3. Results and Discussion
All the fermentations began with the same batch growth of biomass with excess nitrogen. The glucose concentration was used to achieve the correct thickness and covering of biomass on the polypropylene tube [
12]. Once all the glucose was consumed, as indicated by online CO
2 production rates, the medium was drained, and the reactor was then rinsed and filled with the respective production medium in order to remove nitrogen from the reactor and induce the production of fumaric acid. The production fermentations were operated at pH 4, with a constant addition of urea at
−1 −1. These variables were found to greatly affect the production of fumaric acid, with the values being the optimum operating conditions [
17].
To investigate the feasibility of fumaric acid production with a lignocellulosic hydrolysate, it was first necessary to understand the metabolism of xylose and a glucose–xylose mixture. We conducted batch fermentations of glucose, xylose, and then a 50% glucose–xylose mixture that simulated lignocellulosic hydrolysate. The total sugar concentration for these fermentations was 20
−1.
Figure 1 shows the batch fermentation of glucose. It can be seen that the major products were fumaric acid and ethanol. The minor products of malic acid, succinic acid, and pyruvic acid reached maximum concentrations of
−1,
−1, and
−1, respectively.
The production rate of fumaric acid and ethanol proved to be equivalent during the first 10
of the fermentation, producing large amounts of ethanol—which is unfavourable. This production of ethanol was induced by the high glucose concentration.
R. oryzae has been found to be a Crabtree-positive organism, producing ethanol under fully aerobic conditions [
15]. Ethanol is an unwanted by-product because it decreases the yield of fumaric acid and complicates the downstream separation and processing. Although ethanol can be assimilated and metabolised, no fumaric acid is produced from it. Fumaric acid is produced by
R. oryzae through a reductive tricarboxylic acid (TCA) cycle that is present in the cytosol [
28]. Ethanol is likely consumed by its conversion to acetate—and afterwards to acetyl-CoA—from where it can be consumed by the TCA-cycle for the production of energy. Therefore, it offers no benefit to the production of fumaric acid.
Using the equations and integration method outlined in
Section 2.5, the effect of the dilution rate was accounted for to accurately determine the consumption and production rates of the organism. For the batch fermentation of glucose, the final accumulative yield of fumaric acid from glucose was
−1. The yield of ethanol was found to be
−1, which is a large yield for an unwanted by-product. It has been found that the production of ethanol could be avoided by carefully throttling the glucose feed rate on the reactor, which in turn increases the yield of fumaric acid from glucose up to
−1 [
15,
17]. The high yield of ethanol and low yield of fumaric acid in this batch fermentation clearly highlights the advantage of throttling the glucose feed rate in order to avoid the production of ethanol. The overall rate of fumaric acid production was
−1 −1, and the maximum rate was found to be
−1 −1. This maximum rate of fumaric acid production with the co-production of ethanol (
−1 −1) is slightly less than the maximum fumaric acid production rate (
−1 −1) found in a glucose-limited fermentation where no ethanol was produced [
17]. The concentration of biomass and all other parameters were identical between these fermentations. The fumaric acid production can be considered to be equivalent for the two conditions. Because the batch fermentation has an unrestricted glucose intake, the rate of glycolysis increases to a point where the TCA-cycle reaches a limit. The residual carbon that cannot be accommodated through the TCA-cycle or fumaric acid production is directed to the production of ethanol. This illustrates the Crabtree effect.
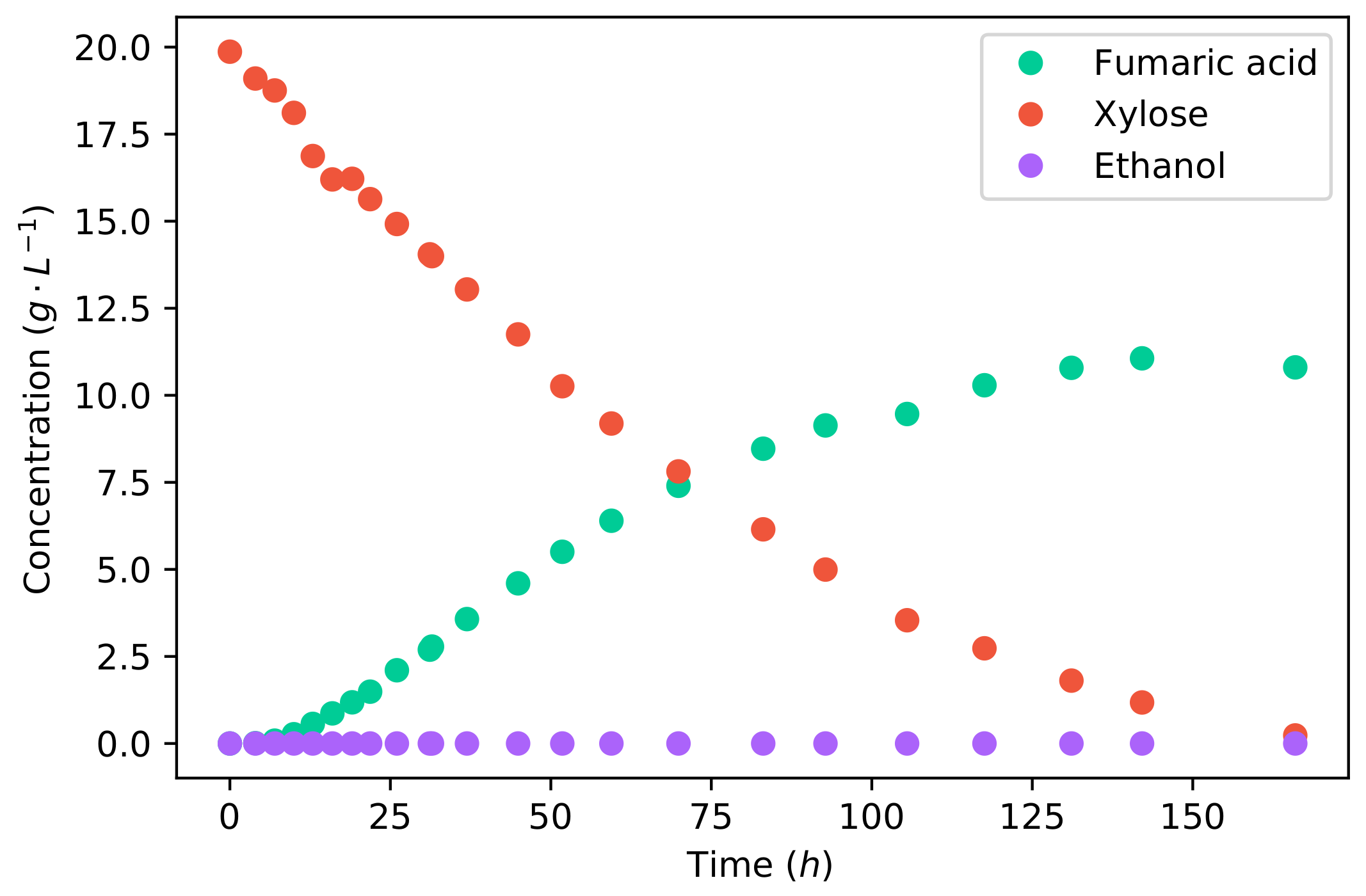
Comparing the fermentation of glucose to that of xylose, as shown in
Figure 2, it can firstly be seen that the duration of fermentation was considerably longer for xylose. Glucose fermentation ended after 58
, whereas the fermentation of xylose took 166
for the same mass of substrate. The average fumaric acid production rate was
−1 −1, and the maximum rate achieved was
−1 −1. Comparing the average production rates, the production rate of fumaric acid from xylose is
% lower than that from glucose.
The metabolism of xylose is largely different to that of glucose. Xylose has to be catabolised to xylitol, then D-xylulose, and followed by D-xylulose-5-P, which enters the Pentose phosphate pathway from which D-glucose-6-P is produced; this is the start of glycolysis [
29]. This is a longer pathway compared to the catabolism of glucose, which undergoes a single enzymatic step to produce D-glucose-6-P. These additional enzymatic steps required for the catabolism of xylose are likely the cause of the slow xylose utilisation and fumaric acid production. In a study on xylose utilisation by a recombinant
Saccharomyces cerevisiae, it was found that the enzymatic route of xylose to glycolysis was the rate-limiting step which resulted in inefficient metabolism affecting the energy balance of the cell [
30].
However, it can be seen in
Figure 2 that there is no ethanol produced from xylose. This is a result of the lower glycolytic flux, which does not saturate the metabolism. All carbon can be accommodated by the reductive TCA cycle, producing fumarate, and the TCA cycle; therefore, no ethanol is produced as an overflow. The final accumulative yield of fumaric acid on xylose is
−1, which is considerably higher than that found for glucose (
Figure 1). The increased yield was caused by the lack of ethanol production. This yield is also the highest yield of fumaric acid from xylose that has been reported in the literature—an improvement resulting from the operating conditions used. All the fermentations in the literature used CaCO
3 for pH control, which did not provide the optimal pH, and the carbon–nitrogen ratio was insufficiently controlled. These results indicate that xylose can be a promising substrate for the production of fumaric acid. However, the co-fermentation of xylose and glucose is of key importance.
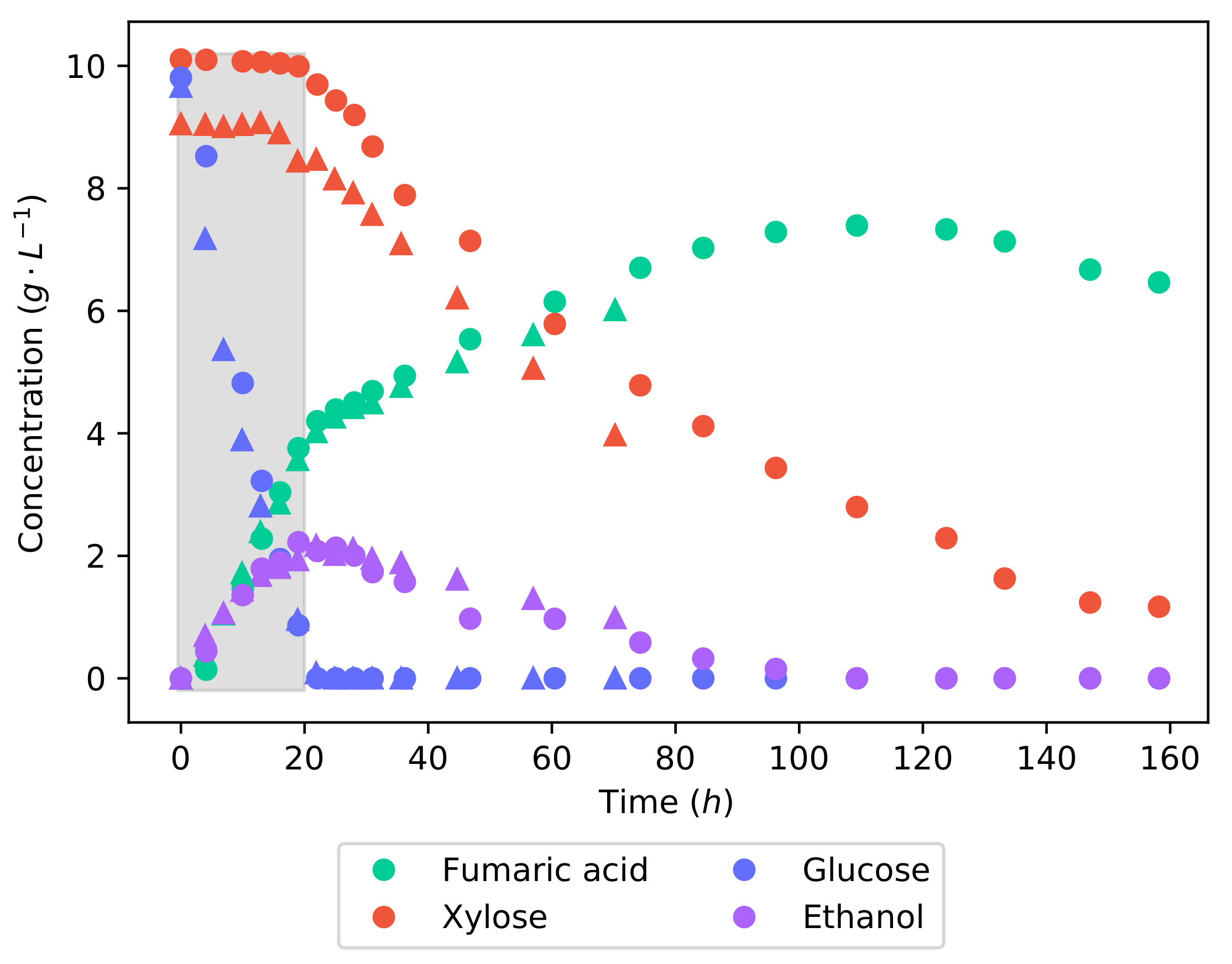
Figure 3 shows the concentration profiles of the fermentation of the synthetic lignocellulosic hydrolysate. Glucose is metabolised preferentially over xylose—in the first 21
, glucose was consumed at a rate of
−1 −1, while there was no consumption of xylose. This illustrates carbon catabolite repression (CCR), a well-known phenomenon that prioritises the most energy efficient substrate in a mixture and leads to a diauxic or two-phase utilisation of the substrate [
18]. Once the glucose concentration was depleted, xylose consumption began and increased to a rate of
−1 −1. In the pure substrate fermentations, the average rates of glucose and xylose consumption were
−1 −1 and
−1 −1, respectively. Thus, it can be seen that the catabolism of glucose was uninhibited by the presence of xylose; only after the complete consumption of glucose did xylose catabolism reach its full capability. The effect of the two-stage substrate utilisation could be plainly seen in the concentration profile of fumaric acid. While glucose was being consumed, the production rate was at
−1 −1, which then dropped to
−1 −1 once only xylose was remaining. It can, however, be seen that the ethanol produced during the catabolism of the glucose is now being consumed during the consumption of xylose.
The fumaric acid yield from the synthetic LH batch fermentation is
−1, which is considerably lower than the yields obtained from either of the pure substrate fermentations. This value is well within the range found in the literature [
22,
23,
24,
25]. The reason for this lower yield is likely a result of the co-fermentation of glucose and xylose. Different metabolic pathways are used to metabolise glucose and xylose; for this reason, the co-fermentation would require the production of more enzymes than necessary if only a single substrate was consumed. It can also be seen that ethanol is produced while glucose is being consumed, certainly contributing to the decreased fumaric acid yield. To make the production of fumaric acid from lignocellulosic hydrolysate viable, the yield will have to be improved. It has been found that minimising the medium glucose concentration negates the production of ethanol and drastically improves the yield of fumaric acid [
15].
An effective method of controlling the glucose concentration is by beginning the production fermentation with a medium void of glucose. All glucose is then fed at a specific rate that is equal to the consumption rate. This allows for the rate of glycolysis to be controlled, and we thus have the ability to negate the production of ethanol.
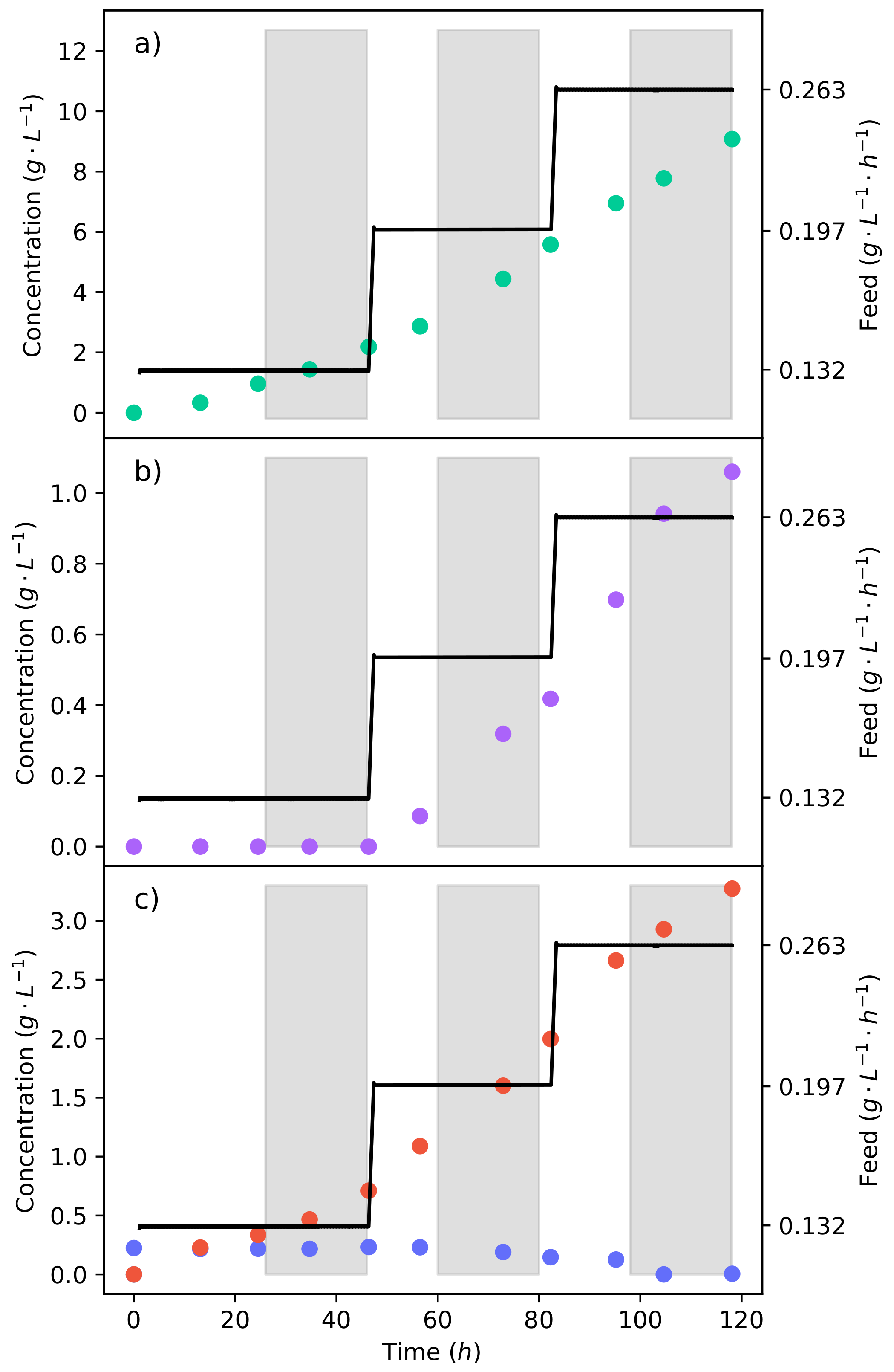
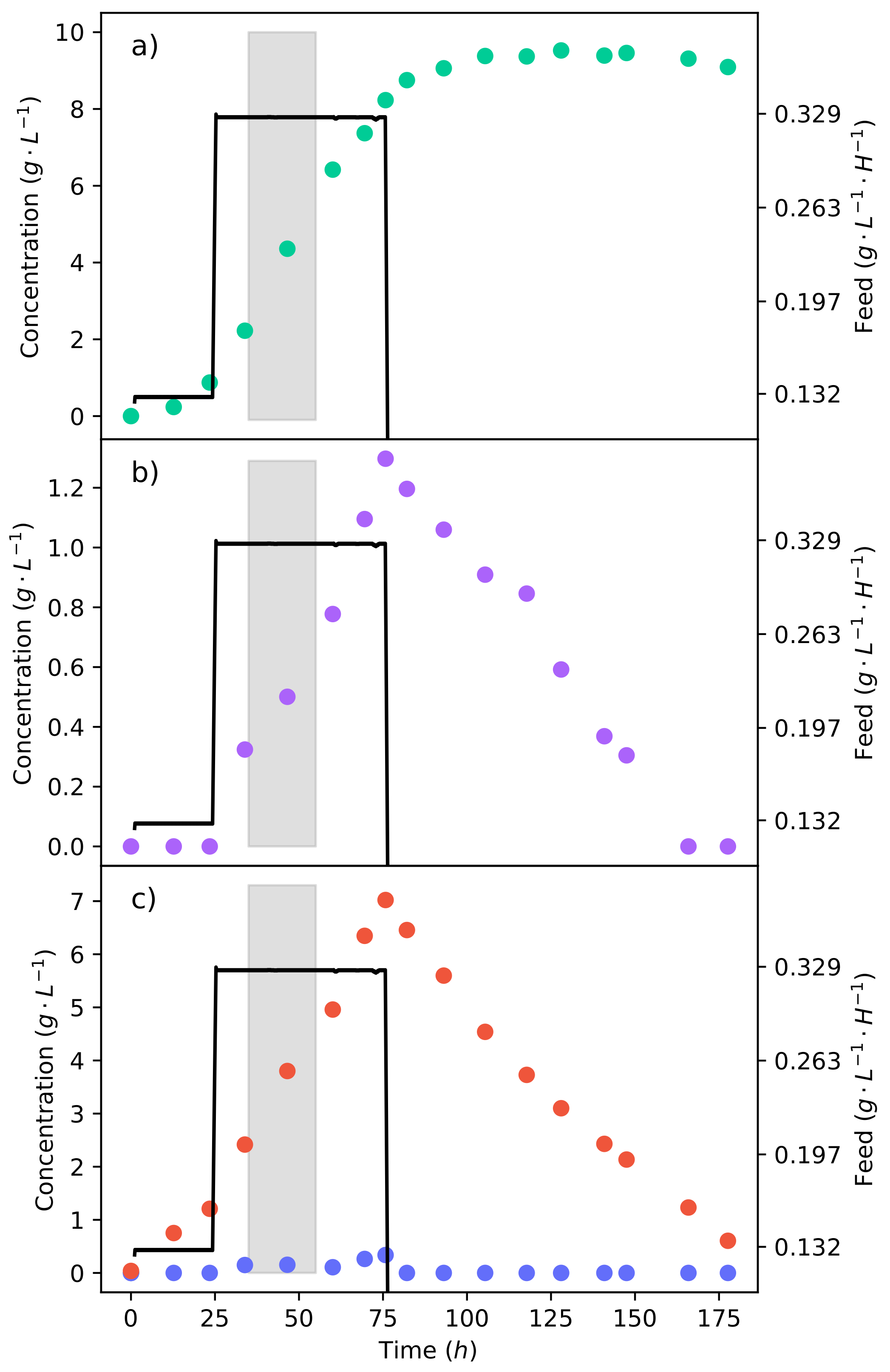
Figure 4a–c shows the concentration profiles and substrate feed rate of the fermentation, where a 50% glucose–xylose mixture was continuously fed into the reactor. The fermentation began at a substrate feed rate (
−1 −1) where an equivalent glucose feed rate did not produce any ethanol [
15]. It can be seen that for 48
, there was no production of ethanol; meanwhile, fumaric acid was still being produced. The substrate feed rate was then stepped up to
−1 −1, immediately triggering the production of ethanol.
This indicates that the ethanol breakthrough rate is between
−1 −1 and
−1 −1. In pure glucose fermentations under the same conditions, it has been found that the ethanol breakthrough rate is between
−1 −1 and
−1 −1 [
17]. The lessening of the ethanol breakthrough point is an unexpected effect, especially since it can be seen from the pure xylose fermentation (
Figure 2) that no ethanol was produced. Ethanol is produced as a result of metabolite overflow (Crabtree effect), or for the production of energy. Adenosine triphosphate (ATP) levels have been found to decrease in the fermentation of xylose as compared to that of glucose [
30]. Therefore, the production of ethanol is likely a response to the lower ATP levels, causing glucose to be directed to ethanol in order to produce nicotinamide adenine dinucleotide (NADH) at a faster rate. Although the production of NADH from ethanol is less efficient, it is faster than the TCA cycle [
27].
It can also be seen in
Figure 4c that there is an accumulation of xylose from the lowest feed rate. There was, however, the complete consumption of glucose at all the feed rates tested. This results from CCR, where glucose is consumed preferentially over xylose. Using a 24
running average at the end of the feed rate, it was found that xylose was consumed at a rate of
−1 −1, translating to
% of the xylose fed being consumed. As the substrate feed rate was increased, the consumption rate of xylose also increased. Because the proportion of glucose to xylose in the feed remained constant, it can be seen that a higher glucose consumption rate enabled a higher xylose consumption rate. This was likely concurrent with the production of ethanol from the glucose, which provided more NADH. The production of ethanol is not a result of xylose accumulation since no ethanol was produced from the batch xylose fermentation.
The calculated yield of fumaric acid produced from the substrate—consumed after the first 48
and at a feed rate of
−1 −1 where no ethanol was produced—was found to be only
−1. The low yield is a result of a large portion of the substrate being directed to the TCA cycle for cell maintenance. The feed rates of
−1 −1 and
−1 −1 achieved yields of
−1 and
−1, respectively. This shows an initial increased yield with an increase in the feed rate. However, there is the production of ethanol. It has been found that the fumaric acid yield increases with an increased feed rate up to the point of ethanol breakthrough [
17], after which the yield decreases. A feed rate of
−1 −1 was selected as a half-way point between
−1 −1 and the upper point of ethanol breakthrough (
−1 −1). The feed rate was tested for 48
and is shown in
Figure 5 by the triangular markers.
Figure 5b shows that for the entire fermentation, no ethanol was produced; this indicates that the ethanol breakthrough point lies between
−1 −1 and
−1 −1. By negating ethanol production, the fumaric acid yield obtained at the end of the fermentation increased to
−1.
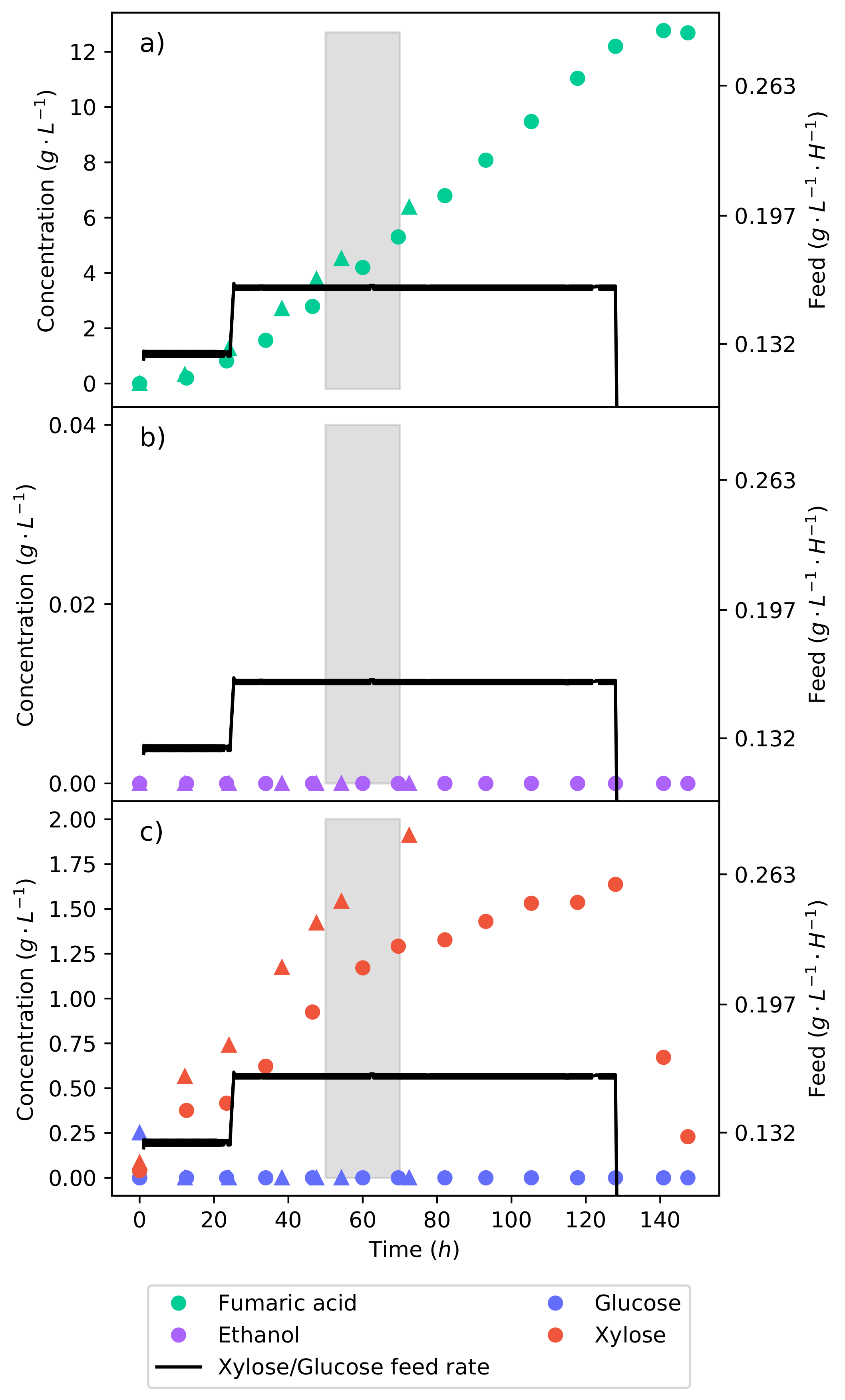
Utilising the information gathered from the continuous fermentations where the feed rate was stepped, a strategy was hypothesised to increase the fumaric acid yield on a lignocellulosic hydrolysate feed. The production of ethanol can be avoided by controlling the feed rate on the reactor; all the glucose will be consumed, and the xylose will be allowed to accumulate. Once all the substrate has been fed, the substrate feed will be stopped, and the accumulated xylose will then be allowed to be metabolised. The same mass of substrate feed in the batch fermentations (20
−1) will be fed over the course of the fermentation.
Figure 5 shows this fermentation.
For the first 24
, the feed rate was at
−1 −1, allowing for the organism to adapt and for an inter-run comparison to be conducted. The feed rate was then increased to
−1 −1 for the remainder of the run until all the substrate had been fed. In
Figure 5, it can be seen that the feed strategy was successful: no ethanol was produced, and once the feed rate stopped, the accumulated xylose was consumed. The fermentation was terminated once the production of fumaric acid ceased. The overall fumaric acid yield on the synthetic lignocellulosic hydrolysate feed was
−1. Considering that the batch fermentation shown in
Figure 3 has the same mass of substrate feed but a fumaric acid yield of
−1, the benefit of controlling the metabolism is clear. Manipulating the substrate feed rate achieved a
% improvement of the fumaric acid yield. The increased yield is a result of the negated ethanol production and the optimal metabolic flux that selects for the production of fumaric acid.
It was then considered whether a higher feed rate would produce a higher yield, as was later found by increasing the feed rate from
−1 −1 to
−1 −1. This increased the selectivity of carbon directed to fumaric acid. It was found that this relationship holds up to a glucose feed rate of
−1 −1, which vastly improves the fumaric acid yield [
17]. At this glucose feed rate, a fumaric acid yield of
−1 was achieved.
A fermentation of synthetic LH with this feed rate was then conducted, as shown in
Figure 6. The feed rate was stepped up to
−1 −1 after the first 24
. The same mass of substrate (20
−1) was to be fed; since the feed rate was far higher, this implied that the substrate would be delivered over a shorter period of time.
Figure 6c shows that there was a considerable accumulation of xylose as a result of the high feed rate, which also had a clear effect on the production of ethanol (
Figure 6b). In contrast, the glucose concentration remained low, indicating that the feed rate was matched by the rate of consumption. It can be seen that the production of fumaric acid slowed down and then ceased 24
later, after the feed rate halted (
Figure 6a). This can also be seen in the lower feed rate fermentation in
Figure 5, suggesting that the organism adapted to the co-fermentation of glucose and xylose in order to produce fumaric acid. Once glucose was no longer present, the production of fumaric acid stopped. The yield could be further improved if one were able to avoid xylose accumulation. However, this would require the ratio of glucose and xylose to be tailored to the respective uptake rates, and this may not be possible with a hydrolysate.
Table 1 summarises the crucial results from the fermentations with equivalent amounts of substrate. It can plainly be seen that the fermentation with the lower LH feed rate that avoided ethanol production outperformed the other strategies.
Considering the repeatability of the fermentations presented, as visible in
Figure 3, a duplicate of the fermentation was conducted. When comparing these two data sets, it can be seen that they are identical with all species following the same concentration profiles. Although the duplicate fermentation did not run to completion, it can still be said that the result is repeatable. The repeatability of the continuous fermentations has been proven in previous studies [
15,
17]; however, it will be discussed here for consistency.
All continuous fermentations were operated with the same conditions and substrate feed rate (
−1 −1) for the first 24
. Comparing the fumaric acid concentrations at the end of the 24
, the mean was found to be
−1, with a standard deviation of 0.200; this resulted in a coefficient of variance of 0.204, which proves repeatability. For the 24
duration of each of these runs, ethanol was expectedly not produced because the feed rate of
−1 −1 was below the ethanol breakthrough point. This illustrates that the organism was operating in the same metabolic state for all four fermentations. Using the procedure outlined in
Section 2.5, a mass balance was conducted over each of the fermentations in order to be certain that all the metabolites were accounted for. The mass balance compared the carbon added to the system to the sum of all the metabolites produced. It was found that the mass balance error for all the fermentations was less than 10%, indicating that the majority of the metabolites were accounted for.
Table 1 reports the errors for the specific runs. The errors found are possibly a result of the outlet CO
2 flow rate that had to be assumed and could not be directly measured.
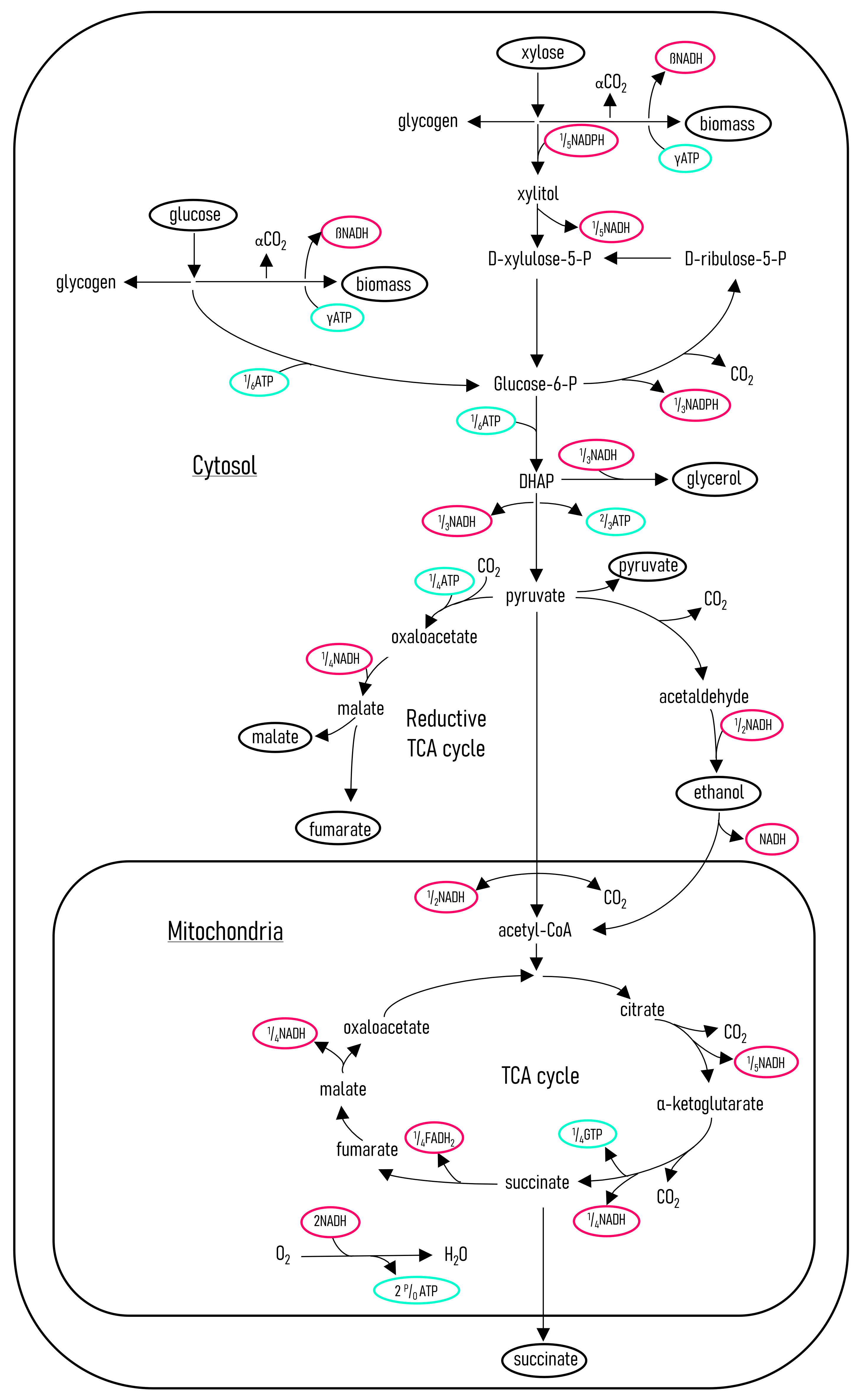
To gain further insight into the metabolism of
R. oryzae, a metabolic flux model was developed for the metabolism of glucose and xylose. The metabolic flux model was verified by comparing the predicted CO
2 rates to that obtained from a mass balance. It was found that the metabolic flux model predicted the CO
2 production rates accurately, using the other known metabolite rates as input.
Figure 7 shows the metabolic pathways determined for
R. oryzae, metabolising glucose and xylose for the predominant production of fumaric acid, ethanol, and CO
2. The flux model was then solved for specific intervals, shown on the previous figures as shaded areas.
The flux model was solved with carbon balances as well as with NADH and NADPH balances. Further information on the development of the metabolic flux model and specific constants determined for
R. oryzae are described by Swart [
17].
Figure 8 shows the metabolic rates determined from the flux model for the batch fermentation of glucose (
Figure 1), synthetic LH (
Figure 3), and the optimal glucose continuous feed fermentation [
17]. The result of a high glucose concentration is clearly demonstrated.
In
Figure 8a, it can be seen that the glucose uptake rates of both the pure glucose batch fermentation and the synthetic LH fermentation are equivalent. As a result of CRC, only glucose is consumed in the synthetic LH fermentation, indicating that xylose has no effect on the metabolism. It was found that the optimal glucose feed rate was below this maximum glucose uptake rate.
Figure 8b shows the glycolytic flux of carbon to pyruvate; this is the metabolic pathway after which the flux is split between the TCA cycle, fumaric acid production, and ethanol production. It can be seen that the glycolytic flux for both of the batch fermentations is higher than that of the continuous fermentation. Now, by comparing the ethanol production rates, it can be seen that the optimal glucose feed rate produced considerably less ethanol. This suggests that the production of ethanol is a result of a glycolytic threshold being surpassed. Once the glycolytic threshold has been passed, the proportion of carbon directed to ethanol increases, while fumaric acid production decreases. Operating below this glycolytic threshold improves both the yield and the rate of fumaric acid production.
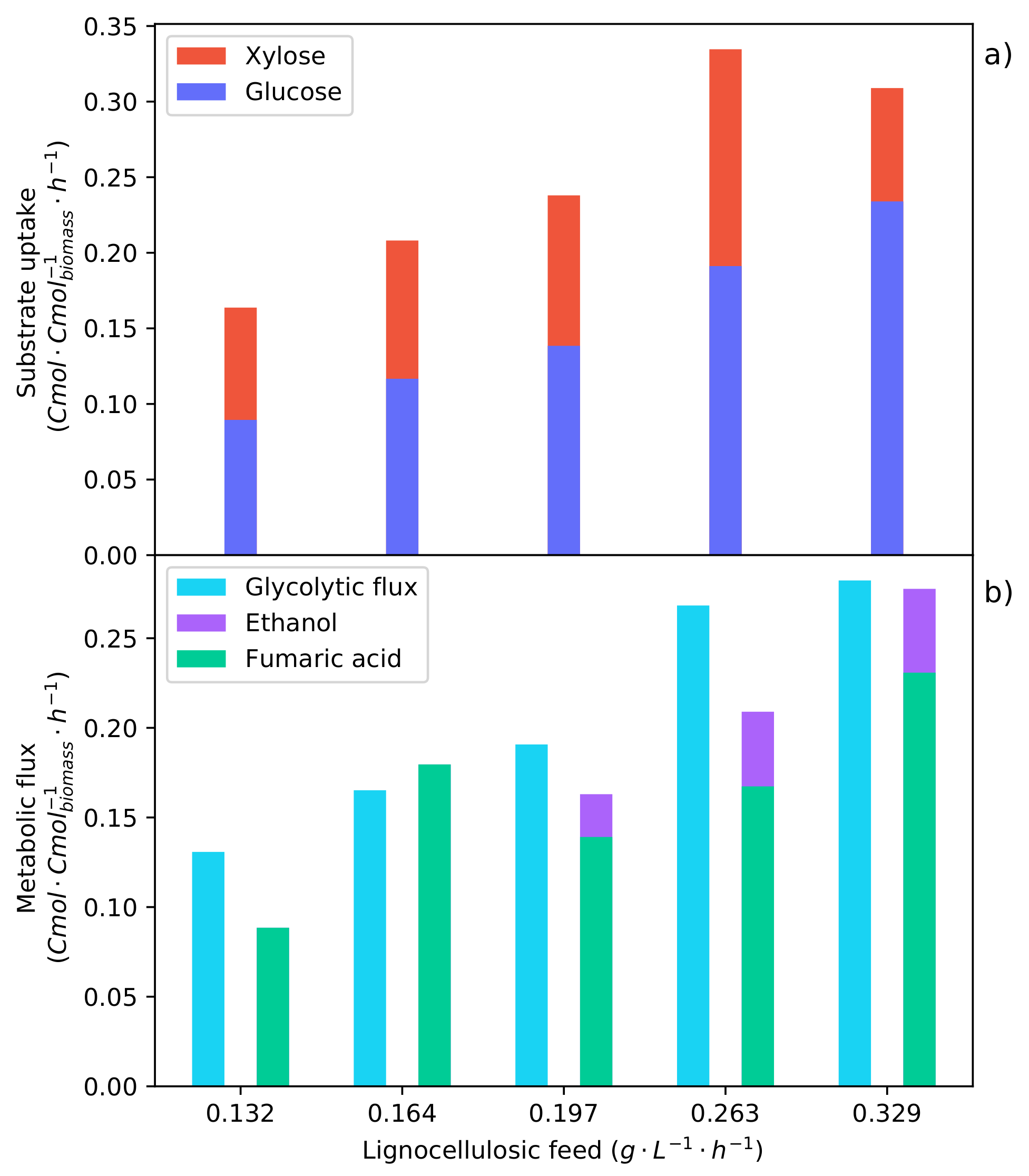
The metabolic flux model was solved for each of the feed rates tested for the continuous synthetic LH fermentations.
Figure 9 shows the metabolic fluxes determined. The co-fermentation of glucose and xylose can be seen for each of the feed rates in
Figure 9a. A comparison of the glucose and xylose uptake rates shows a visible proportionality between the rates. An
value of 0.983 was found for the first four substrate feed rates between
−1 −1 and
−1 −1. This is contrary to what was seen in the synthetic LH batch fermentation, where CRC resulted in the preferential consumption of glucose over xylose. The correlation of the glucose and xylose rates—considering that there was xylose accumulation at each feed rate—suggests that there is a dependency of xylose on glucose. An increased glucose feed rate enables a higher xylose uptake rate. Considering the feed rate of
−1 −1, it can be seen that the proportionality between the glucose and the xylose rates no longer holds. The glucose consumption rate has increased proportionally with the increased feed rate; however, the rate of xylose uptake decreased. Considering the glycolytic flux in
Figure 9b for the substrate feed rates of
−1 −1 and
−1 −1, it can be seen that they are equivalent rates. This suggests an upper limit for the glycolytic flux during the co-fermentation of glucose and xylose. Once the upper limit is reached, glucose is used preferentially over xylose, which results in a decreased xylose consumption rate.
In
Figure 9b, a feed rate of
−1 −1 is shown to be the optimum, directing the highest fraction of carbon consumed to fumaric acid. The feed rate below (
−1 −1) has a high fraction that is directed to the TCA cycle, which results in a low fumaric acid yield. This low yield is overcome when the feed rate is increased, and the fraction of carbon directed to the TCA cycle accordingly decreases. The higher feed rate (
−1 −1) surpasses an upper threshold of the glycolytic flux and induces the production of ethanol, decreasing both the yield and the rate of fumaric acid production. Comparing the glycolytic flux at which the ethanol breakthrough occurs for the pure glucose fermentation (
Figure 8b) and that of the synthetic LH fermentations (
Figure 9b), it can be seen that ethanol production starts at a lower glycolytic flux during the co-fermentation of glucose and xylose. This suggests some effect that xylose has on the glycolytic flux and supports the evidence indicating that xylose causes an inefficient metabolic state, which in turn affects the energy balance [
30]. This energy imbalance would explain why the ethanol breakthrough occurs at a lower substrate uptake rate.