Cultivation of Lactic Acid Bacteria and Evaluation of the Antimicrobial Potential of Partially Purified Bacteriocin-like Inhibitory Substances against Cariogenic and Food Pathogens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Culture Conditions
2.3. Co-Cultures
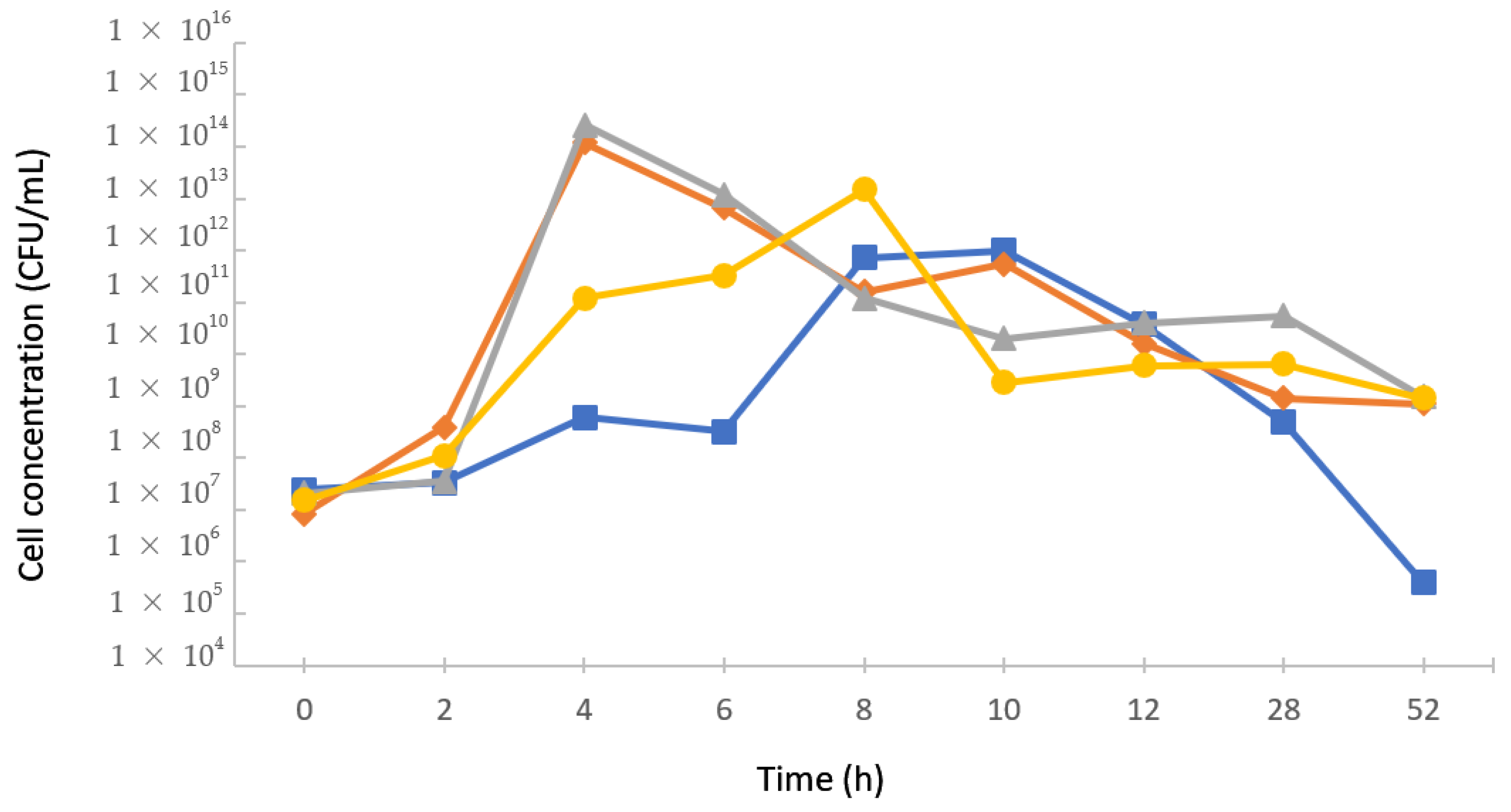
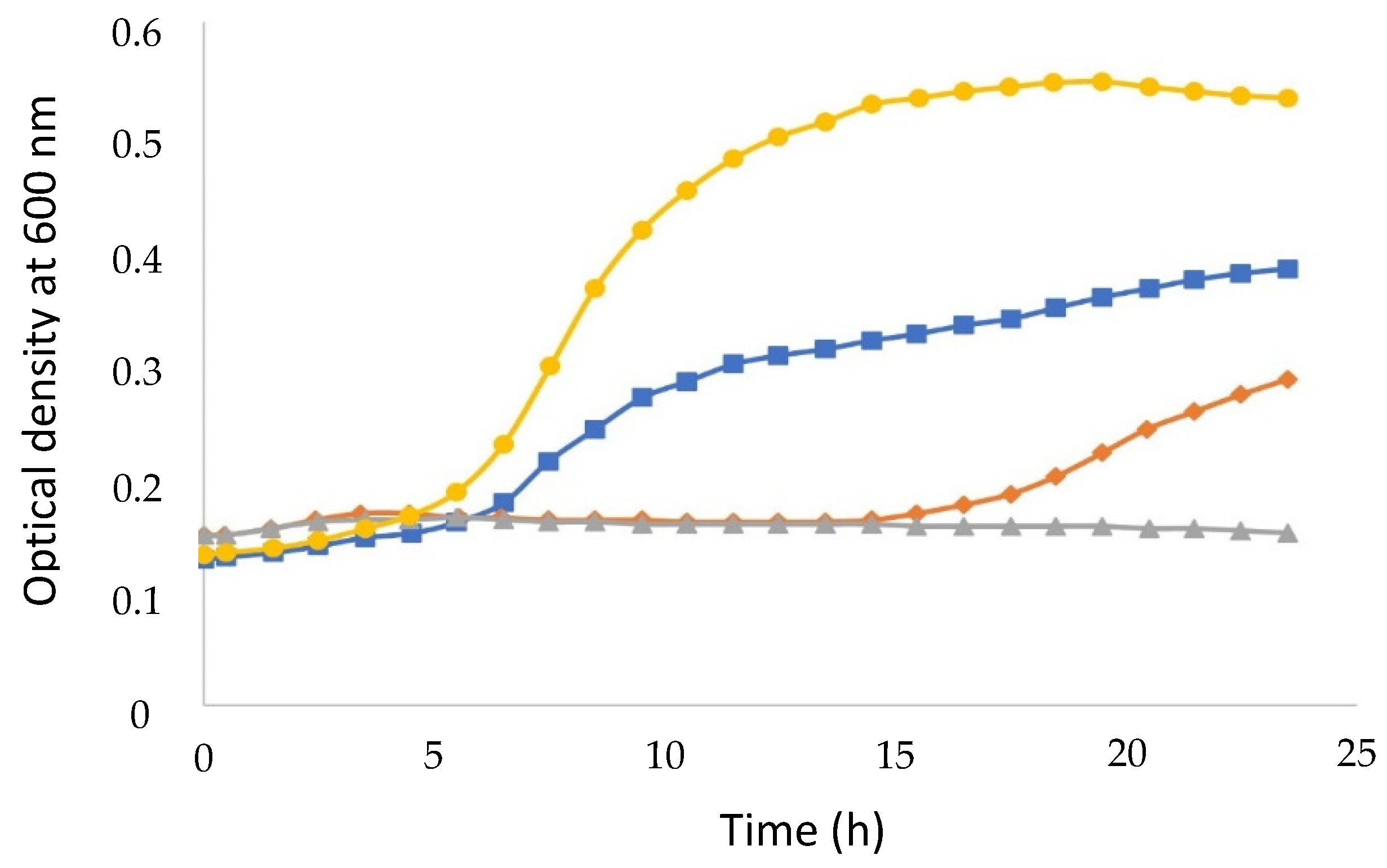
2.4. Kinetic Study of Lactic Acid Bacteria Growth
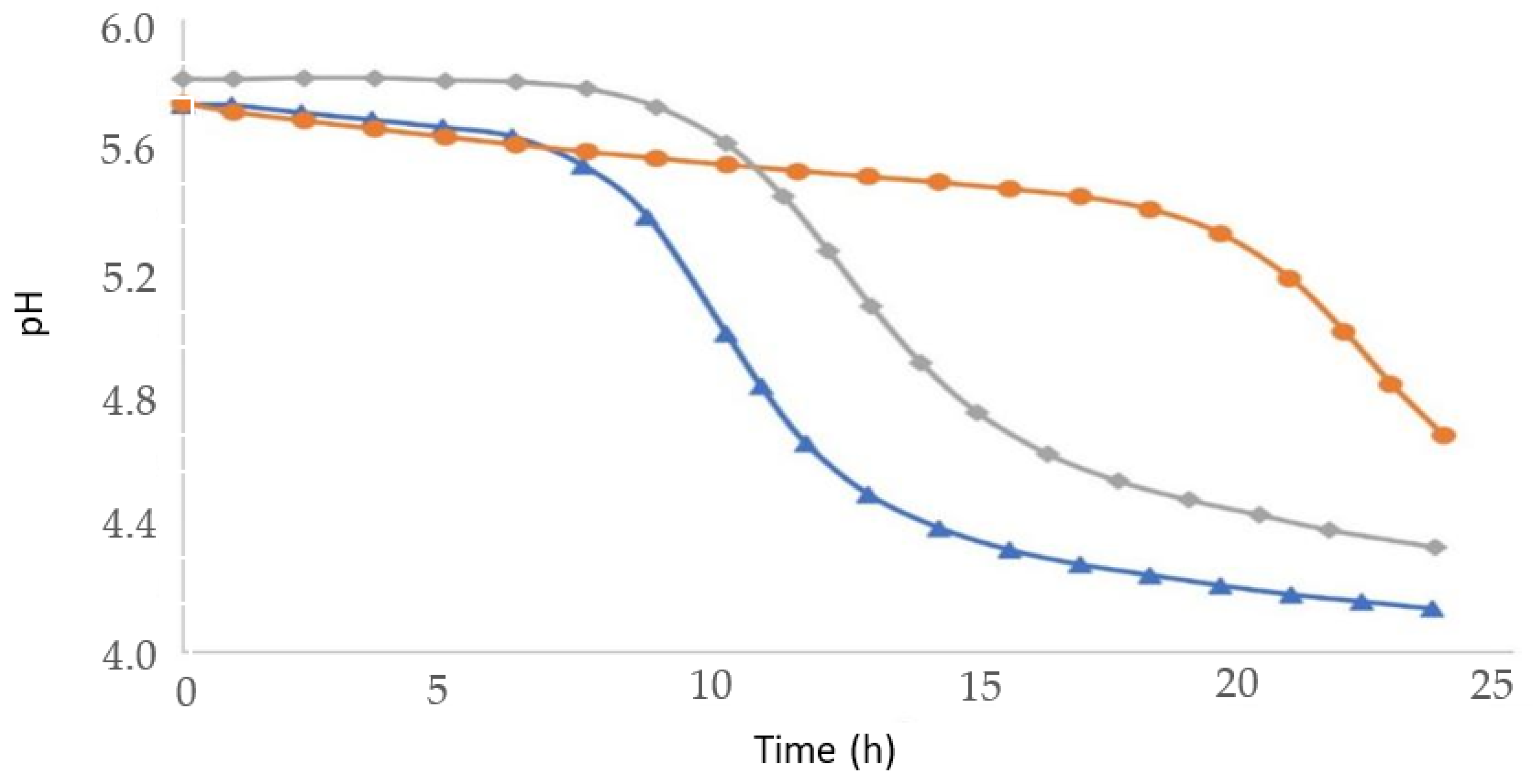
2.5. Acidification Kinetics
2.6. Determination of Antimicrobial Activity by the Critical Dilution Technique
2.7. Determination of Antimicrobial Activity against Cariogenic Microorganism
2.8. Effect of Proteases, Solvents, Salts and Detergents on BLIS
2.9. BLIS Concentration by Precipitation with Ammonium Sulfate
3. Results and Discussion
3.1. Antimicrobial Activity of BLIS by the Critical Dilution Technique
3.2. Kinetics of Lactic Bacteria Growth in Monocultures
3.3. Antimicrobial Activity against Cariogenic Microorganism
3.4. Preliminary Characterization of BLIS
3.5. BLIS Production in Co-Cultures
3.6. BLIS Concentration and Partial Purification by Salting out with Ammonium Sulfate
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Belda-Ferre, P.; Alcaraz, L.D.; Cabrera-Rubio, R.; Romero, H.; Simón-Soro, A.; Pignatelli, M.; Mira, A. The oral metagenome in health and disease. ISME J. 2012, 6, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D.; Zaura, E. Dental biofilm: Ecological interactions in health and disease. J. Clin. Periodontol. 2017, 44, S12–S22. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Gálvez, A.; Abriouel, H.; López, R.L.; Ben Omar, N. Bacteriocin-based strategies for food biopreservation. Int. J. Food Microbiol. 2007, 120, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Lata, S.; Varghese, N.O.; Varughese, J.M. Remineralization potential of fluoride and amorphous calcium phosphate-casein phospho peptide on enamel lesions: An in vitro comparative evaluation. J. Conserv. Dent. 2010, 13, 42–46. [Google Scholar] [CrossRef]
- Rubaba, M.; Muhamma, H.; Amin, N.S.; Deog-Hwan, O. Biosensors for rapid and sensitive detection of Staphylococcus aureus in food. Biosens. Bioelectron. 2018, 105, 49–57. [Google Scholar] [CrossRef]
- Mealey, B.L. Influence of periodontal infections on systemic health. Periodontol. 2000 1999, 21, 197–209. [Google Scholar] [CrossRef]
- Willis, J.R.; Gabaldón, T. The human oral microbiome in health and disease: From sequences to ecosystems. Microorganisms 2020, 8, 308. [Google Scholar] [CrossRef]
- Haque, M.; Sartelli, M.; Haque, S.Z. Dental infection and resistance-global health consequences. Dent. J. 2019, 7, 22. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO) of the United Nations Food Safety and Quality: Probiotics. Available online: http://www.fao.org/food/food-safety-quality/a-z-index/probiotics/en/ (accessed on 24 July 2022).
- Sabatini, S.; Lauritano, D.; Candotto, V.; Silvestre, F.J.; Nardi, G.M. Oral probiotics in the management of gingivitis in diabetic patients: A double blinded randomized controlled study. J. Biol. Regul. Homeost. Agents. 2017, 31, 197–202. [Google Scholar]
- Tekce, M.; Ince, G.; Gursoy, H.; Dirikan Ipci, S.; Cakar, G.; Kadir, T.; Yilmaz, S. Clinical and microbiological effects of probiotic lozenges in the treatment of chronic periodontitis: A 1-year follow-up study. J. Clin. Periodontol. 2015, 42, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.K.; Brandsborg, E.; Holmstrom, K.; Twetman, S. Effect of tablets containing probiotic candidate strains on gingival inflammation and composition of the salivary microbiome: A randomised controlled trial. Benef. Microbes. 2018, 9, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Nadkerny, P.V.; Ravishankar, P.L.; Pramod, V.; Agarwal, L.A.; Bhandari, S. A comparative evaluation of the efficacy of probiotic and chlorhexidine mouthrinses on clinical inflammatory parameters of gingivitis: A randomized controlled clinical study. J. Indian Soc. Periodontol. 2015, 19, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Grusovin, M.G.; Bossini, S.; Calza, S.; Cappa, V.; Garzetti, G.; Scotti, E.; Gherlone, E.F.; Mensi, M. Clinical efficacy of Lactobacillus reuteri-containing lozenges in the supportive therapy of generalized periodontitis stage III and IV, grade C: 1-year results of a double-blind randomized placebo-controlled pilot study. Clin. Oral Investig. 2020, 24, 2015–2024. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.F.; Salvador, S.L.; Silva, P.H.; Furlaneto, F.A.; Figueiredo, L.; Casarin, R.; Ervolino, E.; Palioto, D.B.; Souza, S.L.S.; Taba, M., Jr.; et al. Benefits of Bifidobacterium animalis subsp. lactis probiotic in experimental periodontitis. J. Periodontol. 2017, 88, 197–208. [Google Scholar] [CrossRef]
- Toiviainen, A.; Jalasvuori, H.; Lahti, E.; Gursoy, U.; Salminen, S.; Fontana, M.; Flannagan, S.; Eckert, G.; Kakaras, A.; Paster, B.; et al. Impact of orally administered lozenges with Lactobacillus rhamnosus GG and Bifidobacterium animalis subsp. lactis BB-12 on the number of salivary mutans streptococci, amount of plaque, gingival inflammation and the oral microbiome in healthy adults. Clin. Oral Investig. 2015, 19, 77–83. [Google Scholar] [CrossRef]
- Invernici, M.M.; Salvador, S.L.; Silva, P.H.F.; Soares, M.S.M.; Casarin, R.; Palioto, D.B.; Souza, S.L.S.; Taba, M., Jr.; Navaes, A.B., Jr.; Furlaneto, F.A.C.; et al. Effects of Bifidobacterium probiotic on the treatment of chronic periodontitis: A randomized clinical trial. J. Clin. Periodontol. 2018, 45, 1198–1210. [Google Scholar] [CrossRef]
- Sabo, S.S.; Vitolo, M.; Domínguez González, J.M.; Oliveira, R.P.S. Overview of Lactobacillus plantarum as a promising bacteriocins producer among lactic acid bacteria. Food Res. Int. 2014, 64, 527–536. [Google Scholar] [CrossRef]
- Balciunas, E.M.; Al Arni, S.; Converti, A.; Leblanc, J.G.; Oliveira, R.P.S. Production of bacteriocin-like inhibitory substances (BLIS) by Bifidobacterium lactis using whey as a substrate. Int. J. Dairy Technol. 2016, 69, 236–242. [Google Scholar] [CrossRef]
- Porto, M.C.W.; Kuniyoshi, T.M.; Azevedo, P.O.S.; Vitolo, M.; Oliveira, R.P.S. Pediococcus spp.: An important genus of lactic acid bacteria and pediocin producers. Biotechnol. Adv. 2017, 35, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Seminario-Amez, M.; Lopez-Lopez, J.; Estrugo-Devesa, A.; Ayuso-Montero, R.; Jane-Salas, E. Probiotics and oral health: A systematic review. Med. Oral Patol. Oral Cir. Bucal. 2017, 22, e282–e288. [Google Scholar] [CrossRef] [PubMed]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. A review of the role of probiotic supplementation in dental caries. Probiotics Antimicrob. Proteins 2020, 12, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Veiga, P.; Suez, J.; Derrien, M.; Elinav, E. Moving from probiotics to precision probiotics. Nat. Microbiol. 2020, 5, 878–880. [Google Scholar] [CrossRef]
- Piazentin, A.C.M.; Mendonça, C.M.N.; Vallejo, M.; Mussato, S.I.; Oliveira, R.P.S. Bacteriocin-like inhibitory substances production by Enterococcus faecium 135 in co-culture with Ligilactobacillus salivarius and Limosilactobacillus reuteri. Braz. J. Microbiol. 2022, 53, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Pereira, W.A.; Piazentin, A.C.M.; Oliveira, R.C.; Mendonça, C.M.N.; Tabata, Y.A.; Mendes, M.A.; Fock, R.A.; Makiyama, E.N.; Corrêa, B.; Vallejo, M.; et al. Bacteriocinogenic probiotic bacteria isolated from an aquatic environment inhibit the growth of food and fish pathogens. Sci. Rep. 2022, 12, 5530. [Google Scholar] [CrossRef] [PubMed]
- Todorov, S.D.; Dicks, L.M.T. Screening for bacteriocin-producing lactic acid bacteria from boza, a traditional cereal beverage from Bulgaria. Process. Biochem. 2006, 41, 11–19. [Google Scholar] [CrossRef]
- Martinez, F.A.C.; Domínguez, J.M.; Converti, A.; Oliveira, R.P.S. Production of bacteriocin-like inhibitory substance by Bifidobacterium lactis in skim milk supplemented with additives. J. Dairy Res. 2015, 82, 350–355. [Google Scholar] [CrossRef]
- Carvalho, K.G.; Bambirra, F.H.S.; Nicoli, J.R.; Oliveira, J.S.; Santos, A.M.C.; Bemquerer, M.P.; Miranda, A.; Franco, B.D.G.M. Characterization of multiple antilisterial peptides produced by sakacin P-producing Lactobacillus sakei subsp. sakei 2a. Arch. Microbiol. 2018, 200, 635–644. [Google Scholar] [CrossRef]
- Maria, P.; Sofia, A. Pediocins: The bacteriocins of pediococci. Sources, production, properties and applications. Microb. Cell Fact. 2009, 8, 3. [Google Scholar] [CrossRef]
- Bhalla, M.; Ingle, N.A.; Kaur, N.; Yadav, P. Mutans streptococci estimation in saliva before and after consumption of probiotic curd among school children. J. Int. Soc. Prev. Community Dent. 2015, 5, 31–34. [Google Scholar] [CrossRef]
- Todorov, S.D.; Prévost, H.; Lebois, M.; Dousset, X.; LeBlanc, J.G.; Franco, B.D.G.M. Bacteriocinogenic Lactobacillus plantarum ST16Pa isolated from papaya (Carica papaya)-From isolation to application: Characterization of a bacteriocin. Food Res. Int. 2011, 44, 1351–1363. [Google Scholar] [CrossRef]
- Sudhir, R.; Praveen, P.; Anantharaj, A.; Venkataraghavan, K. Assessment of the effect of probiotic curd consumption on salivary pH and Streptococcus mutans counts. Niger. Med. J. 2012, 53, 135–139. [Google Scholar] [CrossRef]
- Ashwin, D.; Ke, V.; Taranath, M.; Ramagoni, N.K.; Nara, A.; Sarpangala, M. Effect of probiotic containing ice-cream on salivary mutans streptococci (SMS) levels in children of 6–12 years of age: A randomized controlled double blind study with six-months follow up. J. Clin. Diagn. Res. 2015, 9, ZC06–ZC09. [Google Scholar] [CrossRef] [PubMed]
- Alegbeleye, O.O.; Singleton, I.; Sant’Ana, A.S. Sources and contamination routes of microbial pathogens to fresh produce during field cultivation: A review. Food Microbiol. 2018, 73, 177–208. [Google Scholar] [CrossRef] [PubMed]
- Jawan, R.; Abbasiliasi, S.; Mustafa, S.; Kapri, M.R.; Halim, M.; Ariff, A.B. In vitro evaluation of potential probiotic strain Lactococcus lactis Gh1 and its bacteriocin-like inhibitory substances for potential use in the food industry. Probiotics Antimicrob. Proteins 2021, 13, 422–440. [Google Scholar] [CrossRef]
- Nishant, T.; Sathish Kumar, D.; Arun Kumar, R.; Hima Bindu, K.; Raviteja, Y. Bacteriocin producing probiotic lactic acid bacteria. J. Microb. Biochem. Technol. 2011, 3, 121–124. [Google Scholar] [CrossRef]
- Barman, S.; Ghosh, R.; Mandal, D.C. Production optimization of broad spectrum bacteriocin of three strains of Lactococcus lactis isolated from homemade buttermilk. Ann. Agrar. Sci. 2018, 16, 286–296. [Google Scholar] [CrossRef]
- Oliveira, R.P.S.; Perego, P.; Nogueira, M.; Oliveira, D.; Converti, A. Effect of inulin on the growth and metabolism of a probiotic strain of Lactobacillus rhamnosus in co-culture with Streptococcus thermophilus. LWT-Food Sci. Technol. 2012, 47, 358–363. [Google Scholar] [CrossRef]



| BLIS-Producing LAB | Bioindicator Strain | |||
|---|---|---|---|---|
| Listeria innocua 2711 | Carnobacterium maltaromaticum CECT 4020 | Staphylococcus aureus CECT 239 | Escherichia coli ATCC 25922 | |
| Lactobacillus plantarum ST16 Pa | 3200 | 400 | 800 | - 1 |
| Pediococcus pentosaceus ATCC 33316 | - | - | - | - |
| Lactococcus lactis CECT-4434 | 800 | - | - | - |
| Bifidobacterium lactis BL 04 | 6400 | 800 | 800 | 400 |
| Lactobacillus sakei 2a | - | - | - | - |
| Lactobacillus lactis 27 | 1600 | 800 | 3200 | - |
| BLIS-Producing LAB | CFS 1 | CFS1 2 | CFS2 3 |
|---|---|---|---|
| Lactobacillus plantarum ST16 Pa | 1.43 | 0.94 | 0.54 |
| Lactococcus lactis CECT-4434 | 1.46 | 0.73 | 0.27 |
| Bifidobacterium lactis BL 04 | 0.8 | 0.24 | 0.12 |
| Lactobacillus lactis 27 | 1.29 | 0.64 | 0.32 |
| Treatment Agent | Bifidobacterium lactis BL 04 | Lactobacillus lactis 27 |
|---|---|---|
| Acetonitrile | 9.57 ± 0.09 | 9.41 ± 0.09 |
| Isopropanol | 11.29 ± 0.14 | 9.27 ± 0.08 |
| Sodium chloride | 11.45 ± 0.14 | 15.35 ± 0.13 |
| Ammonium sulfate | 7.81 ± 0.08 | 14.35 ± 0.12 |
| Triton 100-X | 15.07 ± 0.13 | 14.12 ± 0.12 |
| EDTA | 11.65 ± 0.11 | 15.92 ± 0.14 |
| Tween 20 | 11.18 ± 0.10 | 13.54 ± 0.12 |
| Tween 80 | 9.83 ± 0.09 | 12.35 ± 0.11 |
| SDS | 10.80 ± 0.10 | 8.23 ± 0.08 |
| Water (control) | 10.04 ± 0.10 | 8.85 ± 0.09 |
| Culture | Antimicrobial Activity (AU/mL) |
|---|---|
| Bifidobacterium lactis BL 04 | 6400 |
| Streptococcus thermophilus TA040 | 1600 |
| Bifidobacterium lactis BL 04 + Streptococcus thermophilus TA040 | 12,800 |
| Ammonium Sulfate Concentration (% w/v) | Bifidobacterium lactis BL 04 | Lactobacillus lactis 27 |
|---|---|---|
| 10 | 3200 | 800 |
| 20 | 6400 | 3200 |
| 30 | 12,800 | 3200 |
| 40 | 25,600 | 3200 |
| 50 | 51,200 | 6400 |
| 60 | 102,400 | 6400 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, A.R.S.; de Souza de Azevedo, P.O.; Converti, A.; de Souza Oliveira, R.P. Cultivation of Lactic Acid Bacteria and Evaluation of the Antimicrobial Potential of Partially Purified Bacteriocin-like Inhibitory Substances against Cariogenic and Food Pathogens. Fermentation 2022, 8, 400. https://doi.org/10.3390/fermentation8080400
da Silva ARS, de Souza de Azevedo PO, Converti A, de Souza Oliveira RP. Cultivation of Lactic Acid Bacteria and Evaluation of the Antimicrobial Potential of Partially Purified Bacteriocin-like Inhibitory Substances against Cariogenic and Food Pathogens. Fermentation. 2022; 8(8):400. https://doi.org/10.3390/fermentation8080400
Chicago/Turabian Styleda Silva, Amanda Romana Santos, Pamela Oliveira de Souza de Azevedo, Attilio Converti, and Ricardo Pinheiro de Souza Oliveira. 2022. "Cultivation of Lactic Acid Bacteria and Evaluation of the Antimicrobial Potential of Partially Purified Bacteriocin-like Inhibitory Substances against Cariogenic and Food Pathogens" Fermentation 8, no. 8: 400. https://doi.org/10.3390/fermentation8080400






