The Impact of Wet Fermentation on Coffee Quality Traits and Volatile Compounds Using Digital Technologies
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Physicochemical Characterisation of Coffee Brew
2.2.1. pH Measurement
2.2.2. Salt Determination
2.2.3. Colour Measurement
2.3. Near-Infrared Spectroscopy (NIR) Analysis
2.4. Identification and Quantification of Volatiles by HS-SPME-GC-MS
2.5. Electronic Nose (E-Nose) and Data Extraction
2.6. Statistical Analysis and Machine Learning (ML) Modelling
3. Results and Discussion
3.1. Physicochemical Estimation
3.1.1. Measurement of pH and Salt Content
3.1.2. Colour Measurement
3.2. Near-Infrared Spectroscopy (NIR) Analysis
3.3. Electronic Nose Outputs
3.4. Identification and Quantification of Volatile Compounds in Coffee Brew
3.5. Machine Learning Modelling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elhalis, H.; Cox, J.; Frank, D.; Zhao, J. The role of wet fermentation in enhancing coffee flavor, aroma and sensory quality. Eur. Food Res. Technol. 2021, 247, 485–498. [Google Scholar] [CrossRef]
- Bhumiratana, N.; Adhikari, K.; Chambers, E., IV. Evolution of sensory aroma attributes from coffee beans to brewed coffee. LWT-Food Sci. Technol. 2011, 44, 2185–2192. [Google Scholar] [CrossRef]
- Pereira, L.L.; Guarçoni, R.C.; Pinheiro, P.F.; Osório, V.M.; Pinheiro, C.A.; Moreira, T.R.; Ten Caten, C.S. New propositions about coffee wet processing: Chemical and sensory perspectives. Food Chem. 2020, 310, 125943. [Google Scholar] [CrossRef] [PubMed]
- Duarte, G.S.; Pereira, A.A.; Farah, A. Chlorogenic acids and other relevant compounds in Brazilian coffees processed by semi-dry and wet post-harvesting methods. Food Chem. 2010, 118, 851–855. [Google Scholar] [CrossRef]
- Silva, C.F.; Schwan, R.F.; Dias, Ë.S.; Wheals, A.E. Microbial diversity during maturation and natural processing of coffee cherries of Coffea arabica in Brazil. Int. J. Food Microbiol. 2000, 60, 251–260. [Google Scholar] [CrossRef]
- Barbosa, M.d.S.G.; dos Santos Scholz, M.B.; Kitzberger, C.S.G.; de Toledo Benassi, M. Correlation between the composition of green Arabica coffee beans and the sensory quality of coffee brews. Food Chem. 2019, 292, 275–280. [Google Scholar] [CrossRef]
- Knopp, S.; Bytof, G.; Selmar, D. Influence of processing on the content of sugars in green Arabica coffee beans. Eur. Food Res. Technol. 2006, 223, 195–201. [Google Scholar] [CrossRef]
- Haile, M.; Kang, W.H. The role of microbes in coffee fermentation and their impact on coffee quality. J. Food Qual. 2019, 2019, 4836709. [Google Scholar] [CrossRef]
- Cao, X.; Wu, H.; Gonzalez Viejo, C.; Dunshea, F.R.; Suleria, H.A. Effects of Postharvest Processing on Aroma Formation in Roasted Coffee—A Review. Int. J. Food Sci. Technol. 2022. [Google Scholar] [CrossRef]
- Zakidou, P.; Plati, F.; Matsakidou, A.; Varka, E.-M.; Blekas, G.; Paraskevopoulou, A. Single origin coffee aroma: From optimized flavor protocols and coffee customization to instrumental volatile characterization and chemometrics. Molecules 2021, 26, 4609. [Google Scholar] [CrossRef]
- Wu, H.; Lu, P.; Liu, Z.; Sharifi-Rad, J.; Suleria, H.A. Impact of roasting on the phenolic and volatile compounds in coffee beans. Food Sci. Nutr. 2022, 10, 2408–2425. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.W.; Cheong, M.W.; Curran, P.; Yu, B.; Liu, S.Q. Coffee fermentation and flavor—An intricate and delicate relationship. Food Chem. 2015, 185, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Toci, A.T.; Farah, A. Volatile fingerprint of Brazilian defective coffee seeds: Corroboration of potential marker compounds and identification of new low quality indicators. Food Chem. 2014, 153, 298–314. [Google Scholar] [CrossRef] [PubMed]
- Steen, I.; Waehrens, S.S.; Petersen, M.A.; Münchow, M.; Bredie, W.L. Influence of serving temperature on flavour perception and release of Bourbon Caturra coffee. Food Chem. 2017, 219, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Cordoba, N.; Pataquiva, L.; Osorio, C.; Moreno, F.L.M.; Ruiz, R.Y. Effect of grinding, extraction time and type of coffee on the physicochemical and flavour characteristics of cold brew coffee. Sci. Rep. 2019, 9, 8440. [Google Scholar] [CrossRef]
- Severini, C.; Derossi, A.; Ricci, I.; Fiore, A.G.; Caporizzi, R. How much caffeine in coffee cup? Effects of processing operations, extraction methods and variables. Quest. Caffeine 2017, 45–85. [Google Scholar]
- Baggenstoss, J.; Perren, R.; Escher, F. Water content of roasted coffee: Impact on grinding behaviour, extraction, and aroma retention. Eur. Food Res. Technol. 2008, 227, 1357–1365. [Google Scholar] [CrossRef]
- Zhang, K.; Cheng, J.; Hong, Q.; Dong, W.; Chen, X.; Wu, G.; Zhang, Z. Identification of changes in the volatile compounds of robusta coffee beans during drying based on HS-SPME/GC-MS and E-nose analyses with the aid of chemometrics. LWT 2022, 161, 113317. [Google Scholar] [CrossRef]
- Dzung, N.H.; Dzuan, L.; Tu, H.D. The role of sensory evaluation in food quality control, food research and development: A case of coffee study. In Proceedings of the 8th Asean Food Conference, Hanoi, Vietnam, 8–11 October 2003; pp. 862–866. [Google Scholar]
- Fuentes, S.; Gonzalez Viejo, C.; Torrico, D.D.; Dunshea, F.R. Development of a biosensory computer application to assess physiological and emotional responses from sensory panelists. Sensors 2018, 18, 2958. [Google Scholar] [CrossRef]
- Kemp, S.E.; Hollowood, T.; Hort, J. Sensory Evaluation: A Practical Handbook; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Czerny, M.; Christlbauer, M.; Christlbauer, M.; Fischer, A.; Granvogl, M.; Hammer, M.; Hartl, C.; Hernandez, N.M.; Schieberle, P. Re-investigation on odour thresholds of key food aroma compounds and development of an aroma language based on odour qualities of defined aqueous odorant solutions. Eur. Food Res. Technol. 2008, 228, 265–273. [Google Scholar] [CrossRef]
- Gonzalez Viejo, C.; Fuentes, S. Low-cost methods to assess beer quality using artificial intelligence involving robotics, an electronic nose, and machine learning. Fermentation 2020, 6, 104. [Google Scholar] [CrossRef]
- Huang, C.; Gu, Y. A Machine Learning Method for the Quantitative Detection of Adulterated Meat Using a MOS-Based E-Nose. Foods 2022, 11, 602. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, S.; Summerson, V.; Gonzalez Viejo, C.; Tongson, E.; Lipovetzky, N.; Wilkinson, K.L.; Szeto, C.; Unnithan, R.R. Assessment of smoke contamination in grapevine berries and taint in wines due to bushfires using a low-cost E-nose and an artificial intelligence approach. Sensors 2020, 20, 5108. [Google Scholar] [CrossRef]
- Rasekh, M.; Karami, H. E-nose coupled with an artificial neural network to detection of fraud in pure and industrial fruit juices. Int. J. Food Prop. 2021, 24, 592–602. [Google Scholar] [CrossRef]
- Xu, M.; Wang, J.; Zhu, L. The qualitative and quantitative assessment of tea quality based on E-nose, E-tongue and E-eye combined with chemometrics. Food Chem. 2019, 289, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Michishita, T.; Akiyama, M.; Hirano, Y.; Ikeda, M.; Sagara, Y.; Araki, T. Gas chromatography/olfactometry and electronic nose analyses of retronasal aroma of espresso and correlation with sensory evaluation by an artificial neural network. J. Food Sci. 2010, 75, S477–S489. [Google Scholar] [CrossRef]
- Romani, S.; Cevoli, C.; Fabbri, A.; Alessandrini, L.; dalla Rosa, M. Evaluation of coffee roasting degree by using electronic nose and artificial neural network for off-line quality control. J. Food Sci. 2012, 77, C960–C965. [Google Scholar] [CrossRef]
- Flambeau, K.J.; Lee, W.-J.; Yoon, J. Discrimination and geographical origin prediction of washed specialty Bourbon coffee from different coffee growing areas in Rwanda by using electronic nose and electronic tongue. Food Sci. Biotechnol. 2017, 26, 1245–1254. [Google Scholar] [CrossRef]
- Gonzalez Viejo, C.; Tongson, E.; Fuentes, S. Integrating a Low-Cost Electronic Nose and Machine Learning Modelling to Assess Coffee Aroma Profile and Intensity. Sensors 2021, 21, 2016. [Google Scholar] [CrossRef]
- Viejo, C.G.; Fuentes, S.; Godbole, A.; Widdicombe, B.; Unnithan, R.R. Development of a low-cost e-nose to assess aroma profiles: An artificial intelligence application to assess beer quality. Sens. Actuators B Chem. 2020, 308, 127688. [Google Scholar] [CrossRef]
- Gonzalez Viejo, C.; Fuentes, S.; Torrico, D.; Howell, K.; Dunshea, F.R. Assessment of beer quality based on foamability and chemical composition using computer vision algorithms, near infrared spectroscopy and machine learning algorithms. J. Sci. Food Agric. 2018, 98, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Duran, C.G.V. The Effect of Bubble Formation within Carbonated Drinks on the Brewage Foamability, Bubble Dynamics and Sensory Perception by Consumers. Ph.D. Thesis, The University of Melbourne, Melbourne, Australia, 2020. [Google Scholar]
- Gonzalez Viejo, C.; Torrico, D.; Dunshea, F.; Fuentes, S. Emerging Technologies Based on Artificial Intelligence to Assess the Quality and Consumer Preference of Beverages. Beverages 2019, 5, 62. [Google Scholar]
- Fuller, M.; Rao, N.Z. The effect of time, roasting temperature, and grind size on caffeine and chlorogenic acid concentrations in cold brew coffee. Sci. Rep. 2017, 7, 17979. [Google Scholar] [CrossRef]
- Rao, N.Z.; Fuller, M. Acidity and antioxidant activity of cold brew coffee. Sci. Rep. 2018, 8, 16030. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Lim, L.-T. Fourier transform infrared and physicochemical analyses of roasted coffee. J. Agric. Food Chem. 2012, 60, 5446–5453. [Google Scholar] [CrossRef]
- Bakke, A.J.; Stubbs, C.A.; McDowell, E.H.; Moding, K.J.; Johnson, S.L.; Hayes, J.E. Mary Poppins was right: Adding small amounts of sugar or salt reduces the bitterness of vegetables. Appetite 2018, 126, 90–101. [Google Scholar]
- Ley, J.P. Masking bitter taste by molecules. Chemosens. Percept. 2008, 1, 58–77. [Google Scholar] [CrossRef]
- Noda, K.; Amano, Y.; Shimamura, Y.; Murata, M. Distribution of pyrrolothiazolate, a pigment formed through the Maillard reaction between cysteine and glucose, in foods and beverages and some of its properties. Food Sci. Technol. Res. 2020, 26, 735–742. [Google Scholar] [CrossRef]
- Rojo-Poveda, O.; Barbosa-Pereira, L.; Mateus-Reguengo, L.; Bertolino, M.; Stévigny, C.; Zeppa, G. Effects of particle size and extraction methods on cocoa bean shell functional beverage. Nutrients 2019, 11, 867. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Ferreira, M.M.; Salva, T. Chemometric models for the quantitative descriptive sensory analysis of Arabica coffee beverages using near infrared spectroscopy. Talanta 2011, 83, 1352–1358. [Google Scholar] [CrossRef]
- Barbin, D.F.; Felicio, A.L.d.S.M.; Sun, D.-W.; Nixdorf, S.L.; Hirooka, E.Y. Application of infrared spectral techniques on quality and compositional attributes of coffee: An overview. Food Res. Int. 2014, 61, 23–32. [Google Scholar] [CrossRef]
- Okubo, N.; Kurata, Y. Nondestructive classification analysis of green coffee beans by using near-infrared spectroscopy. Foods 2019, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Díez, I.; González-Sáiz, J.; Sáenz-González, C.; Pizarro, C. Coffee varietal differentiation based on near infrared spectroscopy. Talanta 2007, 71, 221–229. [Google Scholar] [CrossRef] [PubMed]
- LeBouf, R.F.; Aldridge, M. Carbon monoxide emission rates from roasted whole bean and ground coffee. J. Air Waste Manag. Assoc. 2019, 69, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Newton, J. Carbon monoxide exposure from coffee roasting. Appl. Occup. Environ. Hyg. 2002, 17, 600–602. [Google Scholar] [CrossRef]
- Killian, B.; Rivera, L.; Soto, M.; Navichoc, D. Carbon footprint across the coffee supply chain: The case of Costa Rican coffee. J. Agric. Sci. Technol. B 2013, 3, 151. [Google Scholar]
- Anaya, A.L.; Cruz-Ortega, R.; Waller, G.R. Metabolism and ecology of purine alkaloids. Front. Biosci.-Landmark 2006, 11, 2354–2370. [Google Scholar] [CrossRef]
- Yu, J.-M.; Chu, M.; Park, H.; Park, J.; Lee, K.-G. Analysis of volatile compounds in coffee prepared by various brewing and roasting methods. Foods 2021, 10, 1347. [Google Scholar] [CrossRef]
- Diviš, P.; Pořízka, J.; Kříkala, J. The effect of coffee beans roasting on its chemical composition. Potravin. Slovak J. Food Sci. 2019, 13, 344–350. [Google Scholar] [CrossRef]
- Toledo, P.R.; Pezza, L.; Pezza, H.R.; Toci, A.T. Relationship between the different aspects related to coffee quality and their volatile compounds. Compr. Rev. Food Sci. Food Saf. 2016, 15, 705–719. [Google Scholar] [CrossRef]
- Wang, F.; Shen, H.; Liu, T.; Yang, X.; Yang, Y.; Guo, Y. Formation of pyrazines in Maillard model systems: Effects of structures of lysine-containing dipeptides/tripeptides. Foods 2021, 10, 273. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, N.; Whitworth, M.B.; Cui, C.; Fisk, I.D. Variability of single bean coffee volatile compounds of Arabica and robusta roasted coffees analysed by SPME-GC-MS. Food Res. Int. 2018, 108, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Viejo, C.; Harris, N.M.; Fuentes, S. Quality Traits of Sourdough Bread Obtained by Novel Digital Technologies and Machine Learning Modelling. Fermentation 2022, 8, 516. [Google Scholar] [CrossRef]




| Sample | Size 1 (250 µm) | Size 2 (350 µm) | Size 3 (550 µm) | Size 4 (750 µm) | |
|---|---|---|---|---|---|
| pH | |||||
| Unfermented coffee beans | UFC1 | 4.79 aC ± 0.03 | 4.77 bB ± 0.01 | 4.76 cD ± 0.01 | 4.77 bC ± 0.01 |
| UFC2 | 4.86 abB ± 0.01 | 4.81 bA ± 0.01 | 4.85 aA ± 0.02 | 4.83 bB ± 0.03 | |
| UFC3 | 4.94 aA ± 0.03 | 4.79 bB ± 0.03 | 4.80 bBC ± 0.02 | 4.77 bC ± 0.02 | |
| UFC4 | 4.89 aB ± 0.03 | 4.78 cB ± 0.01 | 4.83 bB ± 0.02 | 4.85 aB ± 0.02 | |
| UFC5 | 4.95 aA ± 0.03 | 4.83 bA ± 0.01 | 4.82 aB ± 0.02 | 4.93 aA ± 0.03 | |
| Average | 4.89 ± 0.03 | 4.80 ± 0.01 | 4.81 ± 0.02 | 4.83 ± 0.02 | |
| Fermented coffee beans | FC1 | 4.83 bB ± 0.03 | 4.82 bA ± 0.02 | 4.75 cD ± 0.01 | 4.87 aB ± 0.02 |
| FC2 | 4.77 aD ± 0.02 | 4.74 aC ± 0.02 | 4.74 aD ± 0.01 | 4.73 aD ± 0.02 | |
| FC3 | 4.80 aC ± 0.03 | 4.83 aA ± 0.02 | 4.79 aC ± 0.01 | 4.82 aB ± 0.01 | |
| FC4 | 4.80 bcC ± 0.01 | 4.79 cB ± 0.01 | 4.81 bB ± 0.02 | 4.84 aB ± 0.01 | |
| FC5 | 4.69 bE ± 0.02 | 4.66 bD ± 0.01 | 4.83 aB ± 0.04 | 4.66 bE ± 0.02 | |
| Average | 4.78 ± 0.02 | 4.77 ± 0.01 | 4.78 ± 0.02 | 4.78 ± 0.02 | |
| Salt content | |||||
| Unfermented coffee beans | UFC1 | 0.11 aBC ± 0.01 | 0.11 aBC ± 0.01 | 0.11 aB ± 0.01 | 0.10 bAB ± 0.01 |
| UFC2 | 0.12 aB ± 0.01 | 0.11 aBC ± 0.01 | 0.09 bC ± 0.01 | 0.08 bBC ± 0.01 | |
| UFC3 | 0.13 aB ± 0.01 | 0.12 bB ± 0.01 | 0.10 cBC ± 0.01 | 0.09 dB ± 0.01 | |
| UFC4 | 0.13 bB ± 0.01 | 0.14 aA ± 0.01 | 0.09 cC ± 0.01 | 0.07 dC ± 0.01 | |
| UFC5 | 0.11 bBC ± 0.01 | 0.13 aB ± 0.01 | 0.10 cBC ± 0.01 | 0.07 dC ± 0.01 | |
| Average | 0.12 ± 0.01 | 0.12 ± 0.01 | 0.10 ± 0.01 | 0.08 ± 0.01 | |
| Fermented coffee beans | FC1 | 0.12 aB ± 0.01 | 0.11 aBC ± 0.01 | 0.09 bC ± 0.01 | 0.09 bB ± 0.01 |
| FC2 | 0.12 ab ± 0.01 | 0.11 bcBC ± 0.01 | 0.13 aA ± 0.01 | 0.10 cAB ± 0.01 | |
| FC3 | 0.10 aC ± 0.01 | 0.10 aC ± 0.01 | 0.10 aBC ± 0.01 | 0.07 bC ± 0.01 | |
| FC4 | 0.19 aA ± 0.01 | 0.14 bA ± 0.01 | 0.11 cB ± 0.01 | 0.11 cA ± 0.01 | |
| FC5 | 0.11 aBC ± 0.01 | 0.10 aC ± 0.01 | 0.09 bC ± 0.01 | 0.09 bB ± 0.01 | |
| Average | 0.13 ± 0.01 | 0.11 ± 0.01 | 0.10 ± 0.01 | 0.09 ± 0.01 | |
| Sample | Size 1 (250 µm) | Size 2 (350 µm) | Size 3 (550 µm) | Size 4 (750 µm) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | L* | a* | b* | L* | a* | b* | ||
| Unfermented coffee beans | UFC1 | 50.24 de ± 0.93 | 8.05 e ± 0.59 | 54.52 ab ± 1.99 | 53.57 d ± 1.53 | 6.24 d ± 1.25 | 53.74 b ± 0.99 | 58.36 bc ± 0.96 | 4.76 ab ± 1.64 | 54.01 ab ± 2.66 | 59.98 bcde ± 1.74 | 5.44 bc ± 1.49 | 56.69 a ± 2.10 |
| UFC2 | 57.56 bc ± 0.18 | 6.71 f ± 1.89 | 52.46 bc ± 1.44 | 60.13 bc ± 0.82 | 4.17 d ± 1.69 | 51.01 b ± 0.94 | 58.57 b ± 1.52 | 5.55 a ± 0.60 | 52.96 ab ± 0.90 | 57.70 def ± 1.20 | 7.57 ab ± 0.88 | 56.76 a ± 1.47 | |
| UFC3 | 49.63 ef ± 1.21 | 12.83 bc ± 1.02 | 47.60 e ± 1.79 | 56.80 cd ± 0.68 | 10.33 bc ± 1.32 | 63.27 a ± 0.79 | 57.80 bc ± 1.70 | 7.03 a ± 1.72 | 58.05 a ± 1.98 | 69.14 a ± 1.35 | 0.64 cd ± 0.73 | 53.24 ab ± 1.59 | |
| UFC4 | 40.07 g ± 0.27 | 13.94 b ± 1.67 | 54.49 ab ± 1.06 | 44.74 e ± 1.47 | 12.29 b ± 1.47 | 37.83 c ± 1.56 | 51.65 c ± 0.93 | 7.30 a ± 1.90 | 52.72 ab ± 1.78 | 55.02 ef ± 2.72 | 3.94 bcd ± 1.68 | 42.67 bc ± 1.31 | |
| UFC5 | 39.58 g ± 0.39 | 18.91 a ± 0.35 | 52.78 bc ± 0.52 | 42.21 e ± 0.63 | 17.19 a ± 0.40 | 53.06 b ± 0.65 | 58.20 bc ± 0.66 | 6.24 a ± 0.26 | 54.65 ab ± 0.29 | 49.98 f ± 1.59 | 11.10 a ± 1.02 | 56.43 a ± 0.17 | |
| Average | 47.42 ± 0.60 | 12.09 ± 1.10 | 52.37 ± 1.36 | 51.49 ± 1.03 | 10.04 ± 1.23 | 51.78 ± 0.99 | 56.92 ± 1.15 | 6.18 ± 1.22 | 54.48 ± 1.52 | 58.36 ± 1.72 | 5.74 ± 1.16 | 53.16 ± 1.33 | |
| Fermented coffee beans | FC1 | 50.31 de ± 0.60 | 8.40 e ± 0.37 | 50.07 d ± 1.53 | 52.45 d ± 0.91 | 7.02 cd ± 0.21 | 52.65 b ± 0.72 | 57.06 bc ± 2.28 | 7.18 a ± 1.64 | 53.18 ab ± 1.28 | 65.03 abcd ± 1.25 | 2.58 bcd ± 0.36 | 45.73 abc ± 1.60 |
| FC2 | 51.93 d ± 0.37 | 10.27 d ± 0.51 | 56.25 a ± 0.87 | 54.44 e ± 1.46 | 8.97 a ± 0.72 | 57.17 ab ± 1.94 | 58.41 bc ± 0.75 | 6.98 a ± 1.05 | 57.01 ab ± 1.70 | 60.42 bcde ± 1.63 | 4.60 bcd ± 0.79 | 54.61 ab ± 1.68 | |
| FC3 | 58.94 a ± 1.22 | 2.36 gh ± 0.61 | 48.92 de ± 2.12 | 64.73 ab ± 0.82 | −0.42 e ± 0.86 | 42.03 c ± 1.82 | 63.16 ab ± 1.40 | −0.36 c ± 0.96 | 42.64 c ± 0.80 | 67.24 ab ± 1.02 | −0.67 d ± 2.12 | 40.49 c ± 1.17 | |
| FC4 | 59.63 a ± 2.18 | 3.40 g ± 1.21 | 49.97 d ± 0.75 | 69.79 a ± 1.24 | −2.40 e ± 0.61 | 39.50 c ± 2.59 | 66.45 a ± 1.30 | 0.48 bc ± 0.96 | 49.04 bc ± 2.67 | 59.28 cde ± 1.20 | 6.46 ab ± 1.51 | 56.94 a ± 1.80 | |
| FC5 | 58.78 ab ± 0.92 | 1.29 h ± 0.04 | 52.38 bc ± 1.37 | 69.92 a ± 1.26 | −0.21 e ± 1.46 | 50.43 b ± 2.92 | 69.92 a ± 2.26 | −0.21 bc ± 1.46 | 50.43 abc ± 2.92 | 66.90 abc ± 0.53 | −0.44 d ± 0.45 | 47.03 abc ± 1.39 | |
| Average | 55.92 ± 1.06 | 5.14 ± 0.55 | 51.52 ± 1.33 | 62.27 ± 1.14 | 2.59 ± 0.77 | 48.36 ± 1.99 | 63.00 ± 1.59 | 2.81 ± 1.21 | 50.46 ± 1.87 | 63.77 ± 1.13 | 2.51 ± 1.05 | 48.96 ± 1.53 | |
| ΔE | 11.01 | 13.54 | 91.1 | 15.92 | |||||||||
| No. | Compound Name | Molecular Formula | Aroma | RT * (min) | Conc. (ng/mL) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Unfermented Coffee | |||||||||
| UFC1 | UFC2 | UFC3 | UFC4 | UFC5 | |||||
| Pyridines | |||||||||
| 1 | Pyridine | C5H5N | Sour/smoky/burnt/coffee | 10.49 | 0.35–0.68 | 0.68–0.83 | 0.45–0.65 | 0.66–0.99 | 0.53–1.00 |
| Pyrazines | |||||||||
| 2 | 2-Methylpyrazine | C5H6N2 | Nutty/cocoa/roasted | 13.01 | 1.98–2.03 | 1.03–1.43 | 1.32–1.75 | 1.20–1.87 | 1.18–2.15 |
| 3 | 2,5-Dimethylpyrazine | C5H6N2 | Nutty/peanut/musty/earthy | 14.73 | 0.67–0.91 | 0.37–0.65 | 0.41–0.74 | 0.50–0.97 | 0.47–1.06 |
| 4 | 2,6-Dimethylpyrazine | C6H8N2 | Chocolate/nutty/roasted | 14.92 | 1.02–1.25 | 0.61–0.96 | 0.66–1.15 | 0.77–1.27 | 0.70–1.44 |
| 5 | 2-Ethylpyrazine | C6H8N2 | Nutty/roasted/cocoa/coffee | 15.05 | 0.71–0.83 | 0.43–0.67 | 0.63–0.73 | 0.56–0.89 | 0.55–0.93 |
| 6 | 2-Ethyl-6-methylpyrazine | C7H10N2 | Roasted potato/roasted hazelnut | 16.55 | 0.77–1.06 | 0.46–0.84 | 0.47–0.91 | 0.61–1.21 | 0.59–1.43 |
| 7 | 2-Ethyl-5-methylpyrazine | C7H10N2 | Coffee/roasted/nutty | 16.72 | 0.68–0.96 | 0.42–0.73 | 0.43–0.82 | 0.58–1.09 | 0.58–1.22 |
| Acids | |||||||||
| 8 | Acetic acid | C2H4O2 | Sour/overripe fruit | 18.34 | 0.80–0.98 | 0.43–0.83 | 0.52–0.77 | 0.52–0.88 | 0.46–0.89 |
| Furan and Furanic compounds | |||||||||
| 9 | Furfural | C5H4O2 | Sweet/woody/bready/caramellic | 18.54 | 5.65–7.13 | 9.10–10.53 | 5.83–8.75 | 8.99–13.08 | 7.24–12.03 |
| 10 | 2-Furanmethanol | C5H6O2 | Sweet/brown caramellic/bready/coffee | 23.62 | 2.74–2.99 | 2.72–3.69 | 2.48–3.48 | 2.71–4.29 | 2.62–4.10 |
| 11 | 5-Methylfurfural | C6H6O2 | Spice/caramel/bready/coffee | 21.37 | 3.10–5.72 | 5.04–7.10 | 3.04–5.61 | 4.33–8.71 | 3.96–8.03 |
| 12 | Furfuryl acetate | C7H8O3 | Fruity/banana/ethereal | 20.52 | 1.48–2.00 | 2.31–3.12 | 1.30–2.20 | 2.21–3.30 | 1.93–3.16 |
| Ketones | |||||||||
| 13 | 2-Acetylfuran | C6H6O2 | Sweet/nutty/roasted/coffee | 19.64 | 1.33–1.52 | 2.05–2.47 | 1.44–2.10 | 1.73–2.67 | 1.15–2.26 |
| Phenols | |||||||||
| 14 | 2-Methoxy-4-vinylphenol | C9H10O2 | Sweet/spicy/clove-like/smoky | 34.77 | 0.33–0.78 | 0.25–0.36 | 0.33–0.43 | 0.31–0.43 | 0.34–0.39 |
| No. | Compound Name | Molecular Formula | Aroma | RT * (min) | Conc. (ng/mL) | ||||
| Fermented Coffee | |||||||||
| FC1 | FC2 | FC3 | FC4 | FC5 | |||||
| Pyridines | |||||||||
| 1 | Pyridine | C5H5N | Sour/smoky/burnt/coffee | 10.49 | 0.37–0.49 | 0.47–0.61 | 0.34–0.42 | 0.57–0.72 | 0.29–0.36 |
| Pyrazines | |||||||||
| 2 | 2-Methylpyrazine | C5H6N2 | Nutty/cocoa/roasted | 13.01 | 2.21–2.63 | 1.59–2.24 | 1.63–2.37 | 1.46–2.05 | 1.61–1.87 |
| 3 | 2,5-Dimethylpyrazine | C5H6N2 | Nutty/peanut/musty/earthy | 14.73 | 0.79–1.17 | 0.53–0.97 | 0.55–0.97 | 0.52–0.99 | 0.59–0.67 |
| 4 | 2,6-Dimethylpyrazine | C6H8N2 | Chocolate/nutty/roasted | 14.92 | 1.19–1.61 | 0.93–1.46 | 0.38–1.44 | 0.87–1.38 | 1.05–1.14 |
| 5 | 2-Ethylpyrazine | C6H8N2 | Nutty/roasted/cocoa/coffee | 15.05 | 0.79–1.06 | 0.61–1.00 | 0.58–0.93 | 0.57–0.91 | 0.63–0.87 |
| 6 | 2-Ethyl-6-methylpyrazine | C7H10N2 | Roasted potato/roasted hazelnut | 16.55 | 0.78–1.30 | 0.71–1.28 | 0.59–1.08 | 0.69–1.26 | 0.84–0.94 |
| 7 | 2-Ethyl-5-methylpyrazine | C7H10N2 | Coffee/roasted/nutty | 16.72 | 0.75–1.19 | 0.58–1.07 | 0.59–1.04 | 0.62–1.13 | 0.72–0.81 |
| Acids | |||||||||
| 8 | Acetic acid | C2H4O2 | Sour/overripe fruit | 18.34 | 0.70–0.75 | 0.37–0.84 | 0.46–0.75 | 0.60–0.76 | 0.39–0.83 |
| Furan and Furanic compounds | |||||||||
| 9 | Furfural | C5H4O2 | Sweet/woody/bready/caramellic | 18.54 | 7.63–8.66 | 7.91–10.32 | 5.09–7.29 | 6.76–9.29 | 5.65–5.89 |
| 10 | 2-Furanmethanol | C5H6O2 | Sweet/brown caramellic/bready/coffee | 23.62 | 3.01–3.46 | 3.40–4.14 | 2.18–3.14 | 2.64–3.84 | 2.51–2.83 |
| 11 | 5-Methylfurfural | C6H6O2 | Spice/caramel/bready/coffee | 21.37 | 3.43–4.70 | 4.84–7.36 | 2.17–3.94 | 3.92–6.49 | 3.63–4.07 |
| 12 | Furfuryl acetate | C7H8O3 | Fruity/banana/ethereal | 20.52 | 1.49–2.04 | 3.82–5.00 | 0.87–1.67 | 2.01–2.99 | 1.88–2.22 |
| Ketones | |||||||||
| 13 | 2-Acetylfuran | C6H6O2 | Sweet/nutty/roasted/coffee | 19.64 | 1.47–1.86 | 1.63–2.30 | 0.91–1.57 | 1.33–2.05 | 1.41–1.45 |
| Phenols | |||||||||
| 14 | 2-Methoxy-4-vinylphenol | C9H10O2 | Sweet/spicy/clove-like/smoky | 34.77 | 0.40–0.53 | 0.36–0.47 | 0.08–0.44 | 0.35–0.42 | 0.37–0.40 |
| Stage | Samples | Accuracy | Error | Performance (MSE) |
|---|---|---|---|---|
| Model 1: Inputs: NIR; Targets: type of coffee | ||||
| Training | 126 | 99.7% | 0.3% | 0.04 |
| Testing | 54 | 91.6% | 9.4% | 0.05 |
| Overall | 180 | 93.9% | 6.1% | - |
| Model 2: Inputs: electronic nose; Targets: type of coffee | ||||
| Training | 420 | 98.4% | 1.6% | 0.02 |
| Validation | 90 | 92.4% | 7.6% | 0.07 |
| Testing | 90 | 92.4% | 7.6% | 0.07 |
| Overall | 600 | 91.2% | 8.8% | - |
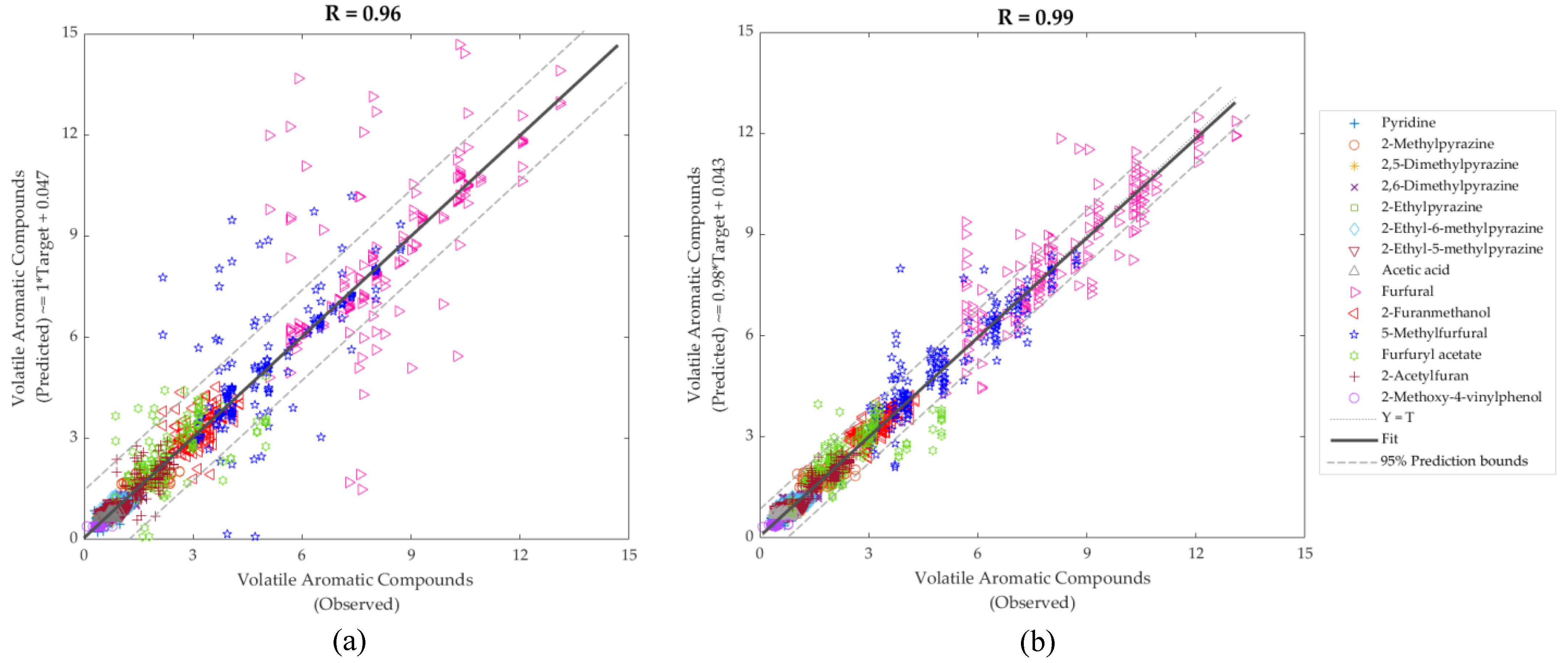
| Stage | Samples | Observations | R | Slope | Performance (MSE) |
|---|---|---|---|---|---|
| Model 3: Inputs: NIR; Targets: volatile aromatic compounds | |||||
| Training | 126 | 1764 | 0.99 | 0.99 | 0.07 |
| Testing | 54 | 756 | 0.89 | 1.00 | 1.34 |
| Overall | 180 | 2520 | 0.96 | 1.00 | - |
| Model 4: Inputs: electronic nose; Targets: volatile aromatic compounds | |||||
| Training | 420 | 5880 | 0.99 | 0.98 | 0.11 |
| Testing | 180 | 2520 | 0.98 | 1.00 | 0.25 |
| Overall | 600 | 8400 | 0.99 | 0.98 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Viejo, C.G.; Fuentes, S.; Dunshea, F.R.; Suleria, H.A.R. The Impact of Wet Fermentation on Coffee Quality Traits and Volatile Compounds Using Digital Technologies. Fermentation 2023, 9, 68. https://doi.org/10.3390/fermentation9010068
Wu H, Viejo CG, Fuentes S, Dunshea FR, Suleria HAR. The Impact of Wet Fermentation on Coffee Quality Traits and Volatile Compounds Using Digital Technologies. Fermentation. 2023; 9(1):68. https://doi.org/10.3390/fermentation9010068
Chicago/Turabian StyleWu, Hanjing, Claudia Gonzalez Viejo, Sigfredo Fuentes, Frank R. Dunshea, and Hafiz A. R. Suleria. 2023. "The Impact of Wet Fermentation on Coffee Quality Traits and Volatile Compounds Using Digital Technologies" Fermentation 9, no. 1: 68. https://doi.org/10.3390/fermentation9010068
APA StyleWu, H., Viejo, C. G., Fuentes, S., Dunshea, F. R., & Suleria, H. A. R. (2023). The Impact of Wet Fermentation on Coffee Quality Traits and Volatile Compounds Using Digital Technologies. Fermentation, 9(1), 68. https://doi.org/10.3390/fermentation9010068










