Abstract
Itaconic acid (ITA) is one of the top 12 platform chemicals. The global ITA market is expanding due to the rising demand for bio-based unsaturated polyester resin and its non-toxic qualities. Although bioconversion using microbes is the main approach in the current industrial production of ITA, ecological production of bio-based ITA faces several issues due to: low production efficiency, the difficulty to employ inexpensive raw materials, and high manufacturing costs. As metabolic engineering advances, the engineering of microorganisms offers a novel strategy for the promotion of ITA bio-production. In this review, the most recent developments in the production of ITA through fermentation and metabolic engineering are compiled from a variety of perspectives, including the identification of the ITA synthesis pathway, the metabolic engineering of natural ITA producers, the design and construction of the ITA synthesis pathway in model chassis, and the creation, as well as application, of new metabolic engineering strategies in ITA production. The challenges encountered in the bio-production of ITA in microbial cell factories are discussed, and some suggestions for future study are also proposed, which it is hoped offers insightful views to promote the cost-efficient and sustainable industrial production of ITA.
1. Introduction
Itaconic acid (ITA) is an important dibasic organic carboxylic acid. It contains two carboxyl groups and an unsaturated carbon-carbon double bond, which makes it an important raw material for the chemical synthesis industry [1]. ITA was selected as one of the 12 most promising platform compounds in the report “High Value Added Chemicals from Biological Sources” by the US Energy Agency in 2004 [2]. It is thus widely used in the fields of coatings, adhesives, plastics, resins, textiles, and polymers [1,3]. The two carboxyl groups in the ITA monomer can form polyesters through esterification reactions, while its double bond structure facilitates cross-linking and curing in subsequent processing to produce various light and thermosets cured polyesters [4,5,6]. Moreover, ITA is also an intermediate for the production of 3-methyltetrahydrofuran, which is a potential biofuel [7,8,9]. Recently, ITA was reported to be a mammalian metabolite during macrophage activation, and 4-octyl itaconate has shown potent antiviral and anti-inflammatory effects against various human pathogenic viral infections, including SARS-CoV2 [10,11]. Thus, ITA has great potential applications in the fabrication of biomaterials and biopharmaceuticals.
Both chemical and biological methods have been explored to produce ITA. Initially, ITA was discovered through the thermal decomposition of citric acid in 1836 [12]. Although several chemical methods have been developed for the synthesis of ITA, the complex separation and purification process limits industrial applications. In addition, chemical synthesis is mostly based on petrochemicals, which leads to high production costs and serious environmental pollution [13,14,15]. Therefore, the current industrial production of ITA mainly depends on biorefineries, in which glucose, starch and other renewable biomass are utilized to produce ITA via the conversion of microbes [16]. With the increasing depletion of fossil fuels, the demand for environmentally friendly bio-based ITA is expanding. The market size of ITA in 2021 has already reached 105 million USD and is expected to reach 135.6 million USD by 2028, which poses a huge challenge for the production of ITA [17,18]. However, the bio-production of ITA still faces problems of low production efficiency, inability to use cheap raw materials, and high production costs, which limit its development [19]. With the development of synthetic biology and metabolic engineering, the biosynthetic pathway of ITA has been clearly identified, and the genetic modification of microbes is regarded as an available and potential way to solve these problems [19,20].
In this review, the recent research progress on the biological production of ITA is summarized from several aspects including the identification of the ITA synthesis pathway, the metabolic engineering of natural ITA producers, the design and construction of the ITA synthesis pathway in model chassis, and the development, as well as application, of new metabolic engineering strategies in the production of ITA. We also discuss the challenges faced in the bio-production of ITA in microbial cell factories, and put forward some views for future research, which it is hoped provide valuable insights to facilitate the industrial bio-production of ITA.
2. The Recent Research on the Bio-Production of ITA
2.1. The Identification of the ITA Synthesis Pathway in Microbes
In 1936, Kinoshita et al. [21] first discovered the biosynthesis of ITA in Aspergillus itaconicus and postulated that the decarboxylation reaction of cis-aconitate was the key process in the biosynthesis of ITA. Subsequently, Bentley and Thiessen et al. [22] identified cis-aconitate dehydrogenase (CadA) in Aspergillus terreus, which converted cis-aconitate to ITA, and confirmed that citric acid was the precursor for ITA synthesis. It was thus proposed that the biosynthesis pathway of ITA originated from the shunt of the tricarboxylic acid cycle (TCA cycle) [23]. Namely, glucose was metabolized to pyruvate via the glycolytic pathway and then transported to the mitochondria where it was converted to acetyl coenzyme A, and entered the TCA cycle to produce cis-aconitate and then generate ITA (Figure 1). It was shown that cis-aconitate was an unstable intermediate, which needed to be transported to the cytosol by the mitochondrial carboxylic acid protein (MttA), and then converted to ITA by the catalysis of CadA [24]. ITA was finally released out of the cell via the function of the major facilitator superfamily protein MfsA [25]. Therefore, these two transporters were essential for ITA production. Moreover, Shin et al. found that the transcription factor Reg also played an important role in the synthesis of ITA, which impacted the activity of CadA, Aco, MttA and MfsA in A. terreus [26].
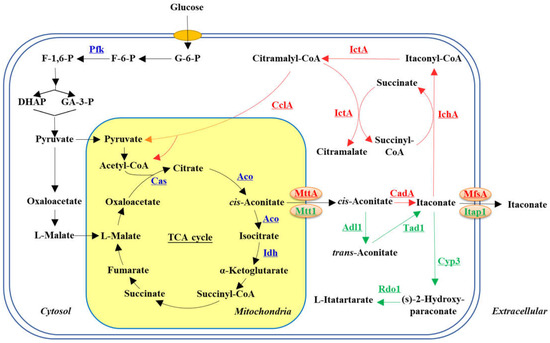
Figure 1.
The synthesis and degradation pathways of ITA in microbes. Pfk, 6-phosphofructo-1-kinase; Cas, citrate synthase; Aco, aconitase; Idh, isocitrate dehydrogenase; CadA, cis-aconitase decarboxylase; MttA/Mtt1, mitochondrial tricarboxylate transport protein; Mfsa, major facilitator superfamily protein; IchA, itaconyl-CoA hydratase; IctA, itaconyl-CoA transferase; CclA, citramalyl-CoA lyase; Adi1, aconitate-Δ-isomerase; Tad1, trans-aconitate decarboxylase; Itap1, itaconate transporter protein; Cyp3, P450 monooxygenase; Rdo1, ring-cleaving dioxygenase; G-6-P, glucose-6-phoshate; F-6-P, fructose-6-phosphate; F-1,6-P, fructose-1,6-bisphosphate; DHAP, dihydroxyacetone phosphate; GA-3-P, glyceraldehyde-3-phosphate. The synthesis pathway identified in A. terreus was present in the red line, while that identified in U. maydis was present in the green line.
Besides the synthesis pathway, the degradation pathway of ITA has also been identified in A. terreus. ITA is first converted to itaconic acyl-CoA by the catalysis of itaconic acyl-Coenzyme A transferase (LctA), and then hydrated to form citric acyl-CoA by itaconic acyl-Coenzyme A hydratase (LchA). Citric acyl-CoA is finally cleaved to pyruvate and acetyl-CoA by citric coenzyme A lyase (CclA), or can be converted to citraconic acid and succinyl-CoA by LctA, which is finally metabolized via the TCA cycle [27].
In addition to A. terreus, Ustilago maydis has also been reported as an excellent ITA producer. However, the synthesis and degradation pathways are completely different from those in A. terreus. Although cis-aconitate is also the precursor in ITA synthesis, it is first converted to trans-aconitate by aconitic acid isomerase (Adi1), and then to ITA by the function of trans-aconitic acid dehydrogenase in U. maydis (Tda1) [28]. Moreover, the protein Itp1 was mainly responsible for the efflux of the ITA, while Ria1 was identified as the transcriptional factor regulating the activity of the ITA synthesis pathway in U. maydis [29]. In the degradation of ITA, P450 monooxygenase (Cyp3) catalyzes the ITA to generate (s)-2-Hydroxy-paraconate, which is then converted to L-itatartarate by the catalysis of cyclic dioxygenase (rod1) (Figure 1) [28,30]. The two completely different ITA metabolic pathways show the diversity of metabolic libraries in wild microbes, and more work needs to be conducted to explore the metabolic mechanisms of ITA in natural producers, which could help to better design microcell factories and optimize fermentation processes.
2.2. Enhancement of ITA Production via the Genetic Modification of Natural Producers
2.2.1. Aspergillus Species
Currently, A. terreus is the main strain used for the industrial production of ITA. Glucose and sucrose are commonly considered to be the best carbon sources for the fermentation of A. terreus to produce ITA [31]. But pure monosaccharides or disaccharides are expensive, which leads to a high production cost of ITA. Therefore, cheaper and renewable carbon sources were explored to replace glucose in the production of ITA, including glycerol, food waste, lignocellulose, etc. [19,20]. Glycerol is a primary by-product in the biodiesel industry, and the utilization of glycerol as a feedstock to produce higher added-value products by microbes is a promising way to improve the re-cycling of the glycerol [32]. Juy et al. explored using glycerol as the carbon source to produce ITA by A. terreus, and the titer reached 27.6 g/L, which suggested that glycerol could be used as a potential substrate in ITA production [33]. Food waste, rich in carbohydrates and protein, is also considered a promising potential feedstock for renewable bio-based chemicals [34,35]. Narisetty et al. screened a thermophilic A. terreus, which could produce 41.1 g/L ITA using the hydrolysates of food waste as a substrate [36]. Lignocellulose has great potential as a non-food carbon source, and it is usually pretreated to form hydrolysates containing glucose and xylose by chemical and biological methods before being used as a feedstock. But the furan derivatives and phenolic compounds in the lignocellulose hydrolysates inhibited the growth of the microbes [37,38,39]. Krull et al. reported pretreating the hydrolysate using a cation exchange resin to obtain the detoxified hydrolysates, which was then used to produce ITA, leading to the production of up to 27.7 g/L [40]. Kerssemakers pretreated eucalyptus fiber pulp with cellulase to obtain hydrolysates, and the ITA production reached 37.5 g/L using the hydrolysates as a feedstock [41]. Sun et al. isolated and identified an ionic liquid tolerant strain A. terreus NRAU-7 with cellulase production ability, which had great potential in the conversion of lignocellulose to ITA [42]. However, the lignocellulose degradation efficiency still requires improvement. Although food waste and lignocellulose can be hydrolyzed into available monosaccharides and disaccharides, the yield of ITA is still low when the hydrolysates are used as the substrate. The main reason, is that more weak acids, furans, phenols and metal ions are formed during the pretreatment process, which are toxic to A. terreus and negatively affect the production of ITA. Therefore, the improvement of the tolerance and productivity of A. terreus is a critical step to solving the problem.
Traditionally, physical and chemical mutagenesis are usually used to screen the mutants with high yields of ITA. For instance, Yahiro et al. reported mutagenizing A. terreus TN-484 with nitrosoguanidine and screened a preferable mutant that produced 82 g/L of ITA from glucose, about 1.3 times higher than that of the original strain [43]. Wu et al. treated A. terreus CICC40205 using UV light, and a 33.4% increase in ITA production was obtained by the mutant using the hydrolysate of wheat bran as a substrate [44]. Similarly, Yang et al. treated A. terreus No. 2433 using UV-LiCl mutagenesis, and 19.35 g/L of ITA production was achieved from the hydrolysate of bamboo by the final mutant [45]. Compared with physical and chemical mutagenesis, the protoplasmic fusion technique is a more rational approach to obtain stable recombinants, which allows the fusant to combine the genetic traits of both parents [46]. Kirimura et al. obtained an ITA producing fusant F-112 via the fusion of the starch glycosylase producing strain Aspergillus usamii IAM 2185 with A. terreus IFO6123. The fusant F-112 was able to produce ITA by direct fermentation of soluble starch with a yield of 35.9 g/L, which was about a five-fold increase compared to that of A. terreus IFO6123 [47]. It has been suggested that protoplasmic fusion is an available route to improve the performance of natural strains. Although the traditional physical and chemical mutagenesis have the advantages of low cost and simple operation, the high randomness and blindness of the mutagenesis makes the process more complex and unpredictable. The lack of high throughput screening methods also limits the screening efficiency and application.
As the synthesis pathway of ITA in A. terreus has been gradually identified, the genetic modification of the pathway has been a hot topic of current research to improve ITA synthesis through metabolic engineering. Glucose is converted to pyruvate via the glycolytic pathway, to enter the tricarboxylic acid cycle to generate citric acid as the precursor of ITA. It has been reported that the activity of 6-phosphofructokinase (PfkA) is negatively affected by the feedback inhibition of citric acid, which is considered to be the rate-limiting step in the synthesis of ITA by A. terreus [48]. To avoid the inhibition, and improve ITA production, Tevz et al. altered the phosphorylation site and deleted the inhibitor-binding region of PfkA. After the overexpression of the PfkA mutant in the wide A. terreus, the production of ITA was increased from 13.5 g/L to 31 g/L (Table 1) [49]. Huang et al. explored the effects on ITA accumulation of overexpressing CadA, MfsA, MttA, GpdA, AcoA, t-PfkA, CitA, ATEG_09969 (putative regulator protein which contains a zinc finger motif characteristic of the eukaryotic transcription factors), and ATEG_01954genes in A. terreus NIH 2624. It was indicated that the overexpression of CadA and MfsA could significantly promote the production of ITA [50]. Similarly, Shin et al. overexpressed the CadA, MfsA, MttA and Reg in A. terreus AN37, respectively. They found that the overexpression of MfsA led to the titer of ITA up to 75 g/L, a 18.3% increase compared to the original strain (Table 1) [51]. Since the natural ITA producer was unable to utilize starch directly, it was needed to prepare the hydrolysate of starch before the fermentation process, which resulted in a large amount of wastewater and improved the production costs. Haung et al. introduced the amylase GlaA, from A. niger, into A. terreus, and achieved ITA production from the recombinant using liquefied starch as a substrate [52]. This laid the foundation for the study of simultaneous saccharification and fermentation of starch for ITA production.
In addition to A. terreus, some other Aspergillus species have also been genetically modified to improve ITA production. Aspergillus pseudoterreus ATCC32359 was considered a good ITA producer, which could rapidly consume hexose and pentose at low pH and had the potential to produce ITA from lignocellulose hydrolysates [53]. Pomraning et al. overexpressed the global regulator LaeA in A. pseudoterreus, which increased ITA production by 13% (Table 1) [54]. However, there were limited genetic manipulation tools available for filamentous fungi, and this impacted the efficiency of the modification of the natural ITA producer. Therefore, the development of new gene editing tools was also the focus. Ku proteins were the DNA binding proteins, and could repair DNA double strand breaks through homologous end joining, which competed with homologous recombination and reduced the gene editing efficiency. Guo et al. improved gene targeting efficiency by knocking out the protein kusA in A. terreus, and successfully obtained the uracil-deficient NIH 2624 strain by knocking out pyrG [55]. On this basis, Huang et al. knocked out ku80 in A. terreus CICC40205, and constructed the uracil nutrient-deficient strain by knocking out the pyrG with the defective marker recovered by the application of the Cre/loxp system [56]. Yao et al. established the CRISPR-Cas9 system in A. terreus RA2905, and improved the knockout efficiency to 71% [57]. Sra-Yh Shih et al. further optimized the CRISPR-Cas9 system in A. terreus ATCC20542, and co-delivering two sgRNA/Cas9 expression plasmids resulted in precise gene deletion in the ku70 and pyrG genes [58]. The development of these new genetic manipulation tools promotes the modification of the natural ITA producer A. terreus. However, the application of these technologies in A. terreus is still not enough, and the optimization of the genetic toolbox needs further study.
2.2.2. Ustilaginaceae Species
The Ustilaginaceae species are pathogenic fungi of plant black powdery mildew and have been extensively studied in plant pathology. Most of them were found to have the ability to produce ITA, such as Ustilago vetiveriae, Ustilago vetiveriae, Ustilago rabenhorstiana, Ustilago cynodonti and U. maydis [59,60,61,62].
The production of ITA by U. maydis was first reported as early as 1955 [62]. U. maydis has both mycelial and yeast-like morphology in cultivation, and the latter has the potential to avoid the problems of high viscosity and low oxygen transfer caused by mycelium during the fermentation process [63]. Upon the control of morphology, and optimization of the fermentation conditions, U. maydis reached a level of ITA production comparable to that of A. terreus [64]. According to the study, U. maydis is a pH-sensitive fungus, and neutral conditions were beneficial for the formation of the yeast-like morphology and ITA synthesis [65,66]. Meanwhile, the production of ITA in U. maydis is also regulated by the content of nitrogen sources. The limited nitrogen source promoted ITA production, but led to the formation of a mycelium morphology and the accumulation of lipids, which caused the diversion of carbon flow and affected ITA productivity [67]. Therefore, the control of the carbon/nitrogen ratio (C/N) and pH is essential for the production of ITA by U. maydis. Maassen et al. optimized the C/N ratio and pH in the cultivation of U. maydis MB215, and 44.5 g/L ITA was produced using glucose as substrate [68]. In addition, U. maydis showed preferable tolerance to lignocellulose hydrolysates. It was reported that U. maydis could be co-cultured with Trichoderma reesei, which was capable of degrading lignocellulose by secreting cellulase. And 34 g/L ITA was produced in the co-culture process using cellulose as a feedstock, which indicated the potential of U. maydis in the production of ITA from renewable biomass [69].
In recent years, the identification of ITA metabolic pathway in U. maydis provided a possible strategy to increase ITA production by metabolic engineering (Table 1). Geiser et al. overexpressed the transporter protein MttA and the regulator Ria1 in U. maydis MB215, which led to the ITA production increase about two folds compared to the original strain. Coupled with the knockout of the P450 monooxygenase (encoded by cyp3) and the inhibition of by-products synthesis, the tier of ITA was increased to over 80 g/L in an acidic condition (pH = 3.5) [70]. On this basis, Becker et al. knocked down the ITA oxidation pathway, the lipids synthesis pathway, and the ustilagic acid synthesis pathway in U. maydis MB215 using CRISPER-Cas9 technique to construct a by-product-free platform. After overexpression of Ria1, 53.5 g/L of ITA was produced from 100 g/L of glucose by the engineered strain U. maydis MB215 ITA (Table 1) [71].
Due to the advantages of yeast-like morphology in large-scale fermentation, some studies have been conducted to understand the mechanism of morphology formation. It was shown that Ustilaginaceae could grow as a yeast-like morphology at neutral pH, and form mycelia morphology at acidic conditions. According to the genomics analysis, Faz7 was a dual specificity serine/threonine and tyrosine kinase, which was identified as the key enzyme regulating the tube formation and filamentous growth of Ustilaginaceae. The knockout of the fuz7 gene allowed Ustilaginaceae to better maintain a yeast-like morphology [72]. U. cynodontis had preferable tolerance to low pH but easily formed the mycelia morphology in the production of ITA. Tehrani et al. knocked out the fuz7 in U. cynodontis NBRC9727 to promote the formation of the yeast-like morphology. Coupled with the deletion of Cyp3 and the overexpression of MttA and Ria1, the titer of ITA was improved by about 6.5 fold and the yeast-like morphology could be well maintained at a lower pH [73]. A similar strategy was also conducted to engineer U. maydis MB215 by knocking out fuz7 to control the morphology, overexpressing MttA and Ria1 to enhance the accumulation of ITA, and deleting Cyp3 to reduce the consumption of ITA, which led to the titer of ITA up to 220 g/L by the engineered strain in fed-batch fermentation (Table 1) [74]. These works indicate that the control of morphology, and the regulation of the metabolic pathways, are conducive to improving the efficiency of ITA synthesis by Ustilaginaceae species. Their modifiable morphology and excellent ITA production ability also demonstrates great potential in industrial application.

Table 1.
Improving ITA production via the genetic modification of natural ITA producers.
Table 1.
Improving ITA production via the genetic modification of natural ITA producers.
| Strains | Genetic Modification Employed | Carbon Sources | Titer (g/L) | Yield (g/g) | Productivity g/(L h) | Reference |
|---|---|---|---|---|---|---|
| A. terreus A156 | Overexpression of truncated and mutated pfkA gene from A. niger | Glucose | 31 | - | 0.31 | [49] |
| A. terreus AN37 | Overexpression of native cadA and mfsA mutants | Glucose | 75 | 0.68 | 0.57 | [51] |
| A. terreus CICC 40205 | Overexpression of glaA from A. niger | Starch | 77.6 | - | 1.07 | [52] |
| A. pseudoterreus ATCC32359 | Overexpression of global regulator LaeA | Glucose | 30.4 | - | - | [54] |
| U. maydis MB215 | Deletion of cyp3 and overexpression of regulator gene ria1 under Petef promoter | Glucose | 54.8 | 0.48 | 0.33 | [70] |
| Deletion of cyp3, MEL, UA, dgat; overexpression of ria1 | Glucose | 53.5 | 0.47 | 0.27 | [71] | |
| Overexpression of native rai1 and mttA; deletion of cyp3 and fuz7 | Glucose | 220 | 0.33 | 0.46 | [74] | |
| Overexpression of native rai1 and mttA; deletion of cyp3, fuz7, MEL, UA, dgat | Glucose | 74.9 | 0.54 | 0.53 | [64] | |
| U. cynodontis NBRC9727 | Overexpression of native rai1 and mttA; deletion of cyp3 and fuz7 | Glucose | 22.3 | 0.42 | 0.07 | [73] |
Current research on the natural ITA producers has mostly focused on Aspergillus and Ustilaginaceae species, while some other widely studied strains have also been found to produce ITA, such as Helicobasidium mompa, Helicobasidium mompa, Rhodotorula sp., Candida speciesa and Pseudozyma antarticaa [75,76,77]. However, few studies have been conducted on these natural ITA producers, and the mechanism of ITA synthesis in these strains needed to be further explored. This could help to understand the metabolic mechanisms of microbial ITA synthesis and provide a rich research library for improving the efficiency of ITA synthesis.
2.3. The Design and Construction of the ITA Synthesis Pathway in Model Hosts
Although the natural producers, A. terreus and U. maydis have a high yield of ITA, they mainly use glucose as a carbon source and are not capable of using low-cost starch and lignocellulose, which leads to the current high production costs. Moreover, A. terreus and U. maydis grow relatively slowly, resulting in a low production efficiency. Also, as filamentous fungi, the difficulty in morphology control, laborious handling of spores, high oxygen demand, low reproducibility of fermentation, and complex genetic background still limit the improvement of ITA production. With the development of synthetic biology and metabolic engineering, the synthesis pathways of ITA have been clearly identified, and the design and construction of ITA synthesis pathways in model hosts with a clear genetic background is thus considered a potential way to promote ITA production and compensate for the deficiency.
2.3.1. Genetic Modification of Different Hosts to Produce ITA
Although A. niger and A. terreus belong to the Aspergillus species, A. niger has a clear genetic background and has been widely used as a chassis for metabolic engineering and the industrial production of a wide range of biochemicals, including citric acid, gluconic acid and amylase [78,79]. Citric acid is an important precursor for the synthesis of ITA, while A. niger has a high capacity for citric acid production (200 g/L) [80]. Therefore, A. niger was considered to be the dominant chassis for the synthesis of ITA. Li et al. heterologously expressed CadA from A. terreus in A. niger AB 1.13, and achieved an engineered ITA producing A. niger [81]. Wang et al. proposed overexpressing the two distinct biosynthesis clusters from A. terreus and U. maydis in A. niger ATCC 1015 to improve ITA production. Coupled with increasing the copy number of CadA, down-regulating the expression of IctA, and enhancing the expression of AcoA, the ITA production achieved 9.08 g/L [82]. Based on the strong ability of amylase secretion, Xie et al. introduced the AcoA and CadA from A. terreus into A. niger YX-1217, and 7.2 g/L ITA was produced from corn flour and crude starch, which indicated that A. niger had the potential to produce ITA by simultaneous saccharification and fermentation of low-cost crude starch [83]. However, the low productivity of ITA needed to be further improved.
The yeasts, Yarrowia lipolytica, and Pichia kudriavzevii, as commonly used chassis, have plenty of advantages, such as a clear genetic background, an abundant genetic manipulation toolbox, a fast growth rate and a good tolerance to low pH [84]. A lot of biochemicals have been produced by engineered yeast cell factories [85,86,87,88]. They were thus modified to synthesize ITA (Table 2). Blazeck et al. first introduced the CadA from Aspergillus oryzae into S. cerevisiae and enhanced its expression via a strong promoter, which led to the final yield of 160 mg/L ITA [89]. Similarly, Sun et al. introduced the CadA from A. terreus into P. kudriavzevii and overexpressed the endogenous mitochondrial tricarboxylic acid transporter gene (MTT) to promote the synthesis of ITA. Coupled with the knockout of isocitrate dehydrogenase, the titer of ITA reached 1.3 g/L by the engineered P. kudriavzevii [90]. Y. lipolytica is oleaginous yeast and it was reported that AMP was the essential cofactor for isocitrate dehydrogenase, and adenosine monophosphate deaminase (AMPD) was activated under nitrogen-limited conditions to promote the degradation of AMP, which resulted in a significant accumulation of citric acid in Y. lipolytica [91]. The highest titer of citric acid currently reached is over 80 g/L, by the engineered Y. lipolytica [92]. Blazeck et al. overexpressed the native aconitase with a 36 amino acid N-terminal truncation to remove its putative mitochondrial localization signal (ACOnoMLS) in Y. lipolytica. Combined with the introduction of CadA from A. terreus, 4.6 g/L ITA was obtained by the engineered strain (Table 2) [93]. Based on this work, Zhao et al. further overexpressed MttA and MfsA in Y. lipolytica, and the final titer of ITA achieved was 22.03 g/L [94]. This was the highest production of ITA obtained in yeast cell factories and also showed the promising potential of yeast in the production of ITA.
Besides yeast, some bacteria have also been developed as proven platforms for the synthesis of biochemicals. E. coli is the most commonly used host in genetic engineering with the advantages of the fast growth of cells, low nutritional requirements, and a well-developed genetic modification toolbox, which has been engineered to produce a variety of bio-products including ITA [95,96]. Harder et al. introduced the gene clusters for ITA synthesis into E. coli and 47 g/L ITA was obtained in fed-batch fermentation based on the regulation of knocking down the by-products’ synthesis pathways (Table 2) [97]. It has been demonstrated that E. coli have a good tolerance to ITA and an excellent synthesis capacity. In addition, E. coli as a proven protein expression platform offers the possibility to construct the conversion pathways of multiple carbon sources, enabling the production of ITA from low-cost feedstocks. Okamoto et al. expressed the α-amylase (SBA) in an engineered ITA producing E. coli, and achieved the ITA yield of 0.15 g/L, using 1% starch as the substrate [98]. Jeon et al. enhanced the metabolic pathway of glycerol in E. coli and overexpressed the codon-optimized CadA to achieve ITA production from glycerol. Coupled with the optimization of the fermentation process, 7.23 g/L ITA was finally obtained by the engineered strain [99]. Similar to E. coli, Corynebacterium glutamicum is a model bacterium for the synthesis of amino acid-like products, and has a relatively good acid tolerance which is capable of growing on a variety of carbon sources [100]. It has also been used as a chassis for ITA synthesis. Otten et al. found that the activity of CadA could be enhanced in C. glutamicum via the fusion expression of CadA with maltose binding protein (MalE) from E. coli. Coupled with the inhibition of isocitrate dehydrogenase (ICD), the final titer of ITA reached 7.8 g/L by the engineered C. glutamicum [101]. Joo et al. co-overexpressed CadA and the transcriptional regulator RamA in C. glutamicum, which resulted in 0.881 g/L ITA produced using rice wine waste as the substrate [102]. Acetate is one of the major products in the electrocatalytic or biocatalytic conversion of CO2, while C. glutamicum is also a promising acetate-converter that alternates the carbon source in bioprocesses. Merkel et al. constructed an engineered C. glutamicum by expressing the Cada from A. terreus and the ICD mutant with a low activity. The production of ITA reached 29.2 g/L using acetate as the substrate [103]. Although C. glutamicum has been suggested as a potential host for ITA production, there have been fewer reports on the synthesis of ITA by the modified C. glutamicum, and more investigations could be conducted to further improve its ITA production capacity.
Lignocellulose is the most abundant biomass in the world and can be used as a potential raw material in biorefinery. But the hydrolysates of lignocellulose contain toxic compounds including aromatic chemicals, acetic acid and furfural, which limit its application in the synthesis of ITA by microcell factories. Therefore, the development of new hosts based on natural lignocellulose-degrading microbes has been proposed as an available way to promote the conversion of lignocellulose to ITA. Neurospora crassa was identified as a natural lignocellulose-degrading microbe. Zhao et al. introduced the codon-optimized CadA into N. crassa FGSC 9720 and yielded 20 mg/L ITA using maize straw as the substrate [104]. Candida lignohabitans was reported to grow normally in the hydrolysates of lignocellulose without detoxification. Bellasio et al. overexpressed CadA in C. lignohabitans CBS 10342, and 2.5 g/L was obtained from the un-pretreated hydrolysates by the engineered strain [105]. Similarly, Pseudomonas putida also showed good tolerance to the aromatic compounds in the hydrolysates of lignocellulose, and was also explored as the host to construct the ITA synthesis pathway by overexpressing CadA from A. terreus. After the regulation of the by-products’ synthesis pathway, 1.4 g/L ITA production was achieved using the hydrolysates from the alkali-treated lignin as the substrate [106]. The development of lignocellulose-degrading microbes as chassis is still in the initial stage, and the yield of ITA is low compared to that of other established hosts. However, upon the promise of lignocellulose in the biorefinery, the lignocellulose-degrading microbes could be potential microbes for future bioproduction of ITA if more research is conducted.

Table 2.
Genetic engineering of model host for ITA production.
Table 2.
Genetic engineering of model host for ITA production.
| Hosts | Genetic Modifications | Carbon Source | Titer (g/L) | Yield (g/g) | Productivityg/(L h) | Reference |
|---|---|---|---|---|---|---|
| A. niger AB1.13 | Heterologous expression of CadA | Glucose | 0.7 | 0.01 | 0.0073 | [25] |
| Heterologous expression of CitB, CadA, MttA and mfsA from A. terreus | Glucose | 26.2 | 0.37 | 0.35 | [93] | |
| A. niger ATCC 1015 | Heterologous expression CadA (multiple copies), MttA, MfsA, Adi1, Tad1, Itp1, AcoA and deletion IctA | Glucose | 9.08 | 0.09 | 0.063 | [82] |
| A. niger YX-1217 | Heterologous expression of AcoA and CadA from A. terreus | Cornmeal | 7.2 | - | 0.7 | [83] |
| S. cerevisiae BY4741 | Heterologous expression of CadA from A. terreus | Glucose | 0.168 | [89] | ||
| P. kudriavzevii YB4010 | Heterologous expression of CadA from A. terreus, overexpression of native Pk, MttA and deletion of Icd | Glucose | 1.23 | 0.029 | 0.051 | [90] |
| Y. lipolytica PO1f | Heterologous expression of CadA, MttA, MfsA and AcoA from A. terreus | Glucose | 22 | 0.056 | 0.111 | [92] |
| Y. lipolytica PO1f | Heterologous expression of CadA from A. terreus, and native AcoA (without mitochondrial signal) | Glucose | 4.6 | 0.06 | 0.045 | [107] |
| E. coli MG1655 | Heterologous expression of CadA from A. terreus; deletion of the enzymes in the by-products synthesis pathway | Glucose | 47 | 0.44 | 0.39 | [108] |
| E. coli BW25113 | Heterologous expression of CadA from A. terreus, and native AcnB | Glucose | 4.34 | - | 0.04 | [109] |
| Heterologous expression of CadA from A. terreus, and SBA (α-amylase) from Streptococcus bovis | Soluble starch | 0.15 | - | 0.002 | [98] | |
| E. coli XL1-Blue | Heterologous expression of CadA from A. terreus | Glycerol | 7.2 | - | 0.1 | [99] |
| E. coli MG1655 | Heterologous expression of cadA; down-regulation of Icd, pykA, and sucCD by CiMS system | Glucose | 3.93 | 0.98 | 0.082 | [110] |
| C. glutamicum | Heterologous fusion expression of CadA from A. terreus with MalE from E. coli, and the isocitrate dehydrogenase mutant | Glucose | 7.8 | 0.29 | 0.27 | [101] |
| C. glutamicum | Heterologous expression of CadA from A. terreus and a low activity mutated Icd | Acetate | 29.2 | 0.16 | 1.01 | [103] |
| N. crassa FGSC 9720 | Heterologous expression of CadA | Corn straw | 0.0204 | - | - | [104] |
| C. lignohabitans CBS 10342 | Heterologous expression of CadA from A. terreus | Lignocellulose hydrolysate | 2.5 | - | 0.04 | [105] |
| P. putida | Heterologous expression of CadA from A. terreus controlled by the biosenser PurtA:T7pol:lysY+; deletion of PHA synthetases | Alkali pretreated lignin | 1.4 | - | - | [106] |
2.3.2. Strategies for the Metabolic Regulation of Itaconic Acid Synthesis
Due to the lack of enzymes in the ITA synthesis pathway in the model strains, the enhancement of the expression and activity of the enzyme CadA was considered a fundamental strategy to improve the ITA synthesis capacity of the engineered strains. The expression of heterologous proteins usually led to the misfolding and the formation of inclusion bodies, while the codon optimization of the sequences was a commonly used method to improve the expression efficiency of heterologous proteins [111]. Vuoristo et al. heterologously overexpressed CadA in E. coli, but found that the protein existed mainly in the form of inclusion bodies and resulted in a low production of ITA. After expressing the codon-optimized CadA at a low temperature, the number of inclusion bodies was decreased and the production of ITA increased 24 fold [112]. Similarly, Otten also found the fusion expression of codon-optimized CadA, with maltose binding protein (MalE) from E. coli, could reduce the formation of inclusion bodies, which resulted in a 2-fold increase in CadA activity in C. glutamicumo [101].
The metabolic pool of precursors directly affected the efficiency of product synthesis, and the enhancement of the supply of precursors was also an available way to promote the accumulation of target products. Cis-aconitate was the precursor in the synthesis of ITA. However, in eukaryotes, cis-aconitate as the relatively unstable intermediate in the TCA cycle was generated in mitochondria, while the key enzymes for ITA synthesis (CadA, Tad1 and Adi1) were expressed in the cytosol. Therefore, the overexpression of the mitochondrial transporter protein MttA/Mtt1 was proven to be the important rate-limiting step for ITA synthesis, by accelerating the transportation of cis-aconitate across the mitochondrial membrane into the cytosol to increase the precursor metabolic pool [90,113]. Cis-aconitate was the product derived from citrate, while some hosts showed weak citrate synthesis ability and thus limited the production of ITA, such as E. coli and P. kudriavzevii. The overexpression of the citrate synthase (GltA), and down-regulation of the isocitrate dehydrogenase (Idh), was usually applied to increase the accumulation of citrate [90,97]. Harder et al. used this strategy to enhance the accumulation of citrate in E. coli and the titer of ITA was improved to 32 g/L, coupled with the regulation of by-product synthesis pathways [103]. Similarly, Hossain et al. also overexpressed GltA in A. niger AB1.13, leading to ITA production increasing to 26.2 g/L [79]. These works indicat that the enhancement of the precursor supply is a feasible approach to improve the production of ITA.
Besides the reinforcement of the ITA synthesis pathway, the inhibition of the by-product synthesis was also a preferable strategy to reduce the diversion of the control carbon flow and facilitate ITA production. For example, the overexpression of the transporter protein MfsA in A. niger led to the formation of the by-product oxalic acid. The deletion of oxaloacetate acetohydrolase (OahA) was reported to reduce the production of oxalic acid, but has a negative effect on cell growth due to the interruption of the TCA cycle [81]. Van et al. proposed specifically mutating the OahA to down-regulate the activity in A. niger. Coupled with the inhibition of the by-products acetate and gluconate synthesis pathways, the production of ITA was significantly improved [114]. In the engineered E. coli, acetic acid and lactic acid were the major by-products during ITA production. Knockout of the phosphate acetyltransferase (Pta) and lactate dehydrogenase (LdhA) reduced the generation of acetic acid and lactic acid, which led to more central carbon flux towards the ITA synthesis pathway [112].
The dynamic regulation of metabolic fluxes is a new strategy proposed in recent years, which is beneficial for the improvement of the carbon flux conversion efficiency. Dynamic metabolic regulation can be achieved through biosensors, to automatically keep the metabolic fluxes in balance during ITA production. Hanko et al. identified the ITA-inducible promoter Pccl, and its corresponding LysR-type transcriptional regulator YpItcR, from the ITA-degrading strain Yersinia pseudotuberculosis, which was composed the YpItcR/Pccl ITA biosensor. Combined with the expression of a fluorescent protein, a fluorescence-based ITA biosensor was formed. It was indicated that the fluorescence output was positively correlated with the concentration of ITA, which laid a foundation for the establishment of a high-throughput method for the screening of high-producing engineered ITA microbes [115]. Zhao et al. constructed a CRISPRi mediated self-inducible system (CiMS) based on the YpItcR/Pccl biosensor in an engineered ITA producing E. coli. Based on the modified system, the engineered E. coli predominantly accumulated biomass in the early stage, while CiMS was induced to activate when the accumulation of ITA increased and Icd, pykA and sucCD were inhibited, which led to the carbon flux being almost toward ITA production [110]. Similarly, Elmoreet al. developed a nitrogen starvation biosensor, PurtA:T7pol:lysY+, in P. putida [106]. PurtA is a nitrogen starvation induced promoter and improved the expression of T7 RNA Polymerase (T7pol) under nitrogen-limited conditions to enhance the strength of the T7 promoter. When the biosensor was applied to control the expression of CadA in P. putida, the production of ITA was dynamically divided into two phases. Namely, the expression of CadA was inhibited, and cells grew rapidly with a sufficient nitrogen source in the initial stage, while cell growth was limited with the consumption of the nitrogen source and CadA was highly expressed by the activation of the PurtA promoter. Compared to the group using the constitutive promoter to control the expression of CadA, the application of the PurtA:T7pol:lysY+ biosensor showed a 40% increase in ITA production [100]. These works indicate that the regulation of the central carbon flux could promote the accumulation of ITA by the engineered microcell factories, while the dynamic regulation of the carbon flux provides a more reasonable way to automatically balance the diversion of the carbon flow between cell growth and ITA production.
3. Conclusions and Prospects
ITA, as an important platform biochemical, is not only an important raw material for biomass polymers, but also has great potential for applications in biomedicine and other fields. According to the report, the market size of ITA in 2021 has already reached 105 million USD and is expected to reach 135.6 million USD by 2028. The increasing demand for bio-based unsaturated polyester resin, and its non-toxic properties, are fueling the growth of the itaconic acid market across the globe. Although the current industrial production of ITA mainly depends on biorefineries, the low production efficiency, inability to use cheap raw materials, and high production costs pose a huge challenge for the production of environmentally friendly bio-based ITA. With the development of metabolic engineering, the metabolic pathways of ITA in microbes have been gradually clarified, which provides a new approach for the improvement of ITA biosynthesis. Our work provides an overview of the bio-based production of ITA via fermentation and metabolic engineering.
A. terreus is the predominant strain used for industrial production of ITA, with yields of up to 160 g/L. But it still suffers from slow growth, shear intolerance of the mycelium, high fermentation viscosity, and a requirement of oxygen supply. U. maydis has recently received attention due to its yeast-like morphology and high yield of itconic acid (220 g/L). However, the poor tolerance of U. maydi to low pH has limited its industrial application. Although these natural ITA producers have an excellent ITA production capacity, some issues still need to be further explored, including the lack of ability to utilize low-cost carbon sources, the control of mycelia morphology, the poor tolerance to low pH conditions, and the unclear genetic background to conduct metabolic modifications, which may lead to the further breakthroughs in the economical production of ITA.
The commonly used model hosts have the advantages of proven genetic manipulation, a wide range of carbon source adaption, and easy fermentation control. At present, several hosts have been developed for ITA production, including A. niger, S. cerevisiae, P. kudriavzevii, Y. lipolytica, C. lignohabitans, P. putida, and E. coli. Multiple strategies have been applied to the metabolic regulation to improve ITA synthesis. However, ITA production is still far from commercial production compared to that of the natural ITA producing strains. This is possibly caused by several factors: (1) the low activities of the enzymes heterologously expressed in non-homologous hosts; (2) the lack of a regulatory mechanism for the ITA synthesis pathway in the model strains; (3) the imbalance in metabolic fluxes leading to the accumulation of by-products after introducing the ITA synthesis pathway. With the development of synthetic biology and metabolic engineering, more and more new biotechnologies and strategies have been established including the CRISPR-Cas/dCas system, the CRISPRA and CRISPRI technologies, the biosensor-based dynamic control strategies, etc., which provide new insights into further exploration of metabolic regulation. This will possibly be the focus of future research, including understanding the expression and regulation metabolism of the heterologous enzymes in microbes, the development of more efficient metabolic engineering strategies, and the investigation of more potential hosts.
Besides the improvement of strain performance, reducing production costs is another focus in the industrial production of ITA. Feedstock and downstream product separation and purification processes are determinants of production costs. More renewable and cheaper raw materials, such as lignocellulose, food waste, and CO2-derived acetic acid could be developed as substrates to reduce the cost of raw materials. On the other hand, the calcium salt-based precipitation method is the commonly used method in the separation process, but leads to a large amount of wastewater, which increases the production cost. Therefore, more environmentally-friendly and cost-efficient separation and purification methods should be explored and applied in the production of ITA, such as ion exchange adsorption, nanofiltration, electrodialysis, etc.. It is believed that the solution to these problems would promote the evolution of the cost-efficient, green and sustainable bio-based production of ITA.
Author Contributions
Writing—original draft preparation, R.Z. and H.L.; re-sources and data curation, R.Z., Y.N. and Y.Y.; writing—review and editing, H.L., project administration and funding acquisition, L.D. and F.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (21978019, 21978020, and 22078013), the National Key Research and Development Program of China (2022YFC2106100, 2021YFC2101000).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declared that they have no conflict of interest to this work.
References
- Teleky, B.-E.; Vodnar, D.C. Recent Advances in Biotechnological Itaconic Acid Production, and Application for a Sustainable Approach. Polymers 2021, 13, 3574. [Google Scholar] [CrossRef] [PubMed]
- Werpy, T.; Gene, P. Top Value Added Chemicals from Biomass: Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; National Renewable Energy Lab.: Golden, CO, USA, 2004. [Google Scholar]
- Kumar, S.; Krishnan, S.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Itaconic acid used as a versatile building block for the synthesis of renewable resource-based resins and polyesters for future prospective: A review. Polym. Int. 2017, 66, 1349–1363. [Google Scholar] [CrossRef]
- Patil, D.M.; Phalak, G.A.; Mhaske, S.T. Design and synthesis of bio-based UV curable PU acrylate resin from itaconic acid for coating applications. Des. Monomers. Polym. 2017, 20, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, M.F.; Marques, N.; Correia, J.; Faria, N.T.; Mira, N.P.; Ferreira, F.C. Integrated perspective on microbe-based production of itaconic acid: From metabolic and strain engineering to upstream and downstream strategies. Process Biochem. 2022, 117, 53–67. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, J.; Zhu, G.; Yu, X.; Hu, Y.; Shang, Q.; Chen, J.; Hu, L.; Zhou, Y.; Liu, C. Self-healing, high-performance, and high-biobased-content UV-curable coatings derived from rubber seed oil and itaconic acid. Prog. Org. Coat. 2021, 159, 106391. [Google Scholar] [CrossRef]
- Hegde, K.; Prabhu, A.; Sarma, S.J.; Brar, S.K.; Dasu, V.V. Potential applications of renewable itaconic acid for the synthesis of 3-methyltetrahydrofuran. In Platform Chemical Biorefinery; Elsevier: Amsterdam, The Netherlands, 2016; pp. 181–200. [Google Scholar]
- Park, D.S.; Abdelrahman, O.A.; Vinter, K.P.; Howe, P.M.; Bond, J.Q.; Reineke, T.M.; Zhang, K.; Dauenhauer, P.J. Multifunctional cascade catalysis of itaconic acid hydrodeoxygenation to 3-methyl-tetrahydrofuran. ACS Sustain. Chem. Eng. 2018, 6, 9394–9402. [Google Scholar] [CrossRef]
- De, S.; Saha, B.; Luque, R. Hydrodeoxygenation processes: Advances on catalytic transformations of biomass-derived platform chemicals into hydrocarbon fuels. Bioresour. Technol. 2015, 178, 108–118. [Google Scholar] [CrossRef]
- Mills, E.L.; Ryan, D.G.; Prag, H.A.; Dikovskaya, D.; Menon, D.; Zaslona, Z.; O’Neill, L.A. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature 2018, 556, 113–117. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Y.; Liu, J.; Chen, A.N.; Xu, J.; Zhang, R.; Wang, F.; Deng, L. One-Step Synthesis of 4-Octyl Itaconate through the Structure Control of Lipase. J. Org. Chem. 2021, 86, 7895–7903. [Google Scholar] [CrossRef]
- Baup, S. Ueber eine neue Pyrogen-Citronensäure, und über Benennung der Pyrogen-Säuren überhaupt. Ann. Pharm. 1836, 19, 29–38. [Google Scholar] [CrossRef]
- Zhao, M.; Lu, X.; Zong, H.; Li, J.; Zhuge, B. Itaconic acid production in microorganisms. Biotechnol. Lett. 2018, 40, 455–464. [Google Scholar] [CrossRef]
- Hossain, M.A.; Shah, M.D. A study on the total phenols content and antioxidant activity of essential oil and different solvent extracts of endemic plant Merremia borneensis. Arab. J. Chem. 2015, 8, 66–71. [Google Scholar] [CrossRef]
- Gopaliya, D.; Kumar, V.; Khare, S.K. Recent advances in itaconic acid production from microbial cell factories. Biocatal. Agric. Biotechnol. 2021, 36, 102130. [Google Scholar] [CrossRef]
- Wierckx, N.; Agrimi, G.; Lübeck, P.S.; Steiger, M.G.; Mira, N.P.; Punt, P.J. Metabolic specialization in itaconic acid production: A tale of two fungi. Curr. Opin. Biotech. 2020, 62, 153–159. [Google Scholar] [CrossRef]
- Marketsandresearch.biz. Global Itaconic Anhydride Market 2022 by Manufacturers, Regions, Type and Application, Forecast to 2028. 2022. Available online: https://www.marketsandresearch.biz/report/262120/global-itaconic-acid-market-2022-by-manufacturers-regions-type-and-application-forecast-to-2028 (accessed on 5 January 2022).
- Cunha da Cruz, J.; Machado de Castro, A. Camporese Sérvulo, E.F. World market and biotechnological production of itaconic acid. 3 Biotech 2018, 8, 138. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jin, Y.; Zhang, R.; Ning, Y.; Yu, Y.; Xu, P.; Deng, L.; Wang, F. Recent advances and perspectives on production of value-added organic acids through metabolic engineering. Biotechnol. Adv. 2022, 62, 108076. [Google Scholar] [CrossRef]
- Yang, J.; Xu, H.; Jiang, J.; Zhang, N.; Xie, J.; Wei, M.; Zhao, J. Production of itaconic acid through microbiological fermentation of inexpensive materials. J. Bioresour. Bioprod. 2019, 4, 135–142. [Google Scholar]
- Kinoshita, K. Über die Produktion von Itaconsäure und Mannit durch einen neuen Schimmelpilz Aspergillus itaconicus. Acta Phytochim. 1932, 5, 271–287. [Google Scholar]
- Bentley, R.; Thiessen, C.P. Biosynthesis of itaconic acid in Aspergillus terreus: I. Tracer studies with C14-labeled substrates. J. Biol. Chem. 1957, 226, 673–687. [Google Scholar] [CrossRef]
- Bonnarme, P.; Gillet, B.; Sepulchre, A.M.; Role, C.; Beloeil, J.C.; Ducrocq, C. Itaconate biosynthesis in Aspergillus terreus. J. Bacteriol. 1995, 177, 3573–3578. [Google Scholar] [CrossRef]
- Jaklitsch, W.M.; Kubicek, C.P.; Scrutton, M.C. The subcellular organization of itaconate biosynthesis in Aspergillus terreus. Microbiology 1991, 137, 533–539. [Google Scholar] [CrossRef]
- Li, A.; van Luijk, N.; ter Beek, M.; Caspers, M.; Punt, P.; van der Werf, M. A clone-based transcriptomics approach for the identification of genes relevant for itaconic acid production in Aspergillus. Fungal Genet. Biol. 2011, 48, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.S.; Park, B.; Lee, D.; Oh, M.K.; Chun, G.T.; Kim, S. Enhanced production of itaconic acid through development of transformed fungal strains of Aspergillus terreus. J. Mcrobiol. Biotechnol. 2017, 27, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Huang, X.; Zhong, C.; Li, J.; Lu, X. Identification of an itaconic acid degrading pathway in itaconic acid producing Aspergillus terreus. Appl. Microbiol. Biotechnol. 2016, 100, 7541–7548. [Google Scholar] [CrossRef]
- Geiser, E.; Przybilla, S.K.; Friedrich, A.; Buckel, W.; Wierckx, N.; Blank, L.M.; Bölker, M. Ustilago maydis produces itaconic acid via the unusual intermediate trans-aconitate. Microb. Biotechnol. 2016, 9, 116–126. [Google Scholar] [CrossRef]
- Geiser, E.; Hosseinpour Tehrani, H.; Meyer, S.; Blank, L.M.; Wierckx, N. Evolutionary freedom in the regulation of the conserved itaconate cluster by Ria1 in related Ustilaginaceae. Fungal Biol. Biotechnol. 2018, 5, 14. [Google Scholar] [CrossRef]
- Steiger, M.G.; Wierckx, N.; Blank, L.M.; Mattanovich, D.; Sauer, M. Itaconic acid—An emerging building block. In Industrial Biotechnology, Products and Processes; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017. [Google Scholar]
- Eimhjellen, K.; Larsen, H. The mechanism of itaconic acid formation by Aspergillus terreus. 2. The effect of substrates and inhibitors. Biochem. J. 1955, 60, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xiao, Q.; Yue, Y.; Huang, X.; Zhang, Y.; Deng, L.; Wang, F. Influence analysis of glycerol in fumaric acid co-fermentation process by Rhizopus arrhizus. J. Environ. Chem. Eng. 2021, 9, 104750. [Google Scholar] [CrossRef]
- Juy, M.I.; Lucca, M.E. Study of itaconic acid production by Aspergillus terrus MJL05 strain with different variable. Rev. Colomb. Biotechnol. 2010, 12, 187–193. [Google Scholar]
- Liu, H.; Ma, J.; Wang, M.; Wang, W.; Deng, L.; Nie, K.; Yue, X.; Wang, F.; Tan, T. Food waste fermentation to fumaric acid by Rhizopus arrhizus RH7-13. Appl. Biochem. Biotechnol. 2016, 180, 1524–1533. [Google Scholar] [CrossRef]
- Fan, T.; Liu, X.; Zhao, R.; Zhang, Y.; Liu, H.; Wang, Z.; Wang, F.; Nie, K.; Deng, L. Hydrolysis of food waste by hot water extraction and subsequent Rhizopus fermentation to fumaric acid. J. Environ. Manag. 2020, 270, 110954. [Google Scholar] [CrossRef]
- Narisetty, V.; Prabhu, A.A.; Al-Jaradah, K.; Gopaliya, D.; Hossain, A.H.; Khare, S.K.; Peter, J.P.; Kumar, V. Microbial itaconic acid production from starchy food waste by newly isolated thermotolerant Aspergillus terreus strain. Bioresour. Technol. 2021, 337, 125426. [Google Scholar] [CrossRef] [PubMed]
- Tippkötter, N.; Duwe, A.M.; Wiesen, S.; Sieker, T.; Ulber, R. Enzymatic hydrolysis of beech wood lignocellulose at high solid contents and its utilization as substrate for the production of biobutanol and dicarboxylic acids. Bioresour. Technol. 2014, 167, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, K.; Lai, C.; Ouyang, J.; Yong, Q. Improved itaconic acid production from undetoxified enzymatic hydrolysate of steam-exploded corn stover using an Aspergillus terreus mutant generated by atmospheric and room temperature plasma. BioResources 2016, 11, 9047–9058. [Google Scholar] [CrossRef]
- Jiménez-Quero, A.; Pollet, E.; Zhao, M.; Marchioni, E.; Avérous, L.; Phalip, V. Itaconic and fumaric acid production from biomass hydrolysates by Aspergillus strains. J. Micorobiol. Biotechnol. 2016, 26, 1557–1565. [Google Scholar] [CrossRef]
- Krull, S.; Eidt, L.; Hevekerl, A.; Kuenz, A.; Prüße, U. Itaconic acid production from wheat chaff by Aspergillus terreus. Process Biochem. 2017, 63, 169–176. [Google Scholar] [CrossRef]
- Kerssemakers, A.A.; Doménech, P.; Cassano, M.; Yamakawa, C.K.; Dragone, G.; Mussatto, S.I. Production of itaconic acid from cellulose pulp: Feedstock feasibility and process strategies for an efficient microbial performance. Energies 2020, 13, 1654. [Google Scholar] [CrossRef]
- Sun, Y.; Li, P.; Sun, Q.; Liu, X.; Wang, Y.; Zhang, B.; Dong, S.; Hu, X. Insights into Ionic liquids-resistance mechanism and lignocellulose-degradation model of Aspergillus terreus in 1-ethyl-3-methylimidazolium acetate. Ind. Crops Prod. 2022, 178, 114593. [Google Scholar] [CrossRef]
- Yahiro, K.; Takahama, T.; Park, Y.S.; Okabe, M. Breeding of Aspergillus terreus mutant TN-484 for itaconic acid production with high yield. J. Ferment. Bioeng. 1995, 79, 506–508. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Q.; Deng, Y.; Li, J.; Chen, X.; Gu, Y.; Lv, X.; Zheng, Z.; Jiang, S.; Li, X. Production of itaconic acid by biotransformation of wheat bran hydrolysate with Aspergillus terreus CICC40205 mutant. Bioresour. Technol. 2017, 241, 25–34. [Google Scholar] [CrossRef]
- Yang, J.; Xu, H.; Jiang, J.; Zhang, N.; Xie, J.; Zhao, J.; Bu, Q.; Wei, M. Itaconic acid production from undetoxified enzymatic hydrolysate of bamboo residues using Aspergillus terreus. Bioresour. Technol. 2020, 307, 123208. [Google Scholar] [CrossRef] [PubMed]
- Savitha, S.; Sadhasivam, S.; Swaminathan, K. Regeneration and molecular characterization of an intergeneric hybrid between Graphium putredinis and Trichoderma harzianum by protoplasmic fusion. Biotechnol. Adv. 2010, 28, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Kirimura, K.; Sato, T.; Nakanishi, N.; Terada, M.; Usami, S. Breeding of starch-utilizing and itaconic-acid-producing koji molds by interspecific protoplast fusion between Aspergillus terreus and Aspergillus usamii. Appl. Microbiol. Biotechnol. 1997, 47, 127–131. [Google Scholar] [CrossRef]
- Karaffa, L.; Kubicek, C.P. Citric acid and itaconic acid accumulation: Variations of the same story? Appl. Microbiol. Biotechnol. 2019, 103, 2889–2902. [Google Scholar] [CrossRef]
- Tevž, G.; Benčina, M.; Legiša, M. Enhancing itaconic acid production by Aspergillus terreus. Appl. Microbiol. Biotechnol. 2010, 87, 1657–1664. [Google Scholar] [CrossRef]
- Huang, X.; Lu, X.; Li, Y.; Li, X.; Li, J.J. Improving itaconic acid production through genetic engineering of an industrial Aspergillus terreus strain. Microb. Cell Factories 2014, 13, 119. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Shin, H.D.; Liu, L.; Du, G.; Chen, J. Protein and metabolic engineering for the production of organic acids. Bioresour. Technol. 2017, 239, 412–421. [Google Scholar] [CrossRef]
- Huang, X.; Chen, M.; Lu, X.; Li, Y.; Li, X.; Li, J.J. Direct production of itaconic acid from liquefied corn starch by genetically engineered Aspergillus terreus. Microb. Cell Factories 2014, 13, 108. [Google Scholar] [CrossRef]
- Deng, S.; Dai, Z.; Swita, M.; Pomraning, K.R.; Hofstad, B.; Panisko, E.; Baker, S.; Magnuson, J. Deletion analysis of the itaconic acid biosynthesis gene cluster components in Aspergillus pseudoterreus ATCC32359. Appl. Microbiol. Biotechnol. 2020, 104, 3981–3992. [Google Scholar] [CrossRef]
- Pomraning, K.R.; Dai, Z.; Munoz, N.; Kim, Y.M.; Gao, Y.; Deng, S.; Magnuson, J.K. Itaconic acid production is regulated by LaeA in Aspergillus pseudoterreus. Metab. Eng. Commun. 2022, 15, e00203. [Google Scholar] [CrossRef]
- Guo, C.J.; Knox, B.P.; Sanchez, J.F.; Chiang, Y.M.; Bruno, K.S.; Wang, C.C. Application of an efficient gene targeting system linking secondary metabolites to their biosynthetic genes in Aspergillus terreus. Org. Lett. 2013, 15, 3562–3565. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chen, M.; Li, J.; Lu, X. Establishing an efficient gene-targeting system in an itaconic-acid producing Aspergillus terreus strain. Biotechnol. Lett. 2016, 38, 1603–1610. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Chen, X.; Han, Y.; Zheng, H.; Wang, Z.; Chen, J. Development of versatile and efficient genetic tools for the marine-derived fungus Aspergillus terreus RA2905. Curr. Genet. 2022, 68, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Shih, S.Y.; Mortensen, U.H.; Chang, F.R.; Hsinyuan, T. Editing Aspergillus terreus using the CRISPR-Cas9 system. Synth. Biol. 2022, 7, ysac031. [Google Scholar] [CrossRef]
- Zambanini, T.; Hosseinpour Tehrani, H.; Geiser, E.; Merker, D.; Schleese, S.; Krabbe, J.; Buescher, J.M.; Meurer, G.; Wierckx, N.; Blank, L.M. Efficient itaconic acid production from glycerol with Ustilago vetiveriae TZ1. Biotechnol. Biofuels 2017, 10, 131. [Google Scholar] [CrossRef]
- Krull, S.; Lünsmann, M.; Prüße, U.; Kuenz, A. Ustilago Rabenhorstiana—An alternative natural itaconic acid producer. Fermentation 2020, 6, 4. [Google Scholar] [CrossRef]
- Hosseinpour Tehrani, H.; Saur, K.; Tharmasothirajan, A.; Blank, L.M.; Wierckx, N. Process engineering of pH tolerant Ustilago cynodontis for efficient itaconic acid production. Microb. Cell Factories 2019, 18, 213. [Google Scholar] [CrossRef]
- Haskins, R.; Thorn, J.; Boothroyd, B. Biochemistry of the Ustilaginales: XI. Metabolic products of Ustilago zeae in submerged culture. Can. J. Microbiol. 1955, 1, 749–756. [Google Scholar] [CrossRef]
- Hajian, H.; Yusoff, W.M.W. Itaconic acid production by microorganisms: A review. Curr. Res. J. Biol. Sci. 2015, 7, 37–42. [Google Scholar] [CrossRef]
- Becker, J.; Hosseinpour Tehrani, H.; Ernst, P.; Blank, L.M.; Wierckx, N. An Optimized Ustilago maydis for Itaconic Acid Production at Maximal Theoretical Yield. J. Fungi 2021, 7, 20. [Google Scholar] [CrossRef]
- Aréchiga-Carvajal, E.T.; Ruiz-Herrera, J. The RIM101/pacC homologue from the basidiomycete Ustilago maydis is functional in multiple pH-sensitive phenomena. Eukaryot. Cell 2005, 4, 999–1008. [Google Scholar] [CrossRef]
- Geiser, E.; Wiebach, V.; Wierckx, N.; Blank, L.M. Prospecting the biodiversity of the fungal family Ustilaginaceae for the production of value-added chemicals. Fungal Biol. Biotechnol. 2014, 1, 2. [Google Scholar] [CrossRef]
- Klement, T.; Milker, S.; Jäger, G.; Grande, P.M.; Domínguez de María, P.; Büchs, J. Biomass pretreatment affects Ustilago maydis in producing itaconic acid. Microb. Cell Factories 2012, 11, 43. [Google Scholar] [CrossRef]
- Maassen, N.; Panakova, M.; Wierckx, N.; Geiser, E.; Zimmermann, M.; Bölker, M.; Kinner, U.; Blank, L.M. Influence of carbon and nitrogen concentration on itaconic acid production by the smut fungus Ustilago maydis. Eng. Life Sci. 2014, 14, 129–134. [Google Scholar] [CrossRef]
- Schlembach, I.; Hosseinpour Tehrani, H.; Blank, L.M.; Büchs, J.; Wierckx, N.; Regestein, L.; Rosenbaum, M.A. Consolidated bioprocessing of cellulose to itaconic acid by a co-culture of Trichoderma reesei and Ustilago maydis. Biotechnol. Biofuels 2020, 13, 207. [Google Scholar] [CrossRef]
- Geiser, E.; Przybilla, S.K.; Engel, M.; Kleineberg, W.; Büttner, L.; Sarikaya, E.; Hartog, T.D.; Klankermayer, J.; Leitner, W.; Bölker, M.; et al. Genetic and biochemical insights into the itaconate pathway of Ustilago maydis enable enhanced production. Metab. Eng. 2016, 38, 427–435. [Google Scholar] [CrossRef]
- Becker, J.; Hosseinpour Tehrani, H.; Gauert, M.; Mampel, J.; Blank, L.M.; Wierckx, N. An Ustilago maydis chassis for itaconic acid production without by-products. Microb. Biotechnol. 2020, 13, 350–362. [Google Scholar] [CrossRef]
- Banuett, F.; Herskowitz, I. Identification of fuz7, a Ustilago maydis MEK/MAPKK homolog required for a-locus-dependent and-independent steps in the fungal life cycle. Genes Dev. 1994, 8, 1367–1378. [Google Scholar] [CrossRef] [PubMed]
- Ehrani, H.H.; Tharmasothirajan, A.; Track, E.; Blank, L.M.; Wierckx, N. Engineering the morphology and metabolism of pH tolerant Ustilago cynodontis for efficient itaconic acid production. Metab. Eng. 2019, 54, 293–300. [Google Scholar]
- Hosseinpour Tehrani, H.; Becker, J.; Bator, I.; Saur, K.; Meyer, S.; Rodrigues Lóia, A.C.M.; Blank, L.; Wierckx, N. Integrated strain-and process design enable production of 220 g L− 1 itaconic acid with Ustilago maydis. Biotechnol. Biofuels 2019, 12, 263. [Google Scholar] [CrossRef]
- Araki, T.; Yamazaki, Y.; Suzuki, N. Production of itaconic acid by Helicobasidium mompa TANAKA. Jpn. J. Phytopathol. 1957, 22, 83–87. [Google Scholar] [CrossRef]
- Tabuchi, T.; Sugisawa, T.; Ishidori, T.; Nakahara, T.; Sugiyama, J. Itaconic acid fermentation by a yeast belonging to the genus Candida. Agric. Biol. Chem. 1981, 45, 475–479. [Google Scholar] [CrossRef]
- Levinson, W.E.; Kurtzman, C.P.; Kuo, T.M. Production of itaconic acid by Pseudozyma antarctica NRRL Y-7808 under nitrogen-limited growth conditions. Enzyme Microb. Technol. 2006, 39, 824–827. [Google Scholar] [CrossRef]
- Cairns, T.C.; Barthel, L.; Meyer, V. Something old, something new: Challenges and developments in Aspergillus niger biotechnology. Essays Biochem. 2021, 65, 213–224. [Google Scholar] [PubMed]
- Hossain, A.H.; Van Gerven, R.; Overkamp, K.M.; Lübeck, P.S.; Taşpınar, H.; Türker, M.; Punt, P.J. Metabolic engineering with ATP-citrate lyase and nitrogen source supplementation improves itaconic acid production in Aspergillus niger. Biotechnol. Biofuels 2019, 12, 233. [Google Scholar] [CrossRef] [PubMed]
- Okabe, M.; Lies, D.; Kanamasa, S.; Park, E.Y. Biotechnological production of itaconic acid and its biosynthesis in Aspergillus terreus. Appl. Microbiol. Biotechnol. 2009, 84, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Pfelzer, N.; Zuijderwijk, R.; Brickwedde, A.; van Zeijl, C.; Punt, P. Reduced by-product formation and modified oxygen availability improve itaconic acid production in Aspergillus niger. Appl. Microbiol. Biotechnol. 2013, 97, 3901–3911. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, Y.; Cao, W.; Liu, H. Synergistic effects on itaconic acid production in engineered Aspergillus niger expressing the two distinct biosynthesis clusters from Aspergillus terreus and Ustilago maydis. Microb. Cell Factories 2022, 21, 158. [Google Scholar] [CrossRef]
- Xie, H.; Ma, Q.; Wei, D.; Wang, F. Metabolic engineering of an industrial Aspergillus niger strain for itaconic acid production. 3 Biotech 2020, 10, 113. [Google Scholar] [CrossRef]
- Porro, D.; Branduardi, P. Production of organic acids by yeasts and filamentous fungi. In Biotechnology of Yeasts and Filamentous Fungi; Springer: Cham, Switzerland, 2017; pp. 205–223. [Google Scholar]
- Yang, L.; Liu, H.; Jin, Y.; Liu, J.; Deng, L.; Wang, F. Recent advances in multiple strategies for the synthesis of terpenes by engineered yeast. Fermentation 2022, 8, 615. [Google Scholar] [CrossRef]
- Xia, F.; Du, J.; Wang, K.; Liu, L.; Ba, L.; Liu, H.; Liu, Y. Application of Multiple Strategies To Debottleneck the Biosynthesis of Longifolene by Engineered Saccharomyces cerevisiae. J. Agric. Food Chem. 2022, 70, 11336–11343. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, F.; Deng, L.; Xu, P. Genetic and bioprocess engineering to improve squalene production in Yarrowia lipolytica. Bioresour. Technol. 2020, 317, 123991. [Google Scholar] [CrossRef]
- Liu, H.; Marsafari, M.; Wang, F.; Deng, L.; Xu, P. Engineering acetyl-CoA metabolic shortcut for eco-friendly production of polyketides triacetic acid lactone in Yarrowia lipolytica. Metab. Eng. 2019, 56, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Blazeck, J.; Miller, J.; Pan, A.; Gengler, J.; Holden, C.; Jamoussi, M.; Alper, H.S. Metabolic engineering of Saccharomyces cerevisiae for itaconic acid production. Appl. Microbiol. Biotechnol. 2014, 98, 8155–8164. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Vila-Santa, A.; Liu, N.; Prozorov, T.; Xie, D.; Faria, N.T.; Ferreia, F.C.; Mira, N.P.; Shao, Z. Metabolic engineering of an acid-tolerant yeast strain Pichia kudriavzevii for itaconic acid production. Metab. Eng. Commun. 2020, 10, e00124. [Google Scholar] [CrossRef] [PubMed]
- Beopoulos, A.; Nicaud, J.M.; Gaillardin, C. An overview of lipid metabolism in yeasts and its impact on biotechnological processes. Appl. Microbiol. Biotechnol. 2011, 90, 1193–1206. [Google Scholar] [CrossRef]
- Urak, S.; Yeniay, O.; Karasu-Yalcin, S. Optimization of citric acid production from a carrot juice-based medium by Yarrowia lipolytica using response surface methodology. Ann. Microbiol. 2015, 65, 639–649. [Google Scholar] [CrossRef]
- Blazeck, J.; Hill, A.; Jamoussi, M.; Pan, A.; Miller, J.; Alper, H.S. Metabolic engineering of Yarrowia lipolytica for itaconic acid production. Metab. Eng. 2015, 32, 66–73. [Google Scholar] [CrossRef]
- Zhao, C.; Cui, Z.; Zhao, X.; Zhang, J.; Zhang, L.; Tian, Y.; Qi, Q.; Liu, J. Enhanced itaconic acid production in Yarrowia lipolytica via heterologous expression of a mitochondrial transporter MTT. Appl. Microbiol. Biotechnol. 2019, 103, 2181–2192. [Google Scholar] [CrossRef]
- Liu, H.; Song, R.; Liang, Y.; Zhang, T.; Deng, L.; Wang, F.; Tan, T. Genetic manipulation of Escherichia coli central carbon metabolism for efficient production of fumaric acid. Bioresour Technol. 2018, 270, 96–102. [Google Scholar] [CrossRef]
- Wang, R.; Zhao, S.; Wang, Z.; Koffas, M.A. Recent advances in modular co-culture engineering for synthesis of natural products. Curr. Opin. Biotech. 2020, 62, 65–71. [Google Scholar] [CrossRef]
- Harder, B.J.; Bettenbrock, K.; Klamt, S. Model-based metabolic engineering enables high yield itaconic acid production by Escherichia coli. Metab. Eng. 2016, 38, 29–37. [Google Scholar] [CrossRef]
- Okamoto, S.; Chin, T.; Nagata, K.; Takahashi, T.; Ohara, H.; Aso, Y. Production of itaconic acid in Escherichia coli expressing recombinant α-amylase using starch as substrate. J. Biosci. Bioeng. 2015, 119, 548–553. [Google Scholar] [CrossRef]
- Jeon, H.G.; Cheong, D.E.; Han, Y.; Song, J.J.; Choi, J.H. Itaconic acid production from glycerol using Escherichia coli harboring a random synonymous codon-substituted 5′-coding region variant of the cadA gene. Biotechnol. Bioeng. 2016, 113, 1504–1510. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Blombach, B.; Gauttam, R.; Eikmasnns, B.J. The RamA regulon: Complex regulatory interactions in relation to central metabolism in Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 2018, 102, 5901–5910. [Google Scholar] [CrossRef] [PubMed]
- Otten, A.; Brocker, M.; Bott, M. Metabolic engineering of Corynebacterium glutamicum for the production of itaconate. Metab. Eng. 2015, 30, 156–165. [Google Scholar] [CrossRef]
- Joo, Y.; You, S.; Shin, S.; Ko, J.; Jung, K. Bio-Based Production of Dimethyl Itaconate from Rice Wine Waste-Derived Itaconic Acid. J. Biotechnol. 2017, 12, 1700114. [Google Scholar] [CrossRef]
- Merkel, M.; Kiefer, D.; Schmollack, M.; Blombach, B.; Lilge, L.; Henkel, M.; Hausmann, R. Acetate-based production of itaconic acid with Corynebacterium glutamicum using an integrated pH-coupled feeding control. Bioresour. Technol. 2022, 351, 126994. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Chen, S.; Fang, H. Consolidated bioprocessing of lignocellulosic biomass to itaconic acid by metabolically engineering Neurospora crassa. Appl. Microbiol. Biotechnol. 2018, 102, 9577–9584. [Google Scholar] [CrossRef] [PubMed]
- Bellasio, M.; Mattanovich, D.; Sauer, M.; Marx, H. Organic acids from lignocellulose: Candida lignohabitans as a new microbial cell factory. J. Ind. Microbiol. Biotechnol. 2015, 42, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Elmore, J.R.; Dexter, G.N.; Salvachúa, D.; Martinez-Baird, J.; Hatmaker, E.A.; Huenemann, J.D.; Klingeman, D.M.; Peabody, G.L.; Peterson, D.J.; Singer, C.; et al. Production of itaconic acid from alkali pretreated lignin by dynamic two stage bioconversion. Nature 2021, 12, 2261. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.H.; Li, A.; Brickwedde, A.; Wilms, L.; Caspers, M.; Overkamp, K.; Punt, P.J. Rewiring a secondary metabolite pathway towards itaconic acid production in Aspergillus niger. Microb. Cell Factories 2016, 15, 130. [Google Scholar] [CrossRef] [PubMed]
- Harder, B.J.; Bettenbrock, K.; Klamt, S. Temperature-dependent dynamic control of the TCA cycle increases volumetric productivity of itaconic acid production by Escherichia coli. Biotechno. Bioeng. 2018, 115, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, S.; Chin, T.; Hiratsuka, K.; Aso, Y.; Tanaka, Y.; Takahashi, T.; Ohara, H. Production of itaconic acid using metabolically engineered Escherichia coli. J. Gen. Appl. Microbiol. 2014, 60, 191–197. [Google Scholar] [CrossRef]
- Zhao, M.; Li, Y.; Wang, F.; Ren, Y.; Wei, D. A CRISPRi mediated self-inducible system for dynamic regulation of TCA cycle and improvement of itaconic acid production in Escherichia coli. Synth. Syst. Biotechnol. 2022, 7, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Lanza, A.M.; Curran, K.A.; Rey, L.G.; Alper, H.S. A condition-specific codon optimization approach for improved heterologous gene expression in Saccharomyces cerevisiae. BMC Syst. Biol. 2014, 8, 33. [Google Scholar] [CrossRef]
- Vuoristo, K.S.; Mars, A.E.; Sangra, J.V.; Springer, J.; Eggink, G.; Sanders, J.P.; Weusthuis, R.A. Metabolic engineering of itaconate production in Escherichia coli. Appl. Microbiol. Biotechnol. 2015, 99, 221–228. [Google Scholar] [CrossRef]
- Klement, T.; Büchs, J. Itaconic acid–a biotechnological process in change. Bioresource Technol. 2013, 135, 422–431. [Google Scholar] [CrossRef] [PubMed]
- van der Straat, L.; Vernooij, M.; Lammers, M.; van den Berg, W.; Schonewille, T.; Cordewener, J.; van der Meer, I.; Koops, A.; de Graaff, L.H. Expression of the Aspergillus terreus itaconic acid biosynthesis cluster in Aspergillus niger. Microb. Cell Factories 2014, 13, 11. [Google Scholar] [CrossRef]
- Hanko, E.K.; Minton, N.P.; Malys, N. A transcription factor-based biosensor for detection of itaconic acid. ACS Synth. Biol. 2018, 7, 1436–1446. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).