Thermosonication of Broccoli Florets Prior to Fermentation Increases Bioactive Components in Fermented Broccoli Puree
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Thermal and Thermosonication Pre-Treatments
2.3. Fermentation Starter Culture Preparation
2.4. Fermentation
2.5. Determination of pH and Titratable Acidity
2.6. Microbiological Analysis
2.7. Glucoraphanin Analysis
2.8. Sulforaphane Analysis
2.8.1. Extraction
2.8.2. Solid Phase Extraction
2.8.3. UPLC Analysis
2.9. Total Phenolic Content
2.10. Data Analysis
3. Results and Discussion
3.1. pH and Titratable Acidity
3.1.1. Prior to Fermentation
3.1.2. Effects on Fermentation
3.2. Microbial Analysis
3.2.1. Prior to Fermentation
3.2.2. After Fermentation
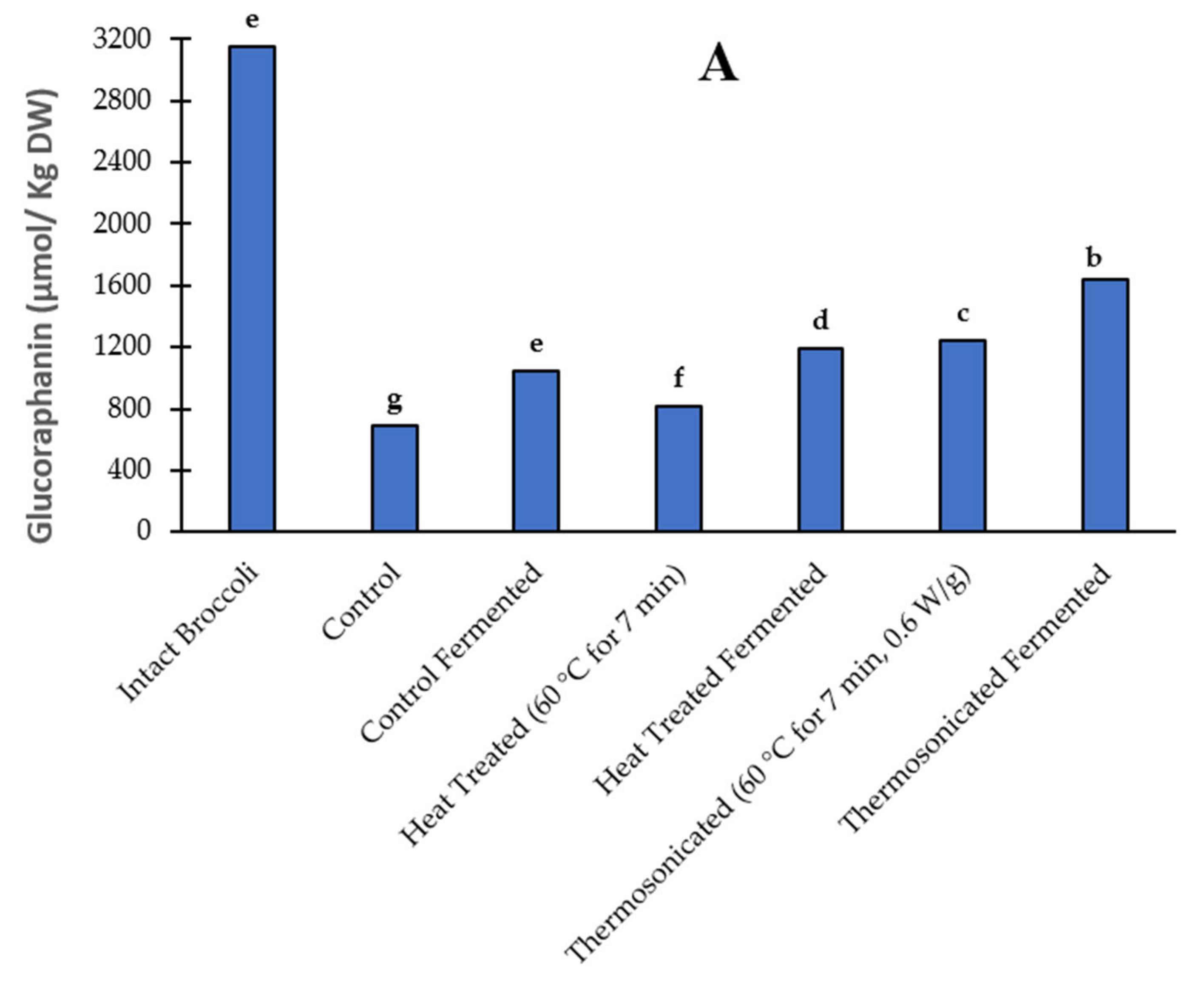
3.3. Glucoraphanin Content
3.3.1. Prior to Fermentation
3.3.2. Post Fermentation
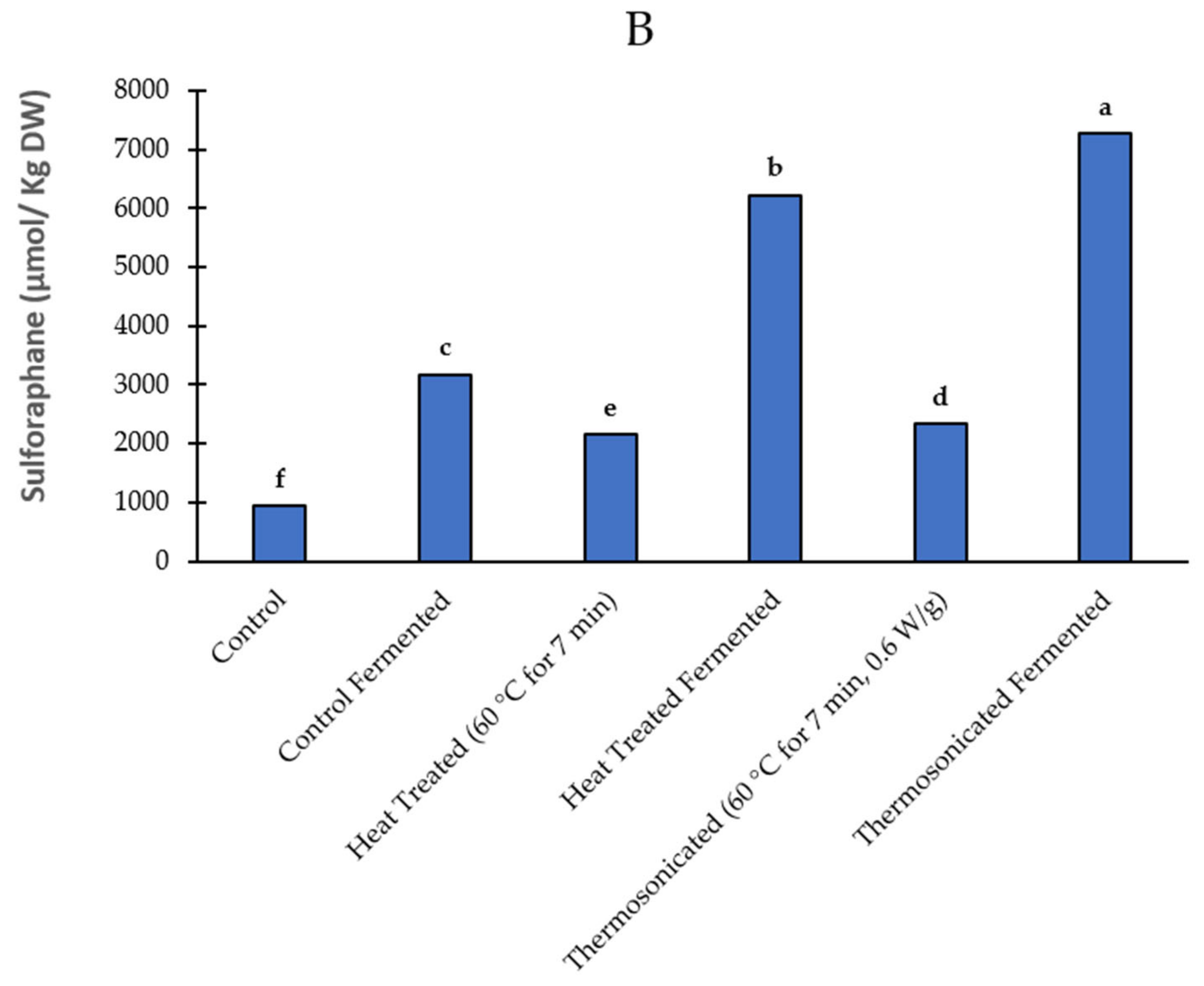
3.4. Sulforaphane Yield
3.4.1. Prior to Fermentation
3.4.2. Post Fermentation
3.5. Total Phenolic Content
3.5.1. Prior to Fermentation
3.5.2. After Fermentation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Terefe, N.S.; Augustin, M.A. Fermentation for tailoring the technological and health related functionality of food products. Crit. Rev. Food Sci. Nutr. 2020, 60, 2887–2913. [Google Scholar] [CrossRef] [PubMed]
- Wuyts, S.; Van Beeck, W.; Allonsius, C.N.; van den Broek, M.F.; Lebeer, S. Applications of plant-based fermented foods and their microbes. Curr. Opin. Biotechnol. 2020, 61, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, L.; Picchi, V.; Tribulato, A.; Cavallaro, C.; Lo Scalzo, R.; Branca, F. The effect of the germination temperature on the phytochemical content of broccoli and rocket sprouts. Int. J. Food Sci. Nutr. 2017, 68, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Pérez, C.; Barrientos, H.; Román, J.; Mahn, A. Optimization of a blanching step to maximize sulforaphane synthesis in broccoli florets. Food Chem. 2014, 145, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Radošević, K.; Srček, V.G.; Bubalo, M.C.; Brnčić, S.R.; Takács, K.; Redovniković, I.R. Assessment of glucosinolates, antioxidative and antiproliferative activity of broccoli and collard extracts. J. Food Compos. Anal. 2017, 61, 59–66. [Google Scholar] [CrossRef]
- Latte, K.P.; Appel, K.E.; Lampen, A. Health benefits and possible risks of broccoli—An overview. Food Chem. Toxicol. 2011, 49, 3287–3309. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Kelleher, M.O.; Eggleston, I.M. The cancer chemopreventive actions of phytochemicals derived from glucosinolates. Eur. J. Nutr. 2008, 47, 73–88. [Google Scholar] [CrossRef]
- Bayat Mokhtari, R.; Baluch, N.; Homayouni, T.S.; Morgatskaya, E.; Kumar, S.; Kazemi, P.; Yeger, H. The role of Sulforaphane in cancer chemoprevention and health benefits: A mini-review. J. Cell. Commun. Signal. 2018, 12, 91–101. [Google Scholar] [CrossRef]
- Matusheski, N.V.; Juvik, J.A.; Jeffery, E.H. Heating decreases epithiospecifier protein activity and increases sulforaphane formation in broccoli. Phytochemistry 2004, 65, 1273–1281. [Google Scholar] [CrossRef]
- Van Eylen, D.; Bellostas, N.; Strobel, B.W.; Oey, I.; Hendrickx, M.; Van Loey, A.; Sørensen, H.; Sørensen, J.C. Influence of pressure/temperature treatments on glucosinolate conversion in broccoli (Brassica oleraceae L. cv Italica) heads. Food Chem. 2009, 112, 646–653. [Google Scholar] [CrossRef]
- Cai, Y.X.; Wang, J.H.; McAuley, C.; Augustin, M.A.; Terefe, N.S. Fermentation for enhancing the bioconversion of glucoraphanin into sulforaphane and improve the functional attributes of broccoli puree. J. Funct. Foods 2019, 61, 103461. [Google Scholar] [CrossRef]
- Cai, Y.X.; Augustin, M.A.; Jegasothy, H.; Wang, J.H.; Terefe, N.S. Mild heat combined with lactic acid fermentation: A novel approach for enhancing sulforaphane yield in broccoli puree. Food Funct. 2020, 11, 779–786. [Google Scholar] [CrossRef]
- Shokri, S.; Jegasothy, H.; Augustin, M.A.; Terefe, N.S. Thermosonication for the Production of Sulforaphane Rich Broccoli Ingredients. Biomolecules 2021, 11, 321. [Google Scholar] [CrossRef]
- Shokri, S.; Terefe, N.S.; Manzari, M. Advances in Food Fermentation: Potential Application of Novel Processing Technologies for Enhancing Fermentation Kinetics and Product Yield. In Innovative Food Processing Technologies: A Comprehensive Review; Elsevier: Amsterdam, The Netherlands, 2021; pp. 135–156. [Google Scholar] [CrossRef]
- Han, D.; Row, K.H. Separation and purification of sulforaphane from broccoli by solid phase extraction. Int. J. Mol. Sci. 2011, 12, 1854–1861. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Del Socorro Cruz-Cansino, N.; Ramírez-Moreno, E.; León-Rivera, J.E.; Delgado-Olivares, L.; Alanís-García, E.; Ariza-Ortega, J.A.; de Jesús Manríquez-Torres, J.; Jaramillo-Bustos, D.P. Shelf life, physicochemical, microbiological and antioxidant properties of purple cactus pear (Opuntia ficus indica) juice after thermoultrasound treatment. Ultrason. Sonochem. 2015, 27, 277–286. [Google Scholar] [CrossRef]
- Alves, L.D.L.; dos Santos, R.L.; Bayer, B.L.; Devens, A.L.M.; Cichoski, A.J.; Mendonça, C.R.B. Thermosonication of tangerine juice: Effects on quality characteristics, bioactive compounds, and antioxidant activity. J. Food Processing Preserv. 2020, 44, e14914. [Google Scholar] [CrossRef]
- Do Amaral Souza, F.D.C.; Moura, L.G.S.; de Oliveira Bezerra, K.; Aguiar, J.P.L.; Mar, J.M.; Sanches, E.A.; Dos Santos, F.F.; Bakry, A.M.; Paulino, B.N.; Campelo, P.H. Thermosonication applied on camu–camu nectars processing: Effect on bioactive compounds and quality parameters. Food Bioprod. Processing 2019, 116, 212–218. [Google Scholar] [CrossRef]
- Yu, Z.; Su, Y.; Zhang, Y.; Zhu, P.; Mei, Z.; Zhou, X.; Yu, H. Potential use of ultrasound to promote fermentation, maturation, and properties of fermented foods: A review. Food Chem. 2021, 357, 129805. [Google Scholar] [CrossRef]
- Shokri, S.; Terefe, N.S.; Shekarforoush, S.S.; Hosseinzadeh, S. Ultrasound-assisted fermentation for enhancing metabolic and probiotic activities of Lactobacillus brevis. Chem. Eng. Processing 2021, 166, 108470. [Google Scholar] [CrossRef]
- Alcántara-Zavala, A.E.; de Dios Figueroa-Cárdenas, J.; Pérez-Robles, J.F.; Arámbula-Villa, G.; Miranda-Castilleja, D.E. Thermosonication as an alternative method for processing, extending the shelf life, and conserving the quality of pulque: A non-dairy Mexican fermented beverage. Ultrason. Sonochem. 2021, 70, 105290. [Google Scholar] [CrossRef]
- Alvarado-Morales, G.; Minjares-Fuentes, R.; Contreras-Esquivel, J.C.; Montañez, J.; Meza-Velázquez, J.A.; Femenia, A. Application of thermosonication for Aloe vera (Aloe barbadensis Miller) juice processing: Impact on the functional properties and the main bioactive polysaccharides. Ultrason. Sonochem. 2019, 56, 125–133. [Google Scholar] [CrossRef]
- Shokri, S.; Shekarforoush, S.S.; Hosseinzadeh, S. Efficacy of low intensity ultrasound on fermentative activity intensification and growth kinetic of Leuconostoc mesenteroides. Chem. Eng. Processing 2020, 153, 107955. [Google Scholar] [CrossRef]
- Shokri, S.; Shekarforoush, S.S.; Hosseinzadeh, S. Stimulatory effects of low intensity ultrasound on the growth kinetics and metabolic activity of Lactococcus lactis subsp. Lactis. Process Biochem. 2020, 89, 1–8. [Google Scholar] [CrossRef]
- Di Cagno, R.; Coda, R.; De Angelis, M.; Gobbetti, M. Exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiol. 2013, 33, 1–10. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; Velázquez-Estrada, R.M.; Roig, A.X.; García-Galindo, H.S.; Sayago-Ayerdi, S.G.; Montalvo-González, E. Thermosonication: An alternative processing for fruit and vegetable juices. Trends Food Sci. Technol. 2017, 61, 26–37. [Google Scholar] [CrossRef]
- Aguilar-Camacho, M.; Welti-Chanes, J.; Jacobo-Velázquez, D.A. Combined effect of ultrasound treatment and exogenous phytohormones on the accumulation of bioactive compounds in broccoli florets. Ultrason. Sonochem. 2019, 50, 289–301. [Google Scholar] [CrossRef]
- Pongmalai, P.; Devahastin, S.; Chiewchan, N.; Soponronnarit, S. Enhancing the recovery of cabbage glucoraphanin through the monitoring of sulforaphane content and myrosinase activity during extraction by different methods. Sep. Purif. Technol. 2017, 174, 338–344. [Google Scholar] [CrossRef]
- Ye, J.H.; Huang, L.Y.; Terefe, N.S.; Augustin, M.A. Fermentation-based biotransformation of glucosinolates, phenolics and sugars in retorted broccoli puree by lactic acid bacteria. Food Chem. 2019, 286, 616–623. [Google Scholar] [CrossRef]
- Claus, H. Extra cellular enzymes and peptides of lactic acid bacteria: Significance for vinification. Dtsch. Lebensm.-Rundsch. 2007, 103, 505–511. [Google Scholar]
- Sarvan, I.; Kramer, E.; Bouwmeester, H.; Dekker, M.; Verkerk, R. Sulforaphane formation and bioaccessibility are more affected by steaming time than meal composition during in vitro digestion of broccoli. Food Chem. 2017, 214, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.B.; Frisina, C.L.; Winkler, S.; Imsic, M.; Tomkins, R.B. Cooking method significantly effects glucosinolate content and sulforaphane production in broccoli florets. Food Chem. 2010, 123, 237–242. [Google Scholar] [CrossRef]
- Ghawi, S.K.; Methven, L.; Niranjan, K. The potential to intensify sulforaphane formation in cooked broccoli (Brassica oleracea var. italica) using mustard seeds (Sinapis alba). Food Chem. 2013, 138, 1734–1741. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, J.; Liu, L.; Awais, M.; Xiao, T.; Wang, L.; Zhou, X.; Tong, L.T.; Zhou, S. Effect of thermosonication pre-treatment on mung bean (Vigna radiata) and white kidney bean (Phaseolus vulgaris) proteins: Enzymatic hydrolysis, cholesterol lowering activity and structural characterization. Ultrason. Sonochem. 2020, 66, 105121. [Google Scholar] [CrossRef]
- Garcia, C.; Guerin, M.; Souidi, K.; Remize, F. Lactic fermented fruit or vegetable juices: Past, present and future. Beverages 2020, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Cheng, D.L.; Hashimoto, K.; Uda, Y. In vitro digestion of sinigrin and glucotropaeolin by single strains of Bifidobacterium and identification of the digestive products. Food Chem. Toxicol. 2004, 42, 351–357. [Google Scholar] [CrossRef]
- Palop, M.L.; Smiths, J.P.; ten Brink, B. Degradation of sinigrin by Lactobacillus agilis strain R16. Int. J. Food Microbiol. 1995, 26, 219–229. [Google Scholar] [CrossRef]
- Nugon-Baudon, L.; Rabot, S.; Wal, J.M.; Szylit, O. Interactions of the intestinal microflora with glucosinolates in rapeseed meal toxicity: First evidence of an intestinal lactobacillus possessing a myrosinase-like activity in vivo. J. Sci. Food Agric. 1990, 52, 547–559. [Google Scholar] [CrossRef]
- Peñas, E.; Pihlava, J.M.; Vidal-Valverde, C.; Frías, J. Influence of fermentation conditions of Brassica oleracea L. var. capitata on the volatile glucosinolate hydrolysis compounds of sauerkrauts. LWT-Food Sci. Technol. 2012, 48, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.Y.; Ediriweera, M.K.; Boo, K.H.; Kim, C.S.; Cho, S.K. Effects of Cooking and Processing Methods on Phenolic Contents and Antioxidant and Anti-Proliferative Activities of Broccoli Florets. Antioxidants 2021, 10, 641. [Google Scholar] [CrossRef]
- Turkmen, N.; Sari, F.; Velioglu, Y.S. The effect of cooking methods on total phenolics and antioxidant activity of selected green vegetables. Food Chem. 2005, 93, 713–718. [Google Scholar] [CrossRef]
- Gliszczyńska-Świgło, A.; Ciska, E.; Pawlak-Lemańska, K.; Chmielewski, J.; Borkowski, T.; Tyrakowska, B. Changes in the content of health-promoting compounds and antioxidant activity of broccoli after domestic processing. Food Addit. Contam. 2006, 23, 1088–1098. [Google Scholar] [CrossRef]
- Şengül, M.; Yildiz, H.; Kavaz, A. The effect of cooking on total polyphenolic content and antioxidant activity of selected vegetables. Int. J. Food Prop. 2014, 17, 481–490. [Google Scholar] [CrossRef]
- Zafra-Rojas, Q.Y.; Cruz-Cansino, N.; Ramírez-Moreno, E.; Delgado-Olivares, L.; Villanueva-Sánchez, J.; Alanís-García, E. Effects of ultrasound treatment in purple cactus pear (Opuntia ficus-indica) juice. Ultrason. Sonochem. 2013, 20, 1283–1288. [Google Scholar] [CrossRef]
- Aadil, R.M.; Khalil, A.A.; Rehman, A.; Khalid, A.; Inam-ur-Raheem, M.; Karim, A.; Gill, A.A.; Abid, M.; Afraz, M.T. Assessing the impact of ultra-sonication and thermo-ultrasound on antioxidant indices and polyphenolic profile of apple-grape juice blend. J. Food Processing Preserv. 2020, 44, e14406. [Google Scholar] [CrossRef]
- Escarpa, A.; González, M. Approach to the content of total extractable phenolic compounds from different food samples by comparison of chromatographic and spectrophotometric methods. Anal. Chim. Acta 2001, 427, 119–127. [Google Scholar] [CrossRef]
- Mason, T.J.; Paniwnyk, L.; Lorimer, J.P. The uses of ultrasound in food technology. Ultrason. Sonochem. 1996, 3, S253–S260. [Google Scholar] [CrossRef]
- Filannino, P.; Bai, Y.; Di Cagno, R.; Gobbetti, M.; Gänzle, M.G. Metabolism of phenolic compounds by Lactobacillus spp. during fermentation of cherry juice and broccoli puree. Food Microbiol. 2015, 46, 272–279. [Google Scholar] [CrossRef]
- De la Fuente, B.; Luz, C.; Puchol, C.; Meca, G.; Barba, F.J. Evaluation of fermentation assisted by Lactobacillus brevis POM, and Lactobacillus plantarum (TR-7, TR-71, TR-14) on antioxidant compounds and organic acids of an orange juice-milk based beverage. Food Chem. 2021, 343, 128414. [Google Scholar] [CrossRef]
- Matthews, A.; Grimaldi, A.; Walker, M.; Bartowsky, E.; Grbin, P.; Jiranek, V. Lactic acid bacteria as a potential source of enzymes for use in vinification. Appl. Environ. Microbiol. 2004, 70, 5715–5731. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Shah, N.P. Changes in antioxidant capacity, isoflavone profile, phenolic and vitamin contents in soymilk during extended fermentation. LWT-Food Sci. Technol. 2014, 58, 454–462. [Google Scholar] [CrossRef]
- Rodríguez, H.; Curiel, J.A.; Landete, J.M.; de las Rivas, B.; de Felipe, F.L.; Gómez-Cordovés, C.; Mancheño, J.M.; Muñoz, R. Food phenolics and lactic acid bacteria. Int. J. Food Microbiol. 2009, 132, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Kwaw, E.; Ma, Y.; Tchabo, W.; Apaliya, M.T.; Wu, M.; Sackey, A.S.; Xiao, L.; Tahir, H.E. Effect of lactobacillus strains on phenolic profile, color attributes and antioxidant activities of lactic-acid-fermented mulberry juice. Food Chem. 2018, 250, 148–154. [Google Scholar] [CrossRef]

| pH | Acidity (g/L) | ||
| Before fermentation | Control | 6.84 ± 0.02 a | 4.30 ± 0.12 a |
| Thermal pre-treated | 6.76 ± 0.05 a | 4.18 ± 0.02 a | |
| TS pre-treated | 6.79 ± 0.03 a | 4.08 ± 0.10 a | |
| After fermentation | Control | 3.80 ± 0.01 b | 10.9 ± 0.27 b |
| Thermal pre-treated | 3.65 ± 0.02 c | 11.7 ± 0.59 c | |
| TS pre-treated | 3.87 ± 0.01 d | 13.1 ± 0.69 d | |
| Microbial Counts (Log CFU/mL) | ||||
|---|---|---|---|---|
| LAB | Enterobacteriaceae | Yeast and Mould | ||
| Before fermentation 1 | Control | 3.85 ± 0.04 a | 3.47 ± 0.04 a | 3.46 ± 0.02 a |
| Thermally pre-treated | 3.25 ± 0.03 b | 2.60 ± 0.09 b | 2.77 ± 0.13 b | |
| TS pre-treated | 2.81 ± 0.05 c | 2.26 ± 0.12 c | 3.42 ± 0.02 c | |
| After fermentation | Control | 7.99 ± 0.04 d | ND 2 | ND |
| Thermally pre-treated | 9.16 ± 0.02 e | ND | ND | |
| TS pre-treated | 9.45 ± 0.02 f | ND | ND | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shokri, S.; Jegasothy, H.; Hliang, M.M.; Augustin, M.A.; Terefe, N.S. Thermosonication of Broccoli Florets Prior to Fermentation Increases Bioactive Components in Fermented Broccoli Puree. Fermentation 2022, 8, 236. https://doi.org/10.3390/fermentation8050236
Shokri S, Jegasothy H, Hliang MM, Augustin MA, Terefe NS. Thermosonication of Broccoli Florets Prior to Fermentation Increases Bioactive Components in Fermented Broccoli Puree. Fermentation. 2022; 8(5):236. https://doi.org/10.3390/fermentation8050236
Chicago/Turabian StyleShokri, Sajad, Hema Jegasothy, Mya Myintzu Hliang, Mary Ann Augustin, and Netsanet Shiferaw Terefe. 2022. "Thermosonication of Broccoli Florets Prior to Fermentation Increases Bioactive Components in Fermented Broccoli Puree" Fermentation 8, no. 5: 236. https://doi.org/10.3390/fermentation8050236
APA StyleShokri, S., Jegasothy, H., Hliang, M. M., Augustin, M. A., & Terefe, N. S. (2022). Thermosonication of Broccoli Florets Prior to Fermentation Increases Bioactive Components in Fermented Broccoli Puree. Fermentation, 8(5), 236. https://doi.org/10.3390/fermentation8050236







