A Method for Improving Microbial Conversion of Diosgenin and Separation and Identification of the Product
Abstract
1. Introduction
2. Experimental
2.1. Identification of Fungal Strains
2.2. Preparation of Spore Solution
2.3. UV-LiCl Combined Mutagenesis Method
2.4. Preparation of Fermented Seed Liquid
2.5. Preparation of Solid-State Medium
2.6. Selection of Significant Factors by the Plackett–Burman Design
2.7. Levels of Each Factor Tested in the Box-Behnken
2.8. Chromatographic Purification
2.9. Thin Layer Chromatography
2.10. High Performance Liquid Chromatography (HPLC)
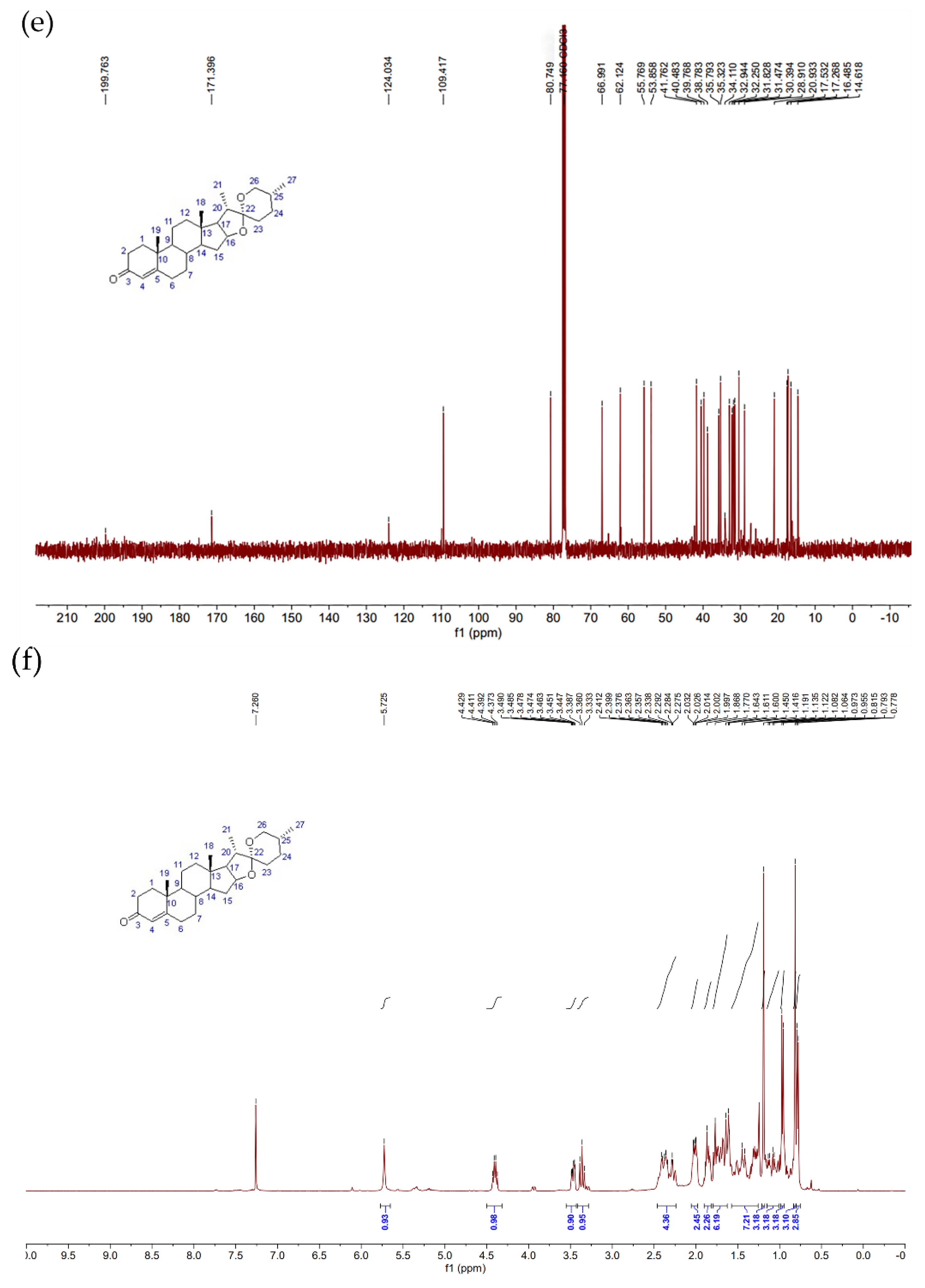
2.11. Nuclear Magnetic Resonance Method
2.12. High Resolution Mass Spectrometry (HR-MS)
3. Results and Discussion
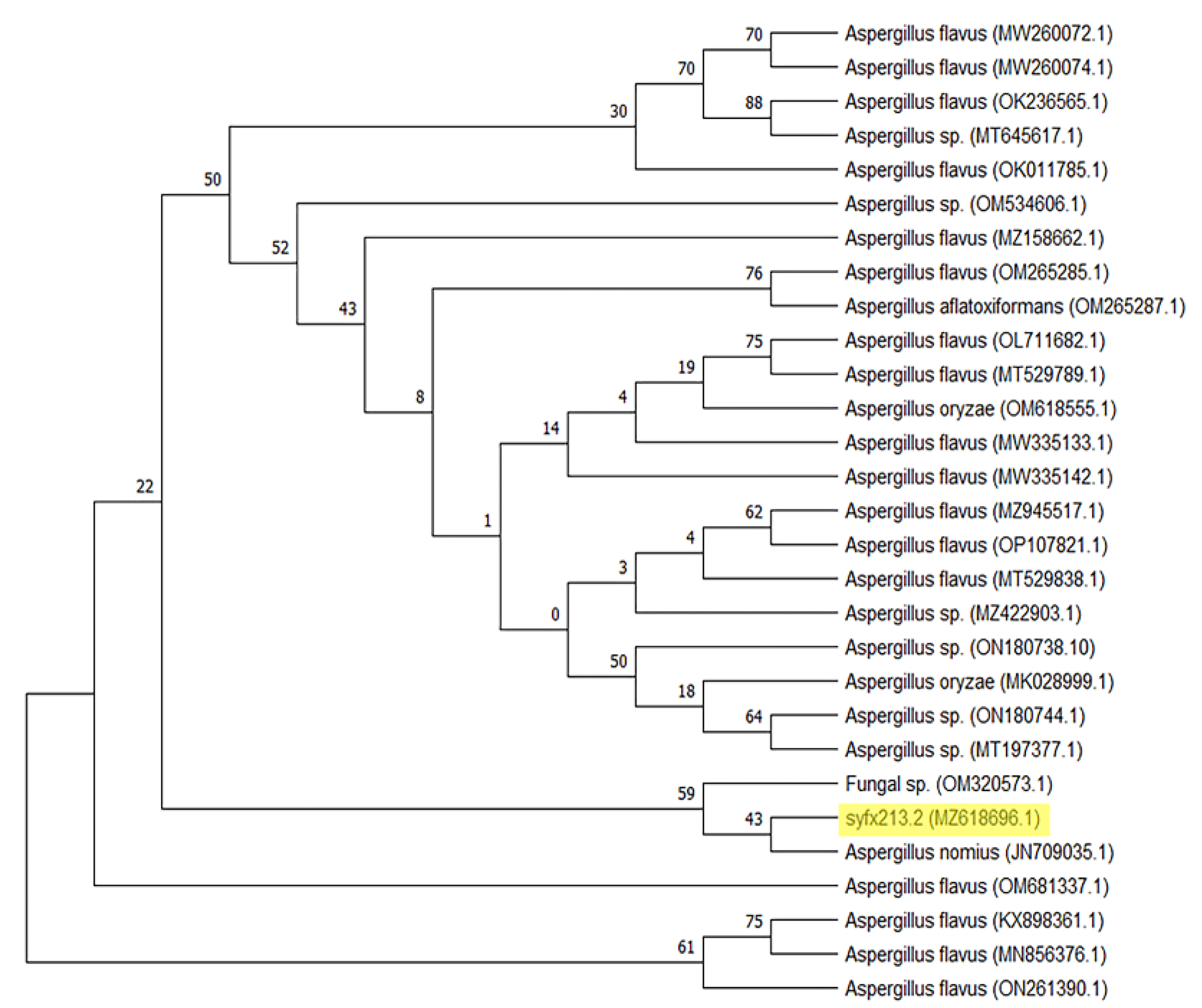
3.1. The Species Identification of the Target Strain
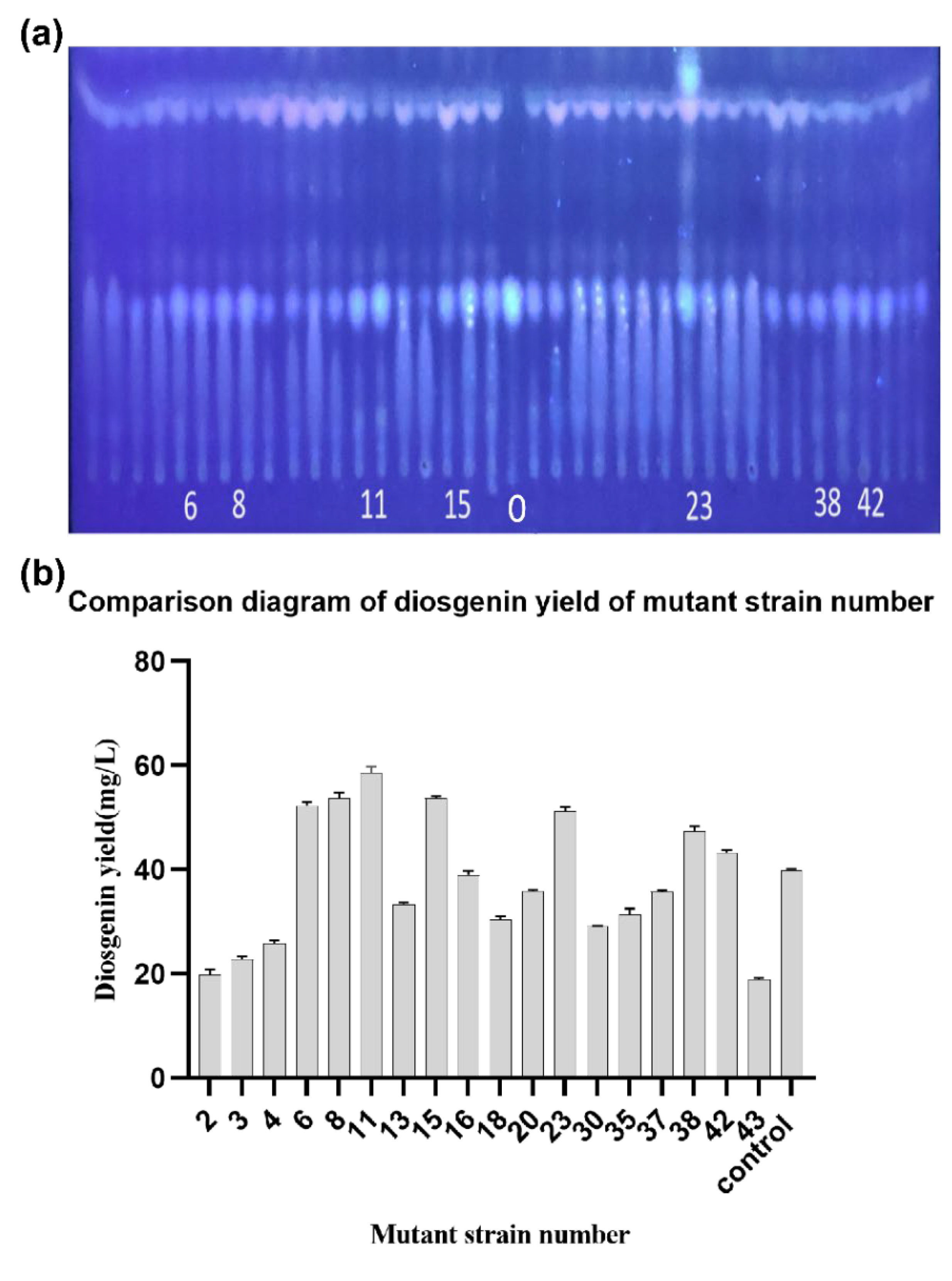
3.2. Screening of High Yield Diosgenin Strains by Compound Mutagenesis
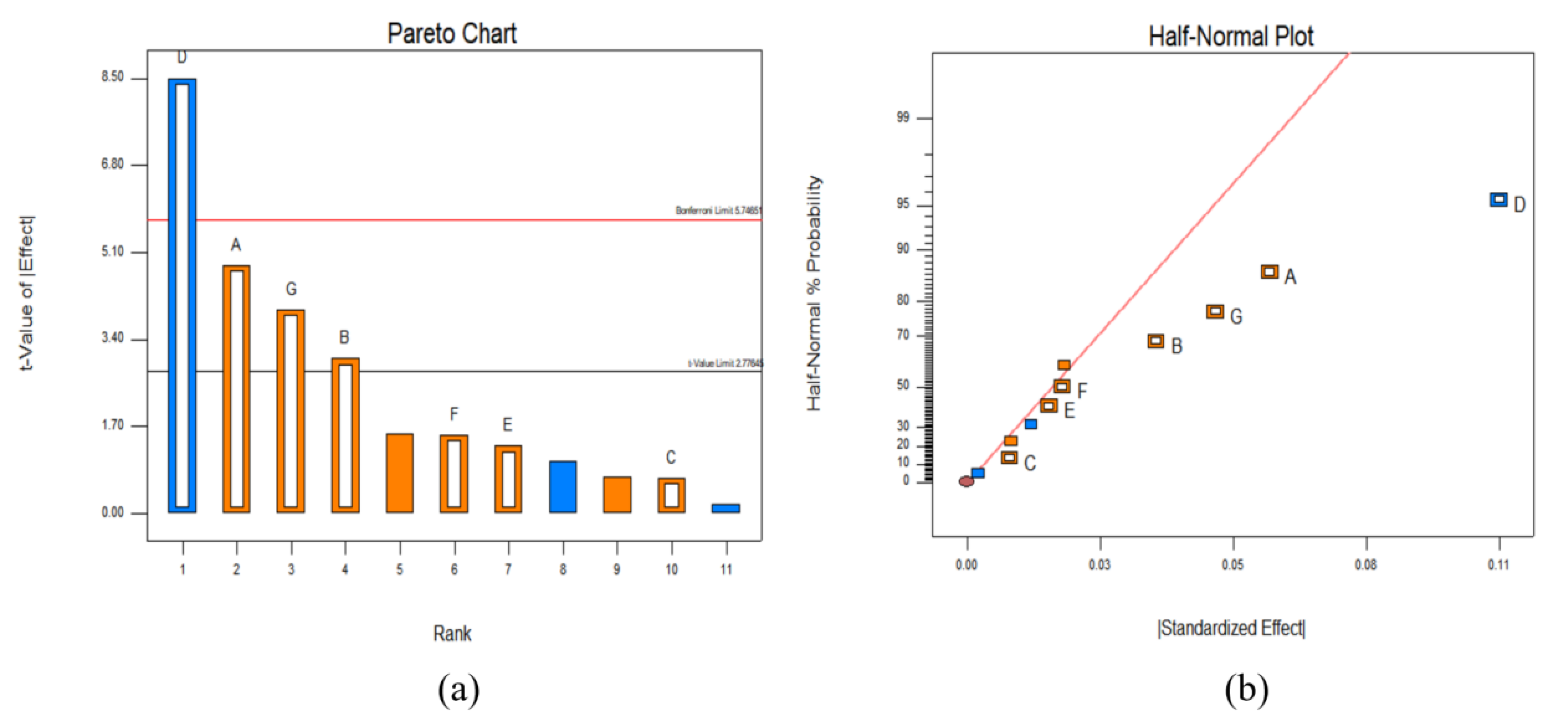
3.2.1. Plackett-Burman Experiment Results
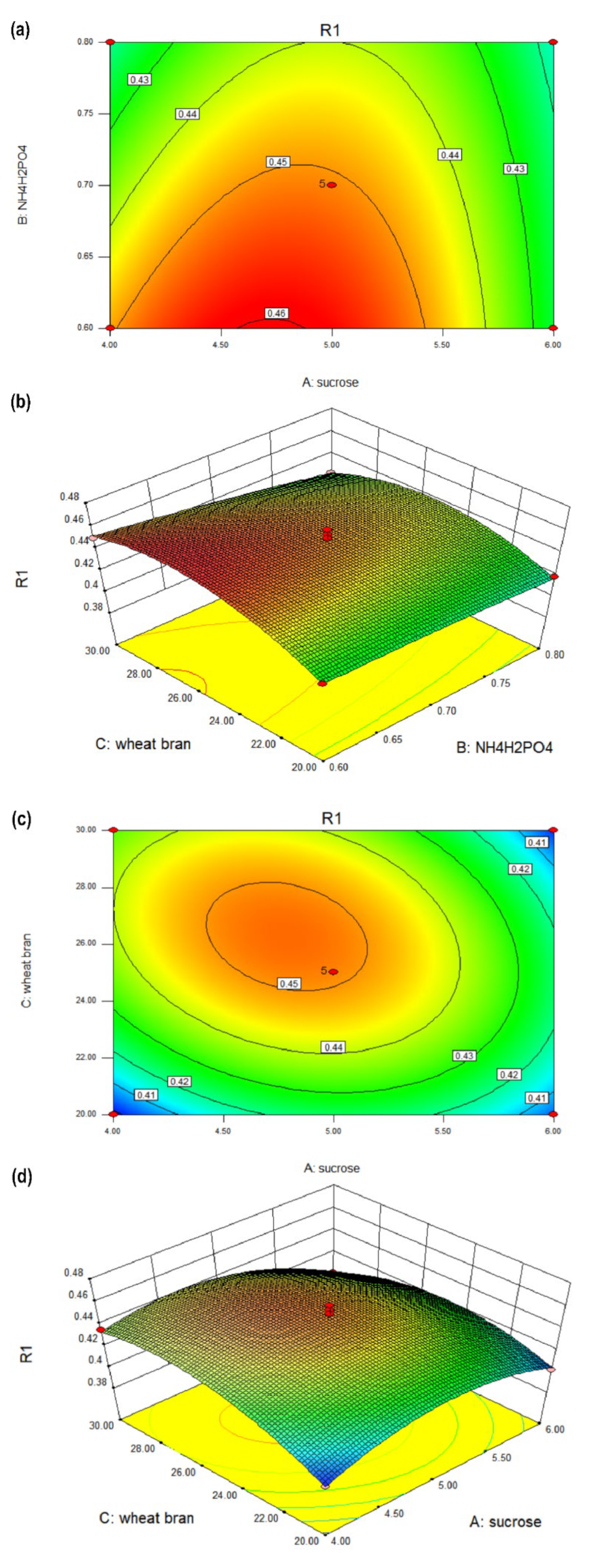
3.2.2. Response Surface Analysis of the Interaction of Various Factors
3.3. Model Validation Experiment
3.4. Separation and Identification of Solid-State Fermentation Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Fan, R.; He, W.; Fan, Y.; Xu, W.; Xu, W.; Yan, G.; Xu, S. Recent advances in chemical synthesis, biocatalysis, and biological evaluation of diosgenin derivatives—A review. Steroids 2022, 180, 108991. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.C.; Jan, C.R.; Liang, W.Z. Exploring the impact of a naturally occurring sapogenin diosgenin on underlying mechanisms of Ca2+ movement and cytotoxicity in human prostate cancer cells. Environ. Toxicol. 2020, 35, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Sirotkin, A.V.; Alexa, R.; Alwasel, S.; Harrath, A.H. The phytoestrogen, diosgenin, directly stimulates ovarian cell functions in two farm animal species. Domest. Anim. Endocrinol. 2019, 69, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Nomoto, K.; Tohda, C. Diosgenin content is a novel criterion to assess memory enhancement effect of yam extracts. J. Nat. Med. 2021, 75, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xu, P.; Wu, S.; Jiang, Z.; Huang, Z.; Li, Q.; Chen, D. Diosgenin Attenuates Lipopolysaccharide-Induced Parkinson’s Disease by Inhibiting the TLR/NF-kappa B Pathway. J. Alzheimers Dis. 2018, 64, 943–955. [Google Scholar] [CrossRef]
- Cai, D.; Qi, J.; Yang, Y.; Zhang, W.; Zhou, F.; Jia, X.; Guo, W.; Huang, X.; Gao, F.; Chen, H.; et al. Design, Synthesis and Biological Evaluation of Diosgenin-Amino Acid Derivatives with Dual Functions of Neuroprotection and Angiogenesis. Molecules 2019, 24, 4025. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, D.; Huang, L. Metabolome Profiling of Eight Chinese Yam (Dioscorea polystachya Turcz.) Varieties Reveals Metabolite Diversity and Variety Specific Uses. Life 2021, 11, 687. [Google Scholar] [CrossRef]
- Zhang, R.; Li, P.; Xu, L.; Chen, Y.; Sui, P.; Zhou, L.; Li, J. Enhancement of Diosgenin Production in Dioscorea zingiberensis Cell Culture by Oligosaccharide Elicitor from its Endophytic Fungus Fusarium oxysporum Dzf17. Nat. Prod. Commun. 2009, 4, 1459–1462. [Google Scholar]
- Li, P.; Mou, Y.; Shan, T.; Xu, J.; Li, Y.; Lu, S.; Zhou, L. Effects of Polysaccharide Elicitors from Endophytic Fusarium oxysporium Dzf17 on Growth and Diosgenin Production in Cell Suspension Culture of Dioscorea zingiberensis. Molecules 2011, 16, 9003–9016. [Google Scholar] [CrossRef]
- Shen, B.W.; Yu, X.J.; Jiang, W.X.; Yuan, H.; Zhao, M.Q.; Zhou, H.; Pan, Z.Q. Green Conversion of Saponins to Diosgenin in an Alcoholysis System Catalyzed by Solid Acid Derived from Phosphorus Tailings. ACS Omega 2021, 6, 5423–5435. [Google Scholar] [CrossRef]
- Zhao, M.Q.; Pan, Z.Q.; Chen, Q.R.; Zhou, H. Catalytic Alcoholysis to Prepare Diosgenin with a Solid Acid Based on Nano TiO2. Catal. Lett. 2022, 152, 3453–3464. [Google Scholar] [CrossRef]
- Cheng, L.Y.; Zhang, H.; Cui, H.Y.; Davari, M.D.; Wei, B.; Wang, W.Y.; Yuan, Q.P. Efficient enzyme-catalyzed production of diosgenin: Inspired by the biotransformation mechanisms of steroid saponins in Talaromyces stollii CLY-6. Green Chem. 2021, 23, 5896–5910. [Google Scholar] [CrossRef]
- Gerocs, A.; Nagy, T.; Nemes-Barnas, K.; Majer, J.; Szoke, B.A.; Kovago, R.; Magalhaes, F.; Gibson, B.; Szekeres, A.; Juhasz, A.; et al. Characterization of Saccharomyces Strains Isolated from “Keknyelu” Grape Must and Their Potential for Wine Production. Fermentation 2022, 8, 416. [Google Scholar] [CrossRef]
- Nunez Perez, J.; Chavez Arias, B.S.; de la Vega Quintero, J.C.; Zarate Baca, S.; Manuel Pais-Chanfrau, J. Multi-Objective Statistical Optimization of Pectinolytic Enzymes Production by an Aspergillus sp. on Dehydrated Coffee Residues in Solid-State Fermentation. Fermentation 2022, 8, 170. [Google Scholar] [CrossRef]
- Banat, I.M.; Carboue, Q.; Saucedo-Castaneda, G.; de Jesus Cazares-Marinero, J. Biosurfactants: The green generation of speciality chemicals and potential production using Solid-State fermentation (SSF) technology. Bioresour. Technol. 2021, 320, 124222. [Google Scholar] [CrossRef]
- Krongrawa, W.; Limmatvapirat, S.; Saibua, S.; Limmatvapirat, C. Optimization of Ultrasound-Assisted Extraction of Yields and Total Methoxyflavone Contents from Kaempferia parviflora Rhizomes. Molecules 2022, 27, 4162. [Google Scholar] [CrossRef]
- Hallol, M.; Helmy, O.; Shawky, A.E.; El-Batal, A.; Ramadan, M. Optimization of Alpha-Amylase Production by a Local Bacillus paramycoides Isolate and Immobilization on Chitosan-Loaded Barium Ferrite Nanoparticles. Fermentation 2022, 8, 241. [Google Scholar] [CrossRef]
- Ghazanfar, M.; Nadeem, M.; Shakir, H.A.; Khan, M.; Ahmad, I.; Franco, M.; Chen, L.; Irfan, M. Valorization of Bombax ceiba Waste into Bioethanol Production through Separate Hydrolysis and Fermentation and Simultaneous Saccharification and Fermentation. Fermentation 2022, 8, 386. [Google Scholar] [CrossRef]
- He, S.; Wang, X.; Chen, J.; Li, X.; Gu, W.; Zhang, F.; Cao, G.; Yu, J. Optimization of the Ultrasonic-Assisted Extraction Technology of Steroidal Saponins from Polygonatum kingianum Collett & Hemsl and Evaluating Its Quality Planted in Different Areas. Molecules 2022, 27, 1463. [Google Scholar] [CrossRef]
- Yao, H.Y.Y.; Wang, J.Q.; Yin, J.Y.; Nie, S.P.; Xie, M.Y. A review of NMR analysis in polysaccharide structure and conformation: Progress, challenge and perspective. Food Res. Int. 2021, 143, 110290. [Google Scholar] [CrossRef]
- Chen, G.; Ma, T.; Ma, Y.; Han, C.; Zhang, J.; Sun, Y. Chemical Composition, Anti-Breast Cancer Activity and Extraction Techniques of Ent-Abietane Diterpenoids from Euphorbia fischeriana Steud. Molecules 2022, 27, 4282. [Google Scholar] [CrossRef] [PubMed]
- Ica, R.; Mlinac-Jerkovic, K.; Ilic, K.; Sajko, T.; Munteanu, C.V.A.; Zamfir, A.D.; Kalanj-Bognar, S. Gangliosidome of a Human Hippocampus in Temporal Lobe Epilepsy Resolved by High-Resolution Tandem Mass Spectrometry. Molecules 2022, 27, 4056. [Google Scholar] [CrossRef] [PubMed]
- Baquiao, A.C.; de Oliveira, M.M.M.; Reis, T.A.; Zorzete, P.; Atayde, D.D.; Correa, B. Polyphasic approach to the identification of Aspergillus section Flavi isolated from Brazil nuts. Food Chem. 2013, 139, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, Y.; Long, H.; Yu, Z.; Huang, M.; Liu, X. Biodiversity and Oenological Property Analysis of Non-Saccharomyces Yeasts Isolated from Korla Fragrant Pears (Pyrus sinkiangensis Yu). Fermentation 2022, 8, 388. [Google Scholar] [CrossRef]
- Lu, F.; Chao, J.; Zhao, X.; Betchem, G.; Ding, Y.; Yang, X.; Li, Y.; Ma, H. Enhancing protease activity of Bacillus subtilis using UV-laser random mutagenesis and high-throughput screening. Process Biochem. 2022, 119, 119–127. [Google Scholar] [CrossRef]
- Wang, Q.L.; Zhang, D.; Li, Y.D.; Zhang, F.M.; Wang, C.; Liang, X.L. Genome Shuffling and Ribosome Engineering of Streptomyces actuosus for High-Yield Nosiheptide Production. Appl. Biochem. Biotechnol. 2014, 173, 1553–1563. [Google Scholar] [CrossRef]
- Widyastuti, W.; Setiawan, F.; Al Afandy, C.; Irawan, A.; Laila, A.; Juliasih, N.; Setiawan, W.A.; Arai, M.; Hendri, J.; Setiawan, A.; et al. Antifungal Agent Chitooligosaccharides Derived from Solid-State Fermentation of Shrimp Shell Waste by Pseudonocardia antitumoralis 18D36-A1. Fermentation 2022, 8, 353. [Google Scholar] [CrossRef]
- De Oliveira, R.L.; da Silva, M.F.; Converti, A.; Porto, T.S. Production of β-fructofuranosidase with transfructosylating activity by Aspergillus tamarii URM4634 Solid-State Fermentation on agroindustrial by-products. Int. J. Biol. Macromol. 2020, 144, 343–350. [Google Scholar] [CrossRef]
- Li, X.Q.; Liu, D.Q. Effects of wheat bran co-fermentation on the quality and bacterial community succession during radish fermentation. Food Res. Int. 2022, 157, 111229. [Google Scholar] [CrossRef]
- Foudah, A.I.; Alshehri, S.; Shakeel, F.; Alqarni, M.H.; Aljarba, T.M.; Alam, P. Simultaneous Estimation of Escitalopram and Clonazepam in Tablet Dosage Forms Using HPLC-DAD Method and Optimization of Chromatographic Conditions by Box-Behnken Design. Molecules 2022, 27, 4209. [Google Scholar] [CrossRef]
- Tanyildizi, M.S.; Selen, V.; Ozer, D. Optimization of alpha-Amylase Production in solid substrate fermentation. Can. J. Chem. Eng. 2009, 87, 493–498. [Google Scholar] [CrossRef]
- Kumar, M.; Dahuja, A.; Tiwari, S.; Punia, S.; Tak, Y.; Amarowicz, R.; Bhoite, A.G.; Singh, S.; Joshi, S.; Panesar, P.S.; et al. Recent trends in extraction of plant bioactives using green technologies: A review. Food Chem. 2021, 353, 129431. [Google Scholar] [CrossRef]
- Chen, Y.; Dong, Y.; Chi, Y.L.; He, Q.; Wu, H.; Ren, Y. Eco-friendly microbial production of diosgenin from saponins in Dioscorea zingiberensis tubers in the presence of Aspergillus awamori. Steroids 2018, 136, 40–46. [Google Scholar] [CrossRef]
- Blunden, G.; Carabot, A.; Cripps, A.L.; Jewers, K. Ruizgenin, a new steroidal sapogenin diol from Agave lecheguilla. Steroids 1980, 35, 503–510. [Google Scholar] [CrossRef]
- Hernandez Linares, M.-G.; Guerrero-Luna, G.; Bernes, S.; Flores-Alamo, M.; Fernandez-Herrera, M.A. Diosgenone: A second P21 polymorph. Acta Crystallogr. Sect. E 2012, 68, o2358. [Google Scholar] [CrossRef]
- Choi, K.R.; Jang, W.D.; Yang, D.; Cho, J.S.; Park, D.; Lee, S.Y. Systems Metabolic Engineering Strategies: Integrating Systems and Synthetic Biology with Metabolic Engineering. Trends Biotechnol. 2019, 37, 817–837. [Google Scholar] [CrossRef]




| Impact Factor | A: Uv Irradiation Time (s) | B: LiCl Concentration (%) |
|---|---|---|
| Level 1 | 180 s | 1.5% |
| Level 2 | 210 s | 2.0% |
| Level 3 | 240 s | 2.5% |
| Code | Factor | −1 | 1 |
|---|---|---|---|
| A | Content of sucrose | 6% | 4% |
| B | Content of NH4H2PO4 | 0.6% | 0.8% |
| C | Content of MgSO4 | 0.4% | 0.8% |
| D | Content of Tween-80 | 1 g/L | 3 g/L |
| E | Content of rice husk | 20% | 30% |
| F | Content of KH2PO4 | 0.5% | 1.5% |
| G | Content of wheat bran | 20% | 30% |
| Number of Trials | A: Uv Irradiation Time (s) | B: LiCl Concentration (%) | Fatality Rate (%) |
|---|---|---|---|
| 1 | 180 s | 1.5% | 70% |
| 2 | 180 s | 2.0% | 80% |
| 3 | 180 s | 2.5% | 90% |
| 4 | 210 s | 1.5% | 70% |
| 5 | 210 s | 2.0% | 90% |
| 6 | 210 s | 2.5% | 100% |
| 7 | 240 s | 1.5% | 90% |
| 8 | 240 s | 2.0% | 90% |
| 9 | 240 s | 2.5% | 100% |
| Number of Tests | A | B | C | D | E | F | G | R1/% |
|---|---|---|---|---|---|---|---|---|
| 1 | −1 | 1 | 1 | −1 | 1 | 1 | 1 | 0.419 |
| 2 | 1 | 1 | 1 | −1 | −1 | −1 | 1 | 0.427 |
| 3 | −1 | 1 | 1 | 1 | −1 | −1 | −1 | 0.203 |
| 4 | −1 | −1 | 1 | −1 | 1 | 1 | −1 | 0.291 |
| 5 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | 0.275 |
| 6 | 1 | −1 | −1 | −1 | 1 | −1 | 1 | 0.392 |
| 7 | 1 | 1 | −1 | −1 | −1 | 1 | −1 | 0.376 |
| 8 | −1 | 1 | −1 | 1 | 1 | −1 | 1 | 0.244 |
| 9 | 1 | −1 | 1 | 1 | 1 | −1 | −1 | 0.258 |
| 10 | −1 | −1 | −1 | 1 | −1 | 1 | 1 | 0.241 |
| 11 | 1 | 1 | −1 | 1 | 1 | 1 | −1 | 0.303 |
| 12 | 1 | −1 | 1 | 1 | −1 | 1 | 1 | 0.285 |
| Quadratic Sum | Degree of Freedom | Variance | F | Prob > F | |
|---|---|---|---|---|---|
| Model | 0.060 | 7 | 8.607 × 10−3 | 17.87 | 0.0072 |
| A-sucrose | 0.011 | 1 | 0.011 | 23.43 | 0.0084 |
| B-NH4H2PO4 | 4.408 × 10−3 | 1 | 4.408 × 10−3 | 9.15 | 0.0390 |
| C-MgSO4 | 2.253 × 10−4 | 1 | 2.253 × 10−4 | 0.47 | 0.5316 |
| D-Tween-80 | 0.035 | 1 | 0.035 | 72.20 | 0.0011 |
| E-rice husk | 8.333 × 10−4 | 1 | 8.333 × 10−4 | 1.73 | 0.2587 |
| F-KH2PO4 | 1.121 × 10−3 | 1 | 1.121 × 10−3 | 2.33 | 0.2018 |
| G-wheat bran | 7.600 × 10−3 | 1 | 7.600 × 10−3 | 15.78 | 0.0165 |
| Residual | 1.927 × 10−3 | 4 | 4.817 × 10−4 | ||
| Cor Total | 0.062 | 11 |
| Number of Tests | A: Content of Sucrose | B: Content of NH4H2PO4 | C: Content of Wheat Bran | R1/% |
|---|---|---|---|---|
| 1 | 5 | 0.6 | 20 | 0.426 |
| 2 | 5 | 0.7 | 25 | 0.452 |
| 3 | 5 | 0.7 | 25 | 0.437 |
| 4 | 5 | 0.8 | 20 | 0.419 |
| 5 | 4 | 0.8 | 25 | 0.418 |
| 6 | 6 | 0.7 | 30 | 0.401 |
| 7 | 4 | 0.7 | 30 | 0.436 |
| 8 | 6 | 0.7 | 20 | 0.402 |
| 9 | 4 | 0.7 | 20 | 0.398 |
| 10 | 5 | 0.7 | 25 | 0.459 |
| 11 | 4 | 0.6 | 25 | 0.449 |
| 12 | 5 | 0.7 | 25 | 0.455 |
| 13 | 5 | 0.7 | 25 | 0.452 |
| 14 | 6 | 0.8 | 25 | 0.417 |
| 15 | 5 | 0.6 | 30 | 0.45 |
| 16 | 6 | 0.6 | 25 | 0.427 |
| 17 | 5 | 0.8 | 30 | 0.423 |
| Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|
| Model | 0.0061 | 9 | 0.0007 | 15.77 | 0.0007 |
| A-sucrose | 0.0004 | 1 | 0.0004 | 8.44 | 0.0228 |
| B-NH4H2PO4 | 0.0007 | 1 | 0.0007 | 16.28 | 0.0050 |
| C-wheat bran | 0.0005 | 1 | 0.0005 | 12.23 | 0.0100 |
| AB | 0.0001 | 1 | 0.0001 | 2.55 | 0.1541 |
| AC | 0.0004 | 1 | 0.0004 | 8.81 | 0.0209 |
| BC | 0.0001 | 1 | 0.0001 | 2.32 | 0.1719 |
| A^2 | 0.0020 | 1 | 0.0020 | 46.13 | 0.0003 |
| B^2 | 9.474 × 10−6 | 1 | 9.474 × 10−6 | 0.2194 | 0.6537 |
| C^2 | 0.0017 | 1 | 0.0017 | 39.01 | 0.0004 |
| The rest of item | 0.0003 | 7 | 0.0000 | ||
| Lack of fit | 0.0000 | 3 | 8.083 × 10−6 | 0.1163 | 0.9459 |
| Pure error | 0.0003 | 4 | 0.0001 | ||
| Total | 0.0064 | 16 |
| 1 | 2 | 3 | ||||
|---|---|---|---|---|---|---|
| The Number of Carbons | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC |
| 1 | 37.3 | 37.2 | 35.7 | |||
| 2 | 31.4 | 37.1 | 34.0 | |||
| 3 | 3.46 (br s) | 71.8 | 213.3 | 199.8 | ||
| 4 | 42.3 | 42.4 | 5.66 (s) | 124.1 | ||
| 5 | 141.0 | 44.3 | 171.4 | |||
| 6 | 5.28 (br s) | 121.5 | 26.1 | 32.9 | ||
| 7 | 32.1 | 26.6 | 32.2 | |||
| 8 | 31.6 | 35.2 | 35.3 | |||
| 9 | 50.1 | 40.8 | 53.8 | |||
| 10 | 36.6 | 35.0 | 38.7 | |||
| 11 | 20.9 | 21.1 | 20.8 | |||
| 12 | 39.8 | 40.1 | 39.7 | |||
| 13 | 40.3 | 40.7 | 40.4 | |||
| 14 | 56.5 | 56.3 | 55.7 | |||
| 15 | 32.1 | 31.8 | 31.7 | |||
| 16 | 4.34 (q, 7.3) | 80.9 | 4.34 (q, 7.2) | 80.8 | 4.34 (q, 7.2) | 80.7 |
| 17 | 62.1 | 62.3 | 62.0 | |||
| 18 | 0.72 (s, 3H) | 16.3 | 0.72 (s, 3H) | 16.6 | 0.75 (s, 3H) | 16.4 |
| 19 | 0.96 (s, 3H) | 19.4 | 0.97 (s, 3H) | 22.7 | 1.13 (s, 3H) | 17.5 |
| 20 | 41.6 | 41.6 | 41.7 | |||
| 21 | 0.90 (d, 6.8, 3H) | 14.5 | 0.91 (d, 6.8, 3H) | 14.6 | 0.90 (d, 6.8, 3H) | 14.6 |
| 22 | 109.3 | 109.3 | 109.4 | |||
| 23 | 31.4 | 31.4 | 31.4 | |||
| 24 | 28.8 | 28.8 | 28.8 | |||
| 25 | 30.3 | 30.3 | 30.3 | |||
| 26 | 3.30 (t, 11.0) 3.41 (dd, 11.0, 3.3) | 66.9 | 3.31 (t, 11.0) 3.41 (dd, 11.0, 3.3) | 66.9 | 3.30 (t, 11.0) 3.41 (dd, 11.0, 3.3) | 66.9 |
| 27 | 0.74 (d, 7.0, 3H) | 17.2 | 0.72 (overlap, 3H) | 17.2 | 0.73 (d, 6.5, 3H) | 17.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mou, F.; Tian, J.; Li, Y.; Han, S.; Shang, R.; Song, Y.; Feng, S.; Zhang, Y.; Cao, R.; Qin, B. A Method for Improving Microbial Conversion of Diosgenin and Separation and Identification of the Product. Fermentation 2023, 9, 70. https://doi.org/10.3390/fermentation9010070
Mou F, Tian J, Li Y, Han S, Shang R, Song Y, Feng S, Zhang Y, Cao R, Qin B. A Method for Improving Microbial Conversion of Diosgenin and Separation and Identification of the Product. Fermentation. 2023; 9(1):70. https://doi.org/10.3390/fermentation9010070
Chicago/Turabian StyleMou, Fangyuan, Junmian Tian, Yulu Li, Shiyao Han, Ruifen Shang, Yuxin Song, Shirong Feng, Yongli Zhang, Rang Cao, and Baofu Qin. 2023. "A Method for Improving Microbial Conversion of Diosgenin and Separation and Identification of the Product" Fermentation 9, no. 1: 70. https://doi.org/10.3390/fermentation9010070
APA StyleMou, F., Tian, J., Li, Y., Han, S., Shang, R., Song, Y., Feng, S., Zhang, Y., Cao, R., & Qin, B. (2023). A Method for Improving Microbial Conversion of Diosgenin and Separation and Identification of the Product. Fermentation, 9(1), 70. https://doi.org/10.3390/fermentation9010070




