Characterization of Bacterial Exopolysaccharides Produced from Different Fruit-Based Solid Media
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Cultivation Conditions
2.2. Fruit Preparation
2.3. Screening for EPS Production
2.4. Recovery of EPS
2.5. Phenol-Sulfuric Colorimetric Determination
2.6. Structural Characterization by NMR Spectroscopy
2.7. Statistical Analysis
3. Results
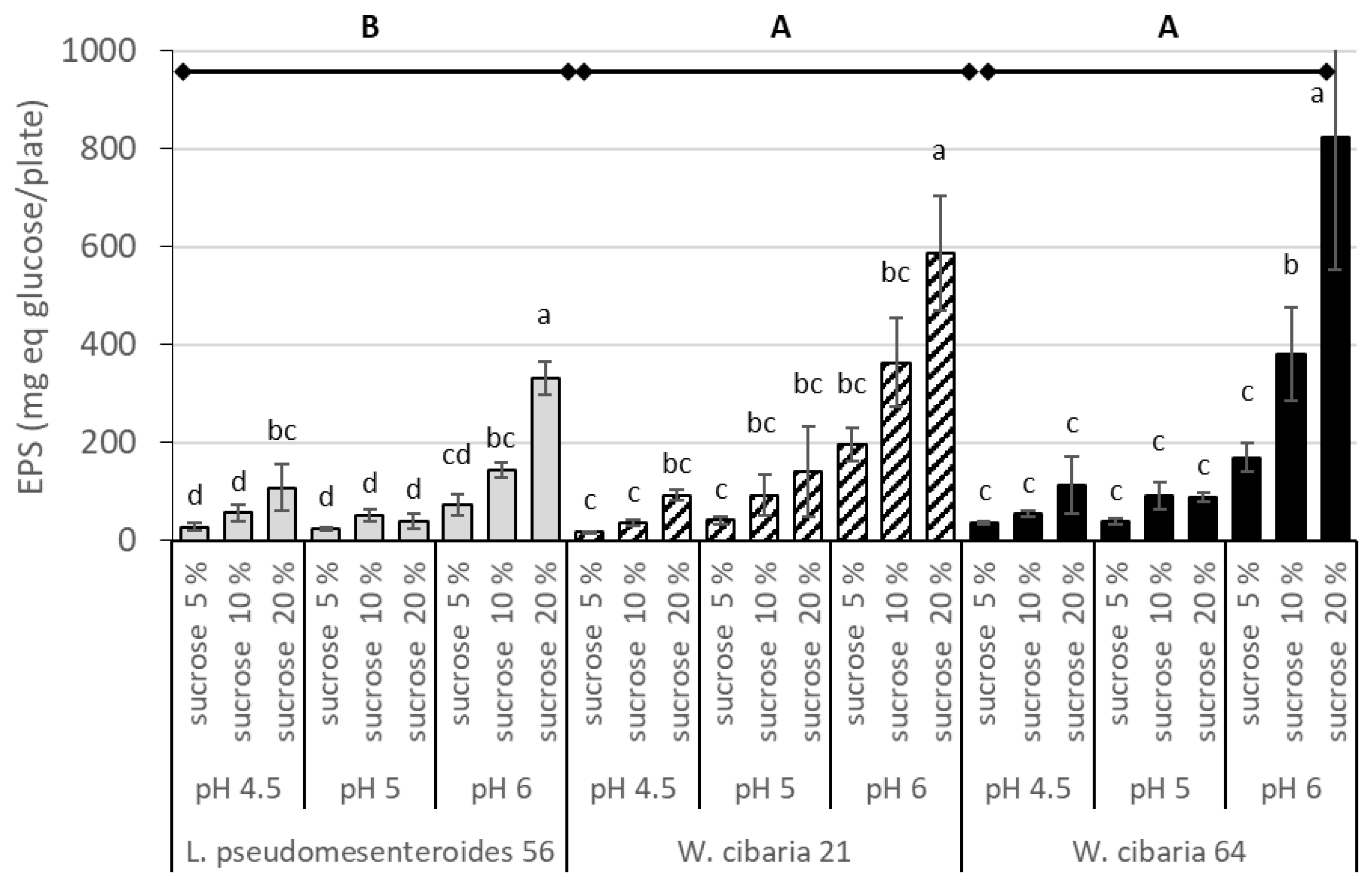
3.1. Determination of EPS Content on MRS Sucrose
3.2. Visual Assessment of EPS Content on Fruit-Based Media
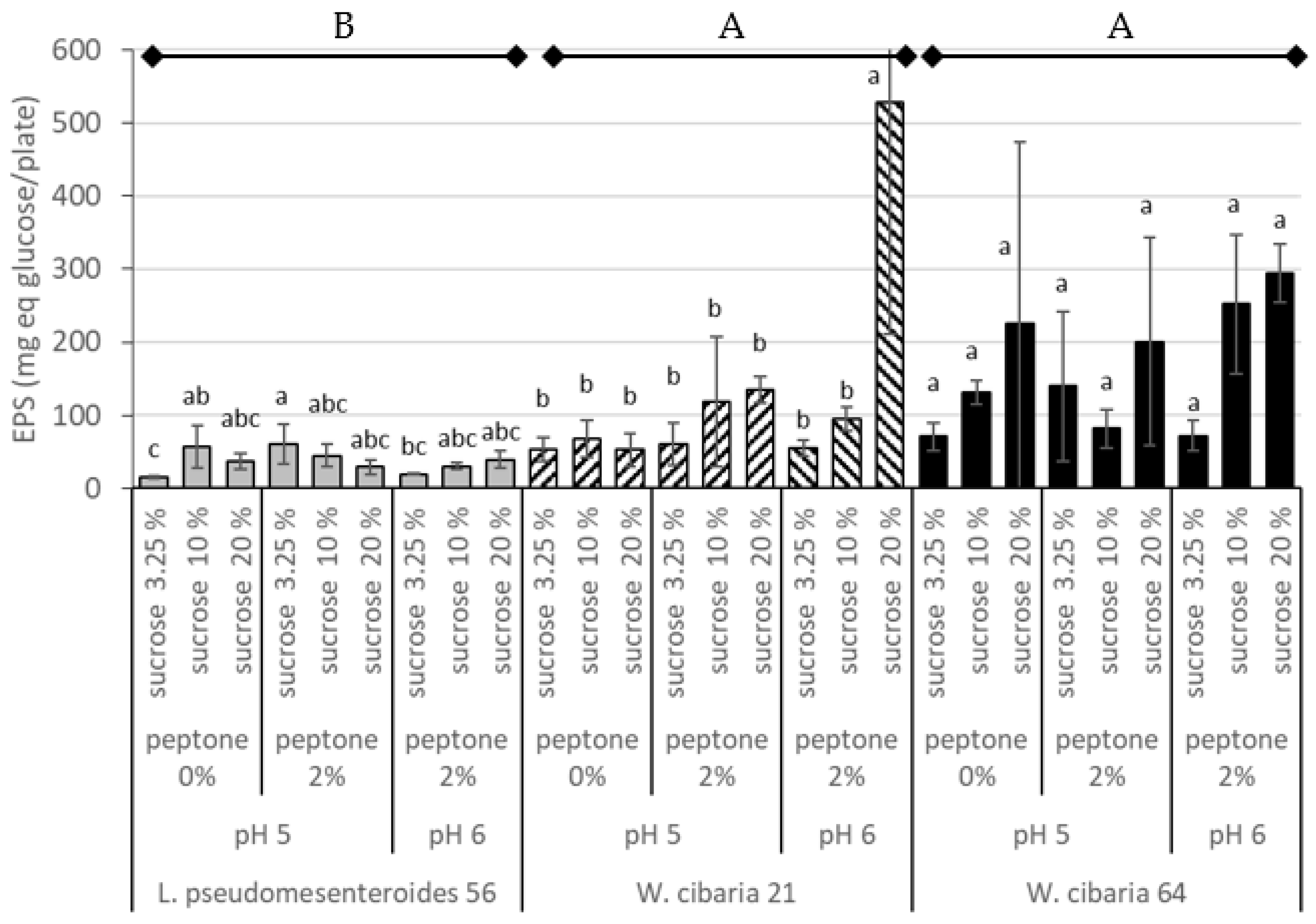
3.3. Determination of EPS Content on Fruit Purée Agar
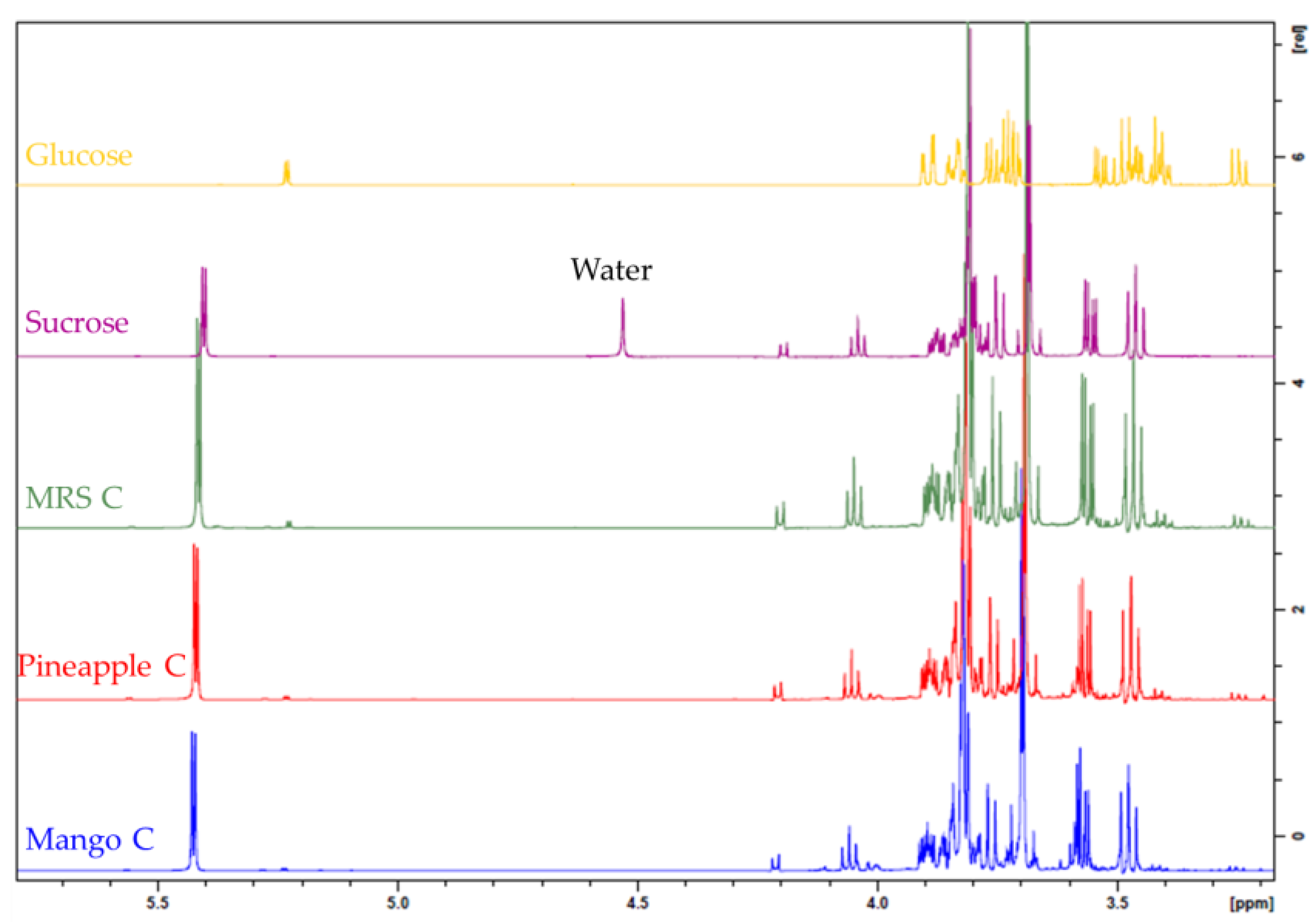
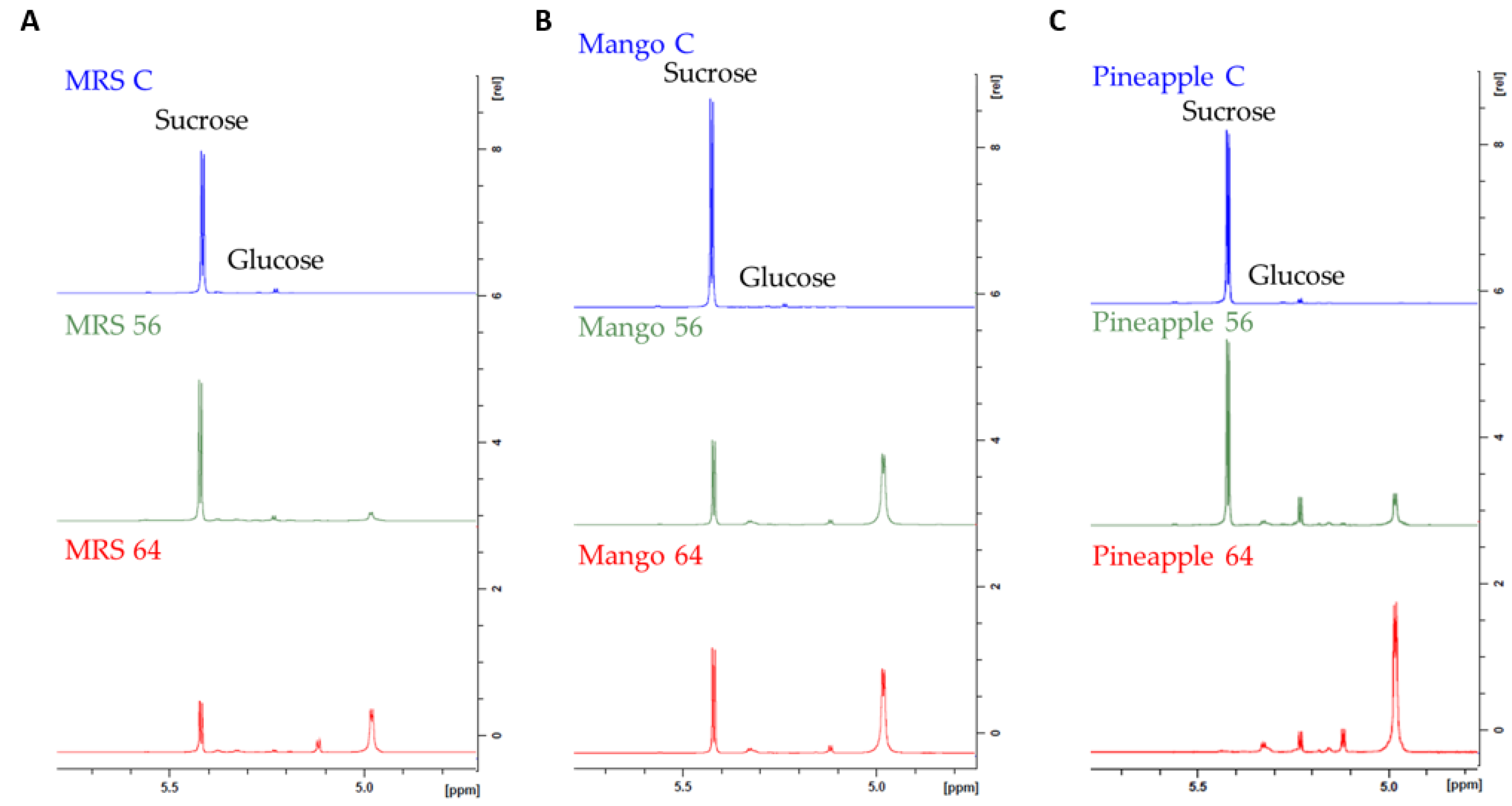
3.4. Structure Determination of EPS by NMR Spectroscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, P.; Saini, P. Food and health potentials of exopolysaccharides derived from Lactobacilli. Microbiol. Res. J. Int. 2017, 22, 1–14. [Google Scholar] [CrossRef]
- Guérin, M.; Silva, C.R.-D.; Garcia, C.; Remize, F. Lactic acid bacterial production of exopolysaccharides from fruit and vegetables and associated benefits. Fermentation 2020, 6, 115. [Google Scholar] [CrossRef]
- Cui, J.; Lian, Y.; Zhao, C.; Du, H.; Han, Y.; Gao, W.; Xiao, H.; Zheng, J. Dietary fibers from fruits and vegetables and their health benefits via modulation of gut microbiota. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1514–1532. [Google Scholar] [CrossRef]
- Nabot, M.; Guérin, M.; Sivakumar, D.; Remize, F.; Garcia, C. Variability of bacterial homopolysaccharide production and properties during food processing. Biology 2022, 11, 171. [Google Scholar] [CrossRef]
- Sørensen, H.M.; Rochfort, K.D.; Maye, S.; MacLeod, G.; Brabazon, D.; Loscher, C.; Freeland, B. Exopolysaccharides of lactic acid bacteria: Production, purification and health benefits towards functional food. Nutrients 2022, 14, 2938. [Google Scholar] [CrossRef] [PubMed]
- Fuller, S.; Beck, E.; Salman, H.; Tapsell, L. New horizons for the study of dietary fiber and health: A review. Plant Foods Hum. Nutr. 2016, 71, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.; Hamilton, J.K.; Rebers, P.A.; Smith, F. A colorimetric method for the determination of sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef]
- Leroy, F.; De Vuyst, L. Advances in production and simplified methods for recovery and quantification of exopolysaccharides for applications in food and health. J. Dairy Sci. 2016, 99, 3229–3238. [Google Scholar] [CrossRef]
- Juvonen, R.; Honkapää, K.; Maina, N.H.; Shi, Q.; Viljanen, K.; Maaheimo, H.; Virkki, L.; Tenkanen, M.; Lantto, R. The impact of fermentation with exopolysaccharide producing lactic acid bacteria on rheological, chemical and sensory properties of pureed carrots (Daucus carota L.). Int. J. Food Microbiol. 2015, 207, 109–118. [Google Scholar] [CrossRef]
- Malang, S.K.; Maina, N.H.; Schwab, C.; Tenkanen, M.; Lacroix, C. Characterization of exopolysaccharide and ropy capsular polysaccharide formation by Weissella. Food Microbiol. 2015, 46, 418–427. [Google Scholar] [CrossRef]
- Velamakanni, R.P.; Vuppugalla, P.; Merugu, R. Techniques Used for characterization of microbial exopolysaccharides. In Microbial Exopolysaccharides as Novel and Significant Biomaterials; Nadda, A.K., Sajna, K.V., Sharma, S., Eds.; Springer Series on Polymer and Composite Materials; Springer International Publishing: Cham, Switzerland, 2021; pp. 19–43. ISBN 978-3-030-75289-7. [Google Scholar]
- Tinzl-Malang, S.K.; Rast, P.; Grattepanche, F.; Sych, J.; Lacroix, C. Exopolysaccharides from co-cultures of Weissella confusa 11GU-1 and Propionibacterium freudenreichii JS15 act synergistically on wheat dough and bread texture. Int. J. Food Microbiol. 2015, 214, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Amezquita, L.E.; Tejada-Ortigoza, V.; Heredia-Olea, E.; Serna-Saldívar, S.O.; Welti-Chanes, J. Differences in the dietary fiber content of fruits and their by-products quantified by conventional and integrated AOAC official methodologies. J. Food Compos. Anal. 2018, 67, 77–85. [Google Scholar] [CrossRef]
- Mashitoa, F.M.; Akinola, S.A.; Manhevi, V.E.; Garcia, C.; Remize, F.; Slabbert, R.M.; Sivakumar, D. Influence of fermentation of pasteurised papaya puree with different lactic acid bacterial strains on quality and bioaccessibility of phenolic compounds during in vitro digestion. Foods 2021, 10, 962. [Google Scholar] [CrossRef] [PubMed]
- Guérin, M.; Remize, F.; Bringart, M.; Planesse, C.; Rondeau, P.; Da Silva, C.R.; Garcia, C. Fermented pineapple juice consumption limits metabolic disorders associated to sugary drinks on high-fat diet-fed mice. Mol. Nutr. Food Res. 2023, 67, 2200670. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Li, G.; Wang, C.; Ling, B.; Yang, R.; Huang, S. Extraction and characterization of dextran from Leuconostoc pseudomesenteroides YB-2 isolated from mango juice. Carbohydr. Polym. 2019, 207, 218–223. [Google Scholar] [CrossRef]
- Cele, N.P.; Akinola, S.A.; Shoko, T.; Manhevi, V.E.; Remize, F.; Sivakumar, D. The bioaccessibility and antioxidant activities of fermented mango cultivar juices after simulated in vitro digestion. Foods 2022, 11, 2702. [Google Scholar] [CrossRef]
- Fessard, A.; Remize, F. Genetic and technological characterization of lactic acid bacteria isolated from tropically grown fruits and vegetables. Int. J. Food Microbiol. 2019, 301, 61–72. [Google Scholar] [CrossRef]
- Ciqual Table de Composition Nutritionnelle des Aliments. Available online: https://ciqual.anses.fr/ (accessed on 24 October 2022).
- FoodData Central. US Department of Agriculture, Agricultural Research Service. Available online: https://fdc.nal.usda.gov/ (accessed on 24 October 2022).
- Li, Y.; Xiao, L.; Tian, J.; Wang, X.; Zhang, X.; Fang, Y.; Li, W. Structural characterization, rheological properties and protection of oxidative damage of an exopolysaccharide from Leuconostoc citreum 1.2461 fermented in soybean whey. Foods 2022, 11, 2283. [Google Scholar] [CrossRef]
- Yilmaz, M.T.; İspirli, H.; Taylan, O.; Taşdemir, V.; Sagdic, O.; Dertli, E. Characterisation and functional roles of a highly branched dextran produced by a bee pollen isolate Leuconostoc mesenteroides BI-20. Food Biosci. 2022, 45, 101330. [Google Scholar] [CrossRef]
- Purama, R.K.; Goswami, P.; Khan, A.T.; Goyal, A. Structural analysis and properties of dextran produced by Leuconostoc mesenteroides NRRL B-640. Carbohydr. Polym. 2009, 76, 30–35. [Google Scholar] [CrossRef]
- Du, R.; Qiao, X.; Zhao, F.; Song, Q.; Zhou, Q.; Wang, Y.; Pan, L.; Han, Y.; Zhou, Z. Purification, characterization and antioxidant activity of dextran produced by Leuconostoc pseudomesenteroides from homemade wine. Carbohydr. Polym. 2018, 198, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Song, Q.; Zhao, F.; Xiao, H.; Zhou, Z.; Han, Y. Purification and characterization of dextran produced by Leuconostoc pseudomesenteroides PC as a potential exopolysaccharide suitable for food applications. Process Biochem. 2019, 87, 187–195. [Google Scholar] [CrossRef]
- Kavitake, D.; Devi, P.B.; Shetty, P.H. Overview of exopolysaccharides produced by Weissella genus—A Review. Int. J. Biol. Macromol. 2020, 164, 2964–2973. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-J.; Chen, Z.; Chen, P.T.; Ng, I.-S. Production, characterization and antibacterial activity of exopolysaccharide from a newly isolated Weissella cibaria under sucrose effect. J. Biosci. Bioeng. 2018, 126, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Bejar, W.; Gabriel, V.; Amari, M.; Morel, S.; Mezghani, M.; Maguin, E.; Fontagné-Faucher, C.; Bejar, S.; Chouayekh, H. Characterization of glucansucrase and dextran from Weissella sp. TN610 with potential as safe food additives. Int. J. Biol. Macromol. 2013, 52, 125–132. [Google Scholar] [CrossRef]
- Tirtaatmadja, V.; Dunstan, D.E.; Boger, D.V. Rheology of dextran solutions. J. Non-Newton. Fluid Mech. 2001, 97, 295–301. [Google Scholar] [CrossRef]
- Li, C.; Li, W.; Chen, X.; Feng, M.; Rui, X.; Jiang, M.; Dong, M. Microbiological, physicochemical and rheological properties of fermented soymilk produced with exopolysaccharide (EPS) producing lactic acid bacteria strains. LWT—Food Sci. Technol. 2014, 57, 477–485. [Google Scholar] [CrossRef]
- Salazar, N.; Gueimonde, M.; de los Reyes-Gavilán, C.G.; Ruas-Madiedo, P. Exopolysaccharides produced by lactic acid bacteria and bifidobacteria as fermentable substrates by the intestinal microbiota. Crit. Rev. Food Sci. Nutr. 2016, 56, 1440–1453. [Google Scholar] [CrossRef]
- Di Cagno, R.; Surico, R.F.; Siragusa, S.; De Angelis, M.; Paradiso, A.; Minervini, F.; De Gara, L.; Gobbetti, M. Selection and use of autochthonous mixed starter for lactic acid fermentation of carrots, french beans or marrows. Int. J. Food Microbiol. 2008, 127, 220–228. [Google Scholar] [CrossRef]
- Garcia, C.; Guerin, M.; Souidi, K.; Remize, F. Lactic fermented fruit or vegetable juices: Past, present and future. Beverages 2020, 6, 8. [Google Scholar] [CrossRef]
- Benhouna, I.S.; Heumann, A.; Rieu, A.; Guzzo, J.; Kihal, M.; Bettache, G.; Champion, D.; Coelho, C.; Weidmann, S. Exopolysaccharide produced by Weissella confusa: Chemical characterisation, rheology and bioactivity. Int. Dairy J. 2019, 90, 88–94. [Google Scholar] [CrossRef]
- Farinazzo, F.S.; Fernandes, M.T.C.; Mauro, C.S.I.; Garcia, S. Statistical optimization of exopolysaccharide production by Leuconostoc pseudomesenteroides JF17 from native Atlantic forest juçara fruit. Prep. Biochem. Biotechnol. 2022, 52, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Wang, Q.; Qu, L.; Liang, L.; Han, Y.; Wang, X.; Zhou, Z. Pilot-scale production of exopolysaccharide from Leuconostoc pseudomesenteroides XG5 and its application in set yogurt. J. Dairy Sci. 2022, 105, 1072–1083. [Google Scholar] [CrossRef]
- Wongsuphachat, W.; H-Kittikun, A.; Maneerat, S. Optimization of exopolysaccharides production by Weissella confusa NH 02 isolated from Thai fermented sausages. Songklanakarin J. Sci. Technol. 2010, 32, 27–35. [Google Scholar]
- Leneveu-Jenvrin, C.; Apicella, A.; Bradley, K.; Meile, J.; Chillet, M.; Scarfato, P.; Incarnato, L.; Remize, F. Effects of maturity level, steam treatment, or active packaging to maintain the quality of minimally processed mango (Mangifera indica Cv. Jose). J. Food Process. Preserv. 2021, 45, e15600. [Google Scholar] [CrossRef]
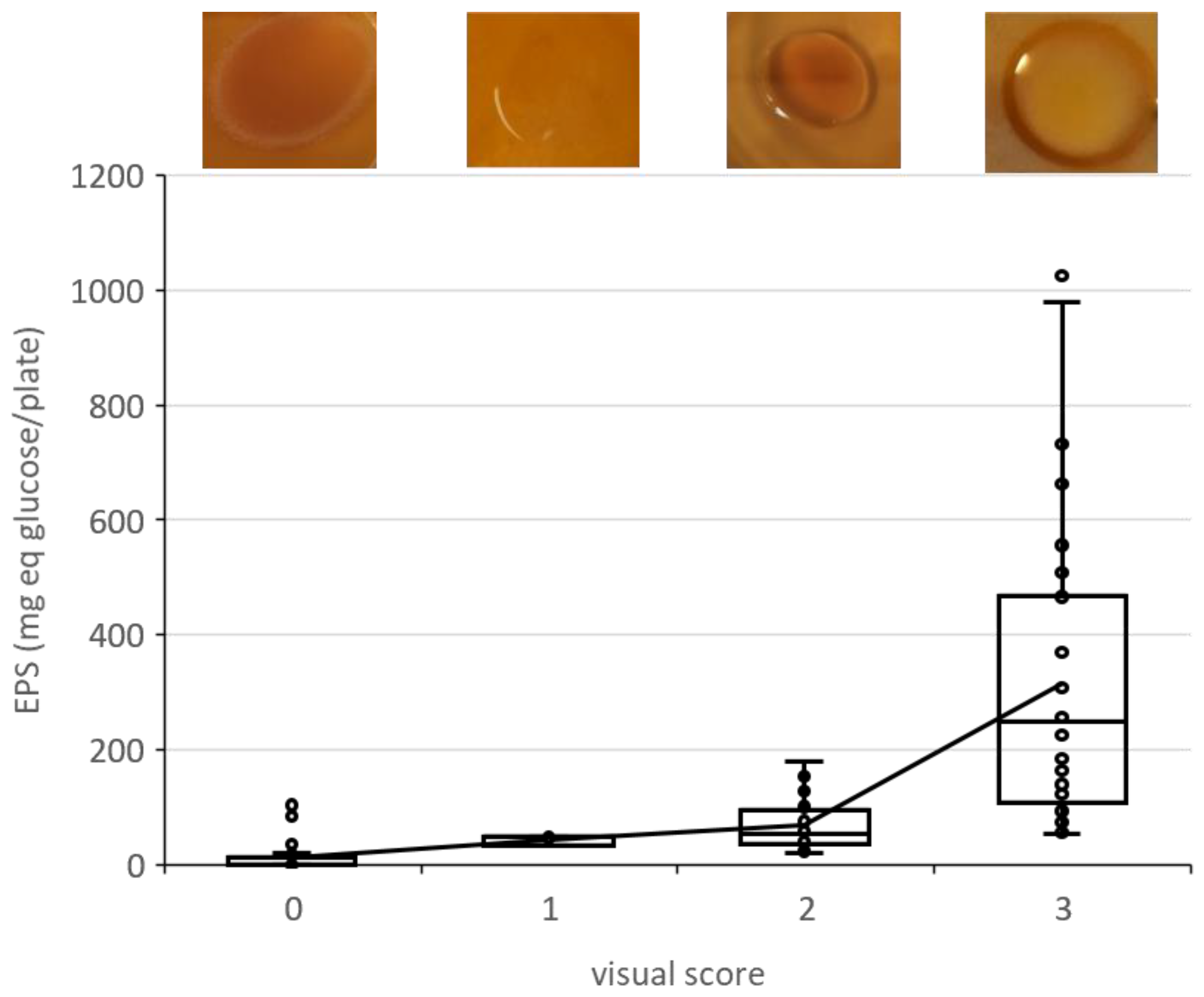

| Fruit | Modification of Condition | L. pseudomesenteroides 56 | W. cibaria 21 | W. cibaria 64 |
|---|---|---|---|---|
| pineapple | none | n.d.(1) | n.d. | n.d. |
| pH adjusted to 6 | 2 | 2 | 2 | |
| sucrose adjusted to 20% | n.d. | n.d. | n.d. | |
| pH 6 and sucrose 20% | 3 | 3 | 3 | |
| mango | none | 2 | 2 | 2 |
| pH adjusted to 6 | 2 | 3 | 3 | |
| sucrose adjusted to 20% | 2 | 2 | 2 | |
| pH 6 and sucrose 20% | 3 | 3 | 3 | |
| papaya | none | 0 | 0 | 0 |
| pH adjusted to 6 | 0 | 0 | 0 | |
| sucrose adjusted to 20% | 0 | 0 | 0 | |
| pH 6 and sucrose 20% | 1 | 1 | 1 |
| Main Saccharide Unit | |||
| Protons | δ (ppm) | Carbons | δ (ppm) |
| H1 | 4.98 | C1 | 100.5 |
| H2 | 3.58 | C2 | 74.2 |
| H3 | 3.73 | C3 | 76.1 |
| H4 | 3.52 | C4 | 72.4 |
| H5 | 3.91 | C5 | 72.9 |
| H6; H6′ | 3.77; 3.98 | C6 | 68.4 |
| Minor saccharide unit | |||
| H1 | 5.12 | C1 | 103.0 |
| H2 | 3.55 | C2 | |
| MRS | Mango | Pineapple | |
|---|---|---|---|
| 56 | 3.23/96.77 | 3.74/96.26 | 4.33/95.67 |
| 64 | 14.68/85.32 | 4.64/95.36 | 8.59/91.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guérin, M.; Garcia, C.; Silva, C.R.-D.; Couprie, J.; Remize, F. Characterization of Bacterial Exopolysaccharides Produced from Different Fruit-Based Solid Media. Fermentation 2023, 9, 657. https://doi.org/10.3390/fermentation9070657
Guérin M, Garcia C, Silva CR-D, Couprie J, Remize F. Characterization of Bacterial Exopolysaccharides Produced from Different Fruit-Based Solid Media. Fermentation. 2023; 9(7):657. https://doi.org/10.3390/fermentation9070657
Chicago/Turabian StyleGuérin, Marie, Cyrielle Garcia, Christine Robert-Da Silva, Joël Couprie, and Fabienne Remize. 2023. "Characterization of Bacterial Exopolysaccharides Produced from Different Fruit-Based Solid Media" Fermentation 9, no. 7: 657. https://doi.org/10.3390/fermentation9070657
APA StyleGuérin, M., Garcia, C., Silva, C. R.-D., Couprie, J., & Remize, F. (2023). Characterization of Bacterial Exopolysaccharides Produced from Different Fruit-Based Solid Media. Fermentation, 9(7), 657. https://doi.org/10.3390/fermentation9070657








