Probiotic and Antifungal Attributes of Lactic Acid Bacteria Isolates from Naturally Fermented Brazilian Table Olives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms and Growth Conditions
2.2. Tolerance to Low pH and Bile Salts
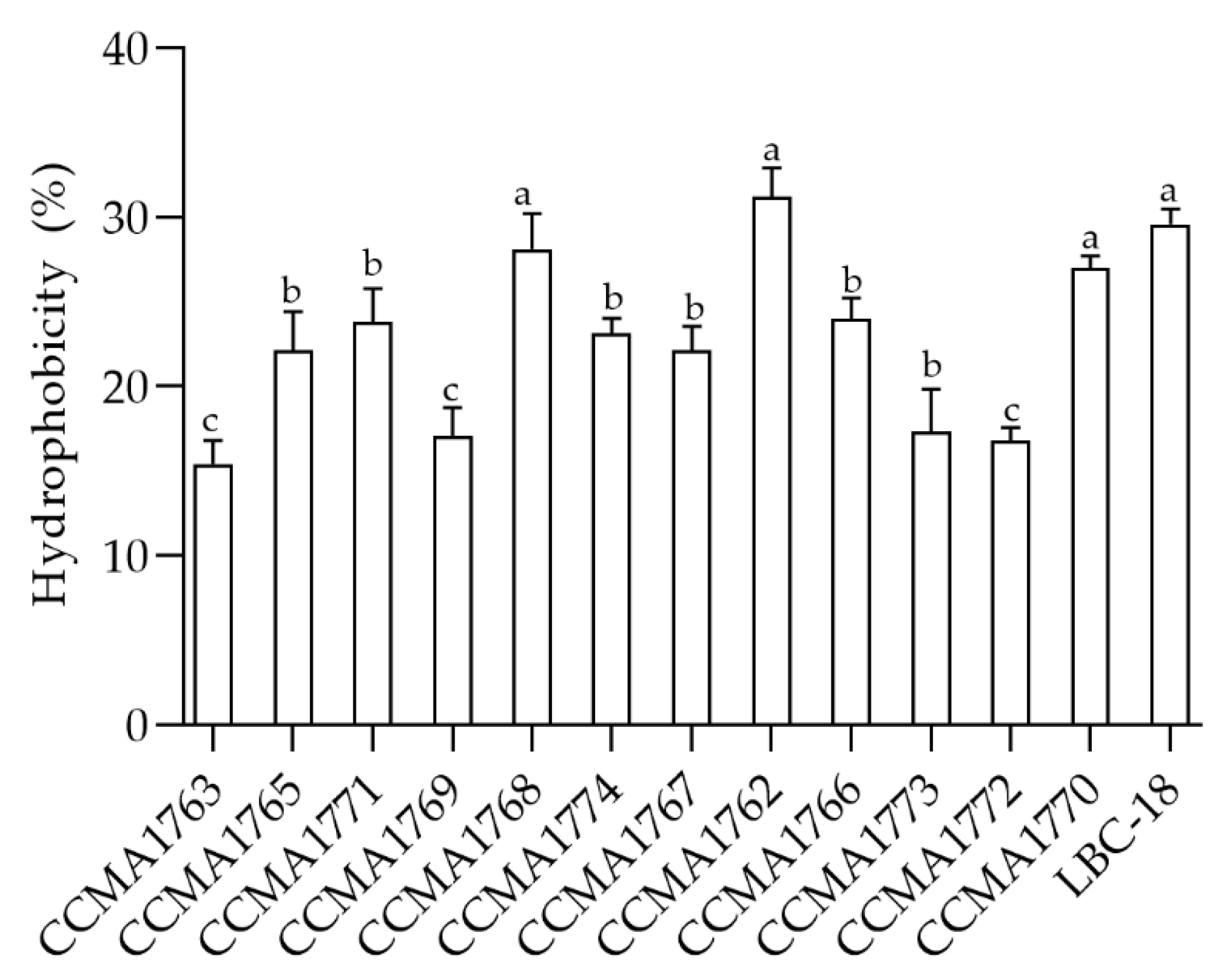
2.3. Cell Surface Hydrophobicity
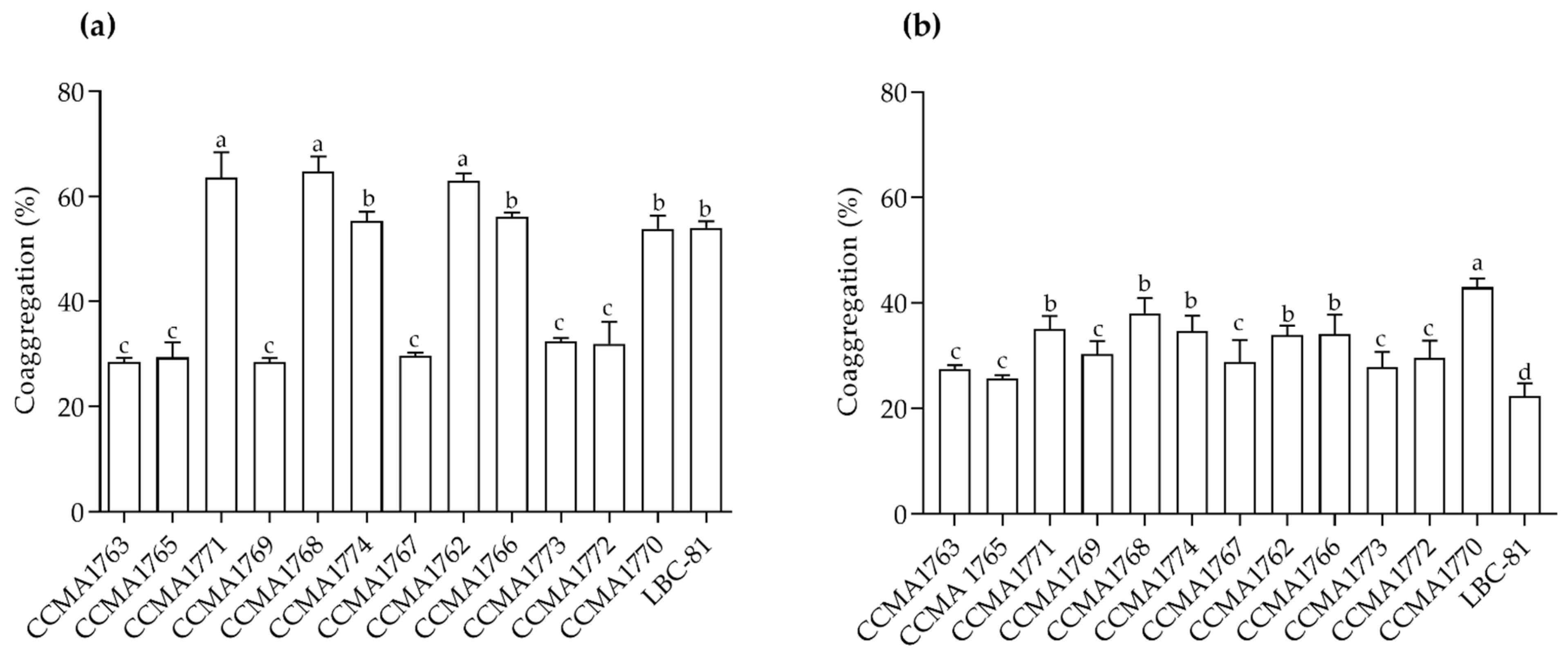
2.4. Auto-Aggregation and Coaggregation Assays
2.5. In Vitro Assessment of Safety Attributes
2.5.1. Gelatinase Activity
2.5.2. DNase Production
2.5.3. Hemolytic Activity
2.6. Antibacterial Activity
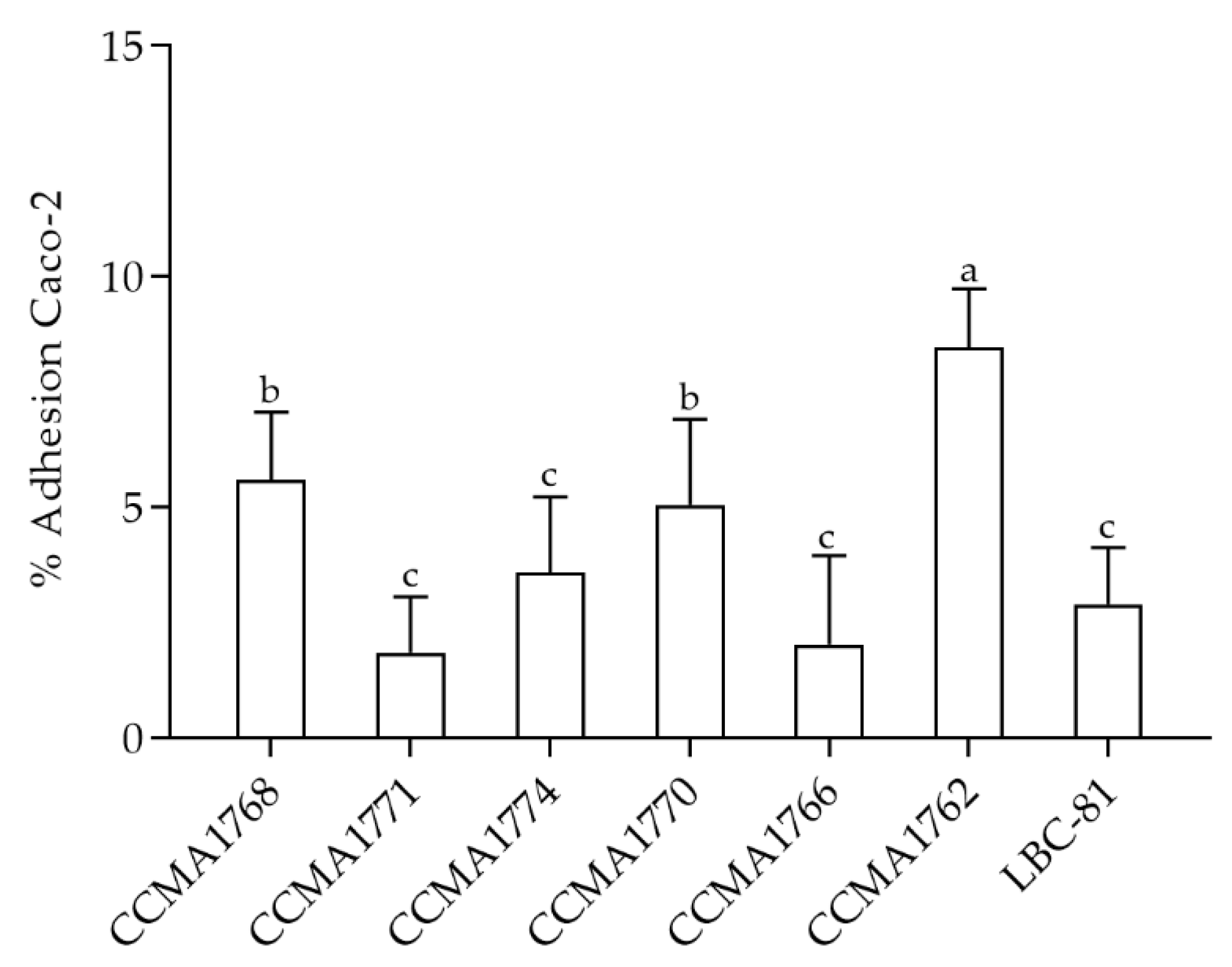
2.7. Adhesion of LAB Strains to Caco-2 Cell Lines
2.7.1. Growth and Maintenance of Caco-2 Cells
2.7.2. Adhesion to Caco-2 Cell Line
2.8. Survival of LAB during In Vitro Digestion
2.9. Antifungal Activity
2.10. Aflatoxin B1 and Ochratoxin A Removal by Selected Lactic Acid Bacteria
2.11. Mycotoxins Analysis
2.12. Statistical Analyses
3. Results
3.1. Screening of LAB Strains
3.2. Acid and Bile Salt Tolerance
3.3. Cell Surface Hydrophobicity
3.4. Auto-Aggregation and Coaggregation of the Pathogens’ Ability
3.5. In Vitro Assessment of Safety Attributes
3.6. Antibacterial Activity
3.7. Caco-2 Cell Line Adhesion Assay
3.8. Survival of LAB during In Vitro Digestion
3.9. Antifungal Activity and Elimination of Mycotoxins
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Tataridou, M.; Kotzekidou, P. Fermentation of table olives by oleuropeinolytic starter culture in reduced salt brines and inactivation of Escherichia coli O157: H7 and Listeria monocytogenes. Int. J. Food Microbiol. 2015, 208, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Ciafardini, G.; Venditti, G.; Zullo, B. Yeast dynamics in the black table olives processing using fermented brine as starter. Food Res. 2021, 5, 92–106. [Google Scholar] [CrossRef]
- IOC (International Olive Council). Table Olives Imports 2020/21 Crop Year. Available online: https://www.internationaloliveoil.org/table-olives-2020-21-crop-year/ (accessed on 16 December 2021).
- Huertas-Alonso, A.J.; Gonzalez-Serrano, D.J.; Hadidi, M.; Salgado-Ramos, M.; Orellana-Palacios, J.C.; Sánchez-Verdú, M.P.; Xia, Q.; Simirgiotis, M.J.; Barba, F.J.; Dar, B.N. Table Olive Wastewater as a Potential Source of Biophenols for Valorization: A Mini Review. Fermentation 2022, 8, 215. [Google Scholar] [CrossRef]
- Bonatsou, S.; Tassou, C.C.; Panagou, E.Z.; Nychas, G.-J.E. Table olive fermentation using starter cultures with multifunctional potential. Microorganisms 2017, 5, 30. [Google Scholar] [CrossRef] [Green Version]
- Peres, C.M.; Peres, C.; Hernández-Mendoza, A.; Malcata, F.X. Review on fermented plant materials as carriers and sources of potentially probiotic lactic acid bacteria–with an emphasis on table olives. Trends Food Sci. Technol. 2012, 26, 31–42. [Google Scholar] [CrossRef]
- Abriouel, H.; Pérez Montoro, B.; de la Fuente Ordoñez, J.J.; Lavilla Lerma, L.; Knapp, C.W.; Benomar, N. New insights into the role of plasmids from probiotic Lactobacillus pentosus MP-10 in Aloreña table olive brine fermentation. Sci. Rep. 2019, 9, 10938. [Google Scholar]
- Saxami, G.; Karapetsas, A.; Lamprianidou, E.; Kotsianidis, I.; Chlichlia, A.; Tassou, C.; Zoumpourlis, V.; Galanis, A. Two potential probiotic lactobacillus strains isolated from olive microbiota exhibit adhesion and antiproliferative effects in cancer cell lines. J. Funct. Foods 2016, 24, 461–471. [Google Scholar] [CrossRef]
- Saxami, G.; Karapetsas, A.; Chondrou, P.; Vasiliadis, S.; Lamprianidou, E.; Kotsianidis, I.; Ypsilantis, P.; Botaitis, S.; Simopoulos, C.; Galanis, A. Potentially probiotic Lactobacillus strains with antiproliferative activity induce cytokine/chemokine production and neutrophil recruitment in mice. Benef. Microbes 2017, 8, 615–623. [Google Scholar] [CrossRef]
- Baccouri, O.; Boukerb, A.M.; Farhat, L.B.; Zébré, A.; Zimmermann, K.; Domann, E.; Cambronel, M.; Barreau, M.; Maillot, O.; Rincé, I. Probiotic potential and safety evaluation of Enterococcus faecalis OB14 and OB15, isolated from traditional tunisian testouri cheese and rigouta, using physiological and genomic analysis. Front. Microbiol. 2019, 10, 881. [Google Scholar] [CrossRef]
- Lee, C.S.; Kim, S.H. Anti-inflammatory and anti-osteoporotic potential of Lactobacillus plantarum A41 and L. fermentum SRK414 as probiotics. Probiotics Antimicrob. Proteins 2020, 12, 623–634. [Google Scholar] [CrossRef]
- Abouloifa, H.; Rokni, Y.; Ghabbour, N.; Karboune, S.; Brasca, M.; D’hallewin, G.; Salah, R.B.; Ktari, N.; Saalaoui, E.; Asehraou, A. Probiotics from fermented olives. In Olives and Olive Oil in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2021; pp. 215–229. [Google Scholar]
- Sanders, M.E.; Akkermans, L.M.; Haller, D.; Hammerman, C.; Heimbach, J.T.; Hörmannsperger, G.; Huys, G. Safety assessment of probiotics for human use. Gut Microbes 2010, 1, 164–185. [Google Scholar] [CrossRef]
- Abouloifa, H.; Rokni, Y.; Bellaouchi, R.; Hasnaoui, I.; Gaamouche, S.; Ghabbour, N.; Chaoui, J.; Brasca, M.; Karboune, S.; Salah, R.B. Technological properties of potential probiotic lactobacillus strains isolated from traditional fermenting green olive. J. Microbiol. Biotechnol. Food Sci. 2020, 9, 884–889. [Google Scholar] [CrossRef]
- Peyer, L.C.; Axel, C.; Lynch, K.M.; Zannini, E.; Jacob, F.; Arendt, E.K. Inhibition of Fusarium culmorum by carboxylic acids released from lactic acid bacteria in a barley malt substrate. Food Control 2016, 69, 227–236. [Google Scholar] [CrossRef]
- Taroub, B.; Salma, L.; Manel, Z.; Ouzari, H.-I.; Hamdi, Z.; Moktar, H. Isolation of lactic acid bacteria from grape fruit: Antifungal activities, probiotic properties, and in vitro detoxification of ochratoxin A. Ann. Microbiol. 2019, 69, 17–27. [Google Scholar] [CrossRef]
- Ilha, E.C.; Da Silva, T.; Lorenz, J.G.; de Oliveira Rocha, G.; Sant’Anna, E.S. Lactobacillus paracasei isolated from grape sourdough: Acid, bile, salt, and heat tolerance after spray drying with skim milk and cheese whey. Eur. Food Res. Technol. 2015, 240, 977–984. [Google Scholar] [CrossRef]
- Fonseca, H.C.; de Sousa Melo, D.; Ramos, C.L.; Dias, D.R.; Schwan, R.F. Probiotic properties of lactobacilli and their ability to inhibit the adhesion of enteropathogenic bacteria to Caco-2 and HT-29 cells. Probiotics Antimicrob. Proteins 2021, 13, 102–112. [Google Scholar] [CrossRef]
- Ramos, C.L.; Thorsen, L.; Schwan, R.F.; Jespersen, L. Strain-specific probiotics properties of Lactobacillus fermentum, Lactobacillus plantarum and Lactobacillus brevis isolates from Brazilian food products. Food Microbiol. 2013, 36, 22–29. [Google Scholar] [CrossRef] [Green Version]
- dos Santos, K.M.O.; Vieira, A.D.S.; Buriti, F.C.A.; do Nascimento, J.C.F.; de Melo, M.E.S.; Bruno, L.M.; de Fátima Borges, M.; Rocha, C.R.C.; de Souza Lopes, A.C.; de Melo Franco, B.D.G. Artisanal Coalho cheeses as source of beneficial Lactobacillus plantarum and Lactobacillus rhamnosus strains. Dairy Sci. Technol. 2015, 95, 209–230. [Google Scholar] [CrossRef]
- Del Re, B.; Sgorbati, B.; Miglioli, M.; Palenzona, D. Adhesion, autoaggregation and hydrophobicity of 13 strains of Bifidobacterium longum. Lett. Appl. Microbiol. 2000, 31, 438–442. [Google Scholar] [CrossRef]
- Handley, P.S.; Harty, D.W.; Wyatt, J.E.; Brown, C.R.; Doran, J.P.; Gibbs, A.C. A comparison of the adhesion, coaggregation and cell-surface hydrophobicity properties of fibrillar and fimbriate strains of Streptococcus salivarius. Microbiology 1987, 133, 3207–3217. [Google Scholar] [CrossRef] [Green Version]
- Gupta, H.; Malik, R.K. Incidence of virulence in bacteriocin-producing enterococcal isolates. Le Lait 2007, 87, 587–601. [Google Scholar] [CrossRef]
- Youssef, N.H.; Duncan, K.E.; Nagle, D.P.; Savage, K.N.; Knapp, R.M.; McInerney, M.J. Comparison of methods to detect biosurfactant production by diverse microorganisms. J. Microbiol. Methods 2004, 56, 339–347. [Google Scholar] [CrossRef]
- Prado, C.; Santos, W.; Carvalho, C.; Moreira, E.; Costa, O. Antimicrobial activity of lactic acid bacteria isolated from Brazilian dry fermented sausages against Listeria monocytogenes. Arq. Bras. Med. Vet. Zootec. 2000, 52, 417–423. [Google Scholar] [CrossRef]
- Madureira, A.R.; Amorim, M.; Gomes, A.M.; Pintado, M.E.; Malcata, F.X. Protective effect of whey cheese matrix on probiotic strains exposed to simulated gastrointestinal conditions. Food Res. Int. 2011, 44, 465–470. [Google Scholar] [CrossRef]
- Guimarães, A.; Santiago, A.; Teixeira, J.A.; Venâncio, A.; Abrunhosa, L. Anti-aflatoxigenic effect of organic acids produced by Lactobacillus plantarum. Int. J. Food Microbiol. 2018, 264, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Taheur, F.B.; Fedhila, K.; Chaieb, K.; Kouidhi, B.; Bakhrouf, A.; Abrunhosa, L. Adsorption of aflatoxin B1, zearalenone and ochratoxin A by microorganisms isolated from Kefir grains. Int. J. Food Microbiol. 2017, 251, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Soares, C.; Rodrigues, P.; Freitas-Silva, O.; Abrunhosa, L.; Venâncio, A. HPLC method for simultaneous detection of aflatoxins and cyclopiazonic acid. World Mycotoxin J. 2010, 3, 225–231. [Google Scholar] [CrossRef]
- Abrunhosa, L.; Inês, A.; Rodrigues, A.I.; Guimarães, A.; Pereira, V.L.; Parpot, P.; Mendes-Faia, A.; Venâncio, A. Biodegradation of ochratoxin A by Pediococcus parvulus isolated from Douro wines. Int. J. Food Microbiol. 2014, 188, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, D.F. Sisvar: A computer statistical analysis system. Ciênc. Agrotecnol. 2011, 35, 1039–1042. [Google Scholar] [CrossRef] [Green Version]
- Kos, B.; Šušković, J.; Vuković, S.; Šimpraga, M.; Frece, J.; Matošić, S. Adhesion and aggregation ability of probiotic strain Lactobacillus acidophilus M92. J. Appl. Microbiol. 2003, 94, 981–987. [Google Scholar] [CrossRef] [Green Version]
- Shah, N. Probiotic bacteria: Selective enumeration and survival in dairy foods. J. Dairy Sci. 2000, 83, 894–907. [Google Scholar] [CrossRef]
- Soni, R.; Jain, N.K.; Shah, V.; Soni, J.; Suthar, D.; Gohel, P. Development of probiotic yogurt: Effect of strain combination on nutritional, rheological, organoleptic and probiotic properties. J. Food Sci. Technol. 2020, 57, 2038–2050. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, M.K.; Giri, S.K. Probiotic functional foods: Survival of probiotics during processing and storage. J. Funct. Foods 2014, 9, 225–241. [Google Scholar] [CrossRef]
- IOC (International Olive Council). Trade Standard Applying to Table Olives; COI/OT/NC No. 1; IOC: Madrid, Spain, 2004. [Google Scholar]
- Lanza, B.; Zago, M.; Di Marco, S.; Di Loreto, G.; Cellini, M.; Tidona, F.; Bonvini, B.; Bacceli, M.; Simone, N. Single and Multiple Inoculum of Lactiplantibacillus plantarum Strains in Table Olive Lab-Scale Fermentations. Fermentation 2020, 6, 126. [Google Scholar] [CrossRef]
- Krausova, G.; Hyrslova, I.; Hynstova, I. In vitro evaluation of adhesion capacity, hydrophobicity, and auto-aggregation of newly isolated potential probiotic strains. Fermentation 2019, 5, 100. [Google Scholar] [CrossRef] [Green Version]
- Sharma, K.; Sharma, N.; Sharma, R. Identification and evaluation of in vitro probiotic attributes of novel and potential strains of lactic acid bacteria isolated from traditional dairy products of North-West Himalayas. J. Clin. Microbiol. Biochem. Technol. 2016, 2, 018–025. [Google Scholar] [CrossRef]
- Cozzolino, A.; Vergalito, F.; Tremonte, P.; Iorizzo, M.; Lombardi, S.J.; Sorrentino, E.; Luongo, D.; Coppola, R.; Di Marco, R.; Succi, M. Preliminary evaluation of the safety and probiotic potential of Akkermansia muciniphila DSM 22959 in comparison with Lactobacillus rhamnosus GG. Microorganisms 2020, 8, 189. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Zhou, T.; Tang, H.; Li, X.; Chen, Y.; Zhang, L.; Zhang, J. Probiotic potential and amylolytic properties of lactic acid bacteria isolated from Chinese fermented cereal foods. Food Control 2020, 111, 107057. [Google Scholar] [CrossRef]
- Kenfack, C.H.M.; Ngoufack, F.Z.; Kaktcham, P.M.; Wang, Y.R.; Zhu, T.; Yin, L. Safety and antioxidant properties of five probiotic Lactobacillus plantarum strains isolated from the digestive tract of honey bees. Am. J. Microbiol. Res. 2018, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Dicks, L.; Botes, M. Probiotic lactic acid bacteria in the gastrointestinal tract: Health benefits, safety and mode of action. Benef. Microbes 2010, 1, 11–29. [Google Scholar] [CrossRef]
- Fontana, L.; Bermudez-Brito, M.; Plaza-Diaz, J.; Munoz-Quezada, S.; Gil, A. Sources, isolation, characterisation and evaluation of probiotics. Br. J. Nutr. 2013, 109, S35–S50. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.-H.; He, L.; Zhou, Y.; Wu, C.-H.; Jong, A. Lactobacillus rhamnosus GG suppresses meningitic E. coli K1 penetration across human intestinal epithelial cells in vitro and protects neonatal rats against experimental hematogenous meningitis. Int. J. Microbiol. 2009, 2009, 647862. [Google Scholar] [CrossRef] [Green Version]
- Cheon, M.-J.; Lim, S.-M.; Lee, N.-K.; Paik, H.-D. Probiotic properties and neuroprotective effects of Lactobacillus buchneri KU200793 isolated from Korean fermented foods. Int. J. Mol. Sci. 2020, 21, 1227. [Google Scholar] [CrossRef] [Green Version]
- MacKenzie, D.A.; Jeffers, F.; Parker, M.L.; Vibert-Vallet, A.; Bongaerts, R.J.; Roos, S.; Walter, J.; Juge, N. Strain-specific diversity of mucus-binding proteins in the adhesion and aggregation properties of Lactobacillus reuteri. Microbiology 2010, 156, 3368–3378. [Google Scholar] [CrossRef] [Green Version]
- Syal, P.; Vohra, A. Probiotic potential of yeasts isolated from traditional Indian fermented foods. Int. J. Microbiol. Res. 2013, 5, 390. [Google Scholar] [CrossRef] [Green Version]
- De Souza, B.M.S.; Borgonovi, T.F.; Casarotti, S.N.; Todorov, S.D.; Penna, A.L.B. Lactobacillus casei and Lactobacillus fermentum strains isolated from mozzarella cheese: Probiotic potential, safety, acidifying kinetic parameters and viability under gastrointestinal tract conditions. Probiotics Antimicrob. Proteins 2019, 11, 382–396. [Google Scholar] [CrossRef]
- Dias, F.S.; Duarte, W.F.; Schwan, R.F. Evaluation of adhesive properties of presumptive probiotic Lactobacillus plantarum strains. Biosci. J. 2013, 29, 1678–1686. [Google Scholar]
- Authority, E.F.S. EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J. 2012, 10, 2740. [Google Scholar]
- Gheziel, C.; Russo, P.; Arena, M.P.; Spano, G.; Ouzari, H.-I.; Kheroua, O.; Saidi, D.; Fiocco, D.; Kaddouri, H.; Capozzi, V. Evaluating the probiotic potential of Lactobacillus plantarum strains from Algerian infant feces: Towards the design of probiotic starter cultures tailored for developing countries. Probiotics Antimicrob. Proteins 2019, 11, 113–123. [Google Scholar] [CrossRef]
- do Carmo, M.S.; itapary dos Santos, C.; Araujo, M.C.; Girón, J.A.; Fernandes, E.S.; Monteiro-Neto, V. Probiotics, mechanisms of action, and clinical perspectives for diarrhea management in children. Food Funct. 2018, 9, 5074–5095. [Google Scholar] [CrossRef]
- Boricha, A.A.; Shekh, S.L.; Pithva, S.P.; Ambalam, P.S.; Vyas, B.R.M. In vitro evaluation of probiotic properties of Lactobacillus species of food and human origin. LWT 2019, 106, 201–208. [Google Scholar] [CrossRef]
- Rowland, I.R.; Capurso, L.; Collins, K.; Cummings, J.; Delzenne, N.; Goulet, O.; Guarner, F.; Marteau, P.; Meier, R. Current level of consensus on probiotic science-Report of an expert meeting-London, 23 November 2009. Gut Microbes 2010, 1, 436–439. [Google Scholar] [CrossRef]
- Somashekaraiah, R.; Mottawea, W.; Gunduraj, A.; Joshi, U.; Hammami, R.; Sreenivasa, M. Probiotic and antifungal attributes of Levilactobacillus brevis MYSN105, isolated from an Indian traditional fermented food Pozha. Front. Microbiol. 2021, 12, 696267. [Google Scholar] [CrossRef]
- Vila-Donat, P.; Marín, S.; Sanchis, V.; Ramos, A. A review of the mycotoxin adsorbing agents, with an emphasis on their multi-binding capacity, for animal feed decontamination. Food Chem. Toxicol. 2018, 114, 246–259. [Google Scholar] [CrossRef] [Green Version]
- Ghadaksaz, A.; Nodoushan, S.M.; Sedighian, H.; Behzadi, E.; Fooladi, A.A.I. Evaluation of the Role of Probiotics As a New Strategy to Eliminate Microbial Toxins: A Review. Probiotics Antimicrob. Proteins 2022, 14, 224–237. [Google Scholar] [CrossRef]
- Liu, L.; Xie, M.; Wei, D. Biological Detoxification of Mycotoxins: Current Status and Future Advances. Int. J. Mol. Sci. 2022, 23, 1064. [Google Scholar] [CrossRef]
- Peltonen, K.D.; El-Nezami, H.S.; Salminen, S.J.; Ahokas, J.T. Binding of aflatoxin B1 by probiotic bacteria. J. Sci. Food Agric. 2000, 80, 1942–1945. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K.; Nowak, A.; Chlebicz, A.; Żbikowski, A.; Pawłowski, K.; Szeleszczuk, P. Probiotic microorganisms detoxify ochratoxin A in both a chicken liver cell line and chickens. J. Sci. Food Agric. 2019, 99, 4309–4318. [Google Scholar] [CrossRef]
- Simões, L.A.; Cristina de Souza, A.; Ferreira, I.; Melo, D.S.; Lopes, L.A.A.; Magnani, M.; Schwan, R.F.; Dias, D.R. Probiotic properties of yeasts isolated from Brazilian fermented table olives. J. Appl. Microbiol. 2021, 131, 1983–1997. [Google Scholar] [CrossRef]
- Mantzourani, I.; Terpou, A.; Bekatorou, A.; Plessas, S. Valorization of Lactic Acid Fermentation of Pomegranate Juice by an Acid Tolerant and Potentially Probiotic LAB Isolated from Kefir Grains. Fermentation 2022, 8, 142. [Google Scholar] [CrossRef]
- Tarrah, A. Probiotics, Prebiotics, and Their Application in the Production of Functional Foods. Fermentation 2022, 8, 154. [Google Scholar] [CrossRef]

| Bacteria Strain | N° of Isolates | Code | Cultivar and Fermentation Time |
|---|---|---|---|
| Levilactobacillus brevis | 3 | CCMA1762 | Ascolano (time 120 days) |
| CCMA1765 | Ascolano (fruit) | ||
| CCMA1766 | Ascolano (time 60 days) | ||
| Lacticaseibacillus paracasei subsp. paracasei | 10 | CCMA1763 | Ascolano (time 120 days) |
| CCMA1764 | Ascolano (time 60 days) | ||
| CCMA1767 | Ascolano (time 30 days) | ||
| CCMA1769 | Ascolano (time 120 days) | ||
| CCMA1770 | Grappolo (time 60 days) | ||
| CCMA1771 | Grappolo (fruit) | ||
| CCMA1772 | Ascolano (time 60 days) | ||
| CCMA1773 | Ascolano (fruit) | ||
| CCMA1774 | Grappolo (time 120 days) | ||
| CCMA1775 | Grappolo (time 30 days) | ||
| Lactiplantibacillus pentosus | 1 | CCMA1768 | Ascolano (time 120 days) |
| Lacticaseibacillus paracasei subsp. paracasei | 1 | LBC-81 | (Danisco A/S, Copenhagen, Denmark) |
| Acid Condition Time of Exposure (h) | Bile Salt Condition Time of Exposure (h) | |||||
|---|---|---|---|---|---|---|
| LAB Strains | T0 | T3 | Survival (%) | T0 | T3 | Survival (%) |
| L. brevis CCMA1766 | 7.92 ± 0.01 aB | 9.15 ± 0.34 aA | 100 | 7.67 ± 0.16 aA | 8.35 ± 0.07 aA | 100 |
| L. paracasei CCMA1763 | 7.52 ± 0.02 aA | 7.97 ± 0.04 bA | 100 | 7.42 ± 0.02 aB | 8.81 ± 0.16 aA | 100 |
| L. paracasei CCMA1764 | 7.86 ± 0.02 aA | 4.25 ± 0.49 dB | 54.07 | 7.80 ± 0.06 aA | 4.09 ± 0.16 cB | 52.43 |
| L. pentosus CCMA1768 | 7.65 ± 0.04 aA | 7.88 ± 0.14 bA | 100 | 7.42 ± 0.06 aA | 7.38 ± 0.45 bA | 99.44 |
| L. paracasei CCMA1769 | 7.63 ± 0.04 aA | 7.73 ± 0.04 bA | 100 | 7.73 ± 0.04 aA | 7.70 ± 0.05 aA | 99.65 |
| L. paracasei CCMA1770 | 7.88 ± 0.04 aA | 7.41 ± 0.34 bA | 94.13 | 7.59 ± 0.08 aA | 8.23 ± 0.11 aA | 100 |
| L. brevis CCMA1762 | 7.96 ± 0.03 aA | 6.73 ± 0.05 cB | 84.55 | 7.56 ± 0.09 aA | 8.36 ± 0.48 aA | 100 |
| L. paracasei CCMA1772 | 7.65 ± 0.06 aA | 6.72 ± 0.03 cB | 87.88 | 7.75 ± 0.05 aA | 7.74 ± 0.19 aA | 99.86 |
| L. paracasei CCMA1771 | 7.85 ± 0.13 aA | 6.64 ± 0.06 cB | 84.56 | 7.84 ± 0.01 aA | 8.44 ± 0.41 aA | 100 |
| L. brevis CCMA1765 | 7.53 ± 0.08 aA | 6.73 ± 0.05 cB | 89.39 | 7.86 ± 0.02 aA | 7.85 ± 0.16 aA | 99.87 |
| L. paracasei CCMA1767 | 7.89 ± 0.02 aA | 6.76 ± 0.08 cB | 85.64 | 7.79 ± 0.12 aA | 7.55 ± 0.27 aA | 97.02 |
| L. paracasei CCMA1773 | 7.87 ± 0.04 aA | 6.64 ± 0.06 cB | 84.38 | 7.81 ± 0.03 aA | 7.91 ± 0.10 aA | 100 |
| L. paracasei CCMA1774 | 7.77 ± 0.05 aA | 6.80 ± 0.16 cB | 87.49 | 7.80 ± 0.06 aB | 8.64 ± 0.29 aA | 100 |
| L. paracasei CCMA1775 | 7.65 ± 0.06 aA | 4.04 ± 0.06 dB | 52.81 | 7.62 ± 0.06 aA | 4.02 ± 0.05 cB | 52.76 |
| L. paracasei LBC-81 | 7.91 ± 0.02 aA | 7.79 ± 0.12 bA | 98.48 | 7.63 ± 0.06 aA | 8.60 ± 0.18 aA | 100 |
| LAB Strains Code | S. aureus ATCC 8702 | S. Enteritidis ATCC 564 | L. monocytogenes ATCC 19117 |
|---|---|---|---|
| L. pentosus CCMA1768 | + | ++ | +++ |
| L. paracasei CCMA1771 | ++ | +++ | ++ |
| L. paracasei CCMA1774 | +++ | ++ | ++ |
| L. paracasei CCMA1770 | +++ | ++ | ++ |
| L. brevis CCMA1766 | +++ | ++ | ++ |
| L. brevis CCMA1762 | ++ | ++ | +++ |
| L. paracasei LBC-81 | ++ | ++ | ++ |
| Organ | Condition | Stirring (rpm) | pH | Time (min) | Viable Cell Counts (Log CFU mL−1) | ||
|---|---|---|---|---|---|---|---|
| LAB Strains Code | |||||||
| L. pentosus CCMA1768 | L. brevis CCMA1762 | L. paracasei CCMA1770 | |||||
| Before simulation | - | - | - | - | 7.04 ± 0.01 aA | 7.03 ± 0.08 aA | 7.21 ± 0.01 aA |
| Mouth | Amylase solution | 200 | 6.9 | 2 | 7.02 ± 0.03 aA | 7.04 ± 0.04 aA | 7.21 ± 0.01 aA |
| Esophagus– Stomach | Pepsin | 130 | 5.5 | 10 | 6.95 ± 0.05 aA | 6.98 ± 0.01 aA | 7.09 ± 0.06 aA |
| 4.6 | 10 | 6.91 ± 0.01 aA | 6.91 ± 0.07 aA | 7.08 ± 0.01 aA | |||
| 3.8 | 10 | 6.88 ± 0.07 aA | 6.88 ± 0.04 aA | 7.07 ± 0.08 aA | |||
| 2.8 | 20 | 6.52 ± 0.02 bB | 6.42 ± 0.03 bB | 6.97 ± 0.01 aA | |||
| 2.3 | 20 | 5.44 ± 0.09 bC | 6.33 ± 0.05 aB | 6.32 ± 0.05 aB | |||
| 2.0 | 20 | 4.37 ± 0.03 cD | 6.04 ± 0.03 aC | 5.18 ± 0.06 bC | |||
| Duodenum | Pancreati + bile salt | 45 | 5.0 | 30 | 4.31 ± 0.08 cD | 5.97 ± 0.01 aC | 4.86 ± 0.04 bD |
| Ileum | - | 45 | 6.5 | 60 | 4.31 ± 0.01 bD | 5.64 ± 0.01 aD | 4.17 ± 0.05 bE |
| Survival (%) | 61.22 | 80.23 | 57.84 | ||||
| Bacteria | Inhibition of Fungi (%) | |||||
|---|---|---|---|---|---|---|
| Aspergillus flavus MUM 08.201 | ||||||
| 2 days | 3 days | 4 days | 5 days | 6 days | 7 days | |
| L. brevis CCMA1762 | 40.7 ± 0.7 cA | 25.4 ± 1.3 bB | 22.9 ± 2.5 bB | 19.8 ± 3.3 bC | 2.5 ± 0.3 bD | 4.7 ± 0.2 aD |
| L. paracasei CCMA1764 | 59.2 ± 0.9 aA | 30.9 ± 1.0 aB | 29.7 ± 3.4 aB | 24.8 ± 0.9 aC | 11.2 ± 0.8 aD | 6.5 ± 0.5 aE |
| L. pentosus CCMA1768 | 51.8 ± 2.4 bA | 29.1 ± 0.8 aB | 25.7 ± 1.5 bC | 19.8 ± 1.0 bD | 5.6 ± 0.4 bE | 3.5 ± 0.8 aE |
| Penicillium nordicum MUM 08.16 | ||||||
| 2 days | 3 days | 4 days | 5 days | 6 days | 7 days | |
| L. brevis CCMA1762 | 100 aA | 100 aA | 100 aA | 100 aA | 67.6 ± 3.1 bB | 64.8 ± 5 bB |
| L. paracasei CCMA1764 | 100 aA | 100 aA | 100 aA | 100 aA | 100 aA | 100 aA |
| L. pentosus CCMA1768 | 100 aA | 100 aA | 100 aA | 100 aA | 100 aA | 100 aA |
| Species | Percentage of Mycotoxin Elimination (%) | |
|---|---|---|
| Aflatoxin B1 | Ochratoxin A | |
| L. brevis CCMA1762 | 45 ± 1.12 | 40 ± 2.05 |
| L. paracasei CCMA1770 | 40 ± 1.40 | 34 ± 1.45 |
| L. pentosus CCMA1768 | 43 ± 1.08 | 38 ± 1.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simões, L.; Fernandes, N.; de Souza, A.; dos Santos, L.; Magnani, M.; Abrunhosa, L.; Teixeira, J.; Schwan, R.F.; Dias, D.R. Probiotic and Antifungal Attributes of Lactic Acid Bacteria Isolates from Naturally Fermented Brazilian Table Olives. Fermentation 2022, 8, 277. https://doi.org/10.3390/fermentation8060277
Simões L, Fernandes N, de Souza A, dos Santos L, Magnani M, Abrunhosa L, Teixeira J, Schwan RF, Dias DR. Probiotic and Antifungal Attributes of Lactic Acid Bacteria Isolates from Naturally Fermented Brazilian Table Olives. Fermentation. 2022; 8(6):277. https://doi.org/10.3390/fermentation8060277
Chicago/Turabian StyleSimões, Luara, Natália Fernandes, Angélica de Souza, Luiz dos Santos, Marciane Magnani, Luís Abrunhosa, José Teixeira, Rosane Freitas Schwan, and Disney Ribeiro Dias. 2022. "Probiotic and Antifungal Attributes of Lactic Acid Bacteria Isolates from Naturally Fermented Brazilian Table Olives" Fermentation 8, no. 6: 277. https://doi.org/10.3390/fermentation8060277
APA StyleSimões, L., Fernandes, N., de Souza, A., dos Santos, L., Magnani, M., Abrunhosa, L., Teixeira, J., Schwan, R. F., & Dias, D. R. (2022). Probiotic and Antifungal Attributes of Lactic Acid Bacteria Isolates from Naturally Fermented Brazilian Table Olives. Fermentation, 8(6), 277. https://doi.org/10.3390/fermentation8060277








