Investigating the Relation between Skin Cell Wall Composition and Phenolic Extractability in Cabernet Sauvignon Wines
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Grape and Pomace Samples
2.3. Winemaking
2.4. Cell Wall Material Isolation
2.5. Cell Wall Material Characterization
2.5.1. Carbohydrate Composition
2.5.2. Uronic Acid Analysis
2.5.3. Phenolic Content
2.5.4. Lipid Analysis
2.5.5. Protein Analysis
2.6. Phenolic Analysis
2.7. Statistical Analysis
3. Results and Discussion
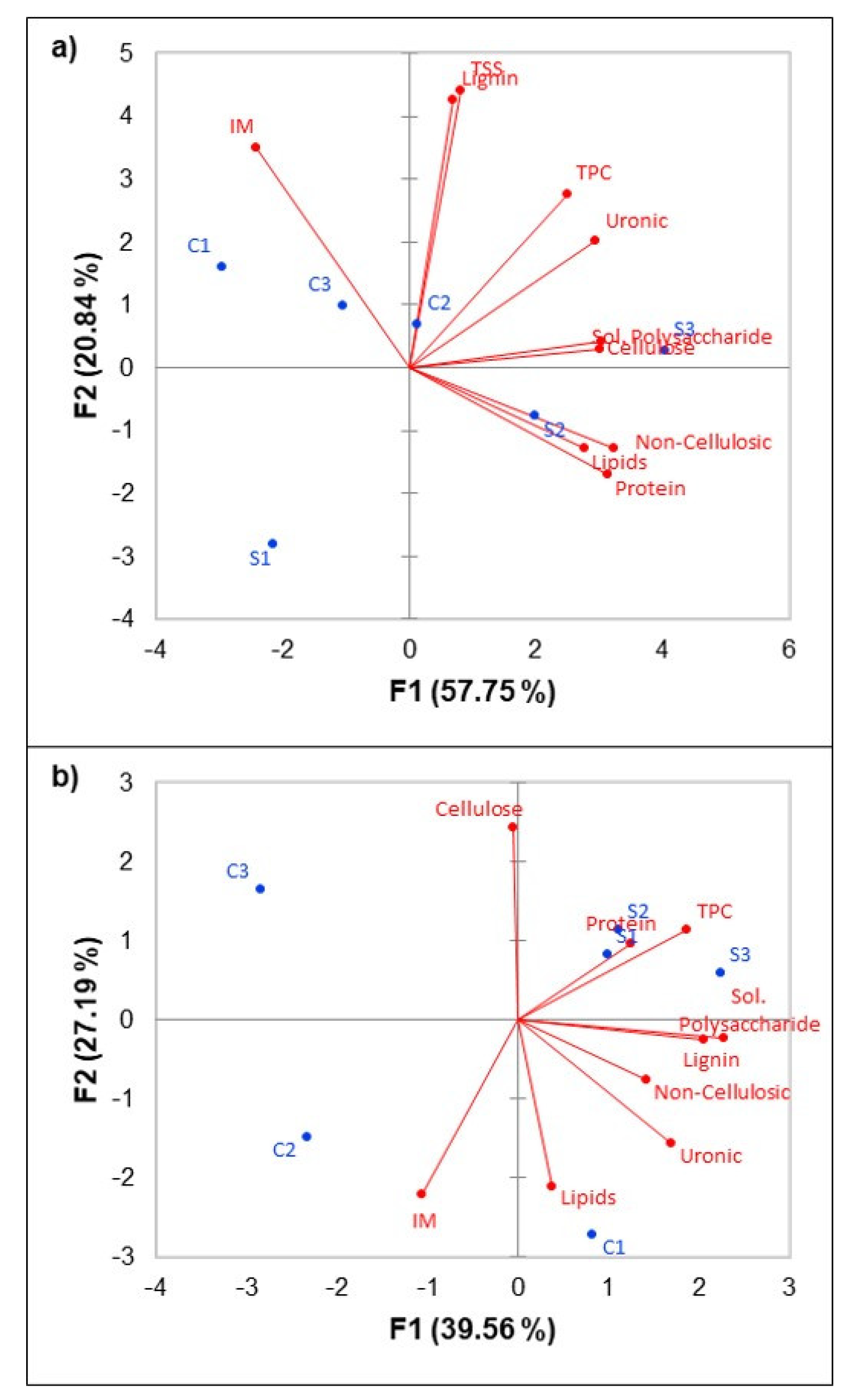
3.1. Cell Wall Material Composition
3.2. Phenolic Composition of Grapes and Wines
3.3. Correlation between CWM Composition and Wine Phenolics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Casassa, L.F.; Huff, R.; Steele, N.B. Chemical consequences of extended maceration and post-fermentation additions of grape pomace in Pinot noir and Zinfandel wines from the Central Coast of California (USA). Food Chem. 2019, 300, 125147. [Google Scholar] [CrossRef]
- Downey, M.O.; Harvey, J.S.; Robinson, S.P. Analysis of tannins in seeds and skins of Shiraz grapes throughout berry development. Aust. J. Grape Wine Res. 2003, 9, 15–27. [Google Scholar] [CrossRef]
- Lerno, L.; Reichwager, M.; Ponangi, R.; Hearne, L.; Block, D.E.; Oberholster, A. Effects of cap and overall fermentation temperature on phenolic extraction in cabernet sauvignon fermentations. Am. J. Enol. Vitic. 2015, 66, 444–453. [Google Scholar] [CrossRef]
- Boulton, R.B. The Copigmentation of Anthocynins and Its Role in the Color of Red Wine: Comments on a Critical Review. Am. J. Enol. Vitic. 2001, 52, 67–87. [Google Scholar]
- Cheynier, V.; Dueñas-Paton, M.; Salas, E.; Maury, C.; Souquet, J.-M.; Sarni-Manchado, P.; Fulcrand, H. Structure and Properties of Wine Pigments and Tannins. Am. J. Enol. Vitic. 2006, 27, 97–105. [Google Scholar]
- Hermosín-Gutierrez, I.; Sánchez-Palomo Lorenzo, E.; Vicario-Espinosa, A. Phenolic composition and magnitude of copigmentation in young and shortly aged red wines made from the cultivars, Cabernet Sauvignon, Cencibel, and Syrah. Food Chem. 2005, 92, 269–283. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.S.; Pinto, T.; Leandro, M.C.; Ricardo-Da-Silva, J.M.; Spranger, M.I. Transfer of catechins and proanthocyanidins from solid parts of the grape cluster into wine. Am. J. Enol. Vitic. 1999, 50, 179–184. [Google Scholar]
- Guidoni, S.; Hunter, J.J. Anthocyanin profile in berry skins and fermenting must/wine, as affected by grape ripeness level of Vitis vinifera cv. Shiraz/R99. Eur. Food Res. Technol. 2012, 235, 397–408. [Google Scholar] [CrossRef]
- Cagnasso, E.; Torchio, F.; Gerbi, V.; Segade, S.R.; Giacosa, S.; Rolle, L. Evolution of the Phenolic Content and Extractability Indices During Ripening of Nebbiolo Grapes from the Piedmont Growing Areas over Six Consecutive Years. South African J. Enol. Vitic. 2011, 32, 229–241. [Google Scholar] [CrossRef][Green Version]
- Romero-Cascales, I.; Ortega-Regules, A.; López-Roca, J.M.; Fernández-Fernández, J.I.; Gómez-Plaza, E. Differences in anthocyanin extractability from grapes to wines according to variety. Am. J. Enol. Vitic. 2005, 53, 54–59. [Google Scholar]
- Río Segade, S.; Soto Vázquez, E.; Díaz Losada, E. Influence of ripeness grade on accumulation and extractability of grape skin anthocyanins in different cultivars. J. Food Compos. Anal. 2008, 21, 599–607. [Google Scholar] [CrossRef]
- Medina-Plaza, C.; Dokoozlian, N.; Ponangi, R.; Blair, T.; Block, D.E.; Oberholster, A. Correlation between skin cell wall composition and polyphenol extractability of pinot noir and cabernet sauvignon grapes. Am. J. Enol. Vitic. 2021, 72, 328–337. [Google Scholar] [CrossRef]
- Apolinar-Valiente, R.; Romero-Cascales, I.; Gómez-Plaza, E.; López-Roca, J.M.; Ros-García, J.M. The composition of cell walls from grape marcs is affected by grape origin and enological technique. Food Chem. 2015, 167, 370–377. [Google Scholar] [CrossRef]
- Joutei, K.A.; Glories, Y.; Mercier, M. Localisation des tanins dans la pellicule de baie de raisin. Vitis 1994, 33, 133–138. [Google Scholar]
- Lecas, M.; Brillouet, J.-M. Cell wall composition of grape berry skins. Phytochemistry 1994, 35, 1241–1243. [Google Scholar] [CrossRef]
- Nunan, K.J.; Sims, I.M.; Bacic, A.; Robinson, S.P.; Fincher, G.B. Isolation and characterization of cell walls from the mesocarp of mature grape berries (Vitis vinifera). Planta 1997, 203, 93–100. [Google Scholar]
- Pinelo, M.; Arnous, A.; Meyer, A.S. Upgrading of grape skins: Significance of plant cell-wall structural components and extraction techniques for phenol release. Trends Food Sci. Technol. 2006, 17, 579–590. [Google Scholar] [CrossRef]
- Ortega-Regules, A.; Ros-García, J.M.; Bautista-Ortín, A.B.; López-Roca, J.M.; Gómez-Plaza, E. Changes in skin cell wall composition during the maturation of four premium wine grape varieties. J. Sci. Food Agric. 2008, 88, 420–428. [Google Scholar] [CrossRef]
- Garrido-Bañuelos, G.; Buica, A.; Schückel, J.; Zietsman, A.J.J.; Willats, W.G.T.; Moore, J.P.; Du Toit, W.J. Investigating the relationship between cell wall polysaccharide composition and the extractability of grape phenolic compounds into Shiraz wines. Part II: Extractability during fermentation into wines made from grapes of different ripeness levels. Food Chem. 2019, 278, 26–35. [Google Scholar] [CrossRef]
- Vicens, A.; Fournand, D.; Williams, P.; Sidhoum, L.; Moutounet, M.; Doco, T. Changes in Polysaccharide and Protein Composition of Cell Walls in Grape Berry Skin (Cv. Shiraz) during Ripening and Over-Ripening. Phytochemistry 2009, 57, 2955–2960. [Google Scholar] [CrossRef] [PubMed]
- Goulao, L.F.; Oliveira, C.M. Cell wall modifications during fruit ripening: When a fruit is not the fruit. Trends Food Sci. Technol. 2008, 19, 4–25. [Google Scholar] [CrossRef]
- Barnavon, L.; Doco, T.; Terrier, N.; Ageorges, A.; Romieu, C.; Pellerin, P. Involvement of pectin methyl-esterase during the ripening of grape berries: Partial cDNA isolation, transcript expression and changes in the degree of methyl-esterification of cell wall pectins. Phytochemistry 2001, 58, 693–701. [Google Scholar] [CrossRef]
- Gao, Y.; Fangel, J.U.; Willats, W.G.T.; Vivier, M.A.; Moore, J.P. Dissecting the polysaccharide-rich grape cell wall changes during winemaking using combined high-throughput and fractionation methods. Carbohydr. Polym. 2015, 133, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Guadalupe, Z.; Ayestarán, B. Polysaccharide profile and content during the vinification and aging of tempranillo red wines. J. Agric. Food Chem. 2007, 55, 10720–10728. [Google Scholar] [CrossRef]
- Miller, K.V.; Noguera, R.; Beaver, J.; Medina-Plaza, C.; Oberholster, A.; Block, D.E. A mechanistic model for the extraction of phenolics from grapes during red wine fermentation. Molecules 2019, 24, 1275. [Google Scholar] [CrossRef]
- Barham, E. Translating terroir: The global challenge of French AOC labeling. J. Rural Stud. 2003, 19, 127–138. [Google Scholar] [CrossRef]
- Morlat, R. Terroirs Viticoles: Étude et Valorisation; Oenoplurimedia: Chaintré, France, 2011. [Google Scholar]
- White, R.E. The Value of Soil Knowledge in Understanding Wine Terroir. Front. Environ. Sci. 2020, 8, 1–6. [Google Scholar] [CrossRef]
- De Vries, J.A.; Voragen, A.G.J.; Rombouts, F.M.; Pilnik, W. Extraction and purification of pectins from alcohol insoluble solids from ripe and unripe apples. Carbohydr. Polym. 1981, 1, 117–127. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.; Hamilton, J.; Rebers, P. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Olle, D.; Lozano, Y.F.; Brillouet, J.M. Isolation and characterization of soluble polysaccharides and insoluble cell wall material of the pulp from four mango (Mangifera indica L) cultivars. J. Agric. Food Chem. 1996, 44, 2658–2662. [Google Scholar] [CrossRef]
- Lurie, S.; Levin, A.; Greve, L.C.; Labavitch, J.M. Pectic polymer changes in nectarines during normal and abnormal ripening. Phytochemistry 1994, 36, 11–17. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Rosselló, C.; Simal, S.; Garau, M.C.; López, F.; Femenia, A. Physico-chemical properties of cell wall materials obtained from ten grape varieties and their byproducts: Grape pomaces and stems. LWT-Food Sci. Technol. 2010, 43, 1580–1586. [Google Scholar] [CrossRef]
- Ahmed, A.E.R.; Labavitch, J.M. A simplified method for accurate determination of cell wall uronide content. J. Food Biochem. 1978, 1, 361–365. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A.J. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Luque de Castro, M.D.; Garcia Ayuso, L.E. Environmental Applications. Soxhlet Extraction. In Encyclopedia of Separation Science; Academic Press: San Diego, CA, USA, 2000; pp. 2701–2709. [Google Scholar]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Peng, Z.; Iland, P.G.; Oberholster, A.; Sefton, M.A.; Waters, E.J. Analysis of pigmented polymers in red wine by reverse phase HPLC. Aust. J. Grape Wine Res. 2002, 8, 70–75. [Google Scholar] [CrossRef]
- Apolinar-Valiente, R.; Romero-Cascales, I.; Gómez-Plaza, E.; López-Roca, J.M.; Ros-García, J.M. Cell wall compounds of red grapes skins and their grape marcs from three different winemaking techniques. Food Chem. 2015, 187, 89–97. [Google Scholar] [CrossRef]
- Saura-Calixto, F.; Goñi, I.; Mañas, E.; Abia, R. Klason lignin, condensed tannins and resistant protein as dietary fibre constituents: Determination in grape pomaces. Food Chem. 1991, 39, 299–309. [Google Scholar] [CrossRef]
- Cassab, G.I. Plant Cell Wall Proteins. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 281–309. [Google Scholar] [CrossRef]
- Gil Cortiella, M.; Peña-Neira, Á. Extracción de polisacáridos solubles procedentes de hollejos de uva. Cienc. e Investig. Agrar. 2017, 44, 83–93. [Google Scholar]
- Bindon, K.A.; Kassara, S.; Smith, P.A. Towards a model of grape tannin extraction under wine-like conditions: The role of suspended mesocarp material and anthocyanin concentration. Aust. J. Grape Wine Res. 2017, 23, 22–32. [Google Scholar] [CrossRef]
- Osete-Alcaraz, A.; Bautista-Ortín, A.B.; Ortega-Regules, A.; Gómez-Plaza, E. Elimination of Suspended Cell Wall Material in Musts Improves the Phenolic Content and Color of Red Wines. Am. J. Enol. Vitic. 2019, 70, 201–204. [Google Scholar] [CrossRef]
- Cohen, S.D.; Tarara, J.M.; Kennedy, J.A. Diurnal temperature range compression hastens berry development and modifies flavonoid partitioning in grapes. Am. J. Enol. Vitic. 2012, 63, 112–120. [Google Scholar] [CrossRef]
- Gouot, J.C.; Smith, J.P.; Holzapfel, B.P.; Barril, C. Grape berry flavonoid responses to high bunch temperatures post véraison: Effect of intensity and duration of exposure. Molecules 2019, 24, 4341. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.D.; Tarara, J.M.; Kennedy, J.A. Assessing the impact of temperature on grape phenolic metabolism. Anal. Chim. Acta 2008, 621, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Coletta, A.; Trani, A.; Faccia, M.; Punzi, R.; Dipalmo, T.; Crupi, P.; Antonacci, D.; Gambacorta, G. Influence of viticultural practices and winemaking technologies on phenolic composition and sensory characteristics of Negroamaro red wines. Int. J. Food Sci. 2013, 48, 2215–2227. [Google Scholar] [CrossRef]
- Downey, M.O.; Dokoozlian, N.K.; Krstic, M.P. Cultural Practice and Environmental Impacts on the Flavonoid Composition of Grapes and Wine: A Review of Recent Research. Am. J. Enol. Vitic. 2006, 3, 257–268. [Google Scholar]
- Teixeira, A.; Eiras-Dias, J.; Castellarin, S.D.; Gerós, H. Phenolics of Grapevine under Challenging Environments. Int. J. Mol. Sci. 2013, 14, 18711–18739. [Google Scholar] [CrossRef]
- Hernández-Hierro, J.M.; Quijada-Morín, N.; Martínez-Lapuente, L.; Guadalupe, Z.; Ayestarán, B.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Relationship between skin cell wall composition and anthocyanin extractability of Vitis vinifera L. cv. Tempranillo at different grape ripeness degree. Food Chem. 2014, 146, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Quijada-Morín, N.; Hernandez-Hierro, J.M.; Rivas-Gonzalo, J.C.; Escribano-Bailon, M.T. Extractability of Low Molecular Mass Flavanols and Flavonols from Red Grape Skins. Relationship to Cell Wall Composition at Different Ripeness Stages. J. Agric. Food Chem. 2015, 63, 7654–7662. [Google Scholar] [CrossRef] [PubMed]



| Region | Code | Latitude | Longitude | Harvest Date | °Brix | pH | TA (g/L) |
|---|---|---|---|---|---|---|---|
| Sonoma County | S1 | 38.75926 | −122.991 | 10/13/2018 | 23.65 | 3.93 | 3.90 |
| S2 | 38.76359 | −122.977 | 10/13/2018 | 26.85 | 3.71 | 4.00 | |
| S3 | 38.64369 | −122.917 | 10/19/2018 | 25.35 | 3.75 | 4.50 | |
| Central Coast | C1 | 35.73251 | −120.652 | 10/25/2018 | 26.60 | 3.86 | 3.50 |
| C2 | 35.87655 | −120.908 | 10/26/2018 | 25.55 | 3.95 | 3.30 | |
| C3 | 35.57229 | −120.601 | 10/30/2018 | 22.90 | 3.63 | 4.40 |
| Berry Composition (mg/g Berry) | Wine Composition (mg/L Wine) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Region | Code | Flavan-3-ol * | Anthocyanin | Polymeric Pigment | Polymeric Phenol * | Flavan-3-ol * | Anthocyanin * | Polymeric Pigment | Polymeric Phenol * |
| Sonoma | S1 | 0.10 ± 0.01 a | 0.89 ± 0.04 b | 0.03 ± 0.008 a | 3.19 ± 0.73 a | 70.24 ± 0.91 a | 460.92 ± 42.50 a | 18.14 ± 1.627 a | 310.31 ± 45.35 a |
| S2 | 0.12 ± 0.01 a | 1.12 ± 0.16 a | 0.04 ± 0.002 a | 3.11 ± 0.06 a | 73.12 ± 3.22 a | 510.25 ± 17.58 a | 19.71 ± 2.25 a | 313.58 ± 13.51 a | |
| S3 | 0.11 ± 0.02 a | 1.23 ± 0.09 a | 0.03 ± 0.007 a | 3.43 ± 0.60 a | 61.38 ± 0.11 b | 525.99 ± 16.86 a | 19.59 ± 0.74 a | 342.97 ± 11.96 a | |
| C. Coast | C1 | 0.19 ± 0.03 a | 1.15 ± 0.02 a | 0.04 ± 0.002 a | 4.01 ± 0.20 ab | 75.90 ± 0.35 a | 422.14 ± 7.37 b | 18.38 ± 2.02 a | 217.73 ± 28.14 a |
| C2 | 0.14 ± 0.01 a | 0.71 ± 0.07 b | 0.03 ± 0.003 a | 3.15 ± 0.49 b | 73.79 ± 5.83 a | 472.91 ± 0.87 a | 17.47 ± 3.20 a | 272.93 ± 35.47 a | |
| C3 | 0.20 ± 0.04 a | 1.21 ± 0.12 a | 0.03 ± 0.005 a | 4.31 ± 0.58 a | 71.44 ± 1.43 a | 417.00 ± 11.63 b | 16.50 ± 1.95 a | 245.62 ± 31.44 a | |
| % Extractability | Flavan-3-ols * | Anthocyanin | Polymeric Pigments * | Polymeric Phenols * |
|---|---|---|---|---|
| S1 | 50.88 ± 5.10 a | 36.14 ± 1.84 b | 42.90 ± 2.58 a | 6.83 ± 0.41 a |
| S2 | 49.89 ± 4.50 a | 30.61 ± 1.82 bc | 39.48 ± 2.39 abc | 7.37 ± 0.43 a |
| S3 | 38.74 ± 4.20 b | 30.77 ± 1.94 bc | 40.38 ± 2.71 ab | 7.18 ± 0.38 a |
| C1 | 29.78 ± 2.34 b | 26.76 ± 0.56 cd | 33.54 ± 1.96 c | 3.97 ± 0.21 c |
| C2 | 30.76 ± 2.14 b | 43.35 ± 3.89 a | 34.46 ± 2.15 bc | 5.62 ± 0.37 b |
| C3 | 33.04 ± 2.96 b | 22.69 ± 1.08 d | 33.71 ± 2.09 c | 3.78 ± 0.26 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina-Plaza, C.; Meade, H.; Dokoozlian, N.; Ponangi, R.; Blair, T.; Block, D.E.; Oberholster, A. Investigating the Relation between Skin Cell Wall Composition and Phenolic Extractability in Cabernet Sauvignon Wines. Fermentation 2022, 8, 401. https://doi.org/10.3390/fermentation8080401
Medina-Plaza C, Meade H, Dokoozlian N, Ponangi R, Blair T, Block DE, Oberholster A. Investigating the Relation between Skin Cell Wall Composition and Phenolic Extractability in Cabernet Sauvignon Wines. Fermentation. 2022; 8(8):401. https://doi.org/10.3390/fermentation8080401
Chicago/Turabian StyleMedina-Plaza, Cristina, Haley Meade, Nick Dokoozlian, Ravi Ponangi, Tom Blair, David E. Block, and Anita Oberholster. 2022. "Investigating the Relation between Skin Cell Wall Composition and Phenolic Extractability in Cabernet Sauvignon Wines" Fermentation 8, no. 8: 401. https://doi.org/10.3390/fermentation8080401
APA StyleMedina-Plaza, C., Meade, H., Dokoozlian, N., Ponangi, R., Blair, T., Block, D. E., & Oberholster, A. (2022). Investigating the Relation between Skin Cell Wall Composition and Phenolic Extractability in Cabernet Sauvignon Wines. Fermentation, 8(8), 401. https://doi.org/10.3390/fermentation8080401








