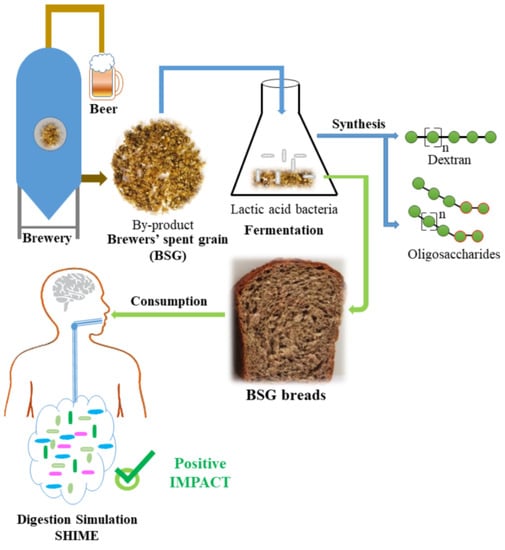
Fermented Brewers’ Spent Grain Containing Dextran and Oligosaccharides as Ingredient for Composite Wheat Bread and Its Impact on Gut Metabolome In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganism and Growth Conditions
2.2. Raw Materials
2.3. Brewers’ Spent Grain Fermentation
2.4. Bacterial Enumeration, pH and Total Titratable Acidity (TTA)
2.5. Quantification of Dextran and Oligosaccharides from Fermented BSG
2.6. Organic Acids and Sugars Analysis of Fermented BSG
2.7. BSG Bread Baking Procedure, Volume and Texture Analysis of Breads
2.8. Determination of pH, TTA, Sugars and Free Amino Acids of Breads
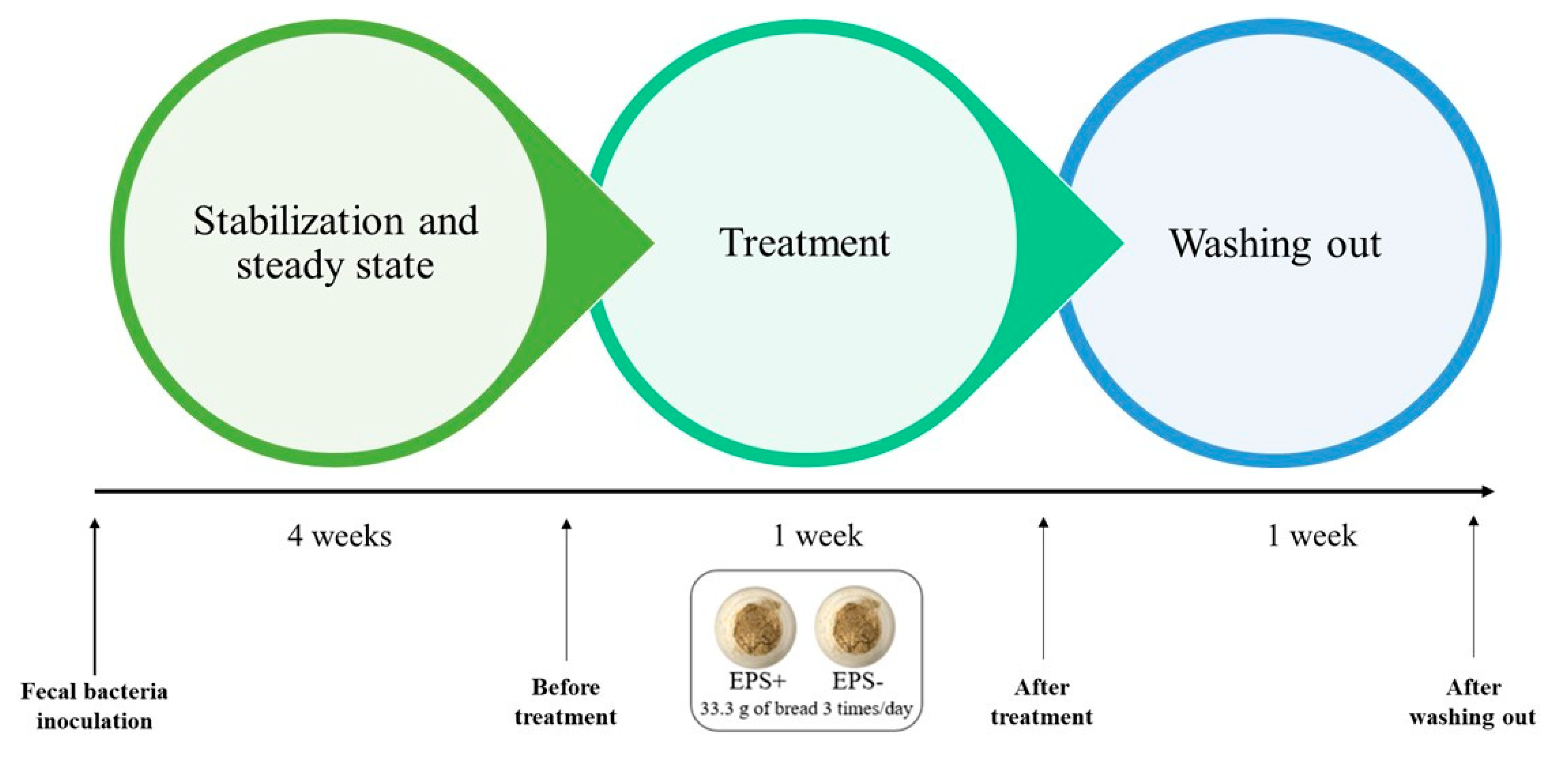
2.9. In Vitro Bread Digestion
2.10. Simulator of Human Intestinal Microbial Ecosystem (SHIME®) Set up and Experiment
2.11. Metabolic Activity Analysis
2.12. Statistical Analysis
3. Results
3.1. Microbial Growth, pH, TTA and Organic Acids of Fermented BSG
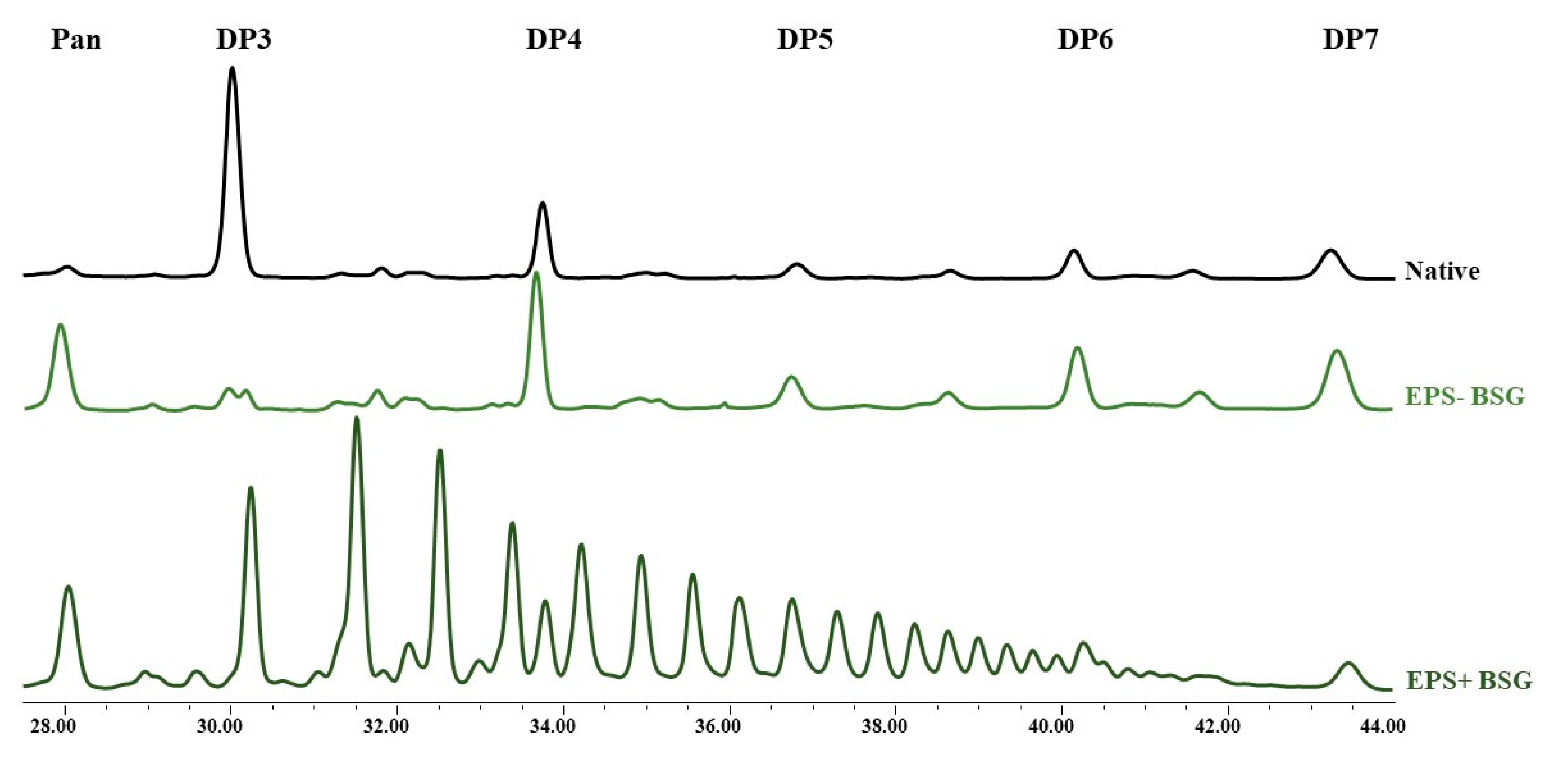
3.2. Sugar, Oligosaccharides and Dextran Analysis of Fermented BSG
3.3. Technological and Chemical Profile of Breads
3.4. SHIME® Lumen Microbial Metabolites
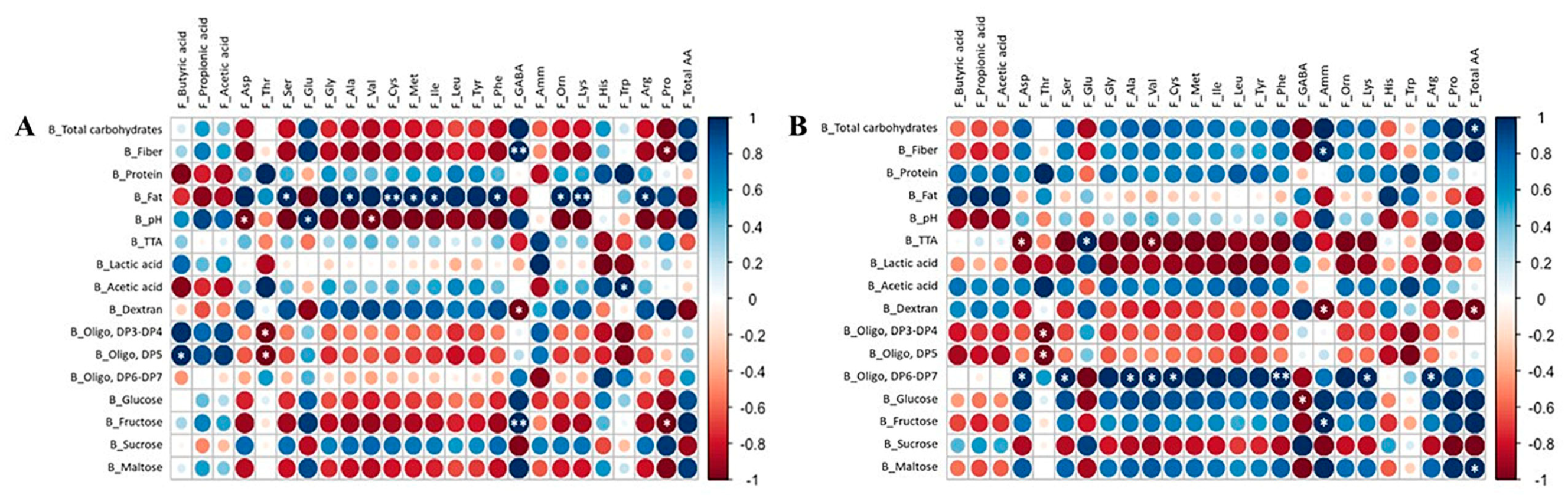
3.5. Correlation between Bread Variables and Microbial Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Brewers’ Spent Grain: Generation, Characteristics and Potential Applications. J. Cereal Sci 2006, 43, 1–14. [Google Scholar] [CrossRef]
- Petrovic, J.; Pajin, B.; Tanackov-Kocic, S.; Pejin, J.; Fistes, A.; Bojanic, N.; Loncarevic, I. Quality Properties of Cookies Supplemented with Fresh Brewer’s Spent Grain. Food Feed Res. 2017, 44, 57–63. [Google Scholar] [CrossRef]
- Schettino, R.; Verni, M.; Acin-albiac, M.; Vincentini, O.; Krona, A.; Knaapila, A.; di Cagno, R.; Gobbetti, M.; Rizzello, C.G.; Coda, R. Bioprocessed Brewers’ Spent Grain Improves Nutritional and Antioxidant Properties of Pasta. Antioxidants 2021, 10, 742. [Google Scholar] [CrossRef] [PubMed]
- Verni, M.; Rizzello, C.G.; Coda, R. Fermentation Biotechnology Applied to Cereal Industry By-Products: Nutritional and Functional Insights. Front. Nutr. 2019, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Ktenioudaki, A.; Alvarez-Jubete, L.; Smyth, T.J.; Kilcawley, K.; Rai, D.K.; Gallagher, E. Application of Bioprocessing Techniques (Sourdough Fermentation and Technological Aids) for Brewer’s Spent Grain Breads. Food Res. Int. 2015, 73, 107–116. [Google Scholar] [CrossRef]
- Neylon, E.; Arendt, E.K.; Zannini, E.; Sahin, A.W. Fermentation as a Tool to Revitalise Brewers’ Spent Grain and Elevate Techno-Functional Properties and Nutritional Value in High Fibre Bread. Foods 2021, 10, 1639. [Google Scholar] [CrossRef] [PubMed]
- Koirala, P.; Maina, N.H.; Nihtilä, H.; Katina, K.; Coda, R. Brewers’ Spent Grain as Substrate for Dextran Biosynthesis by Leuconostoc Pseudomesenteroides DSM20193 and Weissella Confusa A16. Microb. Cell Fact. 2021, 20, 23. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Trani, A.; Knaapila, A.; Hietala, S.; Coda, R.; Katina, K.; Maina, N.H. The Effect of in Situ Produced Dextran on Flavour and Texture Perception of Wholegrain Sorghum Bread. Food Hydrocoll. 2020, 106, 105913. [Google Scholar] [CrossRef]
- Wang, Y.; Compaoré-Sérémé, D.; Sawadogo-Lingani, H.; Coda, R.; Katina, K.; Maina, N.H. Influence of Dextran Synthesized in Situ on the Rheological, Technological and Nutritional Properties of Whole Grain Pearl Millet Bread. Food Chem. 2019, 285, 221–230. [Google Scholar] [CrossRef]
- Slavin, J. Fiber and Prebiotics: Mechanisms and Health Benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef]
- Jana, U.K.; Kango, N.; Pletschke, B. Hemicellulose-Derived Oligosaccharides: Emerging Prebiotics in Disease Alleviation. Front. Nutr. 2021, 8, 670817. [Google Scholar] [CrossRef]
- Sarbini, S.R.; Kolida, S.; Deaville, E.R.; Gibson, G.R.; Rastall, R.A. Potential of Novel Dextran Oligosaccharides as Prebiotics for Obesity Management through in Vitro Experimentation. Br. J. Nutr. 2014, 112, 1303–1314. [Google Scholar] [CrossRef]
- Dalile, B.; van Oudenhove, L.; Vervliet, B.; Verbeke, K. The Role of Short-Chain Fatty Acids in Microbiota–Gut–Brain Communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of Short Chain Fatty Acids by the Gut Microbiota and Their Impact on Human Metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.J. Review Article: The Role of Butyrate on Colonic Function. Aliment. Pharm. 2008, 27, 104–119. [Google Scholar] [CrossRef]
- Kumar, J.; Rani, K.; Datt, C. Molecular Link between Dietary Fibre, Gut Microbiota and Health. Mol. Biol. Rep. 2020, 47, 6229–6237. [Google Scholar] [CrossRef]
- Lynch, K.M.; Strain, C.R.; Johnson, C.; Patangia, D.; Stanton, C.; Koc, F.; Gil-Martinez, J.; O’Riordan, P.; Sahin, A.W.; Ross, R.P.; et al. Extraction and Characterisation of Arabinoxylan from Brewers Spent Grain and Investigation of Microbiome Modulation Potential. Eur. J. Nutr. 2021, 60, 4393–4411. [Google Scholar] [CrossRef]
- Costabile, A.; Walton, G.E.; Tzortzis, G.; Vulevic, J.; Charalampopoulos, D.; Gibson, G.R. Development of a Bread Delivery Vehicle for Dietary Prebiotics to Enhance Food Functionality Targeted at Those with Metabolic Syndrome. Gut Microbes 2015, 6, 300–309. [Google Scholar] [CrossRef]
- Wang, Y.; Maina, N.H.; Coda, R.; Katina, K. Challenges and Opportunities for Wheat Alternative Grains in Breadmaking: Ex-Situ- versus in-Situ-Produced Dextran. Trends Food Sci. Technol. 2021, 113, 232–244. [Google Scholar] [CrossRef]
- Da Ros, A.; Polo, A.; Rizzello, C.G.; Acin-Albiac, M.; Montemurro, M.; Di Cagno, R.; Gobbetti, M. Feeding with Sustainably Sourdough Bread Has the Potential to Promote the Healthy Microbiota Metabolism at the Colon Level. Microbiol. Spectr. 2021, 9, e00494-21. [Google Scholar] [CrossRef]
- Katina, K.; Maina, N.H.; Juvonen, R.; Flander, L.; Johansson, L.; Virkki, L.; Tenkanen, M.; Laitila, A. In Situ Production and Analysis of Weissella Confusa Dextran in Wheat Sourdough. Food Microbiol. 2009, 26, 734–743. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Coda, R.; Säde, E.; Tuomainen, P.; Tenkanen, M.; Katina, K. In Situ Synthesis of Exopolysaccharides by Leuconostoc Spp. and Weissella Spp. and Their Rheological Impacts in Fava Bean Flour. Int. J. Food Microbiol. 2017, 248, 63–71. [Google Scholar] [CrossRef]
- Wang, Y.; Sorvali, P.; Laitila, A.; Maina, N.H.; Coda, R.; Katina, K. Dextran Produced in Situ as a Tool to Improve the Quality of Wheat-Faba Bean Composite Bread. Food Hydrocoll. 2018, 84, 396–405. [Google Scholar] [CrossRef]
- Weiss, W.; Vogelmeier, C.; Görg, A. Electrophoretic Characterization of Wheat Grain Allergens from Different Cultivars Involved in Bakers’ Asthma. Electrophoresis 1993, 14, 805–816. [Google Scholar] [CrossRef]
- de Pasquale, I.; Verni, M.; Verardo, V.; Gómez-Caravaca, A.M.; Rizzello, C.G. Nutritional and Functional Advantages of the Use of Fermented Black Chickpea Flour for Semolina-Pasta Fortification. Foods 2021, 10, 182. [Google Scholar] [CrossRef]
- Mackie, A.; Rigby, N. InfoGest Consensus Method. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- van den Abbeele, P.; Duysburgh, C.; Jiang, T.A.; Rebaza, M.; Pinheiro, I.; Marzorati, M. A Combination of Xylooligosaccharides and a Polyphenol Blend Affect Microbial Composition and Activity in the Distal Colon Exerting Immunomodulating Properties on Human Cells. J. Funct. Foods 2018, 47, 163–171. [Google Scholar] [CrossRef]
- de Boever, P.; Deplancke, B.; Verstraete, W. Fermentation by Gut Microbiota Cultured in a Simulator of the Human Intestinal Microbial Ecosystem Is Improved by Supplementing a Soygerm Powder. J. Nutr. 2000, 130, 2599–2606. [Google Scholar] [CrossRef]
- van Herreweghen, F.; van den Abbeele, P.; de Mulder, T.; de Weirdt, R.; Geirnaert, A.; Hernandez-Sanabria, E.; Vilchez-Vargas, R.; Jauregui, R.; Pieper, D.H.; Belzer, C.; et al. In Vitro Colonisation of the Distal Colon by Akkermansia Muciniphila Is Largely Mucin and PH Dependent. Benef. Microbes 2017, 8, 81–96. [Google Scholar] [CrossRef]
- Lotti, C.; Rubert, J.; Fava, F.; Tuohy, K.; Mattivi, F.; Vrhovsek, U. Development of a Fast and Cost-Effective Gas Chromatography--Mass Spectrometry Method for the Quantification of Short-Chain and Medium-Chain Fatty Acids in Human Biofluids. Anal. Bioanal. Chem. 2017, 409, 5555–5567. [Google Scholar] [CrossRef]
- McKenna, S.; Meyer, M.; Gregg, C.; Gerber, S. S-CorrPlot: An Interactive Scatterplot for Exploring Correlation. J. Comput. Graph. Stat. 2016, 25, 445–463. [Google Scholar] [CrossRef]
- Lynch, K.M.; Steffen, E.J.; Arendt, E.K. Brewers’ Spent Grain: A Review with an Emphasis on Food and Health. J. Inst. Brew. 2016, 122, 553–568. [Google Scholar] [CrossRef]
- Kajala, I.; Mäkelä, J.; Coda, R.; Shukla, S.; Shi, Q.; Maina, N.H.; Juvonen, R.; Ekholm, P.; Goyal, A.; Tenkanen, M.; et al. Rye Bran as Fermentation Matrix Boosts in Situ Dextran Production by Weissella Confusa Compared to Wheat Bran. Appl. Microbiol. Biotechnol. 2016, 100, 3499–3510. [Google Scholar] [CrossRef] [PubMed]
- Kothari, D.; Das, D.; Patel, S.; Goyal, A. Dextran and Food Application. In Polysaccharides: Bioactivity and Biotechnology; Springer: Cham, Switzerland, 2015; pp. 735–752. [Google Scholar] [CrossRef]
- Galle, S.; Schwab, C.; Arendt, E.; Gänzle, M. Exopolysaccharide-Forming Weissella Strains as Starter Cultures for Sorghum and Wheat Sourdoughs. J. Agric. Food Chem. 2010, 58, 5834–5841. [Google Scholar] [CrossRef] [PubMed]
- Wisselink, H.W.; Weusthuis, R.A.; Eggink, G.; Hugenholtz, J.; Grobben, G.J. Mannitol Production by Lactic Acid Bacteria: A Review. Int. Dairy J. 2002, 12, 151–161. [Google Scholar] [CrossRef]
- Monsan, P.; Bozonnet, S.; Ecile Albenne, C.; Joucla, G. Homopolysaccharides from Lactic Acid Bacteria. Int. Dairy J. 2001, 11, 675–685. [Google Scholar] [CrossRef]
- Galle, S.; Arendt, E.K. Exopolysaccharides from Sourdough Lactic Acid Bacteria. Crit. Rev. Food Sci. Nutr. 2014, 54, 891–901. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Müller, M.; Hermes, G.D.A.; Emanuel E, C.; Holst, J.J.; Zoetendal, E.G.; Smidt, H.; Troost, F.; Schaap, F.G.; Damink, S.O.; Jocken, J.W.E.; et al. Effect of Wheat Bran Derived Prebiotic Supplementation on Gastrointestinal Transit, Gut Microbiota, and Metabolic Health: A Randomized Controlled Trial in Healthy Adults with a Slow Gut Transit. Gut Microbes 2020, 12, 1704141. [Google Scholar] [CrossRef]
- Olano-Martin, E.; Mountzouris, K.C.; Gibson, G.R.; Rastall, R.A. In Vitro Fermentability of Dextran, Oligodextran and Maltodextrin by Human Gut Bacteria. Br. J. Nutr. 2000, 83, 247–255. [Google Scholar] [CrossRef]
- Jang, E.Y.; Hong, K.B.; Chang, Y.B.; Shin, J.; Jung, E.Y.; Jo, K.; Suh, H.J. In Vitro Prebiotic Effects of Malto-Oligosaccharides Containing Water-Soluble Dietary Fiber. Molecules 2020, 25, 5201. [Google Scholar] [CrossRef]
- Hughes, R.L.; Alvarado, D.A.; Swanson, K.S.; Holscher, H.D. The Prebiotic Potential of Inulin-Type Fructans: A Systematic Review. Adv. Nutr. 2021, 13, 492–529. [Google Scholar] [CrossRef]
- van de Wiele, T.; van den Abbeele, P.; Ossieur, W.; Possemiers, S.; Marzorati, M. The Simulator of the Human Intestinal Microbial Ecosystem (SHIME®). In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Springer: Cham, Switzerland, 2015; pp. 305–317. [Google Scholar] [CrossRef]
- Venegas, D.P.; de La Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol 2019, 10, 277. [Google Scholar] [CrossRef]
- Duncan, S.H.; Holtrop, G.; Lobley, G.E.; Calder, A.G.; Stewart, C.S.; Flint, H.J. Contribution of Acetate to Butyrate Formation by Human Faecal Bacteria. Br. J. Nutr. 2004, 91, 915–923. [Google Scholar] [CrossRef]
- Pryde, S.E.; Duncan, S.H.; Hold, G.L.; Stewart, C.S.; Flint, H.J. The Microbiology of Butyrate Formation in the Human Colon. FEMS Microbiol. Lett. 2002, 217, 133–139. [Google Scholar] [CrossRef]
- Marinelli, L.; Martin-Gallausiaux, C.; Bourhis, J.M.; Béguet-Crespel, F.; Blottière, H.M.; Lapaque, N. Identification of the Novel Role of Butyrate as AhR Ligand in Human Intestinal Epithelial Cells. Sci. Rep. 2019, 9, 643. [Google Scholar] [CrossRef]
- Gonzalez-Garcia, R.A.; McCubbin, T.; Navone, L.; Stowers, C.; Nielsen, L.K.; Marcellin, E. Microbial Propionic Acid Production. Fermentation 2017, 3, 21. [Google Scholar] [CrossRef]
- Hald, S.; Schioldan, A.G.; Moore, M.E.; Dige, A.; Lærke, H.N.; Agnholt, J.; Knudsen, K.E.B.; Hermansen, K.; Marco, M.L.; Gregersen, S.; et al. Effects of Arabinoxylan and Resistant Starch on Intestinal Microbiota and Short-Chain Fatty Acids in Subjects with Metabolic Syndrome: A Randomised Crossover Study. PLoS ONE 2016, 11, e0159223. [Google Scholar] [CrossRef]
- Akagawa, S.; Akagawa, Y.; Nakai, Y.; Yamagishi, M.; Yamanouchi, S.; Kimata, T.; Chino, K.; Tamiya, T.; Hashiyada, M.; Akane, A.; et al. Fiber-Rich Barley Increases Butyric Acid-Producing Bacteria in the Human Gut Microbiota. Metabolites 2021, 11, 559. [Google Scholar] [CrossRef]
- Flint, H.J. Gut Microbial Metabolites in Health and Disease. Gut Microbes 2016, 7, 187–188. [Google Scholar] [CrossRef]
- Scott, K.P.; Gratz, S.W.; Sheridan, P.O.; Flint, H.J.; Duncan, S.H. The Influence of Diet on the Gut Microbiota. Pharm. Res. 2013, 69, 52–60. [Google Scholar] [CrossRef]
- Wallace, R.J.; McKain, N. Peptidase Activity of Human Colonic Bacteria. Anaerobe 1997, 3, 251–257. [Google Scholar] [CrossRef]
- Turroni, F.; Ribbera, A.; Foroni, E.; van Sinderen, D.; Ventura, M. Human Gut Microbiota and Bifidobacteria: From Composition to Functionality. Antonie van Leeuwenhoek. Int. J. Gen. Mol. Microbiol. 2008, 94, 35–50. [Google Scholar] [CrossRef]
- Neis, E.P.J.G.; Dejong, C.H.C.; Rensen, S.S. The Role of Microbial Amino Acid Metabolism in Host Metabolism. Nutrients 2015, 7, 2930–2946. [Google Scholar] [CrossRef]
- Smith, E.A.; MacFarlane, G.T. Enumeration of Amino Acid Fermenting Bacteria in the Human Large Intestine: Effects of PH and Starch on Peptide Metabolism and Dissimilation of Amino Acids. FEMS Microbiol. Ecol. 1998, 25, 355–368. [Google Scholar] [CrossRef]
- Diether, N.E.; Willing, B.P. Microbial Fermentation of Dietary Protein: An Important Factor in Diet–Microbe–Host Interaction. Microorganisms 2019, 7, 19. [Google Scholar] [CrossRef]
- Macfarlane, G.T.; Gibson, G.R.; Cummings, J.H. Comparison of Fermentation Reactions in Different Regions of the Human Colon. J. Appl. Bacteriol. 1992, 72, 57–64. [Google Scholar] [CrossRef]
- Cárdenas-Castro, A.P.; Bianchi, F.; Tallarico-Adorno, M.A.; Montalvo-González, E.; Sáyago-Ayerdi, S.G.; Sivieri, K. In Vitro Colonic Fermentation of Mexican “Taco” from Corn-Tortilla and Black Beans in a Simulator of Human Microbial Ecosystem (SHIME®) System. Food Res. Int. 2019, 118, 81–88. [Google Scholar] [CrossRef]
- Epps, H.M.R.; Gale, E.F. The Influence of the Presence of Glucose during Growth on the Enzymic Activities of Escherichia Coli: Comparison of the Effect with That Produced by Fermentation Acids. Biochem. J. 1942, 36, 619. [Google Scholar] [CrossRef] [PubMed]
- Al-Khafaji, A.H.; Jepsen, S.D.; Christensen, K.R.; Vigsnæs, L.K. The Potential of Human Milk Oligosaccharides to Impact the Microbiota-Gut-Brain Axis through Modulation of the Gut Microbiota. J. Funct Foods 2020, 74, 104176. [Google Scholar] [CrossRef]
- Bourassa, M.W.; Alim, I.; Bultman, S.J.; Ratan, R.R. Butyrate, Neuroepigenetics and the Gut Microbiome: Can a High Fiber Diet Improve Brain Health? Neurosci. Lett. 2016, 625, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Turck, D.; Bohn, T.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Knutsen, H.K.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Tolerable Upper Intake Level for Dietary Sugars. EFSA J. 2022, 20, e07074. [Google Scholar] [CrossRef]
- Ose, R.; Hirano, K.; Maeno, S.; Nakagawa, J.; Salminen, S.; Tochio, T.; Endo, A. The Ability of Human Intestinal Anaerobes to Metabolize Different Oligosaccharides: Novel Means for Microbiota Modulation? Anaerobe 2018, 51, 110–119. [Google Scholar] [CrossRef]
- Sanchez, J.I.; Marzorati, M.; Grootaert, C.; Baran, M.; van Craeyveld, V.; Courtin, C.M.; Broekaert, W.F.; Delcour, J.A.; Verstraete, W.; van de Wiele, T. Arabinoxylan-Oligosaccharides (AXOS) Affect the Protein/Carbohydrate Fermentation Balance and Microbial Population Dynamics of the Simulator of Human Intestinal Microbial Ecosystem. Microb Biotechnol. 2009, 2, 101–113. [Google Scholar] [CrossRef]
- de Filippo, C.; Cavalieri, D.; di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of Diet in Shaping Gut Microbiota Revealed by a Comparative Study in Children from Europe and Rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef]

| WB | BB | EPS-BB | EPS+BB | |||||
|---|---|---|---|---|---|---|---|---|
| g * | % d.w. ** | g | % d.w. | g | % d.w. | g | % d.w. | |
| BSG | 523.3 | 33.32 | 523.3 | 526.0 | 33.49 | |||
| Sucrose | 58.4 | 3.72 | ||||||
| Fermented BSG | 523.3 | 33.32 | 584.4 | |||||
| Wheat flour | 900 | 57.31 | 798 | 50.81 | 798 | 50.81 | 739 | 47.06 |
| Water | 540 | 34.38 | 118.7 | 7.56 | 118.7 | 7.56 | 116.6 | 7.42 |
| Salt | 13.5 | 0.86 | 13.5 | 0.86 | 13.5 | 0.86 | 13.5 | 0.86 |
| Sugar | 18.0 | 1.15 | 18.0 | 1.15 | 18.0 | 1.15 | 18.0 | 1.15 |
| Yeast | 45.0 | 2.87 | 45.0 | 2.87 | 45.0 | 2.87 | 45.0 | 2.87 |
| Fat | 54.0 | 3.44 | 54.0 | 3.44 | 54.0 | 3.44 | 54.0 | 3.44 |
| Total | 1570.5 | 100.0 | 1570.5 | 100.0 | 1570.5 | 100.0 | 1570.5 | 100.0 |
| Timepoints | BSG | pH | TTA | Lactic Acid | Acetic Acid |
|---|---|---|---|---|---|
| mL of 0.1 N NaOH | mg/100 g Fermented BSG | ||||
| T0 | EPS- | 5.9 ± 0.0 a | 2.4 ± 0.1 a | nd * | nd * |
| EPS+ | 1.9 ± 0.1 a | ||||
| T24 | EPS- | 4.6 ± 0.4 b | 7.1 ± 1.4 b | 246.2 ± 24.5 a | 106.4 ± 6.9 a |
| EPS+ | 4.3 ± 0.1 c | 7.2 ± 0.3 b | 398.9 ± 7.8 b | 91.8 ± 1.9 a | |
| Fructose | Glucose | Sucrose | Maltose | |
|---|---|---|---|---|
| Native | 0.5 ± 0.01 a | 0.4 ± 0.01 a | nd * | 2.3 ± 0.01 a |
| EPS- | 0.5 ± 0.01 a | 0.5 ± 0.01 a | nd * | 2.1 ± 0.1 b |
| EPS+ | 21.0 ± 1.0 b | nd *,b | nd * | 1.1 ± 0.1 c |
| Dextran | Oligosaccharides | |||
|---|---|---|---|---|
| DP3-DP4 ** | DP5 ** | DP6-DP7 ** | ||
| Native | nd * | 0.6 ± 0.02 a | 0.5 ± 0.04 a | 0.4 ± 0.01 a |
| EPS- | 0.3 ± 0.04 b | 1.2 ± 0.11 b | 1.1 ± 0.06 b | |
| EPS+ | 7.2 ± 0.3 | 2.5 ± 0.13 c | 4.8 ± 0.39 c | 2.6 ± 0.25 c |
| Bread Types | pH | TTA (mL) | Sugars | |||
|---|---|---|---|---|---|---|
| Glucose | Fructose | Sucrose | Maltose | |||
| WB | 5.9 ± 0.1 a | 3.2 ± 0.1 a | nd * | |||
| BB | 5.8 ± 0.1 a | 3.4 ± 0.1 a | 2.3 ± 0.07 ab | 11.6 ± 0.07 b | 0.6 ± 0.07 a | 32.4 ± 1.02 a |
| EPS-BB | 5.3 ± 0.1 b | 5.0 ± 0.4 b | 1.3 ± 0.07 b | 8.5 ± 0.01 c | 0.5 ± 0.04 b | 24.9 ± 0.95 b |
| EPS+BB | 5.1 ± 0.1 b | 5.5 ± 0.1 b | 3.3 ± 0.08 a | 29.02 ± 0.02 a | 0.3 ± 0.00 c | 22.8 ± 0.91 b |
| SCFA | Short Chain Fatty Acids (mM) | ||||
|---|---|---|---|---|---|
| Treatments | PC1 (EPS+BB) | PC2 (EPS-BB) | DC1 (EPS+BB) | DC2 (EPS-BB) | |
| Acetic acid | Before treatment | 20.86 ± 1.78 b | 22.56 ± 0.67 b | 30.72 ± 1.65 b | 33.73 ± 2.23 a |
| After treatment | 38.47 ± 2.14 a/* | 33.91 ± 1.85 a | 45.19 ± 3.71 a | 43.00 ± 2.10 a | |
| After washing out | 24.15 ± 0.05 b/* | 21.75 ± 0.22 b | 35.45 ± 0.19 b/* | 34.94 ± 2.91 a | |
| Propionic acid | Before treatment | 2.71 ± 0.17 a | 3.02 ± 0.12 a | 3.45 ± 0.20 a | 3.73 ± 0.05 b |
| After treatment | 3.09 ± 0.27 a | 3.21 ± 0.20 a | 4.33 ± 0.58 a | 4.66 ± 0.40 a | |
| After washing out | 2.80 ± 0.28 a | 3.06 ± 0.07 a | 3.71 ± 0.08 a | 3.97 ± 0.09 b/* | |
| Butyric acid | Before treatment | 8.88 ± 0.17 b | 9.23 ± 0.15 b | 9.62 ± 0.22 b | 9.91 ± 0.04 b |
| After treatment | 11.16 ± 0.32 a | 12.05 ± 0.41 a/* | 13.39 ± 0.92 a | 13.46 ± 0.48 a | |
| After washing out | 9.02 ± 0.17 b | 9.24 ± 0.09 b | 10.43 ± 0.10 b/* | 9.95 ± 0.07 b | |
| FAA | Free Amino Acids (mg/Kg) | ||||
| Treatments | PC1 (EPS+BB) | PC2 (EPS-BB) | DC1 (EPS+BB) | DC2 (EPS-BB) | |
| Asp | Before treatment | 3.44 ± 0.24 b | 2.89 ± 0.20 b | 1.91 ± 0.12 c | 1.83 ± 0.13 b |
| After treatment | 72.67 ± 5.13 a/* | 33.26 ± 2.35 a | 3.63 ± 0.26 a/* | 2.86 ± 0.20 a | |
| After washing out | 5.11 ± 0.36 b/* | 3.85 ± 0.27 b | 2.55 ± 0.18 b/* | 1.89 ± 0.13 b | |
| Thr | Before treatment | 0.79 ± 0.01 c/* | 0.52 ± 0.01 b | 0.12 ± 0.01 c | 0.17 ± 0.01 b/* |
| After treatment | 80.43 ± 0.53 a/* | 11.33 ± 0.07 a | 0.32 ± 0.01 a | 0.52 ± 0.01 a/* | |
| After washing out | 2.78 ± 0.02 b/* | 0.26 ± 0.01 c | 0.16 ± 0.01 b/* | 0.11 ± 0.01 c | |
| Ser | Before treatment | 1.09 ± 0.05 b/* | 0.69 ± 0.03 b | 0.40 ± 0.02 b/* | 0.20 ± 0.01 b |
| After treatment | 15.14 ± 0.76 a/* | 4.41 ± 0.22 a | 0.64 ± 0.03 a | 0.74 ± 0.04 a | |
| After washing out | 1.20 ± 0.06 b/* | 0.75 ± 0.04 b | 0.22 ± 0.01 c/* | 0.15 ± 0.01 b | |
| Glu | Before treatment | 220.41 ± 36.13 b | 180.89 ± 29.65 b | 8.54 ± 1.36 b | 9.26 ± 1.52 b |
| After treatment | 497.21 ± 81.50 a | 488.63 ± 80.10 a | 23.50 ± 3.85 a | 23.62 ± 3.87 a | |
| After washing out | 205.78 ± 33.73 b | 185.34 ± 30.38 b | 8.78 ± 1.44 b | 7.86 ± 1.29 b | |
| Gly | Before treatment | 78.36 ± 2.24 c/* | 65.54 ± 1.87 b | 0.16 ± 0.01 b | 0.15 ± 0.01 c |
| After treatment | 147.59 ± 4.22 a/* | 142.98 ± 4.08 a | 12.93 ± 0.37 a/* | 1.41 ± 0.04 a | |
| After washing out | 92.11 ± 2.63 b/* | 38.31 ± 1.09 c | 0.17 ± 0.01 b | 0.29 ± 0.01 b/* | |
| Ala | Before treatment | 7.95 ± 0.29 b/* | 5.08 ± 0.18 b | 1.24 ± 0.05 b/* | 0.66 ± 0.02 b |
| After treatment | 166.07 ± 5.97 a/* | 113.19 ± 4.06 a | 43.14 ± 1.55 a/* | 2.91 ± 0.10 a | |
| After washing out | 7.40 ± 0.27 b/* | 1.51 ± 0.05 c | 1.18 ± 0.04 b/* | 0.45 ± 0.02 c | |
| Val | Before treatment | 12.29 ± 0.67 b | 15.30 ± 0.84 b/* | 0.31 ± 0.20 b | 0.34 ± 0.02 b |
| After treatment | 117.78 ± 6.45 a/* | 80.75 ± 4.42 a | 5.09 ± 0.29 a/* | 1.55 ± 0.09 a | |
| After washing out | 8.71 ± 0.48 b | 9.25 ± 0.50 c | 0.34 ± 0.02 b/* | 0.02 ± 0.01 c | |
| Cys | Before treatment | 5.85 ± 0.90 b | 8.09 ± 1.24 b | 0.27 ± 0.04 b | 0.63 ± 0.10 b/* |
| After treatment | 33.50 ± 5.14 a/* | 13.61 ± 2.09 a | 1.56 ± 0.24 a | 1.81 ± 0.28 a | |
| After washing out | 0.61 ± 0.09 c | 3.14 ± 0.48 c/* | 0.54 ± 0.08 b | 0.53 ± 0.08 b | |
| Met | Before treatment | 3.03 ± 0.78 b | 2.72 ± 0.29 b | 0.16 ± 0.01 b | 0.15 ± 0.01 a |
| After treatment | 9.87 ± 0.61 a | 10.64 ± 0.65 a | 5.92 ± 0.36 a/* | 0.16 ± 0.01 a | |
| After washing out | 0.72 ± 0.18 c | 0.62 ± 0.21 c | 0.12 ± 0.01 b | 0.11 ± 0.01 b | |
| Ile | Before treatment | 8.50 ± 0.52 b | 10.44 ± 0.63 b/* | 2.63 ± 0.18 b | 2.57 ± 0.16 b |
| After treatment | 76.70 ± 4.66 a/* | 38.36 ± 2.33 a | 5.47 ± 0.33 a/* | 3.30 ± 0.20 a | |
| After washing out | 6.19 ± 0.38 b | 6.40 ± 0.39 c | 2.26 ± 0.14 b/* | 0.01 ± 0.01 c | |
| Leu | Before treatment | 5.79 ± 0.36 b | 6.87 ± 0.43 b | 1.82 ± 0.13 b/* | 0.89 ± 0.06 b |
| After treatment | 73.69 ± 4.58 a/* | 58.89 ± 3.66 a | 2.91 ± 0.18 a/* | 1.61 ± 0.10 a | |
| After washing out | 4.45 ± 0.28 b/* | 3.45 ± 0.21 c | 1.27 ± 0.08 c/* | 0.01 ± 0.01 c | |
| Tyr | Before treatment | 4.69 ± 0.34 b/* | 1.63 ± 0.12 b | 0.81 ± 0.07 b/* | 0.52 ± 0.04 b |
| After treatment | 53.20 ± 3.86 a/* | 14.14 ± 1.03 a | 10.25 ± 0.74 a/* | 2.36 ± 0.17 a | |
| After washing out | 6.84 ± 0.50 b/* | 1.45 ± 0.11 b | 0.64 ± 0.05 b/* | 0.34 ± 0.02 b | |
| Phe | Before treatment | 6.07 ± 0.43 c/* | 1.78 ± 0.13 b | 1.46 ± 0.12 b/* | 1.03 ± 0.07 b |
| After treatment | 134.13 ± 9.54 a/* | 44.27 ± 3.15 a | 75.52 ± 5.37 a/* | 6.10 ± 0.43 a | |
| After washing out | 10.62 ± 0.76 b/* | 0.99 ± 0.07 b | 0.99 ± 0.07 b | 0.83 ± 0.06 b | |
| GABA | Before treatment | 5.91 ± 0.04 a/* | 4.14 ± 0.03 c | 3.93 ± 0.04 b | 4.64 ± 0.03 b/* |
| After treatment | 5.82 ± 0.04 a | 10.93 ± 0.08 a/* | 8.72 ± 0.06 a/* | 8.43 ± 0.06 a | |
| After washing out | 4.83 ± 0.03 b/* | 4.68 ± 0.03 b | 2.01 ± 0.01 c/* | 1.78 ± 0.01 c | |
| Ammonia | Before treatment | 387.23 ± 11.97 a | 369.68 ± 25.86 a | 597.77 ± 41.82 b | 632.95 ± 44.28 b |
| After treatment | 287.21 ± 20.09 b | 408.31 ± 28.56 a/* | 779.79 ± 54.55 a | 811.31 ± 56.76 a | |
| After washing out | 361.28 ± 25.28 a | 422.16 ± 29.53 a | 606.98 ± 42.46 b | 688.60 ± 48.17 b | |
| Orn | Before treatment | 1.43 ± 0.09 c | 3.96 ± 0.25 b/* | 1.16 ± 0.08 b/* | 0.51 ± 0.03 c |
| After treatment | 6.27 ± 0.40 a | 25.76 ± 1.66 a/* | 2.14 ± 0.14 a | 5.55 ± 0.36 a/* | |
| After washing out | 2.29 ± 0.15 b | 3.59 ± 0.23 b/* | 0.46 ± 0.03 c | 1.31 ± 0.08 b/* | |
| Lys | Before treatment | 8.62 ± 0.70 c | 33.24 ± 2.73 b/* | 1.66 ± 0.15 a | 1.41 ± 0.12 b |
| After treatment | 23.64 ± 1.94 a | 70.59 ± 5.80 a/* | 1.94 ± 0.16 a | 2.44 ± 0.20 a | |
| After washing out | 13.55 ± 1.11 b | 30.73 ± 2.52 b/* | 1.60 ± 0.13 a | 1.62 ± 0.13 b | |
| His | Before treatment | 29.25 ± 5.74 b | 23.55 ± 4.62 b | 0.12 ± 0.01 a | 0.18 ± 0.01 a |
| After treatment | 44.96 ± 8.82 a | 47.48 ± 9.32 a | 0.17 ± 0.03 a | 0.17 ± 0.03 a | |
| After washing out | 23.27 ± 4.57 b | 23.73 ± 4.66 b | 0.13 ± 0.03 a | 0.07 ± 0.01 b | |
| Trp | Before treatment | 14.21 ± 1.47 b/* | 7.17 ± 0.74 c | 1.14 ± 0.21 b/* | 0.47 ± 0.04 b |
| After treatment | 35.30 ± 3.65 a | 36.43 ± 3.76 a | 2.31 ± 0.24 a/* | 1.07 ± 0.11 a | |
| After washing out | 15.31 ± 1.58 b | 13.27 ± 1.37 b | 0.82 ± 0.09 b/* | 0.48 ± 0.05 b | |
| Arg | Before treatment | 1.60 ± 0.17 b | 3.46 ± 0.36 c/* | NF | NF |
| After treatment | 13.06 ± 1.34 a | 19.85 ± 2.04 a/* | 0.93 ± 0.09 a | 1.51 ± 0.16 a/* | |
| After washing out | 2.92 ± 0.30 b | 6.62 ± 0.68 b/* | 0.01 ± 0.01 b | 0.32 ± 0.03 b/* | |
| Pro | Before treatment | 1.35 ± 0.05 b/* | 0.93 ± 0.03 c | 0.90 ± 0.03 b | 0.98 ± 0.03 b |
| After treatment | 12.90 ± 0.47 a/* | 2.02 ± 0.07 a | 1.76 ± 0.06 a/* | 1.44 ± 0.05 a | |
| After washing out | 0.82 ± 0.03 b | 1.47 ± 0.05 b/* | 0.86 ± 0.03 b/* | 0.77 ± 0.03 c | |
| Total | Before treatment | 807.87 ± 19.35 b | 748.57 ± 45.42 b | 626.51 ± 42.33 b | 659.55 ± 43.58 b |
| After treatment | 1907.15 ± 24.32 a/* | 1675.85 ± 21.01 a | 988.65 ± 58.68 a | 880.88 ± 55.18 a | |
| After washing out | 776.82 ± 25.24 b | 761.57 ± 10.18 b | 632.11 ± 43.10 b | 707.52 ± 48.87 b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koirala, P.; Costantini, A.; Maina, H.N.; Rizzello, C.G.; Verni, M.; Beni, V.D.; Polo, A.; Katina, K.; Cagno, R.D.; Coda, R. Fermented Brewers’ Spent Grain Containing Dextran and Oligosaccharides as Ingredient for Composite Wheat Bread and Its Impact on Gut Metabolome In Vitro. Fermentation 2022, 8, 487. https://doi.org/10.3390/fermentation8100487
Koirala P, Costantini A, Maina HN, Rizzello CG, Verni M, Beni VD, Polo A, Katina K, Cagno RD, Coda R. Fermented Brewers’ Spent Grain Containing Dextran and Oligosaccharides as Ingredient for Composite Wheat Bread and Its Impact on Gut Metabolome In Vitro. Fermentation. 2022; 8(10):487. https://doi.org/10.3390/fermentation8100487
Chicago/Turabian StyleKoirala, Prabin, Alice Costantini, Henry N. Maina, Carlo Giuseppe Rizzello, Michela Verni, Valentina De Beni, Andrea Polo, Kati Katina, Raffaella Di Cagno, and Rossana Coda. 2022. "Fermented Brewers’ Spent Grain Containing Dextran and Oligosaccharides as Ingredient for Composite Wheat Bread and Its Impact on Gut Metabolome In Vitro" Fermentation 8, no. 10: 487. https://doi.org/10.3390/fermentation8100487
APA StyleKoirala, P., Costantini, A., Maina, H. N., Rizzello, C. G., Verni, M., Beni, V. D., Polo, A., Katina, K., Cagno, R. D., & Coda, R. (2022). Fermented Brewers’ Spent Grain Containing Dextran and Oligosaccharides as Ingredient for Composite Wheat Bread and Its Impact on Gut Metabolome In Vitro. Fermentation, 8(10), 487. https://doi.org/10.3390/fermentation8100487