Effects of Fermentation on the Apparent Metabolizable Energy and Standardized Ileal Digestibility of Amino Acids in Soybean Meal Fed to Broiler Chickens
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Preparation of FSBM
2.3. Experiment 1: AME Measurements
2.4. Experiment 2: Ileal Digestibility Measurements
2.5. Chemical Analysis
2.6. Scanning Electron Microscopy of SBM and FSBM
2.7. Calculations
2.8. Statistical Analysis
3. Results
3.1. Effects of Fermentation on the Chemical Composition and Total Amino Acids of SBM
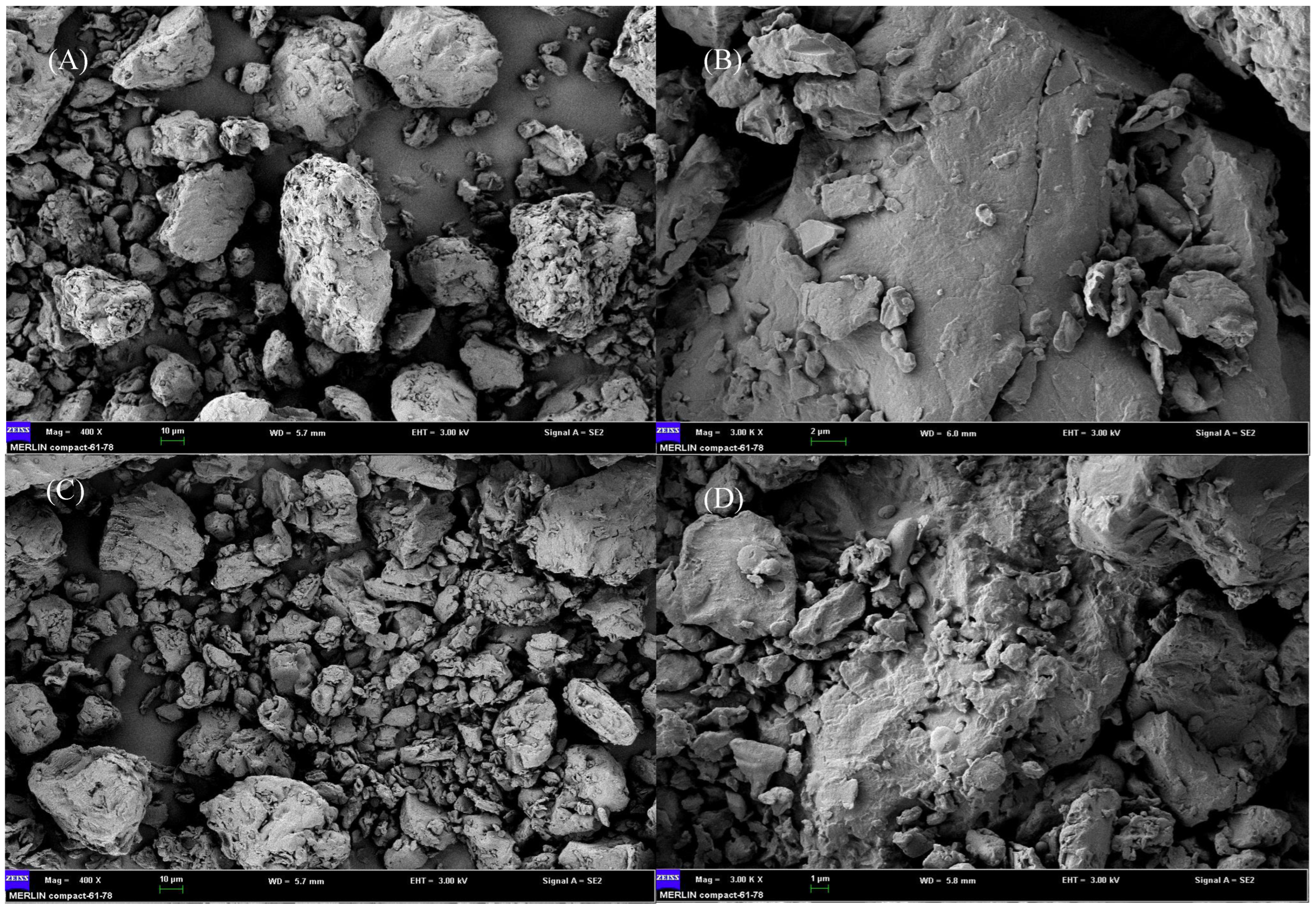
3.2. Microscopic Observation
3.3. Apparent Metabolizable Energy of SBM and FSBM
3.4. Ileal Digestibility of Amino Acids in SBM and FSBM
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, H.; Qu, Y.; Li, J.; Liu, X.; Wu, R.; Wu, J. Improvement of the protein quality and degradation of allergens in soybean meal by combination fermentation and enzymatic hydrolysis. LWT 2020, 128, 109442. [Google Scholar] [CrossRef]
- Ravindran, V.; Abdollahi, M.R.; Bootwalla, S.M. Nutrient analysis, metabolizable energy, and digestible amino acids of soybean meals of different origins for broilers. Poult. Sci. 2014, 93, 2567–2577. [Google Scholar] [CrossRef]
- Liu, W.; Liu, G.H.; Liao, R.B.; Chang, Y.L.; Huang, X.Y.; Wu, Y.B.; Yang, H.M.; Yan, H.J.; Cai, H.Y. Apparent metabolizable and net energy values of corn and soybean meal for broiler breeding cocks. Poult. Sci. 2017, 96, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Hsiao, F.S.; Wen, C.; Wu, C.; Dybus, A.; Yu, Y.; Yu, Y. Mixed fermentation of soybean meal by protease and probiotics and its effects on the growth performance and immune response in broilers. J. Appl. Anim. Res. 2019, 47, 339–348. [Google Scholar] [CrossRef]
- Soumeh, E.A.; Mohebodini, H.; Toghyani, M.; Shabani, A.; Ashayerizadeh, A.; Jazi, V. Synergistic effects of fermented soybean meal and mannan-oligosaccharide on growth performance, digestive functions, and hepatic gene expression in broiler chickens. Poult. Sci. 2019, 98, 6797–6807. [Google Scholar] [CrossRef]
- Catalán, N.; Villasante, A.; Wacyk, J.; Ramírez, C.; Romero, J. Fermented Soybean Meal Increases Lactic Acid Bacteria in Gut Microbiota of Atlantic Salmon (Salmo salar). Probiotics Antimicrob. Proteins 2018, 10, 566–576. [Google Scholar] [CrossRef]
- Peng, C.; Cao, C.; He, M.; Shu, Y.; Tang, X.; Wang, Y.; Zhang, Y.; Xia, X.; Li, Y.; Wu, J. Soybean Glycinin- and β-Conglycinin-Induced Intestinal Damage in Piglets via the p38/JNK/NF-κB Signaling Pathway. J. Agric. Food Chem. 2018, 66, 9534–9541. [Google Scholar] [CrossRef]
- Wu, J.J.; Zhang, Y.; Dong, J.H.; Cao, C.M.; Li, B.; Feng, S.B.; Ding, H.Y.; Ma, L.Y.; Wang, X.C.; Li, Y. Allergens and intestinal damage induced by soybean antigen proteins in weaned piglets. Ital. J. Anim. Sci. 2016, 15, 437–445. [Google Scholar] [CrossRef]
- Medeiros, S.; Xie, J.; Dyce, P.W.; Cai, H.Y.; Delange, K.; Zhang, H.; Li, J. Isolation of bacteria from fermented food and grass carp intestine and their efficiencies in improving nutrient value of soybean meal in solid state fermentation. J. Anim. Sci. Biotechnol. 2018, 9, 29. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, C.; Wang, C.; Lu, Z.; Wang, F.; Feng, J.; Wang, Y. Effect of soybean meal fermented with Bacillus subtilis BS12 on growth performance and small intestinal immune status of piglets. Food Agric. Immunol. 2018, 29, 133–146. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, Z.; Yu, W.; Zheng, L.; Li, L.; Gu, W.; Xu, H.; Wei, B.; Yan, X. Nutritional quality improvement of soybean meal by Bacillus velezensis and Lactobacillus plantarum during two-stage solid- state fermentation. AMB Express 2021, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.F.; Lin, L.J.; Wang, C.H.; Tsai, C.S.; Chang, S.C.; Lee, T.T. Assessment of Intestinal Immunity and Permeability of Broilers on Partial Replacement Diets of Two-Stage Fermented Soybean Meal by Bacillus velezensis and Lactobacillus brevis ATCC 367. Animals 2021, 11, 2336. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Cho, S. Changes in allergenic and antinutritional protein profiles of soybean meal during solid-state fermentation with Bacillus subtilis. LWT 2016, 70, 208–212. [Google Scholar] [CrossRef]
- Wongputtisin, P.; Khanongnuch, C.; Khongbantad, W.; Niamsup, P.; Lumyong, S. Screening and selection of Bacillus spp. for fermented corticate soybean meal production. J. Appl. Microbiol. 2012, 113, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Upadhaya, S.D.; Kim, I.H. Ileal digestibility of nutrients and amino acids in unfermented, fermented soybean meal and canola meal for weaning pigs. Anim. Sci. J. 2015, 86, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Sharawy, Z.; Goda, A.M.A.S.; Hassaan, M.S. Partial or total replacement of fish meal by solid state fermented soybean meal with Saccharomyces cerevisiae in diets for Indian prawn shrimp, Fenneropenaeus indicus, Postlarvae. Anim. Feed. Sci. Technol. 2016, 212, 90–99. [Google Scholar] [CrossRef]
- Yang, A.; Zuo, L.; Cheng, Y.; Wu, Z.; Li, X.; Tong, P.; Chen, H. Degradation of major allergens and allergenicity reduction of soybean meal through solid-state fermentation with microorganisms. Food Funct. 2018, 9, 1899–1909. [Google Scholar] [CrossRef]
- Wu, P.; Guo, Y.; Golly, M.K.; Ma, H.; He, R.; Luo, S.; Zhang, C.; Zhang, L.; Zhu, J. Feasibility study on direct fermentation of soybean meal by Bacillus stearothermophilus under non-sterile conditions. J. Sci. Food Agric. 2019, 99, 3291–3298. [Google Scholar] [CrossRef]
- Jeong, J.S.; Park, J.W.; Lee, S.I.; Kim, I.H. Apparent ileal digestibility of nutrients and amino acids in soybean meal, fish meal, spray-dried plasma protein and fermented soybean meal to weaned pigs. Anim. Sci. J. 2016, 87, 697–702. [Google Scholar] [CrossRef]
- Sinn, S.M.; Gibbons, W.R.; Brown, M.L.; Derouchey, J.M.; Levesque, C.L. Evaluation of microbially enhanced soybean meal as an alternative to fishmeal in weaned pig diets. Animal 2017, 11, 784–793. [Google Scholar] [CrossRef]
- Jeong, J.S.; Kim, I.H. Comparative efficacy of up to 50% partial fish meal replacement with fermented soybean meal or enzymatically prepared soybean meal on growth performance, nutrient digestibility and fecal microflora in weaned pigs. Anim. Sci. J. 2015, 86, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, C.D.; Oliveira, M.S.F.; Lagos, L.V.; Weeden, T.L.; Mercado, A.J.; Stein, H.H. Nutritional value of a new source of fermented soybean meal fed to growing pigs. J. Anim. Sci. 2020, 98, skaa357. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Pahm, S.K.; Stein, H.H. Ileal digestibility of amino acids in conventional, fermented, and enzyme-treated soybean meal and in soy protein isolate, fish meal, and casein fed to weanling pigs. J. Anim. Sci. 2010, 88, 2674–2683. [Google Scholar] [CrossRef] [PubMed]
- Premathilaka, K.T.; Nawarathne, S.R.; Nambapana, M.N.; Macelline, S.P.; Wickramasuriya, S.S.; Ang, L.; Jayasena, D.D.; Heo, J.M. Partial or complete replacement of fishmeal with fermented soybean meal on growth performance, fecal composition, and meat quality in broilers. J. Anim. Sci. Technol. 2020, 62, 824–839. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhang, Y.; Yin, Y.; Wang, C.; Lu, Z.; Wang, F.; Feng, J.; Wang, Y. Amino acid and phosphorus digestibility of fermented corn-soybean meal mixed feed with Bacillus subtilis and Enterococcus faecium fed to pigs. J. Anim. Sci. 2017, 95, 3996–4004. [Google Scholar] [CrossRef]
- Azam, F.; Qaisrani, S.N.; Khalique, A.; Bibi, F.; Akram, C.A.; Naveed, S.; Pasha, T.N. Exploring nutritive profile, metabolizable energy, protein, and digestible amino acids contents of indigenous protein sources of different locations for male broilers. Poult. Sci. 2019, 98, 4664–4672. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2006. [Google Scholar]
- Titgemeyer, E.C.; Armendariz, C.K.; Bindel, D.J.; Greenwood, R.H.; L Est, C.A. Evaluation of titanium dioxide as a digestibility marker for cattle. J. Anim. Sci. 2001, 79, 1059. [Google Scholar] [CrossRef]
- Mukherjee, R.; Chakraborty, R.; Dutta, A. Role of Fermentation in Improving Nutritional Quality of Soybean Meal—A Review. Asian Austral. J. Anim. 2016, 29, 1523–1529. [Google Scholar] [CrossRef]
- Li, Y.; Guo, B.; Wu, Z.; Wang, W.; Li, C.; Liu, G.; Cai, H. Effects of Fermented Soybean Meal Supplementation on the Growth Performance and Cecal Microbiota Community of Broiler Chickens. Animals 2020, 10, 1098. [Google Scholar] [CrossRef]
- Zhang, Y.; Ishikawa, M.; Koshio, S.; Yokoyama, S.; Dossou, S.; Wang, W.; Zhang, X.; Shadrack, R.S.; Mzengereza, K.; Zhu, K.; et al. Optimization of Soybean Meal Fermentation for Aqua-Feed with Bacillus subtilis natto Using the Response Surface Methodology. Fermentation 2021, 7, 306. [Google Scholar] [CrossRef]
- Gilbert, E.R.; Wong, E.A.; Webb, K.E., Jr. Board-invited review: Peptide absorption and utilization: Implications for animal nutrition and health. J. Anim. Sci. 2008, 86, 2135–2155. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, B.; Li, C.; Wang, W.; Wu, Z.; Liu, G.; Cai, H. Isolation of a Highly Efficient Antigenic-Protein-Degrading Bacillus amyloliquefaciens and Assessment of Its Safety. Animals 2020, 10, 1144. [Google Scholar] [CrossRef] [PubMed]
- Olukomaiya, O.; Fernando, C.; Mereddy, R.; Li, X.; Sultanbawa, Y. Solid-state fermented plant protein sources in the diets of broiler chickens: A review. Anim. Nutr. 2019, 5, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Nieto, R.; Miranda, A.; García, M.A.; Aguilera, J.F. The effect of dietary protein content and feeding level on the rate of protein deposition and energy utilization in growing Iberian pigs from 15 to 50 kg body weight. Brit. J. Nutr. 2002, 88, 39–49. [Google Scholar] [CrossRef]
- Perryman, K.R.; Dozier, W.A. Apparent metabolizable energy and apparent ileal amino acid digestibility of low and ultra-low oligosaccharide soybean meals fed to broiler chickens. Poultry Sci. 2012, 91, 2556–2563. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, J.; Chen, J.; Pirzado, S.A.; Li, Y.; Cai, H.; Liu, G. Effects of Fermentation on Standardized Ileal Digestibility of Amino Acids and Apparent Metabolizable Energy in Rapeseed Meal Fed to Broiler Chickens. Animals 2020, 10, 1774. [Google Scholar] [CrossRef]
- Golian, A.; Guenter, W.; Hoehler, D.; Jahanian, H.; Nyachoti, C.M. Comparison of various methods for endogenous ileal amino acid flow determination in broiler chickens. Poult. Sci. 2008, 87, 706–712. [Google Scholar] [CrossRef]
- Barua, M.; Abdollahi, M.R.; Zaefarian, F.; Wester, T.J.; Girish, C.K.; Chrystal, P.V.; Ravindran, V. Basal ileal endogenous amino acid flow in broiler chickens as influenced by age. Poult. Sci. 2021, 100, 101269. [Google Scholar] [CrossRef]
- Kong, C.; Adeola, O. Ileal endogenous amino acid flow response to nitrogen-free diets with differing ratios of corn starch to dextrose in broiler chickens. Poult. Sci. 2013, 92, 1276–1282. [Google Scholar] [CrossRef]
- de Coca-Sinova, A.; Valencia, D.G.; Jiménez-Moreno, E.; Lázaro, R.; Mateos, G.G. Apparent Ileal Digestibility of Energy, Nitrogen, and Amino Acids of Soybean Meals of Different Origin in Broilers. Poult. Sci. 2008, 87, 2613–2623. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Yi, J.Q.; Piao, X.S.; Li, P.F.; Zeng, Z.K.; Wang, D.; Liu, L.; Wang, G.Q.; Han, X. The metabolizable energy value, standardized ileal digestibility of amino acids in soybean meal, soy protein concentrate and fermented soybean meal, and the application of these products in early-weaned piglets. Asian Australas. J. Anim. Sci. 2013, 26, 691. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Begum, M.; Park, J.H.; Lee, S.J.; Jang, K.H.; Hong, Y.H.; Cho, S.J.; Kim, I.H. Apparent total tract digestibility and ileal digestibility of dry matter, nitrogen, energy and amino acids in conventional, Bacillus subtilis-fermented and enzyme-treated soybean meal fed to weanling pigs. Vet. Med. 2016, 12, 669–680. [Google Scholar] [CrossRef]
- Kim, D.H.; Heo, P.S.; Jang, J.C.; Jin, S.S.; Hong, J.S.; Kim, Y.Y. Effect of different soybean meal type on ileal digestibility of amino acid in weaning pigs. J. Anim. Sci. Technol. 2015, 57, 11. [Google Scholar] [CrossRef] [PubMed]
| Items | Basal Diet | Test Diets | |
|---|---|---|---|
| SBM | FSBM | ||
| Ingredient | |||
| Corn | 59.21 | 40.68 | 40.68 |
| Soybean meal | 28.99 | 19.91 | 19.91 |
| Fermented soybean meal | 0 | 30.00 | 30.00 |
| Corn gluten meal | 3.35 | 2.30 | 2.30 |
| Soybean oil | 4.28 | 2.94 | 2.94 |
| Dicalcium phosphate | 1.85 | 1.85 | 1.85 |
| DL-Methionine | 0.14 | 0.14 | 0.14 |
| L-Lysine | 0.13 | 0.13 | 0.13 |
| Limestone | 1.05 | 1.05 | 1.05 |
| Salt | 0.28 | 0.28 | 0.28 |
| 50% Choline Chloride | 0.10 | 0.10 | 0.10 |
| Premix 1 | 0.22 | 0.22 | 0.22 |
| TiO2 | 0.40 | 0.40 | 0.40 |
| Total | 100.00 | 100.00 | 100.00 |
| Nutrient level 2 | |||
| AME (MJ/kg) | 12.96 | 11.95 | 11.95 |
| Crude protein, % | 20.00 | 27.73 | 29.86 |
| Calcium, % | 0.90 | 0.87 | 0.87 |
| Available phosphorous, % | 0.40 | 0.38 | 0.38 |
| Lysine, % | 1.00 | 1.53 | 1.53 |
| Methionine, % | 0.40 | 0.51 | 0.51 |
| Items | NFD | SBM | FSBM |
|---|---|---|---|
| Soybean meal | 0 | 42.62 | 0 |
| Fermented soybean meal | 0 | 0 | 36.86 |
| Corn starch | 68.10 | 40.18 | 45.94 |
| Sucrose | 19.98 | 10.00 | 10.00 |
| Crystallitic cellulose | 5.00 | 0 | 0 |
| Soybean oil | 3.00 | 3.00 | 3.00 |
| Dicalcium phosphate | 1.90 | 1.90 | 1.90 |
| Limestone | 1.00 | 1.00 | 1.00 |
| Salt | 0.30 | 0.30 | 0.30 |
| 50% Choline | 0.10 | 0.10 | 0.10 |
| TiO2 | 0.40 | 0.40 | 0.40 |
| Vitamin Premix 1 | 0.02 | 0.02 | 0.02 |
| Mineral Premix | 0.20 | 0.20 | 0.20 |
| Zeolite Powder | 0 | 0.28 | 0.28 |
| Total | 100.00 | 100.00 | 100.00 |
| Nutrients levels 2 | |||
| ME (MJ/kg) | 13.31 | 12.32 | 12.50 |
| Crude protein, % | 0.20 | 19.89 | 19.92 |
| Calcium, % | 0.80 | 0.89 | 0.89 |
| Available phosphorous, % | 0.35 | 0.39 | 0.38 |
| Lysine, % | 0.01 | 1.30 | 1.19 |
| Methionine, % | 0.01 | 0.23 | 0.25 |
| Items | SBM | FSBM | Change, % |
|---|---|---|---|
| Trypsion inhibitors, mg/g | 25.87 | 8.33 | −67.80 |
| Crude fiber, % | 6.22 | 5.75 | −7.56 |
| Essential amino acid | |||
| Arg | 3.65 | 2.85 | −21.73 |
| His | 1.31 | 1.33 | 1.73 |
| Lys | 3.06 | 3.12 | 1.80 |
| Met | 0.47 | 0.55 | 17.47 |
| Thr | 2.08 | 1.94 | −6.79 |
| Ile | 2.38 | 2.50 | 5.04 |
| Leu | 4.03 | 4.25 | 5.42 |
| Phe | 2.55 | 2.83 | 11.03 |
| Val | 2.68 | 2.92 | 9.05 |
| Non-essential amino acids | |||
| Cys | 0.89 | 0.56 | −37.76 |
| Gly | 2.21 | 2.32 | 5.01 |
| Ala | 2.26 | 2.57 | 13.64 |
| Tyr | 1.63 | 1.72 | 5.52 |
| Asp | 5.99 | 6.05 | 0.97 |
| Ser | 2.75 | 2.14 | −22.04 |
| Pro | 2.54 | 2.84 | 11.47 |
| Glu | 9.92 | 11.21 | 12.98 |
| EAA | 22.20 | 22.28 | 0.39 |
| NEAA | 28.20 | 29.40 | 4.27 |
| Total | 50.39 | 51.68 | 2.56 |
| Items | SBM | FSBM | SEM | p-Value |
|---|---|---|---|---|
| AME | 10.29 | 10.62 | 0.17 | 0.12 |
| AMEn | 9.09 | 9.23 | 0.13 | 0.29 |
| AME/AMEn | 0.88 | 0.87 | - | - |
| Amino Acid | Ileal Endogenous Amino Acid Concentration (mg/kg, Dry Matter Intake) |
|---|---|
| Essential amino acids | |
| Arg | 153.80 |
| His | 80.64 |
| Lys | 157.07 |
| Met | 65.45 |
| Thr | 315.31 |
| Ile | 156.47 |
| Leu | 248.29 |
| Phe | 144.80 |
| Val | 235.77 |
| Non-essential amino acids | |
| Cys | 80.85 |
| Gly | 219.51 |
| Ala | 177.46 |
| Tyr | 154.69 |
| Asp | 401.29 |
| Ser | 304.23 |
| Pro | 253.66 |
| Glu | 459.58 |
| Items | AID, % | SID, % | ||||||
|---|---|---|---|---|---|---|---|---|
| SBM | FSBM | SEM | p-Value | SBM | FSBM | SEM | p-Value | |
| Essential amino acids | ||||||||
| Arg | 85.44 | 87.55 | 1.02 | 0.07 | 86.41 | 88.52 | 1.02 | 0.07 |
| His | 82.58 | 83.59 | 1.10 | 0.38 | 84.70 | 85.01 | 0.92 | 0.75 |
| Lys | 84.13 | 85.12 | 0.98 | 0.35 | 85.96 | 86.31 | 0.92 | 0.71 |
| Met | 85.92 | 87.94 | 1.08 | 0.09 | 88.13 | 90.15 | 1.12 | 0.09 |
| Thr | 75.23 | 77.17 | 1.38 | 0.19 | 78.27 | 80.75 | 1.39 | 0.11 |
| Ile | 80.78 | 85.17 | 1.18 | 0.04 | 83.10 | 86.74 | 1.04 | <0.01 |
| Leu | 81.31 | 84.71 | 1.12 | <0.01 | 83.51 | 86.15 | 0.96 | 0.02 |
| Phe | 82.93 | 87.31 | 0.94 | <0.01 | 84.19 | 88.58 | 0.94 | <0.01 |
| Val | 79.29 | 83.58 | 1.20 | <0.01 | 82.40 | 85.90 | 1.01 | <0.01 |
| Non-essential amino acids | ||||||||
| Cys | 67.84 | 72.15 | 1.85 | 0.04 | 71.27 | 75.58 | 1.89 | 0.04 |
| Gly | 75.16 | 77.52 | 1.31 | 0.10 | 77.46 | 79.82 | 1.31 | 0.13 |
| Ala | 81.06 | 82.55 | 1.08 | 0.20 | 83.55 | 84.35 | 0.91 | 0.41 |
| Tyr | 80.72 | 83.64 | 1.22 | 0.03 | 83.61 | 85.73 | 0.99 | 0.06 |
| Asp | 76.09 | 79.67 | 1.36 | 0.03 | 77.65 | 81.23 | 1.36 | 0.02 |
| Ser | 78.51 | 77.52 | 1.30 | 0.47 | 81.11 | 80.13 | 1.30 | 0.47 |
| Pro | 78.09 | 80.44 | 1.14 | 0.13 | 80.36 | 82.70 | 1.41 | 0.13 |
| Glu | 81.84 | 80.58 | 1.31 | 0.36 | 82.33 | 81.66 | 1.26 | 0.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Li, S.; Li, C.; Chang, W.; Cai, H.; Liu, G. Effects of Fermentation on the Apparent Metabolizable Energy and Standardized Ileal Digestibility of Amino Acids in Soybean Meal Fed to Broiler Chickens. Fermentation 2023, 9, 23. https://doi.org/10.3390/fermentation9010023
Li Y, Li S, Li C, Chang W, Cai H, Liu G. Effects of Fermentation on the Apparent Metabolizable Energy and Standardized Ileal Digestibility of Amino Acids in Soybean Meal Fed to Broiler Chickens. Fermentation. 2023; 9(1):23. https://doi.org/10.3390/fermentation9010023
Chicago/Turabian StyleLi, Yang, Shuzhen Li, Chong Li, Wenhuan Chang, Huiyi Cai, and Guohua Liu. 2023. "Effects of Fermentation on the Apparent Metabolizable Energy and Standardized Ileal Digestibility of Amino Acids in Soybean Meal Fed to Broiler Chickens" Fermentation 9, no. 1: 23. https://doi.org/10.3390/fermentation9010023
APA StyleLi, Y., Li, S., Li, C., Chang, W., Cai, H., & Liu, G. (2023). Effects of Fermentation on the Apparent Metabolizable Energy and Standardized Ileal Digestibility of Amino Acids in Soybean Meal Fed to Broiler Chickens. Fermentation, 9(1), 23. https://doi.org/10.3390/fermentation9010023








