend, it became obvious that lactic acid fermentation occurred. The initial pH value of 3.80 decreased to 3.73 after 24 h of fermentation and ended up to 3.35 after 4 weeks of refrigerated storage at 4 °C. These findings are in accordance with other researchers’ findings [31] who indi-cated that after 24 h of fermentation of carrot juice, the reducing sugars (glucose and fructose) produce mainly lactic acid. Hashemi et al. [32] also observed a similar trend in pH values after fermentation of bergamot juice with different lactobacilli strains. Espirito et al. [33] also recorded the same trend in pH values in their study. It has been established that several lactobacilli strains have the ability to be acid-tolerant, and thus, exhibit survivability under the acidic environment of fruit and vegetable juices, with pH values ranging from 4.3 to 3.7 [34]. Furthermore, investigations have demon-strated that at lower pH values, some lactobacilli strains show increased viability, a fact that was not in line with our findings, where the microbial counts of L. paracasei SP5 decreased during refrigerated storage and lower pH values [35].
It has been reported that the malolactic fermentation may take place in fermented mixed carrot–orange juice, especially if bacteria, such as Leuconostoc spp., Lactobacillus spp. and Pediococcus spp. are responsible for the fermentation [36]. This spontaneous process of malolactic fermentation has been found to reduce the high acidity in apple juice and results in a less markedly sour taste in the final juice product [36]. The lactic acid production is accompanied by the decrease of malic acid, evidence that malolactic fermentation occurred during the fermentation process. It is known that lactic acid is mainly produced by the transformation of malic acid and the conversion of pyruvic acid from the Embden–Meyerhof pathway [37].
Furthermore, this accumulation of organic acids creates unfavorable conditions for the growth of undesirable microorganisms [38]. This fact was established in our research by the fact that no yeast or fungi were detected. Moreover, fruit and vegetable juices, in their fermented or unfermented form, are able to operate as substrates for the proliferation of lactobacilli strains over the critical limit of 6 LogCFU/mL during fer-mentation and cold storage serving the probiotication of the juice/beverage [14]. These data are in accordance with our findings, where the availability of L. paracasei SP5 was maintained over 9 LogCFU/mL even after the 4th week of refrigerated storage.
The volatiles’ profile in apple–orange–carrot juice before and after fermentation revealed qualitative and quantitative differences. It is already known from the literature that the lactic acid fermentation of fruit juices develops a volatile profile combining both volatiles from lactic acid fermentation and fruit aroma with a mild formation or degradation of some compounds [39]. In our study, esters, alcohols, organic acids, aldehydes, terpenes and sesquiterpenes were the predominant compounds identified. These findings are in line with other researchers who identified esters in fermented fruit juices contributing to fruity notes, and high alcohol and terpenes contents may be responsible for the intense floral and fruity notes [13].
More specifically, lactic acid and acetic acid can be involved in the formation of esters, and linalool, geraniol and α-terpineol, which were also detected in our study, can be synthesized from precursors by LAB glycosidases during fermentation or by cleavage under acidic conditions. Terpenes have been correlated to pine and citrus; in particular, copaene is responsible for citrus, spicy and woody notes, limonene for fruity and lemon characters, a-terpineol for citrus and chemical odors and linalool for floral, fruity and lemon notes [40]. Furthermore, limonene, which provides a lemon, orange, citrus and sweet aroma, was found to decrease after fermentation [41]. This result is in line with Peng et al.’s [42] findings in a respective volatile profile determination of fermented apple juices.
On the other hand, alcohols may be emitted by microorganisms and be derived from the reduction of aldehydes or other alcohols [43]. This may explain the great number of alcohols identified in the fermented and unfermented apple–orange–carrot juice. The quantity is greater in the fermented juice in the majority of alcohols. They are responsible for the sensation of sweetness and their quantity is usually increased after fermentation, likely due to the degradation of glucose and amino acids’ catabolism [44]. Furthermore, aldehydes exhibit unstable behavior in real food systems, and as a consequence, they may be reduced to alcohols or oxidized to acids [45]. Moreover, es-ters exhibited as predominant compounds 3-methylbutyl-acetate, hexyl-acetate and ethyl-2-methyl-butyrate, which were reduced throughout the fermentation probably because the volatilization or hydrolysis of esters was greater than their formation [46].
As far as the sensory evaluation of the fermented apple–orange–carrot juice is concerned, it was established that compounds including esters, phenols and ketones contribute to the acceptability of fermented juices by consumers [38]. This may explain why the fermented juice achieved relatively high scores in aroma and overall acceptability. Nevertheless, high concentrations of aldehydes may cause off-flavors and negative impact on consumers’ preferences [47]. In our results, we only detected two aldehydes (decanal and 4-ethyl-benzaldehyde), which decreased dramatically after fermentation and almost disappeared after 4 weeks of refrigerated storage. Fermentation time is a factor that modifies metabolic activity and alters flavor attributes [47]. Additionally, lactic and malic acid are already applied in the food industry as acidulants and flavor enhancers, and their concentrations in the fermented juice samples may explain the raised overall acceptability by consumers. Finally, it should be stated that the sensorial impact of fermentation in fermented products, and particularly, in fruit juices, is strain-dependent [14].
5. Conclusions
Apple–orange–carrot juice fermented with a wild type potential probiotic L. paracasei SP5 strain provided satisfying results as a novel probiotic non-dairy product. Respectable amounts of lactic acid were produced in all the studied periods, while several esters, alcohols, terpenes and sesquiterpenes were detected in the fermented juice samples, as well as in the unfermented one. The pH values decreased after fer-mentation and during storage while the measured TTA simultaneously increased. The viability L. paracasei SP5 cells slightly decreased during storage and definitely maintained high levels over 8 Log CFU/mL in all the studied periods. Sensory evaluation results also indicated a market potential for this probiotic juice, since the overall ac-ceptability of the fermented juices scored at higher levels by the panellists than the re-spective unfermented ones in every week of storage. Nevertheless, forthcoming exer-tion is necessary regarding the assessment of the nutritional and technological proper-ties of the final product.
Author Contributions
Conceptualization: I.M.; formal analysis: I.M. and A.A.; investigation: I.M.; data curation: I.M. and A.A.; writing-original draft: I.M.; review and editing: I.M. and S.P.; visualization: I.M.; supervision: I.M. and S.P.; project administration: I.M.; Validation: A.N.; methodology: Y.K. and S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
The authors declare no any conflict of interest.
References
- Khezri, S.; Kia, E.M.; Seyedsaleh, M.M.; Abedinzadeh, S.; Dastras, M. Application of nanotechnology in food industry and related health concern challenges. Int. J. Adv. Biotechnol. Res. 2016, 7, 1370–1382. [Google Scholar]
- Luvián-Morales, J.; Varela-Castillo, F.O.; Flores-Cisneros, L.; Cetina-Pérez, L.; Castro-Eguiluz, D. Functional Foods Modulating Inflammation and Metabolism in Chronic Diseases: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2021, 62, 4371–4392. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Castillo, P.A.; González-Vázquez, R.; Torres-Maravilla, E.; Tello, M.; Bermúdez-Humarán, L.G.; Mayorga-Reyes, L. Probiotics against Viral Infections: Current Clinical Trials and Future Perspectives. Immuno 2021, 1, 468–498. [Google Scholar] [CrossRef]
- FAO/WHO. Probiotics in Food: Health and Nutritional Properties and Guidelines for Evaluation; FAO Food and Nutrition Paper 85. 2006. Available online: https://www.fao.org/3/a0512e/a0512e.pdf (accessed on 12 May 2023).
- Tamminen, M.; Salminen, S.; Ouwehand, A.C. Fermentation of carrot juice by probiotics: Viability and preservation of adhesion. Int. J. Biotechnol. Wellness Ind. 2013, 2, 10–15. [Google Scholar] [CrossRef]
- Rakin, M.; Vukasinovic, M.; Siler-Marinkovic, S.; Maksimovic, M. Contribution of lactic acid fermentation to improved nutritive quality vegetable juices enriched with brewer’s yeast autolysate. Food Chem. 2007, 100, 599–602. [Google Scholar] [CrossRef]
- Kim, M.H.; Yun, C.H.; Lee, C.H.; Ha, J.K. The effects of fermented soybean meal on immunophysiological and stress-related parameters in Holstein calves after weaning. J. Dairy Sci. 2012, 95, 5203–5212. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, M.K.; Giri, S.K. Probiotic functional foods: Survival of probiotics during processing and storage. J. Funct. Foods 2014, 9, 225–241. [Google Scholar] [CrossRef]
- Costa, M.G.M.; Fonteles, T.V.; de Jesus, A.L.T.; Rodrigues, S. Sonicated pineapple juice as substrate for L. casei cultivation for probiotic beverage development: Process optimisation and product stability. Food Chem. 2013, 139, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.B.; Ferrari, J. Development of probiotic grape juice and Lactobacillus paracasei viability under cold storage. In Proceedings of the X CIGR Section IV International Technical Symposium, XXV Congresso Brasileiro de Ciência e Tecnologia de Alimentos, Gramado, Brazil, 24 October 2016; Available online: https://www.researchgate.net/publication/312042441_Development_of_probiotic_grape_juice_and_Lactobacillus_paracasei_viability_under_cold_storage/citations (accessed on 17 May 2023).
- Mousavi, Z.E.; Mousavi, S.M.; Razavi, S.H.; Emam-Djomeh, Z.; Kiani, H. Fermentation of pomegranate juice by probiotic lactic acid bacteria. World J. Microbiol. Biotechnol. 2011, 27, 123–128. [Google Scholar] [CrossRef]
- Panda, S.H.; Parmanick, M.; Ray, R.C. Lactic acid fermentation of sweet potato (Ipomoea batatas L.) into pickles. J. Food Process Preserv. 2007, 31, 83–101. [Google Scholar] [CrossRef]
- Di Cagno, R.; Filannino, P.; Gobbetti, M. Lactic acid fermentation of smoothies and juices. In Lactic Acid Fermentation of Fruits and Vegetables; Paramithiotis, S., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 267–282. [Google Scholar]
- Mantzourani, I.; Terpou, A.; Bekatorou, A.; Mallouchos, A.; Alexopoulos, A.; Kimbaris, A.; Plessas, S. Functional pomegranate beverage production by fermentation with a novel synbiotic L. paracasei biocatalyst. Food Chem. 2020, 308, 125658. [Google Scholar] [CrossRef]
- Wellala, C.K.D.; Bi, J.; Liu, X.; Liu, J.; Lyu, J.; Zhou, M.; Trych, U. Effect of high pressure homogenization combined with juice ratio on water-soluble pectin characteristics, functional properties and bioactive compounds in mixed juices. Innov. Food Sci. Emerg. Technol. 2020, 60, 102279. [Google Scholar] [CrossRef]
- Jakopic, J.; Zupan, A.; Eler, K.; Schmitzer, V.; Stampar, F.; Veberic, R. It’s great to be the King: Apple fruit development affected by the position in the cluster. Sci. Hortic. 2015, 194, 18–25. [Google Scholar] [CrossRef]
- Kalemba-Drożdż, M.; Kwiecień, I.; Szewczyk, A.; Cierniak, A.; Grzywacz-Kisielewska, A. Fermented vinegars from apple peels, raspberries, rosehips, lavender, mint, and rose petals: The composition, antioxidant power, and genoprotective abilities in comparison to acetic macerates, decoctions, and tinctures. Antioxidants 2020, 9, 1121. [Google Scholar] [CrossRef] [PubMed]
- Tounsi, M.S.; Wannes, W.A.; Ouerghemmi, I.; Jegham, S.; Njima, Y.B.; Hamdaoui, G.; Marzouk, B. Juice components and antioxidant capacity of four Tunisian Citrus varieties. J. Sci. Food Agric. 2011, 91, 142–151. [Google Scholar] [CrossRef]
- De la Fuente, B.; Luz, C.; Puchol, C.; Meca, G.; Barba, F.J. Evaluation of fermentation assisted by Lactobacillus brevis POM, and Lactobacillus plantarum (TR-7, TR-71, TR-14) on antioxidant compounds and organic acids of an orange juice-milk based beverage. Food Chem. 2021, 343, 128414. [Google Scholar] [CrossRef]
- Riganakos, K.A.; Karabagias, I.K.; Gertzou, I.; Stahl, M. Comparison of UV-C and thermal treatments for the preservation of carrot juice. Innov. Food Sci. Emerg. Technol. 2017, 42, 165–172. [Google Scholar] [CrossRef]
- Han, Z.; Deng, L.; Xu, Y.; Feng, Y.; Geng, Q.; Xiong, K. Image processing method for detection of carrot green-shoulder, fibrous roots and surface cracks. TCSAE 2013, 29, 156–161. [Google Scholar]
- Liang, J.R.; Deng, H.; Hu, C.Y.; Zhao, P.T.; Meng, Y.H. Vitality, fermentation, aroma profile, and digestive tolerance of the newly selected Lactiplantibacillus plantarum and Lacticaseibacillus paracasei in fermented apple juice. Front. Nutr. 2022, 9, 1045347. [Google Scholar] [CrossRef]
- Kun, S.; Rezessy-Szabó, J.M.; Nguyen, Q.D.; Hoschke, Á. Changes of microbial population and some components in carrot juice during fermentation with selected Bifidobacterium strains. Process Biochem. 2008, 43, 816–821. [Google Scholar] [CrossRef]
- Sadler, G.D.; Murphy, P.A. pH and titratable acidity. In Food Analysis; Nielsen, S.S., Ed.; Springer: Boston, MA, USA, 2010; pp. 219–238. [Google Scholar]
- Bontsidis, C.; Mallouchos, A.; Terpou, A.; Nikolaou, A.; Batra, G.; Mantzourani, I.; Plessas, S. Microbiological and chemical properties of chokeberry juice fermented by novel lactic acid bacteria with potential probiotic properties during fermentation at 4 °C for 4 weeks. Foods 2021, 10, 768. [Google Scholar] [CrossRef]
- Nikolaou, A.; Tsakiris, A.; Kanellaki, M.; Bezirtzoglou, E.; Akrida-Demertzi, K.; Kourkoutas, Y. Wine production using free and immobilized kefir culture on natural supports. Food Chem. 2019, 272, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Plessas, S.; Bekatorou, A.; Gallanagh, J.; Nigam, P.; Koutinas, A.A.; Psarianos, C. Evolution of aroma volatiles during storage of sourdough breads made by mixed cultures of Kluyveromyces marxianus and Lactobacillus delbrueckii ssp. bulgaricus or Lactobacillus helveticus. Food Chem. 2008, 107, 883–889. [Google Scholar]
- Plessas, S.; Bosnea, L.; Alexopoulos, A.; Bezirtzoglou, E. Potential effects of probiotics in cheese and yogurt production: A review. Eng. Life Sci. 2012, 1, 433–440. [Google Scholar] [CrossRef]
- Nualkaekul, S.; Charalampopoulos, D. Survival of Lactobacillus plantarum in model solutions and fruit juices. Int. J. Food Microbiol. 2011, 146, 111–117. [Google Scholar] [CrossRef]
- Rodríguez, H.; Curiel, J.A.; Landete, J.M.; de las Rivas, B.; de Felipe, F.L.; Gómez-Cordovés, C.; Mancheño, J.M.; Muñoz, R. Food phenolics and lactic acid bacteria. Int. J. Food Microbiol. 2009, 132, 79–90. [Google Scholar] [CrossRef]
- Minervini, F.; Calasso, M. Lactobacillus casei group. In Encyclopedia of Dairy Sciences, 3rd ed.; McSweeney, P.L., McNamara, J.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 275–286. [Google Scholar]
- Hashemi, S.M.B.; Khaneghah, A.M.; Barba, F.J.; Nemati, Z.; Shokofti, S.S.; Alizadeh, F. Fermented sweet lemon juice (Citrus limetta) using Lactobacillus plantarum LS5: Chemical composition, antioxidant and antibacterial activities. J. Funct. Foods 2017, 38, 409–414. [Google Scholar] [CrossRef]
- Espirito-Santo, A.P.; Carlin, F.; Renard, C.M. Apple, grape or orange juice: Which one offers the best substrate for lactobacilli growth? A screening study on bacteria viability, superoxide dismutase activity, folates production and hedonic characteristics. Food Res. Int. 2015, 78, 352–360. [Google Scholar] [CrossRef]
- Patel, A.R. Probiotic fruit and vegetable juices-recent advances and future perspective. Int. Food Res. J. 2017, 24, 1850–1857. [Google Scholar]
- Pino, A.; Bartolo, E.; Caggia, C.; Cianci, A.; Randazzo, C.L. Detection of vaginal lactobacilli as probiotic candidates. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Rahman, M.S. (Ed.) Handbook of Food Preservation; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Zhang, C.; Yang, H.; Yang, F.; Ma, Y. Current progress on butyric acid production by fermentation. Curr. Microbiol. 2009, 59, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Valero-Cases, E.; Frutos, M.J. Effect of inulin on the viability of L. plantarum during storage and in vitro digestion and on composition parameters of vegetable fermented juices. Plant Foods Hum. Nutr. 2017, 72, 161–167. [Google Scholar] [CrossRef] [PubMed]
- De Godoy Alves Filho, E.; Rodrigues, T.H.S.; Fernandes, F.A.N.; Pereira, A.L.F.; Narain, N.; de Brito, E.S.; Rodrigues, S. Chemometric evaluation of the volatile profile of probiotic melon and probiotic cashew juice. Food Res. Int. 2017, 99, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Siebert, T.E.; Barter, S.R.; de Barros Lopes, M.A.; Herderich, M.J.; Francis, I.L. Investigation of ‘stone fruit’ aroma in Chardonnay, Viognier and botrytis Semillon wines. Food Chem. 2018, 256, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Andreu-Sevilla, A.J.; Mena, P.; Martí, N.; Viguera, C.G.; Carbonell-Barrachina, Á.A. Volatile composition and descriptive sensory analysis of pomegranate juice and wine. Food Res. Int. 2013, 54, 246–254. [Google Scholar] [CrossRef]
- Peng, W.; Meng, D.; Yue, T.; Wang, Z.; Gao, Z. Effect of the apple cultivar on cloudy apple juice fermented by a mixture of Lactobacillus acidophilus, Lactobacillus plantarum, and Lactobacillus fermentum. Food Chem. 2021, 340, 127922. [Google Scholar] [CrossRef]
- Chen, C.; Lu, Y.; Yu, H.; Chen, Z.; Tian, H. Influence of 4 lactic acid bacteria on the flavor profile of fermented apple juice. Food Biosci. 2019, 27, 30–36. [Google Scholar] [CrossRef]
- Lee, P.R.; Saputra, A.; Yu, B.; Curran, P.; Liu, S.Q. Effects of pure and mixed-cultures of Saccharomyces cerevisiae and Williopsis saturnus on the volatile profiles of grape wine. Food Biotechnol. 2012, 26, 307–325. [Google Scholar] [CrossRef]
- Xu, X.; Bao, Y.; Wu, B.; Lao, F.; Hu, X.; Wu, J. Chemical analysis and flavor properties of blended orange, carrot, apple and Chinese jujube juice fermented by selenium-enriched probiotics. Food Chem. 2019, 289, 250–258. [Google Scholar] [CrossRef]
- Zheng, J.; Elangovan, S.; Valyaev, D.A.; Brousses, R.; Cesar, V.; Sortais, J.B.; Lavigne, G. Hydrosilylation of Aldehydes and Ketones Catalyzed by Half-Sandwich Manganese (I) N-Heterocyclic Carbene Complexes. Adv. Synth. Catal. 2014, 356, 1093–1097. [Google Scholar] [CrossRef]
- Kati, K.; Kaisa, P.; Karin, A. Influence and interactions of processing conditions and starter culture on formation of acids, volatile compounds, and amino acids in wheat sourdoughs. Cereal Chem. 2004, 81, 598–610. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).